Advances in the Food Packaging Production from Agri-Food Waste and By-Products: Market Trends for a Sustainable Development
Abstract
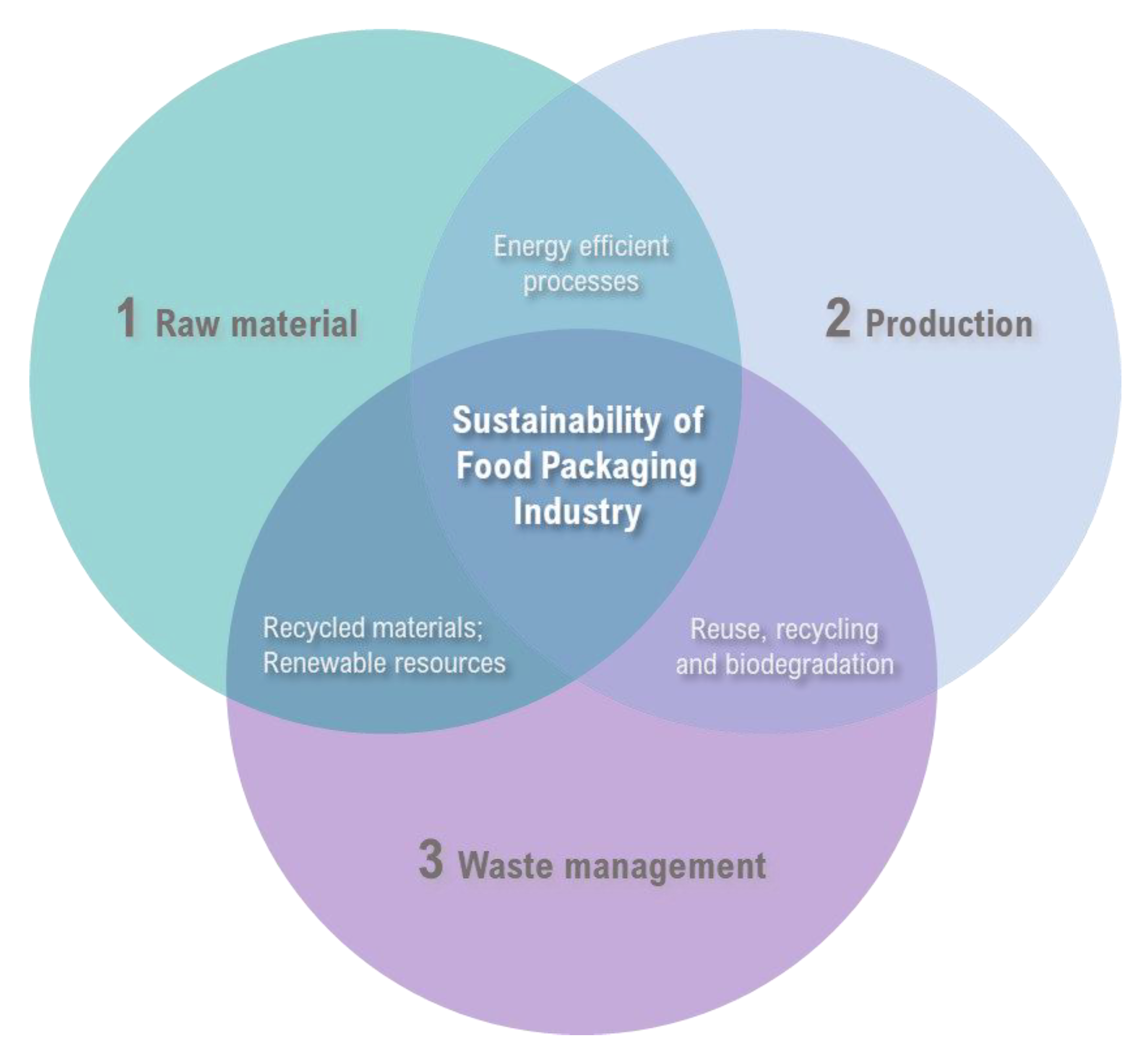
:1. Introduction
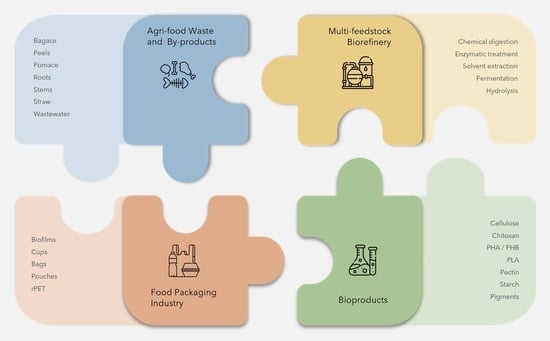
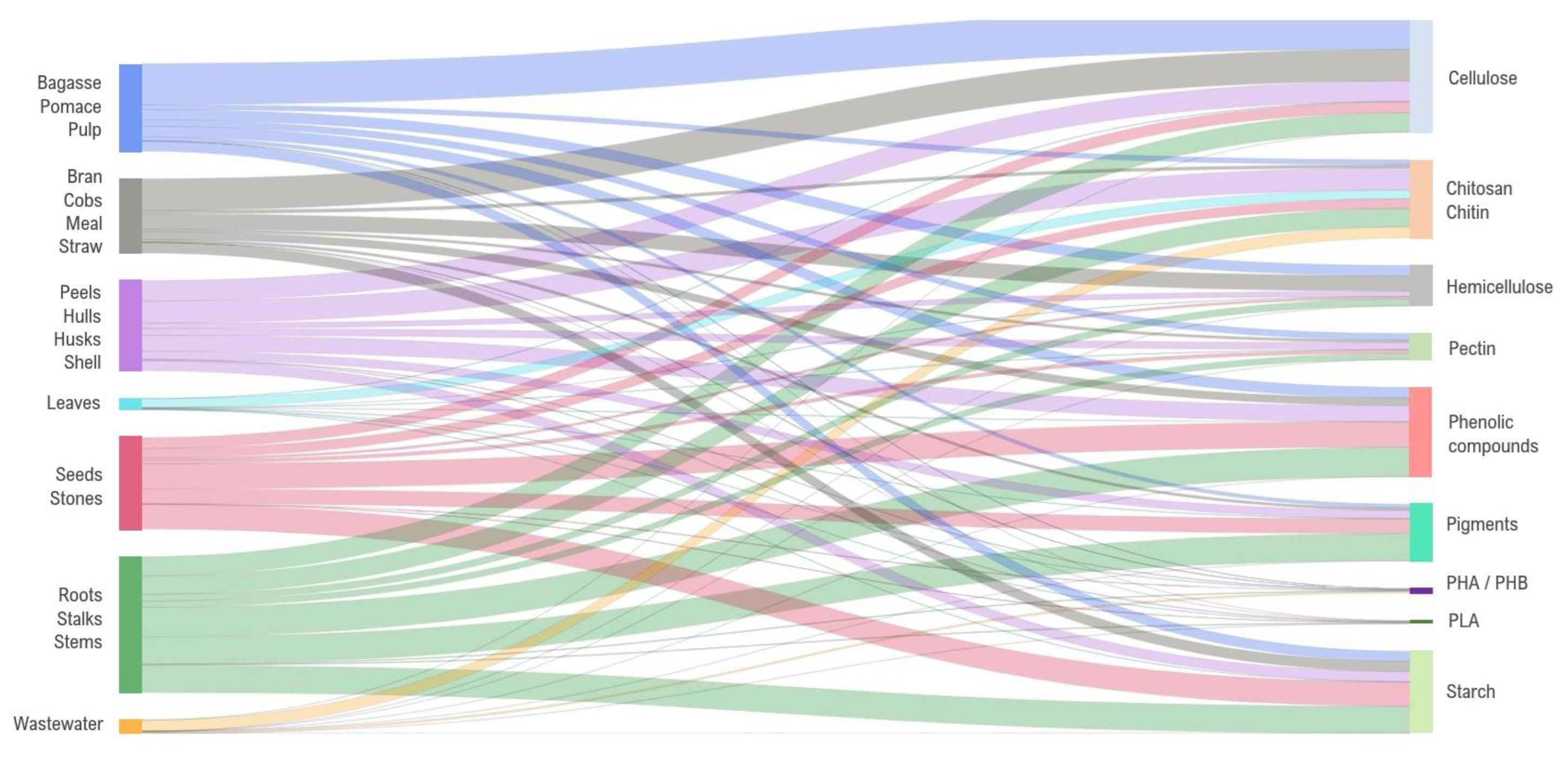
2. From Food Waste and By-Products to Packaging
2.1. Biopolymers for Food Packaging
2.1.1. Protein-Based Biopolymer Packaging
2.1.2. Starch-Based Biopolymer Packaging
