Effects of Different Materials on Biogas Production during Anaerobic Digestion of Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Substrates and Inoculum
2.2. Synthesis of Powders
2.3. Biogas—Methane Potential Experiments
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saravanan, A.P.; Mathimani, T.; Deviram, G.; Rajendran, K.; Pugazhendhi, A. Biofuel policy in India: A review of policy barriers in sustainable marketing of biofuel. J. Clean. Prod. 2018, 193, 734–747. [Google Scholar] [CrossRef]
- Hussein, A.Κ. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Miltner, M.; Makaruk, A.; Harasek, M. Review on available biogas upgrading technologies and innovations towards advanced solutions. J. Clean. Prod. 2017, 161, 1329–1337. [Google Scholar] [CrossRef]
- Hobbs, S.R.; Landis, A.E.; Rittmann, B.E.; Young, M.N.; Parameswaran, P. Enhancing anaerobic digestion of food waste through biochemical methane potential assays at different substrate: Inoculum ratios. Waste Manag. 2018, 71, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liu, G.; Xue, L.i.; Zuo, J.; Chen, T.; Vuppaladadiyam, A.; Duan, H. Anaerobic digestion based waste-to-energy technologies can halve the climate impact of China’s fast-growing food waste by 2040. J. Clean. Prod. 2020, 277, 123490. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Z.; Fu, L.; Han, Y.; Zheng, W.; Yu, F.; Chen, H.; Feng, L.U.; Li, Y.; Ping, W. Novel anaerobic digestion and carbon dioxide emissions efficiency analysis of food waste treatment based on SBM-DEA model. J. Clean. Prod. 2021, 328, 129591. [Google Scholar] [CrossRef]
- Munesue, Y.; Masui, T.; Fushima, T. The effects of reducing food losses and food waste on global food insecurity, natural resources, and greenhouse gas emissions. Environ. Econ. Policy Stud. 2015, 17, 43–77. [Google Scholar] [CrossRef]
- Deena, S.R.; Vickram, A.S.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Ravindran, B.; Chang, S.W.; Awasthi, M.K. Enhanced biogas production from food waste and activated sludge using advanced techniques—A review. Bioresour. Technol. 2022, 355, 127234. [Google Scholar] [CrossRef]
- Li, L.; Xua, Y.; Dai, X.; Dai, L. Principles and advancements in improving anaerobic digestion of organic waste via direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 148, 111367. [Google Scholar] [CrossRef]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef] [Green Version]
- Harirchi, S.; Wainaina, S.; Sar, T.; Nojoumi, S.A.; Parchami, M.; Parchami, M.; Varjani, S.; Khanal, S.K.; Wong, J.; Awasthi, M.K.; et al. Microbiological insights into anaerobic digestion for biogas, hydrogen or volatile fatty acids (VFAs): A review. Bioengineered 2022, 13, 6521–6557. [Google Scholar] [CrossRef]
- Ueki, T.; Nevin, K.P.; Rotaru, A.E.; Wang, L.Y.; Ward, J.E.; Woodard, T.L.; Lovley, D.R. Geobacter Strains Expressing Poorly Conductive Pili Reveal Constraints on Direct Interspecies Electron Transfer Mechanisms. mBio 2018, 9, e01273-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Tan, G.A.; Aslam, M.; Kim, J.; Lee, P.H. Metatranscriptomic evidence for classical and RuBisCO-mediated CO2 reduction to methane facilitated by direct interspecies electron transfer in a methanogenic system. Sci. Rep. 2019, 9, 4116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, R.; Nobu, M.K.; Narihiro, T.; Yu, J.; Sathyagal, A.; Willman, E.; Liu, W.-T. Novel Geobacter species and diverse methanogens contribute to enhanced methane production in media-added methanogenic reactors. Water Res. 2018, 147, 403–412. [Google Scholar] [CrossRef]
