Environmental and Economic Evaluation of Downflow Hanging Sponge Reactors for Treating High-Strength Organic Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Characteristics
2.2. Downflow Hanging Sponge (DHS) Reactor Configuration
2.3. Experimental Setup
2.4. Analytical Analysis
2.5. Kinetic Models
2.5.1. First-Order Substrate Removal Model
2.5.2. Stover–Kincannon Model
3. Results and Discussion
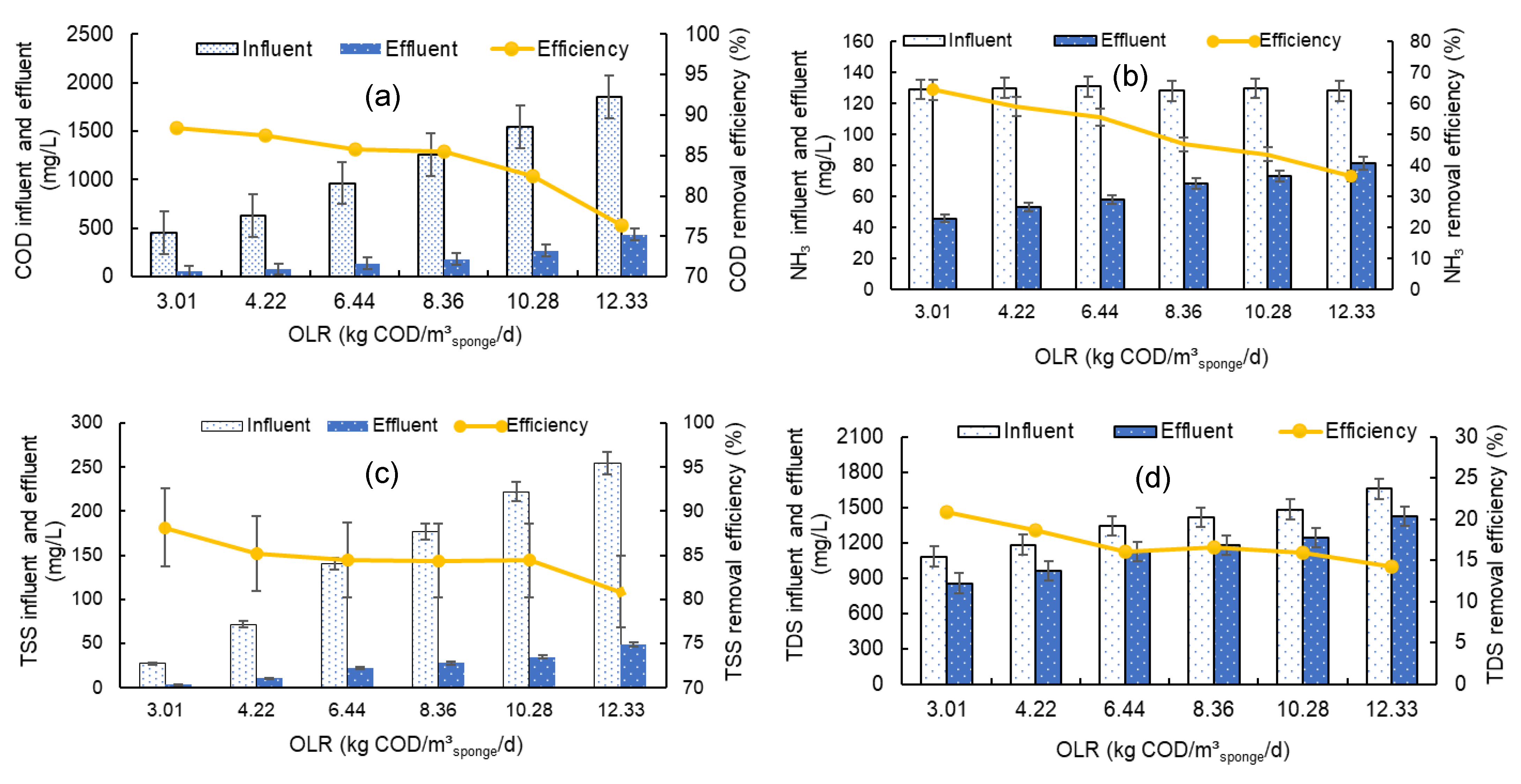
3.1. Effect of Organic Loading Rate on DHS Performance
3.1.1. Effect of Organic Loading Rate on COD Removal
3.1.2. Effect of Organic Loading Rate on NH3 Removal
3.1.3. Effect of Organic Loading Rate on TSS Removal
3.1.4. Effect of Organic Loading Rate on TDS Removal
3.2. DHS Profile
3.2.1. DHS Profile for COD Removal
3.2.2. DHS Profile for NH3 Removal
3.2.3. DHS Profile for TSS Removal
3.2.4. DHS Profile for TDS Removal
3.3. Predicting Effluent COD Using the Kinetic Coefficients of Substrate Removal Models
3.3.1. First-Order Substrate Removal Model Kinetic Parameters
3.3.2. Stover–Kincannon Model Kinetic Parameters
3.3.3. Model Testing
3.4. Characterization of Sponge before and after Wastewater Treatment
3.4.1. SEM
3.4.2. XRD
3.4.3. FTIR
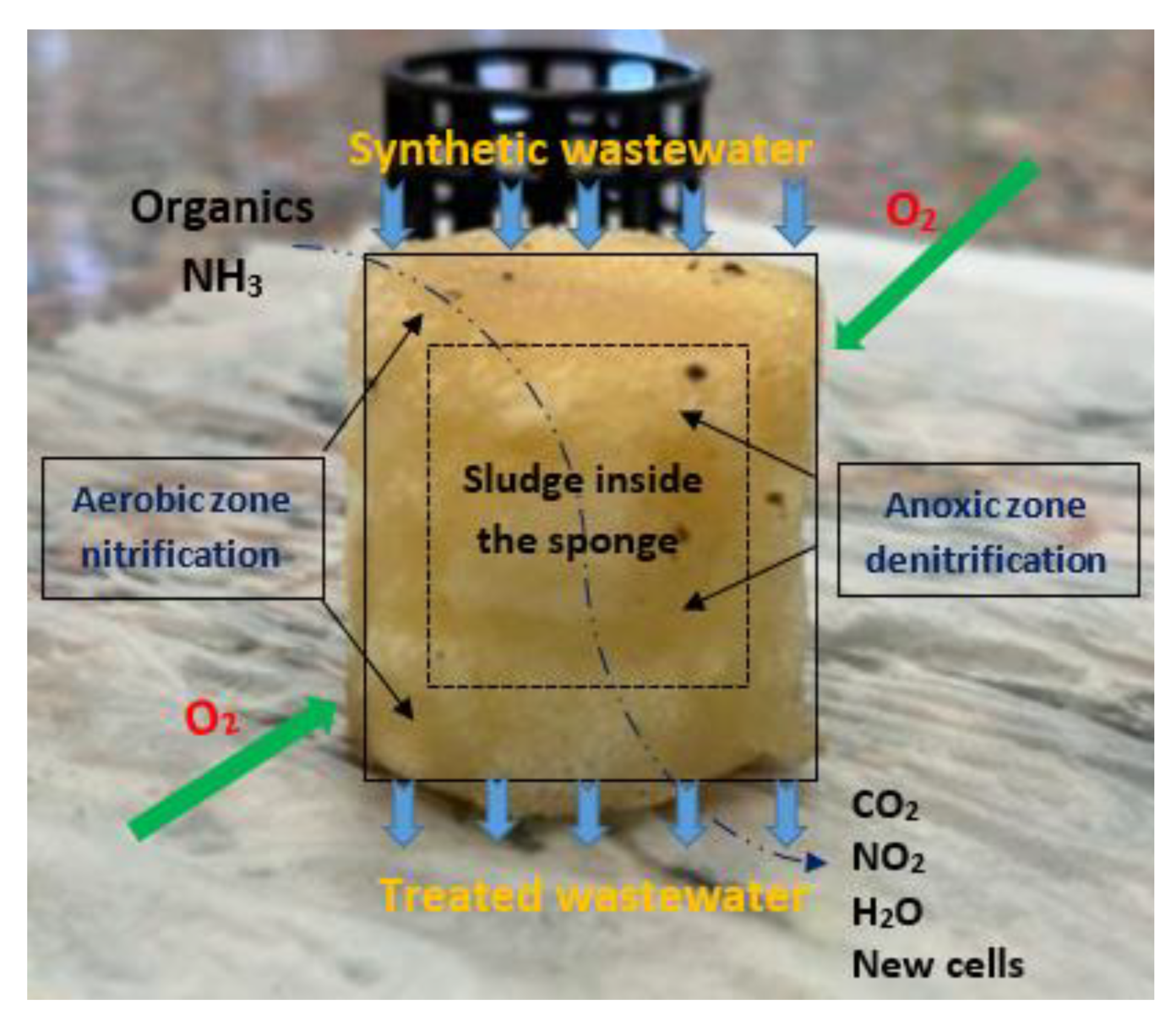
3.5. Suggested Pollutant-Removal Mechanisms via DHS Based on Experimental Results and Literature Survey
3.6. Performance of Sequential DHS System for Wastewater Treatment
3.7. Economic Consideration for DHS Implementation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamza, R.A.; Iorhemen, O.T.; Zaghloul, M.S.; Tay, J.H. Rapid formation and characterization of aerobic granules in pilot-scale sequential batch reactor for high-strength organic wastewater treatment. J. Water Process. Eng. 2018, 22, 27–33. [Google Scholar] [CrossRef]
- Hiep, N.T.; Hong, L.; Nga, D.; Tuan, P.D. A research on the performance of down-flow hanging sponge (DHS) reactor treating domestic wastewater. Vietnam. J. Sci. Technol. 2018, 56, 482–492. [Google Scholar]
- Gao, M.; Guo, B.; Zhang, L.; Zhang, Y.; Yu, N.; Liu, Y. Biomethane recovery from source-diverted household blackwater: Impacts from feed sulfate. Process. Saf. Environ. Prot. 2020, 136, 28–38. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D. A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem. Eng. J. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Batstone, D.; Hülsen, T.; Mehta, C.; Keller, J. Platforms for energy and nutrient recovery from domestic wastewater: A review. Chemosphere 2014, 140, 2–11. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamaguchi, T.; Kuramoto, Y.; Nagano, A.; Shimozaki, S.; Sumino, H.; Araki, N.; Yamazaki, S.; Kawakami, S.; Harada, H. Performance of a pilot-scale sewage treatment: An up-flow anaerobic sludge blanket (UASB) and a down-flow hanging sponge (DHS) reactors combined system by sulfur-redox reaction process under low-temperature conditions. Bioresour. Technol. 2011, 102, 753–757. [Google Scholar] [CrossRef]