2.1.3. Lignocellulosic-Based Biopolymer Packaging
2.1.4. Microbial Biopolymer Packaging
2.1.5. Chitin-Based Biopolymer Packaging
2.1.6. Lipid-Based Biopolymer Packaging
2.1.7. Biodegradable Foams
2.2. Current Production Technologies
2.2.1. Solvent Casting
2.2.2. Tape Casting
2.2.3. Melt Extrusion
2.2.4. Thermopressing/Thermoforming
2.2.5. Compression Molding
2.2.6. Layer-By-Layer (LBL) Assembly
2.2.7. Electrospinning/Electrospraying
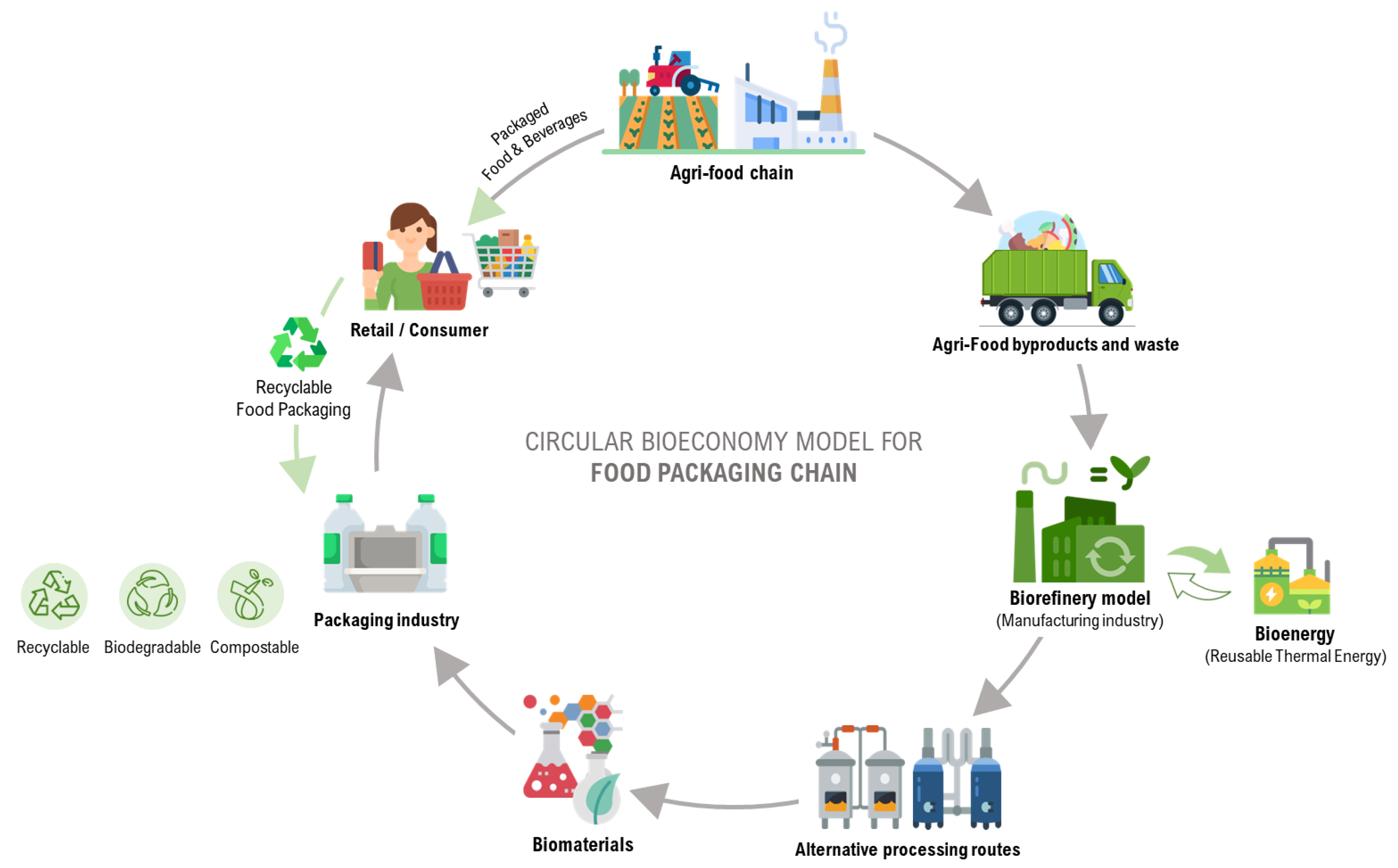
3. The Business of the Sustainable Food Packaging
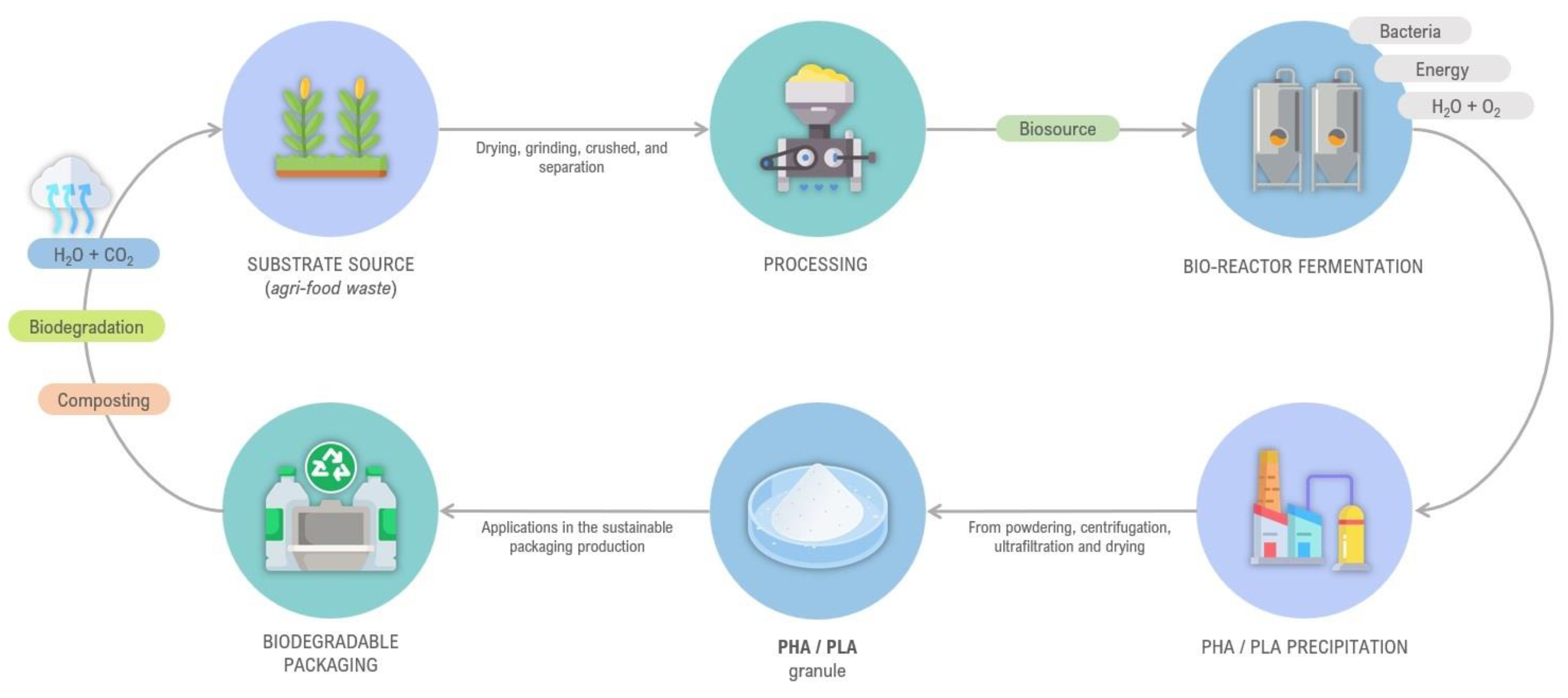
3.1. Biorefinery Model of Agri-Food Waste and Contribution to Bioeconomy
3.1.1. Case Studies on Spent Coffee Grounds (SCGs)
3.1.2. Case Studies on Banana-Biomass-Based Refineries
3.2. Market Opportunities
4. Sustainable Prospects
4.1. The Turning Point of the Food Packaging Industry
4.2. The Scientific Approach to the Sustainable Food Packaging
4.3. Sustainable Strategies of the Food Packaging Chain
4.4. LCA as a Tool for the Food Packaging Industry
5. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO Food Waste Footprint: Impacts on Natural Resources. 2013. Available online: https://www.fao.org/sustainable-food-value-chains/library/details/en/c/266219/ (accessed on 15 December 2022).