- Chen, L.; Fang, W.; Chang, J.; Liang, J.; Zhang, P.; Zhang, G. Improvement of Direct Interspecies Electron Transfer via Adding Conductive Materials in Anaerobic Digestion: Mechanisms, Performances, and Challenges. Front. Microbiol. 2022, 13, 860749. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, D.H.; Yun, M. Effect of operation temperature on anaerobic digestion of food waste: Performance and microbial analysis. Fuel 2017, 209, 598–605. [Google Scholar] [CrossRef]
- Mohammed, T.A.; Fodah, A.A.E.M. Utilization of nanoparticles for biogas production focusing on process stability and effluent quality. Appl. Sci. 2022, 4, 332. [Google Scholar]
- Romero-Güiza, M.S.; Mata-Alvarez, J.; Chimenos Rivera, J.M.; Astals Garcia, S. Nutrient recovery technologies for anaerobic digestion systems: An overview. Revista ION 2016, 29, 7–26. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, Z.; Zhang, Y.; Xiang, Y.; Xu, R.; Jia, M.; Cao, J. Effects of different conductive nanomaterials on anaerobic digestion process and microbial community of sludge Weiping Xiong. Bioresour. Technol. 2020, 304, 123016. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Chen, Y.; Dai, X. Effects of metal nanoparticles on methane production from waste-activated sludge and microorganism community shift in anaerobic granular sludge. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Baniameriana, H.; Tsapekos, P.; Alvarado-Morales, M.; Kasama, T.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Effect of metal oxide based TiO2 nanoparticles on anaerobic digestion process of lignocellulosic substrate. Energy 2020, 191, 116580. [Google Scholar] [CrossRef]
- Baniamerian, H.; Ghofrani Isfahani, P.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of nano-structured materials in anaerobic digestion: Current status and perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Shi, X.; Guo, G.; Zhao, A.; Zhao, Y. Stabilization of sewage sludge in the presence of nanoscale zero-valent iron (nZVI): Abatement of odor and improvement of biogas production. J. Mater. Cycles Waste Manag. 2013, 15, 461–468. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, S.; Chen, Y.; Zhou, F.; Yan, C. Diatomite coated with Fe2O3 as an efficient heterogeneous catalyst for degradation of organic pollutant. J. Taiwan Inst. Chem. Eng. 2015, 49, 105–112. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, L.; Chen, Y.; Su, Y.; Wan, R.; Liu, K.; Huang, H. Effects of titanium dioxide and zinc oxide nanoparticles on methane production from anaerobic co-digestion of primary and excess sludge. J. Environ. Sci. Health Part A 2015, 50, 913–921. [Google Scholar]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.; Zhou, J. Nano-graphene induced positive effects on methanogenesis digestion. Biosource Technol. 