- Juneidi, S.J.; Sorour, M.T.; Aly, S.A. Proposed systematic approach for assessing different wastewater treatment plants alternatives: Case study of Aqaba city (South Jordan). Alex. Eng. J. 2022, 61, 12567–12580. [Google Scholar] [CrossRef]
- Tandukar, M.; Ohashi, A.; Harada, H. Performance comparison of a pilot-scale UASB and DHS system and activated sludge process for the treatment of municipal wastewater. Water Res. 2007, 41, 2697–2705. [Google Scholar] [CrossRef]
- Ware, A.J.; Pescod, M.B.; Storch, B. Evaluation of Alternatives to Conventional Disc Support Media for Rotating Biological Contactors. Water Sci. Technol. 1990, 22, 113–117. [Google Scholar] [CrossRef]
- Abyar, H.; Younesi, H.; Bahramifar, N.; Zinatizadeh, A.A.; Amini, M. Kinetic evaluation and process analysis of COD and nitrogen removal in UAASB bioreactor. J. Taiwan Inst. Chem. Eng. 2017, 78, 272–281. [Google Scholar] [CrossRef]
- Okubo, T.; Onodera, T.; Uemura, S.; Yamaguchi, T.; Ohashi, A.; Harada, H. On-site evaluation of the performance of a full-scale down-flow hanging sponge reactor as a post-treatment process of an up-flow anaerobic sludge blanket reactor for treating sewage in India. Bioresour. Technol. 2015, 194, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Watari, T.; Hata, Y.; Hirakata, Y.; Nguyet, P.N.; Nguyen, T.H.; Maki, S.; Hatamoto, M.; Sutani, D.; Setia, T.; Yamaguch, T. Performance evaluation of down-flow hanging sponge reactor for direct treatment of actual textile wastewater; Effect of effluent recirculation to performance and microbial community. J. Water Process. Eng. 2020, 39, 101724. [Google Scholar] [CrossRef]
- Ahammad, S.; Bereslawski, J.; Dolfing, J.; Mota, C.; Graham, D. Anaerobic–aerobic sequencing bioreactors improve energy efficiency for treatment of personal care product industry wastes. Bioresour. Technol. 2013, 139, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Fleifle, A.; Tawfik, A.; Saavedra, O.; Yoshimura, C.; Elzeir, M. Modeling and profile analysis of a down-flow hanging sponge system treating agricultural drainage water. Sep. Purif. Technol. 2013, 116, 87–94. [Google Scholar] [CrossRef]