- Ashokkumar, V.; Flora, G.; Venkatkarthick, R.; SenthilKannan, K.; Kuppam, C.; Mary Stephy, G.; Kamyab, H.; Chen, W.H.; Thomas, J.; Ngamcharussrivichai, C. Advanced Technologies on the Sustainable Approaches for Conversion of Organic Waste to Valuable Bioproducts: Emerging Circular Bioeconomy Perspective. Fuel 2022, 324, 124313. [Google Scholar] [CrossRef]
- Gonçalves, M.L.M.B.B.; Maximo, G.J. Circular Economy in the Food Chain: Production, Processing and Waste Management. Circ. Econ. Sustain. 2022. [Google Scholar] [CrossRef] [PubMed]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-Food Wastes for Bioplastics: European Prospective on Possible Applications in Their Second Life for a Circular Economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, W.; Ye, S.; Batista, L. Packaging Design for the Circular Economy: A Systematic Review. Sustain. Prod. Consum. 2022, 32, 817–832. [Google Scholar] [CrossRef]
- European Commission. An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Petit-Boix, A.; Leipold, S. Circular Economy in Cities: Reviewing How Environmental Research Aligns with Local Practices. J. Clean. Prod. 2018, 195, 1270–1281. [Google Scholar] [CrossRef]
- Zhu, B.; Nguyen, M.; Sarm Siri, N.; Malik, A. Towards a Transformative Model of Circular Economy for SMEs. J. Bus. Res. 2022, 144, 545–555. [Google Scholar] [CrossRef]
- Girard, G. Does Circular Bioeconomy Contain Singular Social Science Research Questions, Especially Regarding Agriculture–Industry Nexus? Clean. Circ. Bioeconomy 2022, 3, 100030. [Google Scholar] [CrossRef]
- Mouzakitis, Y.; Adamides, E.D. Techno-Economic Assessment of an Olive Mill Wastewater (OMWW) Biorefinery in the Context of Circular Bioeconomy. Eng 2022, 3, 488–503. [Google Scholar] [CrossRef]
- Salvador, R.; Puglieri, F.N.; Halog, A.; de Andrade, F.G.; Piekarski, C.M.; De Francisco, A.C. Key Aspects for Designing Business Models for a Circular Bioeconomy. J. Clean. Prod. 2021, 278, 124341. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Dahiya, S.; Amulya, K.; Katakojwala, R.; Vanitha, T.K. Can Circular Bioeconomy Be Fueled by Waste Biorefineries—A Closer Look. Bioresour. Technol. Rep. 2019, 7, 100277. [Google Scholar] [CrossRef]
- Carus, M.; Dammer, L. The Circular Bioeconomy–Concepts, Opportunities, and Limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- Giampietro, M. On the Circular Bioeconomy and Decoupling: Implications for Sustainable Growth. Ecol. Econ. 2019, 162, 143–156. [Google Scholar] [CrossRef]
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased Composites from Agro-Industrial Wastes and by-Products. Emergent Mater. 2021, 1–49. [Google Scholar] [CrossRef]
- Cristóbal, J.; Caldeira, C.; Corrado, S.; Sala, S. Techno-Economic and Profitability Analysis of Food Waste Biorefineries at European Level. Bioresour. Technol. 2018, 259, 244–252. [Google Scholar] [CrossRef]
- Jorissen, T.; Oraby, A.; Recke, G.; Zibek, S. A Systematic Analysis of Economic Evaluation Studies of Second-Generation Biorefineries Providing Chemicals by Applying Biotechnological Processes. Biofuels Bioprod. Biorefining 2020, 14, 1028–1045. [Google Scholar] [CrossRef]
- Banerjee, S.; Munagala, M.; Shastri, Y.; Vijayaraghavan, R.; Patti, A.F.; Arora, A. Process Design and Techno-Economic Feasibility Analysis of an Integrated Pineapple Processing Waste Biorefinery. ACS Eng. Au 2022, 2, 208–218. [Google Scholar] [CrossRef]
- Zhang, H.; Sablani, S. Biodegradable Packaging Reinforced with Plant-Based Food Waste and by-Products. Curr. Opin. Food Sci. 2021, 42, 61–68. [Google Scholar] [CrossRef]
- Baetge, S.; Martin, K. Rice Straw and Rice Husks as Energy Sources—Comparison of Direct Combustion and Biogas Production. Biomass Convers. Biorefinery 2018, 8, 719–737. [Google Scholar] [CrossRef]