2017, 224, 41–47. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Eljamal, O.; Amen, T.W.M.; Sugihara, Y.; Matsunaga, N. Optimized nano-scale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem. Eng. J. 2017, 309, 349–365. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Comparison of nanoparticles effects on biogas and methane production from anaerobic digestion of cattle dung slurry. Renew. Energy 2016, 87, 592–598. [Google Scholar] [CrossRef]
- Kumar, A.N.; Dissanayake, P.D.; Masek, O.; Priya, A.; Ki Lin, C.S.; Ok, Y.S.; Kim, S.-H. Recent trends in biochar integration with anaerobic fermentation: Win-win strategies in a closed-loop. Renew. Sustain. Energy Rev. 2021, 149, 111371. [Google Scholar] [CrossRef]
- Baek, G.; Rossi, R.; Saikaly, P.E.; Logan, B.E. The impact of different types of high surface area brush fibers with different electrical conductivity and biocompatibility on the rates of methane generation in anaerobic digestion. Sci. Total Environ. 2021, 787, 147683. [Google Scholar] [CrossRef] [PubMed]
- Kenanakis, G.; Androulidaki, M.; Vernardou, D.; Katsarakis, N.; Koudoumas, E. Photoluminescence study of ZnO structures grown by aqueous chemical growth. Thin Solid Films 2011, 520, 1353–1357. [Google Scholar] [CrossRef]
- Vernardou, D.; Stratakis, E.; Kenanakis, G.; Yates, H.M.; Couris, S.; Pemble, M.E.; Koudoumas, E.; Katsarakis, N. One pot direct hydrothermal growth of photoactive TiO2 films on glass. J. Photochem. Photobiol. A Chem. 2009, 202, 81–85. [Google Scholar] [CrossRef]
- Jeyakumar, R.B.; Vincent, G.S. Recent advances and perspectives of nanotechnology in anaerobic digestion: A new paradigm towards sludge biodegradability. Sustainability 2022, 14, 7191. [Google Scholar] [CrossRef]
- Jin, S.E.; Jin, H.E. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef] [Green Version]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Orooji, Y. Facile synthesis of yttria-promoted nickel catalysts supported on MgO-MCM-41 for syngas production from greenhouse gases. Renew Sustain. Energy Rev. 2020, 134, 110130. [Google Scholar] [CrossRef]
- Orooji, Y.; Pakzad, K.; Nasrollahzadeh, M. Lignosulfonate valorization into a Cu containing magnetically recyclable photocatalyst for treating wastewater pollutants in aqueous media. Chemosphere 2022, 305, 135180. [Google Scholar] [CrossRef]
- Keyikoglu, R.; Khataee, A.; Lin, H.; Orooji, Y. Vanadium (V)-doped ZnFe layered double hydroxide for enhanced sonocatalytic degradation of pymetrozine. Chem. Eng. J. 2022, 434, 134730. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Amari, A.; Tahoon, M.A.; Alsaiari, N.S.; Ben, R.F. Removal of meloxicam, piroxicam and Cd+2 by Fe3O4/SiO2/glycidyl methacrylate-S-SH nanocomposite loaded with laccase. Alex. Eng. J. 2020, 59, 905–914. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: A review. J. Ind. Eng. Chem. 2022, 107, 20–30. [Google Scholar] [CrossRef]
- Jajuli, M.N.; Mohamed, N.; Suah, F.B.M. Electrochemical removal of cadmium from a sulphate solution using a three-dimensional electrode. Alex. Eng. J. 2020, 59, 4237–4245. [Google Scholar] [CrossRef]