- Beas, R.E.Y.; Roeleveld, K.K.; Zeeman, G.n; Van Lier, J.B. A downflow hanging sponge (DHS) reactor for faecal coliform removal from an upflow anaerobic sludge blanket (UASB) effluent. Water Sci. Technol. 2015, 72, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Suzuki, S.; Abe, K.; Kubota, K.; Yamaguchi, T.; Ohashi, A.; Takemura, Y.; Harada, H. Removal of organic substances and oxidation of ammonium nitrogen by a down-flow hanging sponge (DHS) reactor under high salinity conditions. Bioresour. Technol. 2010, 101, 5180–5185. [Google Scholar] [CrossRef]
- Tandukar, M.; Machdar, I.; Uemura, S.; Ohashi, A.; Harada, H. Potential of a Combination of UASB and DHS Reactor as a Novel Sewage Treatment System for Developing Countries: Long-Term Evaluation. J. Environ. Eng. 2006, 132, 166–172. [Google Scholar] [CrossRef]
- Machdar, I.; Sekiguchi, Y.; Sumino, H.; Ohashi, A.; Harada, H. Combination of a UASB reactor and a curtain type DHS (downflow hanging sponge) reactor as a cost-effective sewage treatment system for developing countries. Water Sci. Technol. 2000, 42, 83–88. [Google Scholar] [CrossRef]
- Tawfik, A.; Wahab, R.A.; Al-Asmer, A.; Matary, F. Effect of hydraulic retention time on the performance of down-flow hanging sponge system treating grey wastewater. Bioprocess Biosyst. Eng. 2011, 34, 767–776. [Google Scholar] [CrossRef]
- Tawfik, A.; El-Gohary, F.; Ohashi, A.; Harada, H. Optimization of the performance of an integrated anaerobic–aerobic system for domestic wastewater treatment. Water Sci. Technol. 2008, 58, 185–194. [Google Scholar] [CrossRef]
- Tawfik, A.; Ohashi, A.; Harada, H. Sewage treatment in a combined up-flow anaerobic sludge blanket (UASB)–down-flow hanging sponge (DHS) system. Biochem. Eng. J. 2006, 29, 210–219. [Google Scholar] [CrossRef]
- Kapdan, I.K. Kinetic analysis of dyestuff and COD removal from synthetic wastewater in an anaerobic packed column reactor. Process. Biochem. 2005, 40, 2545–2550. [Google Scholar] [CrossRef]
- Kincannon, D.F.; Stover, E.L. Design Methodology for Fixed Film Reaction-Rbcs and Biological Towers. Chemistry 1982, 2, 27–29. [Google Scholar]
- Diamantis, V.; Aivasidis, A. Kinetic analysis and simulation of uasb anaerobic treatment of a synthetic fruit wastewater. Glob. Nest J. 2010, 12, 175–180. [Google Scholar]
- Shahzad, H.M.A.; Khan, S.J.; Habib, Z. Performance evaluation and substrate removal kinetics in a thermophilic anaerobic moving bed biofilm reactor for starch degradation. Water Pr. Technol. 2021, 17, 157–166. [Google Scholar] [CrossRef]