- Rezaei, M.; Liu, B. Food Loss and Waste in the Food Supply Chain. Nutfruit 2017, 2017, 26–27. [Google Scholar]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Abotbina, W.; Sapuan, S.M.; Ilyas, R.A.; Sultan, M.T.H.; Alkbir, M.F.M.; Sulaiman, S.; Harussani, M.M.; Bayraktar, E. Recent Developments in Cassava (Manihot Esculenta) Based Biocomposites and Their Potential Industrial Applications: A Comprehensive Review. Materials 2022, 15, 6992. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Mishra, R.; Kumar, A.; Shukla, S.K.; Lo, S.L.; Kumar, S. Circular Economy-Based Environmental Management Using Biochar: Driving towards Sustainability. Process Saf. Environ. Prot. 2022, 163, 585–600. [Google Scholar] [CrossRef]
- Gupta, H.; Kumar, H.; Kumar, M.; Gehlaut, A.K.; Gaur, A.; Sachan, S.; Park, J.-W. Synthesis of Biodegradable Films Obtained from Rice Husk and Sugarcane Bagasse to Be Used as Food Packaging Material. Environ. Eng. Res. 2020, 25, 506–514. [Google Scholar] [CrossRef]
- Jonglertjunya, W.; Juntong, T.; Pakkang, N.; Srimarut, N.; Sakdaronnarong, C. Properties of Lignin Extracted from Sugarcane Bagasse and Its Efficacy in Maintaining Postharvest Quality of Limes during Storage. LWT-Food Sci. Technol. 2014, 57, 116–125. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Hayat, N.A.; Binti, M.; Nor, M.S.M. Development and Characterization of Food Packaging Bioplastic Film from Cocoa Pod Husk Cellulose Incorporated with Sugarcane Bagasse Fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Berthet, M.-A.; Angellier-Coussy, H.; Chea, V.; Guillard, V.; Gastaldi, E.; Gontard, N. Sustainable Food Packaging: Valorising Wheat Straw Fibres for Tuning PHBV-Based Composites Properties. Compos. Part A Appl. Sci. Manuf. 2015, 72, 139–147. [Google Scholar] [CrossRef]
- Castrillón, H.D.C.; Aguilar, C.M.G.; Álvarez, B.E.A. Circular Economy Strategies: Use of Corn Waste to Develop Biomaterials. Sustainability 2021, 13, 8356. [Google Scholar] [CrossRef]
- Bishop, G.; Styles, D.; Lens, P.N.L. Environmental Performance of Bioplastic Packaging on Fresh Food Produce: A Consequential Life Cycle Assessment. J. Clean. Prod. 2021, 317, 128377. [Google Scholar] [CrossRef]
- Manrich, A.; Moreira, F.K.V.; Otoni, C.G.; Lorevice, M.V.; Martins, M.A.; Mattoso, L.H.C. Hydrophobic Edible Films Made up of Tomato Cutin and Pectin. Carbohydr. Polym. 2017, 164, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Gorrasi, G.; Brachi, P.; Bugatti, V.; Viscusi, G. Valorization of Tomato Processing Residues Through the Production of Active Bio-Composites for Packaging Applications. Front. Mater. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Follonier, S.; Goyder, M.S.; Silvestri, A.C.; Crelier, S.; Kalman, F.; Riesen, R.; Zinn, M. Fruit Pomace and Waste Frying Oil as Sustainable Resources for the Bioproduction of Medium-Chain-Length Polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 71, 42–52. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Zea-Redondo, L.; Moates, G.K.; Wellner, N.; Cross, K.; Waldron, K.W.; Azeredo, H.M.C. Pomegranate Peel Pectin Films as Affected by Montmorillonite. Food Chem. 2016, 198, 107–112. [Google Scholar] [CrossRef]