- Yuan, S.; Fan, W.; Jin, Y.; Wang, D.; Liu, T. Free-standing flexible graphene-based aerogel film with high energy density as an electrode for supercapacitors. Nano Mater. Sci. 2021, 3, 68–74. [Google Scholar] [CrossRef]
- Pushparaj, K.; Liu, W.-C.; Meyyazhagan, A.; Orlacchio, A.; Pappusamy, M.; Vadivalagan, C.; Robert, A.A.; Arumugam, V.A.; Kamyab, H.; Klemeš, J.J.; et al. Nano- from nature to nurture: A comprehensive review on facets, trends, perspectives and sustainability of nanotechnology in the food sector. Energy 2022, 240, 122732. [Google Scholar] [CrossRef]
- Moshfegh, A.; Abouei Mehrizi, A.; Javadzadegan, A.; Joshaghani, M.; Ghasemi-Fare, O. Numerical investigation of various nanofluid heat transfers in microchannel under the effect of partial magnetic field: Lattice Boltzmann approach. J. Therm. Anal. Calorim. 2020, 140, 773–787. [Google Scholar] [CrossRef]
- Li, X.; Yuan, F.; Tian, W.; Dai, C.; Yang, X.; Wang, D.; Du, J.; Yu, W.; Yuan, H. Heat Transfer Enhancement of Nanofluids with Non-Spherical Nanoparticles: A Review. Appl. Sci. 2022, 12, 4767. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 2022, 291, 132928. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Fu, L.; Sanati, A.L.; Alizadeh, M.; Karaman, C.; Orooji, Y. Cyanazine herbicide monitoring as a hazardous substance by a DNA nanostructure biosensor. J. Hazard. Mater. 2022, 423, 127058. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, S.; Golikand, A.N.; ZareNezhad, B. Bimetallic-metal oxide nanoparticles of Pt-M (M: W, Mo, and V) supported on reduced graphene oxide (rGO): Radiolytic synthesis and methanol oxidation electrocatalysis. J. Nanostruct. Chem. 2021, 11, 287–299. [Google Scholar] [CrossRef]
- Sheikh-Mohseni, M.H.; Sedaghat, S.; Derakhshi, P.; Safekordi, A. Electrochemical activity of Ni-montmorillonite/Vulcan XC-72R carbon black nano-catalyst for the oxidation of methanol in acidic medium. J. Nanostruct. Chem. 2019, 9, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Orooji, Y.; Han, N.; Nezafat, Z.; Shafiei, N.; Shen, Z.; Nasrollahzadeh, M.; Karimi-Maleh, H.; Luque, R.; Bokhari, A.; Klemeš, J.J. Valorisation of nuts biowaste: Prospects in sustainable bio(nano)catalysts and environmental applications. J. Clean Prod. 2022, 347, 131220. [Google Scholar] [CrossRef]
- Ansarian, Z.; Khataee, A.; Arefi-Oskoui, S.; Orooji, Y.; Lin, H. Ultrasound-assisted catalytic activation of peroxydisulfate on Ti3GeC2 MAX phase for efficient removal of hazardous pollutants. Mater. Today Chem. 2022, 24, 100818. [Google Scholar] [CrossRef]
- de Vasconcellos, A.; Miller, A.H.; Aranda, D.A.G.; Nery, J.G. Biocatalysts based on nanozeolite-enzyme complexes: Effects of alkoxysilane surface functionalization and biofuel production using microalgae lipids feedstock. Colloids Surf. B Biointerfaces 2018, 165, 150–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of nanoparticles in biofuels: An overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Gonzalez-Estrella, J.; Sierra-Alvarez, R.; Field, J.A. Toxicity assessment of inorganic nanoparticles to acetoclastic and hydrogenotrophic methanogenic activity in anaerobic granular sludge. J. Hazard. Mater. 2013, 260, 278–285. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of anaerobic biodegradability of macropollutants. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington DC, USA, 2005. [Google Scholar]