- Debik, E.; Coskun, T. Use of the Static Granular Bed Reactor (SGBR) with anaerobic sludge to treat poultry slaughterhouse wastewater and kinetic modeling. Bioresour. Technol. 2009, 100, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Faridnasr, M.; Ghanbari, B.; Sassani, A. Optimization of the moving-bed biofilm sequencing batch reactor (MBSBR) to control aeration time by kinetic computational modeling: Simulated sugar-industry wastewater treatment. Bioresour. Technol. 2016, 208, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, P.; Ghangrekar, M. Analysis, evaluation, and optimization of kinetic parameters for performance appraisal and design of UASB reactors. Bioresour. Technol. 2008, 99, 2132–2140. [Google Scholar] [CrossRef]
- Tra, V.-T.; Dang, B.-T.; Binh, Q.A.; Nguyen, Q.-H.; Nguyen, P.-T.; Nguyen, H.-H.; Nguyen, T.-T.; Le, T.-H.; Le, D.-T.; Itayama, T.; et al. Influence of hydraulic loading rate on performance and energy-efficient of a pilot-scale down-flow hanging sponge reactor treating domestic wastewater. Environ. Technol. Innov. 2020, 21, 101273. [Google Scholar] [CrossRef]
- Bundy, C.A.; Wu, D.; Jong, M.-C.; Edwards, S.R.; Ahammad, Z.S.; Graham, D.W. Enhanced denitrification in Downflow Hanging Sponge reactors for decentralised domestic wastewater treatment. Bioresour. Technol. 2017, 226, 1–8. [Google Scholar] [CrossRef]
- Dinh, N.T.; Nguyen, T.H.; Mungray, A.K.; Duong, L.D.; Phuong, N.-T.; Nguyen, D.D.; Chung, W.J.; Chang, S.; Tuan, P.D. Biological treatment of saline domestic wastewater by using a down-flow hanging sponge reactor. Chemosphere 2021, 283, 131101. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.-S.; Yao, X.-D.; Shu, L.; Yan, Y.-J.; Wang, Z.; Li, N.; Cui, X.-T.; Lin, Y.-M.; Kong, Q. Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with synthetic domestic wastewater. Int. Biodeterior. Biodegradation 2016, 113, 120–125. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR spectroscopy of nanodiamonds: Methods and interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Ahmadi, M.; Benis, K.Z.; Faraji, M.; Shakerkhatibi, M.; Aliashrafi, A. Process performance and multi-kinetic modeling of a membrane bioreactor treating actual oil refinery wastewater. J. Water Process. Eng. 2019, 28, 115–122. [Google Scholar] [CrossRef]
- El-Kamah, H.; Mahmoud, M.; Tawfik, A. Performance of down-flow hanging sponge (DHS) reactor coupled with up-flow anaerobic sludge blanket (UASB) reactor for treatment of onion dehydration wastewater. Bioresour. Technol. 2011, 102, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Zaki, D.F.; Zahran, M.K. Degradation of reactive dyes wastewater supplemented with cationic polymer (Organo Pol.) in a down flow hanging sponge (DHS) system. J. Ind. Eng. Chem. 2014, 20, 2059–2065. [Google Scholar] [CrossRef]