- Ginting, M.H.S.; Hasibuan, R.; Lubis, M.; Alanjani, F.; Winoto, F.A.; Siregar, R.C. Utilization of Avocado Seeds as Bioplastic Films Filler Chitosan and Ethylene Glycol Plasticizer. Asian J. Chem. 2018, 30, 1569–1573. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Pisuchpen, S.; Osako, K. Mechanical, Thermal and Heat Sealing Properties of Fish Skin Gelatin Film Containing Palm Oil and Basil Essential Oil with Different Surfactants. Food Hydrocoll. 2016, 56, 93–107. [Google Scholar] [CrossRef]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological Conversion of Spent Coffee Grounds into Polyhydroxyalkanoates and Carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. A Study of the Recovery of the Dietary Fibres from Olive Mill Wastewater and the Gelling Ability of the Soluble Fibre Fraction. LWT -Food Sci. Technol. 2010, 43, 1009–1017. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Preserving Apple (Malus Domestica Var. Anna) Fruit Bioactive Substances Using Olive Wastes Extract-Chitosan Film Coating. Inf. Process. Agric. 2017, 4, 90–99. [Google Scholar] [CrossRef]
- Licciardello, F.; Wittenauer, J.; Saengerlaub, S.; Reinelt, M.; Stramm, C. Rapid Assessment of the Effectiveness of Antioxidant Active Packaging-Study with Grape Pomace and Olive Leaf Extracts. Food Packag. Shelf Life 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Kaisangsri, N.; Kerdchoechuen, O.; Laohakunjit, N. Biodegradable Foam Tray from Cassava Starch Blended with Natural Fiber and Chitosan. Ind. Crops Prod. 2012, 37, 542–546. [Google Scholar] [CrossRef]
- Torres-León, C.; Vicente, A.A.; Flores-López, M.L.; Rojas, R.; Serna-Cock, L.; Alvarez-Pérez, O.B.; Aguilar, C.N. Edible Films and Coatings Based on Mango (Var. Ataulfo) by-Products to Improve Gas Transfer Rate of Peach. LWT 2018, 97, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Chollakup, R.; Kongtud, W.; Sukatta, U.; Premchookiat, M.; Piriyasatits, K.; Nimitkeatkai, H.; Jarerat, A. Eco-Friendly Rice Straw Paper Coated with Longan (Dimocarpus Longan) Peel Extract as Bio-Based and Antibacterial Packaging. Polymers 2021, 13, 3096. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Paiva, A.; Lisboa, P.; Freitas, F.; Alves, V.D.; Simões, P.; Barreiros, S.; Reis, M.A.M. Production of Polyhydroxyalkanoates from Spent Coffee Grounds Oil Obtained by Supercritical Fluid Extraction Technology. Bioresour. Technol. 2014, 157, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Boccalon, E.; Gorrasi, G. Functional Bioplastics from Food Residual: Potentiality and Safety Issues. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3177–3204. [Google Scholar] [CrossRef]
- George, N.; Debroy, A.; Bhat, S.; Singh, S.; Bindal, S. Biowaste to Bioplastics: An Ecofriendly Approach for A Sustainable Future. J Appl. Biotechnol. Rep. 2021, 8, 221–233. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Permeability in Clay/Polyesters Nano-Biocomposites. In Environmental Silicate Nano-Biocomposites; Avérous, L., Pollet, E., Eds.; Green Energy and Technology; Springer: London, UK, 2012; pp. 237–264. ISBN 978-1-4471-4108-2. [Google Scholar]
- Hernández-Muñoz, P.; Kanavouras, A.; Ng, P.; Gavara, R. Development and Characterization of Biodegradable Films Made from Wheat Gluten Protein Fractions. J. Agric. Food Chem. 2004, 51, 7647–7654. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Felix, M.; Guerrero, A. Protein-Based Bioplastics from Biowastes: Sources, Processing, Properties and Applications. In Bioplastics for Sustainable Development; Kuddus, M., Roohi, Eds.; Springer: Singapore, 2021; pp. 137–176. ISBN 9789811618239. [Google Scholar]
- Park, H.-Y.; Kim, S.-J.; Kim, K.M.; You, Y.-S.; Kim, S.Y.; Han, J. Development of Antioxidant Packaging Material by Applying Corn-Zein to LLDPE Film in Combination with Phenolic Compounds. J. Food Sci. 2012, 77, E273–E279. [Google Scholar] [CrossRef]
- Gaona-Sánchez, V.A.; Calderón-Domínguez, G.; Morales-Sánchez, E.; Chanona-Pérez, J.J.; Velázquez-de la Cruz, G.; Méndez-Méndez, J.V.; Terrés-Rojas, E.; Farrera-Rebollo, R.R. Preparation and Characterisation of Zein Films Obtained by Electrospraying. Food Hydrocoll. 2015, 49, 1–10. [Google Scholar] [CrossRef]