- Xue, S.; Zhao, N.; Song, J.; Wang, X. Interactive Effects of Chemical Composition of Food Waste during Anaerobic Co-Digestion under Thermophilic Temperature. Sustainability 2019, 11, 2933. [Google Scholar] [CrossRef] [Green Version]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zielinski, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Farghali, M.; Andriamanohiarisoamanana, F.; Ahmed, M.; Kotb, S.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Impacts of iron oxide and titanium dioxide nanoparticles on biogas production: Hydrogen sulfide mitigation, process stability, and prospective challenges. J. Environ. Manag. 2019, 240, 160–167. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Ida, J.; Toda, T.; Cuevas-Rodríguez, G. Effects and fate of TiO2 nanoparticles in the anaerobic treatment of wastewater and waste sludge. J. Environ. Manag. 2018, 222, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Quan, X.; Zhao, H. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell. Bioresour. Technol. 2016, 200, 6235–6244. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Feng, H.; Gao, A.; Hao, Q.; Jin, W.; Peng, X.; Li, W.; Wu, G.; Chu, P.K. Extracellular electron transfer from aerobic bacteria to Au-loaded TiO2 semiconductor without light: A new bacteria-killing mechanism other than localized surface plasmon resonance or microbial fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 24509–24516. [Google Scholar] [CrossRef]
- Li, H.; Cui, F.; Liu, Z.; Li, D. Transport, fate, and long-term impacts of metal oxide nanoparticles on the stability of an anaerobic methanogenic system with anaerobic granular sludge. Bioresour. Technol. 2017, 234, 448–455. [Google Scholar] [CrossRef]
- Hassaan, M.; Pantaleo, A.; Tedone, L.; Elkatory, M.; Ali, R.; El Nemr, A.; De Mastro, G. Enhancement of biogas production via green ZnO nanoparticles: Experimental results of selected herbaceous crops. Chem. Eng. Commun. 2019, 208, 242–255. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Z.; Tian, L.; Zhang, L.; Cheng, S.; Li, Z.; Cang, D. Mechanistic investigation of toxicological change in ZnO and TiO2 multi-nanomaterial systems during anaerobic digestion and the microorganism response. Biochem. Eng. J. 2019, 147, 62–71. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Yang, H.; Liud, Z. Influence of zinc oxide nanoparticles on anaerobic digestion of waste activated sludge and microbial communities. RSC Advences 2021, 10, 5580–5589. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Hafeez, F.Y.; Zafar, Y.; Majeed, S.; Leng, X.; Zhao, S.; Saif, I.; Malik, K.; Li, X. A Review on Nanoparticles as Boon for Biogas Producers—Nano Fuels and Biosensing Monitoring. Appl. Sci. 2019, 9, 59. [Google Scholar] [CrossRef] [Green Version]