- Mahmoud, M.; Tawfik, A.; El-Gohary, F. Use of down-flow hanging sponge (DHS) reactor as a promising post-treatment system for municipal wastewater. Chem. Eng. J. 2011, 168, 535–543. [Google Scholar] [CrossRef]
- Kubota, K.; Hayashi, M.; Matsunaga, K.; Iguchi, A.; Ohashi, A.; Li, Y.-Y.; Yamaguchi, T.; Harada, H. Microbial community composition of a down-flow hanging sponge (DHS) reactor combined with an up-flow anaerobic sludge blanket (UASB) reactor for the treatment of municipal sewage. Bioresour. Technol. 2014, 151, 144–150. [Google Scholar] [CrossRef]
- Araki, N.; Ohashi, A.; Machdar, I.; Harada, H. Behaviors of nitrifiers in a novel biofilm reactor employing hanging sponge-cubes as attachment site. Water Sci. Technol. 1999, 39, 23–31. [Google Scholar] [CrossRef]
- Onodera, T.; Yoochatchaval, W.; Sumino, H.; Mizuochi, M.; Okadera, T.; Fujita, T.; Banjongproo, P.; Syutsubo, K. Pilot-scale experiment of down-flow hanging sponge for direct treatment of low-strength municipal wastewater in Bangkok, Thailand. Bioprocess Biosyst. Eng. 2014, 37, 2281–2287. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Tanong, K.; Chiemchaisri, C.; Vigneswaran, S. Feasibility study of a cyclic anoxic/aerobic two-stage MBR for treating ABS resin manufacturing wastewater. Bioresour. Technol. 2011, 102, 5325–5330. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Kumar, K.; Gross, M.; Kunetz, T.; Wen, Z. Removal of total dissolved solids from wastewater using a revolving algal biofilm reactor. Water Environ. Res. 2019, 92, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, E.; Gholami, M.; Farzadkia, M.; Nabizadeh, R.; Azari, A. Study of moving bed biofilm reactor in diethyl phthalate and diallyl phthalate removal from synthetic wastewater. Bioresour. Technol. 2015, 183, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Tang, S. Extracellular polymeric substances (EPS) properties and their effects on membrane fouling in a submerged membrane bioreactor. Water Res. 2009, 43, 2504–2512. [Google Scholar] [CrossRef]
- Duan, L.; Jiang, W.; Song, Y.; Xia, S.; Hermanowicz, S.W. The characteristics of extracellular polymeric substances and soluble microbial products in moving bed biofilm reactor-membrane bioreactor. Bioresour. Technol. 2013, 148, 436–442. [Google Scholar] [CrossRef]
- Shao, Y.-Q.; DU, C.-W.; Shen, Y.-Z.; Ma, F.; Zhou, J.-M. Rapid Determination of N Isotope Labeled Nitrate Using Fourier Transform Infrared Attenuated Total Reflection Spectroscopy. Chin. J. Anal. Chem. 2014, 42, 747–752. [Google Scholar] [CrossRef]
- Nomoto, N.; Hatamoto, M.; Hirakata, Y.; Ali, M.; Jayaswal, K.; Iguchi, A.; Okubo, T.; Takahashi, M.; Kubota, K.; Tagawa, T.; et al. Defining microbial community composition and seasonal variation in a sewage treatment plant in India using a down-flow hanging sponge reactor. Appl. Microbiol. Biotechnol. 2018, 102, 4381–4392. [Google Scholar] [CrossRef]
- Mahmoud, M.; Tawfik, A.; Samhan, F.; El-Gohary, F. Sewage treatment using an integrated system consisting of anaerobic hybrid reactor (AHR) and downflow hanging sponge (DHS). Desalination Water Treat. 2009, 4, 168–176. [Google Scholar] [CrossRef]
- Tanikawa, D.; Nakamura, Y.; Tokuzawa, H.; Hirakata, Y.; Hatamoto, M.; Yamaguchi, T. Effluent treatment in an aquaponics-based closed aquaculture system with single-stage nitrification–denitrification using a down-flow hanging sponge reactor. Int. Biodeterior. Biodegradation 2018, 132, 268–273. [Google Scholar] [CrossRef]
- Yoochatchaval, W.; Onodera, T.; Sumino, H.; Yamaguchi, T.; Mizuochi, M.; Okadera, T.; Syutsubo, K. Development of a down-flow hanging sponge reactor for the treatment of low strength sewage. Water Sci. Technol. 2014, 70, 656–663. [Google Scholar] [CrossRef]
- Tanikawa, D.; Sonaka, H.; Kadotani, M.; Sugimori, F.; Motokawa, D.; Ihara, S.; Itoiri, Y.; Kimura, Z.-I. Estimation of microbial community for denitrification in the down-flow hanging sponge (DHS) reactor. Int. Biodeterior. Biodegradation 2020, 153, 105022. [Google Scholar] [CrossRef]
- Uemura, S.; Suzuki, S.; Maruyama, Y.; Harada, H. Direct Treatment of Settled Sewage by DHS Reactors with Different Sizes of Sponge Support Media. Int. J. Environ. Res. 2012, 6, 25–32. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Yang, F.; Xue, Y.; Wang, T. The development of simultaneous partial nitrification, ANAMMOX and denitrification (SNAD) process in a single reactor for nitrogen removal. Bioresour. Technol. 2009, 100, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, N.; Nomoto, N.; Tagawa, T.; Okubo, T.; Uemura, S.; Khalil, N.; Hatamoto, M.; Yamaguchi, T.; Harada, H. Assessment of UASB–DHS technology for sewage treatment: A comparative study from a sustainability perspective. Environ. Technol. 2018, 40, 2825–2832. [Google Scholar] [CrossRef]
- Tare, V.; Gupta, S.; Bose, P. Case studies on biological treatment of tannery effluents in India. J. Air Waste Manag. Assoc. 2003, 53, 976–982. [Google Scholar] [CrossRef]



| Parameter | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | Phase 6 |