- Olaya, W.; Dilawar, H.; Eskicioglu, C. Comparative response of thermophilic and mesophilic sludge digesters to zinc oxide nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 24521–24534. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Z.; Zaidi, A.A.; Naseer, M.N.; AlMohamadi, H. Nanomaterials for biogas augmentation towards renewable and sustainable energy production: A critical review. Front. Bioeng. Biotechnol. 2022, 10, 868454. [Google Scholar] [CrossRef]
- Nguyen, D.; Visvanathan, C.; Jacob, P.; Jegatheesan, V. Effects of nano cerium (IV) oxide and zinc oxide particles on biogas production. Int. Biodeterior. Biodegrad. 2015, 102, 165–171. [Google Scholar] [CrossRef]
- Beiki, H.; Keramati, M. Improvement of Methane Production from Sugar Beet Wastes Using TiO2 and Fe3O4 Nanoparticles and Chitosan Micropowder Additives. Appl. Biochem. Biotechnol. 2019, 189, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.; El Nemr, A.; Elkatory, M.R.; Ragab, S.; El-Nemr, M.A.; Pantaleo, A. Synthesis, Characterization, and Synergistic Effects of Modified Biochar in Combination with α-Fe2O3 NPs on Biogas Production from Red Algae Pterocladia capillacea. Sustainability 2021, 13, 9275. [Google Scholar] [CrossRef]
- Silva, A.R.; Alves, M.M.; Pereira, L. Progress and prospects of applying carbon-based materials (and nanomaterials) to accelerate anaerobic bioprocesses for the removal of micropollutants. Microb. Biotechnol. 2022, 15, 1073–1100. [Google Scholar] [CrossRef]
- Hassanein, A.; Keller, E.; Lansing, S. Effect of metal nanoparticles in anaerobic digestion production and plant uptake from efuent fertilizer. Bioresour. Technol. 2021, 321, 124455. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019, 275, 200–206. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Inoculum | FW |
|---|---|---|
| pH | 7.6 ± 0.0 | 4.9 ± 0.1 |
| TS—total solids (g/kg) | 28.99 ± 1.09 | 226.21 ± 2.31 |
| VS—volatile solids (g/kg) | 16.68 ± 0.80 | 216.89 ± 1.95 |
| TCOD—total chemical oxygen demand (g/L) | - | 151.3 ± 10.0 |
| N—nitrogen (g/L) | - | 2.32 ± 0.04 |
| Parameter | FW | FW & ZnO/Ag | FW & TiO2 | |
|---|---|---|---|---|
| Total biogas production (mL/grVS) | 435 ± 11 | 486 ± 21 | 628 ± 17 | |
| Biogas increment (mL/grVS) & (%) | - | 51 (12%) | 193 (44%) | |
| Biogas composition (%) | CH4 | 71.2 ± 2.1 | 71.2 ± 4 | 71.4 ± 2.1 |
| Total biomethane production (mL/grVS) | 310 | 346 | 448 | |
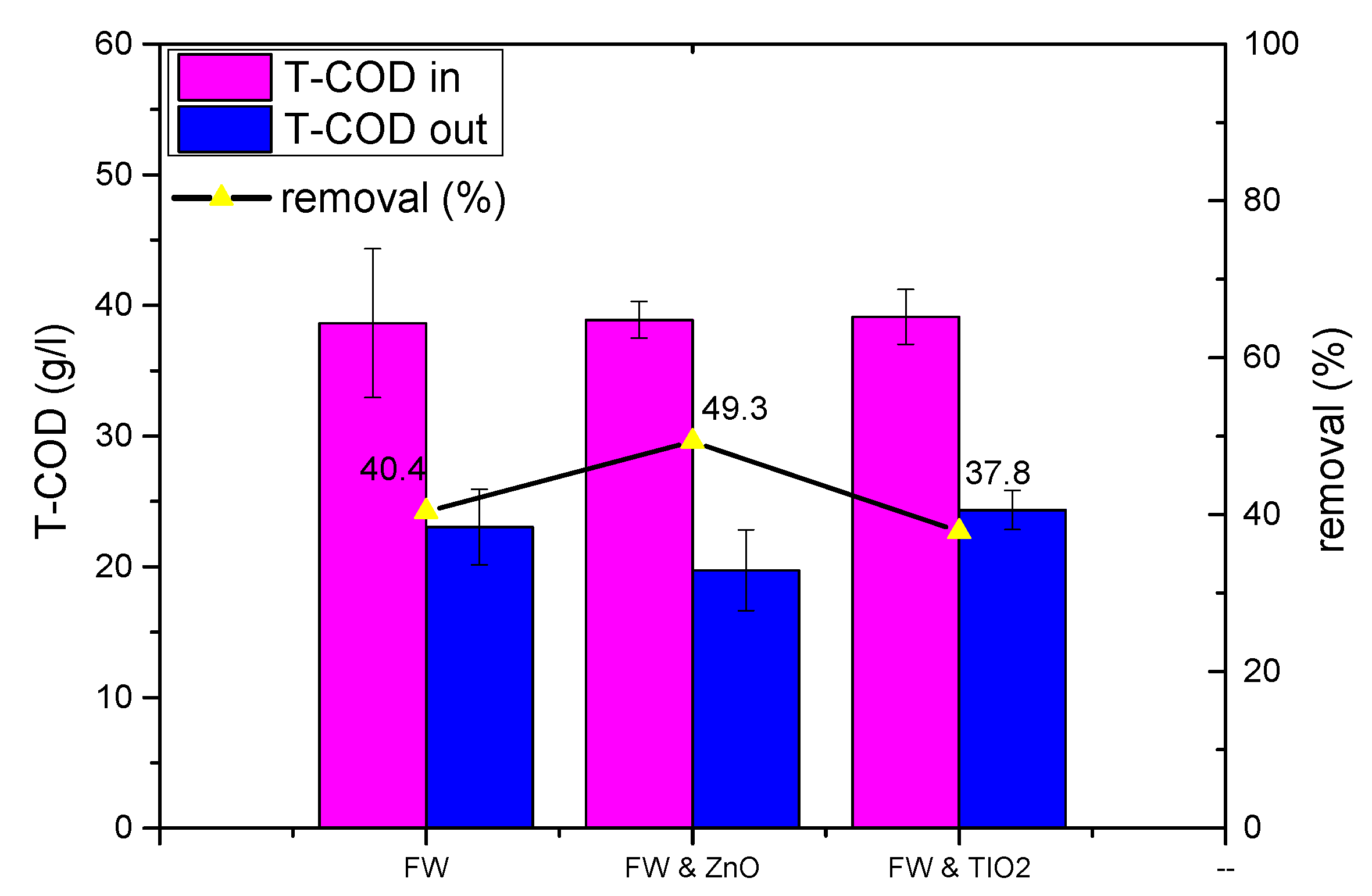
| TCOD removal (%) | 40.4 | 49.3 | 37.8 | |
| VS removal (%) | 40.8 | 39.6 | 46.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dompara, I.; Maragkaki, A.; Papastefanakis, N.; Floraki, C.; Vernardou, D.; Manios, T. Effects of Different Materials on Biogas Production during Anaerobic Digestion of Food Waste. Sustainability 2023, 15, 5698. https://doi.org/10.3390/su15075698
Dompara I, Maragkaki A, Papastefanakis N, Floraki C, Vernardou D, Manios T. Effects of Different Materials on Biogas Production during Anaerobic Digestion of Food Waste. Sustainability. 2023; 15(7):5698. https://doi.org/10.3390/su15075698
Chicago/Turabian StyleDompara, Iliana, Angeliki Maragkaki, Nikolaos Papastefanakis, Christina Floraki, Dimitra Vernardou, and Thrassyvoulos Manios. 2023. "Effects of Different Materials on Biogas Production during Anaerobic Digestion of Food Waste" Sustainability 15, no. 7: 5698. https://doi.org/10.3390/su15075698