|---|---|---|---|---|---|---|
| COD (mg/L) | 451.2 ± 97.0 | 632.6 ± 116.0 | 965.3 ± 98.0 | 1254.7 ± 231.0 | 1542.8 ± 246.0 | 1849.7 ± 366.0 |
| NH3 (mg/L) | 129.0 ± 3.1 | 130.0 ± 1.7 | 130.0 ± 1.6 | 128.0 ± 2.8 | 129.0 ± 1.7 | 128.0 ± 3.6 |
| pH | 7.7 ± 0.3 | 8.2 ± 0.2 | 8.3 ± 0.4 | 8.4 ± 0.8 | 8.6 ± 0.3 | 8.8 ± 0.5 |
| TSS (mg/L) | 27.0 ± 3.5 | 71.5 ± 24.0 | 140.3 ± 39.0 | 176.7 ± 58.0 | 221.8 ± 79.0 | 254.0 ± 93.0 |
| TDS (mg/L) | 1080 ± 49 | 1180 ± 83 | 1340 ± 65 | 1410 ± 67 | 1480 ± 59 | 1650 ± 84 |
| Conductivity (μS) | 1630 ± 260 | 1750 ± 290 | 2020 ± 280 | 2130 ± 250 | 2230 ± 280 | 2380 ± 260 |
| Temperature (°C) | Room temperature (24–34 °C) | |||||
| Kinetic Model | Reactor Segment | Kinetic Parameter | Unit | Value | R2 |
|---|---|---|---|---|---|
| First-order | Segment-1 | K1 | 1/d | 3.773 | 0.991 |
| Segment-2 | K1 | 1/d | 4.914 | 0.982 | |
| Segment-3 | K1 | 1/d | 6.001 | 0.910 | |
| Total reactor | K1 | 1/d | 27.397 | 0.927 | |
| Stover–Kincannon | Segment-1 | KB | g/L/d | 56.444 | 0.992 |
| Umax | g/L/d | 24.272 | |||
| Segment-2 | KB | g/L/d | 20.144 | 0.997 | |
| Umax | g/L/d | 11.249 | |||
| Segment-3 | KB | g/L/d | 14.231 | 0.991 | |
| Umax | g/L/d | 9.025 | |||
| Total reactor | KB | g/L/d | 83.808 | 0.999 | |
| Umax | g/L/d | 76.923 |
| Wastewater Type | Sponge Used | Operation | Influent Wastewater Characteristics (mg/L) Removal Efficiency (R%) | OLR (kg COD/m3/d) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| COD (mg/L) | R% | Ammonia-N (mg/L) | R% | TSS (mg/L) | R% | |||||
| Domestic | 5 cm height × 3.5 cm diameter | HRT: 5.83 h | 118.2 ± 37.5 | 60.0 ± 11.4 | 44 ± 5.1 | 88.4 ± 0.9 | 38.4 ± 4.1 | 69.7 ± 6.1 | 1.2 | [29] |
| HRT: 2.91 h | 114.0 ± 24.6 | 51.5 ± 20.4 | 28.2 ± 8.9 | 80.7 ± 15.4 | 34.1 ± 5.8 | 75.6 ± 6.9 | 1.2 | |||
| Domestic | Combinations of sponge density (fine vs. coarse) | HRT: 1.2 d 100% recirculation | 172.6 ± 49.5 | 86.6 | 30.2 ± 4.7 | 98.6 | NA | NA | 0.2 | [30] |
| HRT: 1.2 d 50% recirculation | 180.4 ± 27.6 | 82.3 | 29.0 ± 5.8 | 91.2 | NA | NA | 0.2 | |||
| HRT: variable 50% recirculation | 174 ± 36.2 | 65.2 | 25.1 ± 4.4 | 68.5 | NA | NA | 0.2 | |||
| HRT: 0.6 d 30% recirculation | 216.4 ± 40.7 | 84.2 | 36.8 ± 8.7 | 81.3 | NA | NA | 0.4 | |||
| Low strength sewage | 3.3 cm height × 3.3 cm diameter | HRT: 4 h | 67 ± 18.1 | 67.14 | 7 ± 1.4 | 85.71 | 36 ± 24.1 | 97.22 | 1.34 | [53] |
| HRT: 2 h | 63 ± 20.7 | 60.31 | 6.9 ± 0.8 | 98.55 | 33 ± 22.8 | 96.97 | ||||
| HRT: 1.5 h | 46 ± 10.8 | 60.87 | 5.7 ± 2.6 | 96.49 | 27 ± 9.7 | 96.30 | ||||
| HRT: 1 h | 56 ± 17.9 | 57.14 | 7.4 ± 1.7 | 97.30 | 27 ± 21.2 | 44.44 | ||||
| Low-strength municipal wastewater | 3.3 cm height × 3.3 cm diameter | HRT: 4 h | 66.8 ± 18.1 | 67.1 | 7.0 ± 1.6 | 98.6 | 36.3 ± 24.1 | 94.8 | NA | [42] |
| HRT: 2 h | 62.6 ± 20.7 | 59.4 | 6.9 ± 0.8 | 98.6 | 33.4 ± 22.8 | 96.1 | NA | |||
| HRT: 1 h | 60.5 ± 16.6 | 65.1 | 7.3 ± 1.7 | 97.3 | 29.4 ± 18.4 | 82.7 | NA | |||
| Agricultural drainage water | 5.0 cm height × 2.0 cm diameter | HRT: 2 h | 249.4 ± 100.2 | 83.7 | 15.8 ± 6 | 85.0 | 159.7 ± 63 | 88.9 | 3.0 | [14] |
| Synthetic natural rubber wastewater | Polyurethane sponge | HRT: 11.8 h | 236 ± 281 | 75.4 ± 11.7 | 187 ± 104 | 59.9 ± 20.4 | NA | NA | NA | [54] |
| Settled sewage | Plastic plate (height 200 cm × width 7 cm) | HRT: 2 h | 106.2 ± 32.3 | 85.2 ± 11.4 | 18.6 ± 7.8 | 94.7 ± 9.2 | NA | NA | NA | [55] |
| Domestic wastewater | 3.3 cm height × 3.3 cm diameter | HRT: 3.6 h | 451.24 ± 97 | 94.02 ± 4.57 | 129.04 ± 3.1 | 88.13 ± 3.26 | 27.02 ± 3.5 | 88.94 ± 6.16 | 3.01 | This study |
| 632.56 ± 116 | 95.38 ± 5.11 | 130.24 ± 1.7 | 84.62 ± 2.89 | 71.49 ± 24.1 | 89.91 ± 7.42 | 4.22 | ||||
| 965.33 ± 98 | 96.29 ± 5.23 | 129.91 ± 1.6 | 77.08 ± 3.48 | 140.3 ± 39.1 | 85.44 ± 5.25 | 6.44 | ||||
| 1254.72 ± 231 | 95.16 ± 4.27 | 128.24 ± 2.8 | 74.66 ± 4.11 | 176.71 ± 58.5 | 89.39 ± 4.16 | 8.36 | ||||
| 1542.83 ± 246 | 94.89 ± 6.9 | 129.73 ± 1.7 | 67.04 ± 5.76 | 221.86 ± 79.8 | 89.62 ± 5.29 | 10.28 | ||||
| 1849.72 ± 366 | 92.58 ± 8.37 | 128.15 ± 3.6 | 63.57 ± 5.08 | 254.03 ± 93.7 | 90.34 ± 3.91 | 12.33 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zidan, A.; Nasr, M.; Fujii, M.; Ibrahim, M.G. Environmental and Economic Evaluation of Downflow Hanging Sponge Reactors for Treating High-Strength Organic Wastewater. Sustainability 2023, 15, 6038. https://doi.org/10.3390/su15076038
Zidan A, Nasr M, Fujii M, Ibrahim MG. Environmental and Economic Evaluation of Downflow Hanging Sponge Reactors for Treating High-Strength Organic Wastewater. Sustainability. 2023; 15(7):6038. https://doi.org/10.3390/su15076038
Chicago/Turabian StyleZidan, Abdelsalam, Mahmoud Nasr, Manabu Fujii, and Mona G. Ibrahim. 2023. "Environmental and Economic Evaluation of Downflow Hanging Sponge Reactors for Treating High-Strength Organic Wastewater" Sustainability 15, no. 7: 6038. https://doi.org/10.3390/su15076038
APA StyleZidan, A., Nasr, M., Fujii, M., & Ibrahim, M. G. (2023). Environmental and Economic Evaluation of Downflow Hanging Sponge Reactors for Treating High-Strength Organic Wastewater. Sustainability, 15(7), 6038. https://doi.org/10.3390/su15076038