- Wittaya, T. Protein-Based Edible Films: Characteristics and Improvement of Properties. In Structure and Function of Food Engineering; Amer Eissa, A., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0695-1. [Google Scholar]
- Jerez, A.; Partal, P.; Martínez, I.; Gallegos, C.; Guerrero, A. Protein-Based Bioplastics: Effect of Thermo-Mechanical Processing. Rheol. Acta 2007, 46, 711–720. [Google Scholar] [CrossRef]
- Dilshad, E.; Waheed, H.; Ali, U.; Amin, A.; Ahmed, I. General Structure and Classification of Bioplastics and Biodegradable Plastics. In Bioplastics for Sustainable Development; Kuddus, M., Roohi, Eds.; Springer: Singapore, 2021; pp. 61–82. ISBN 9789811618239. [Google Scholar]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics Based on Wheat Gluten Processed by Extrusion. J. Clean. Prod. 2019, 239, 117994. [Google Scholar] [CrossRef]
- Patni, N.; Yadava, P.; Agarwal, A.; Maroo, V. An Overview on the Role of Wheat Gluten as a Viable Substitute for Biodegradable Plastics. Rev. Chem. Eng. 2014, 30. [Google Scholar] [CrossRef]
- Onyeaka, H.; Obileke, K.; Makaka, G.; Nwokolo, N. Current Research and Applications of Starch-Based Biodegradable Films for Food Packaging. Polymers 2022, 14, 1126. [Google Scholar] [CrossRef]
- Abral, H.; Hartono, J. Moisture Absorption of Starch Based Biocomposites Reinforced with Water Hyacinth Fibers. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 12035. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-Based Biodegradable Materials: Challenges and Opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Nasir, N.; Othman, S. The Physical and Mechanical Properties of Corn-Based Bioplastic Films with Different Starch and Glycerol Content. J. Phys. Sci. 2021, 32, 89–101. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R.A. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef]
- Li, M.-C.; Lee, J.K.; Cho, U.R. Synthesis, Characterization, and Enzymatic Degradation of Starch-Grafted Poly(Methyl Methacrylate) Copolymer Films. J. Appl. Polym. Sci. 2012, 125, 405–414. [Google Scholar] [CrossRef]
- Fadeyibi, A.; Osunde, Z.D.; Egwim, E.C.; Idah, P.A. Performance Evaluation of Cassava Starch-Zinc Nanocomposite Film for Tomatoes Packaging. J. Agric. Eng. 2017, 48. [Google Scholar] [CrossRef] [Green Version]
- Fitch-Vargas, P.R.; Camacho-Hernández, I.L.; Martínez-Bustos, F.; Islas-Rubio, A.R.; Carrillo-Cañedo, K.I.; Calderón-Castro, A.; Jacobo-Valenzuela, N.; Carrillo-López, A.; Delgado-Nieblas, C.I.; Aguilar-Palazuelos, E. Mechanical, Physical and Microstructural Properties of Acetylated Starch-Based Biocomposites Reinforced with Acetylated Sugarcane Fiber. Carbohydr. Polym. 2019, 219, 378–386. [Google Scholar] [CrossRef]
- Travalini, A.P.; Lamsal, B.; Magalhães, W.L.E.; Demiate, I.M. Cassava Starch Films Reinforced with Lignocellulose Nanofibers from Cassava Bagasse. Int. J. Biol. Macromol. 2019, 139, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Karlovits, I. Lignocellulosic Bio-Refinery Downstream Products in Future Packaging Applications. Int. Symp. Graph. Eng. Des. 2020, 39–53. [Google Scholar] [CrossRef]
- Tajeddin, B. Cellulose-Based Polymers for Packaging Applications. In Lignocellulosic Polymer Composites: Processing, Characterization, and Properties; Scrivener Publishing LLC: Beverly, MA, USA, 2014; pp. 477–498. ISBN 978-1-118-77357-4. [Google Scholar]
- Shaghaleh, H.; Xu, X.; Wang, S. Current Progress in Production of Biopolymeric Materials Based on Cellulose, Cellulose Nanofibers, and Cellulose Derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and Surface Modification of Cellulose Bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable Carboxymethyl Cellulose Based Material for Sustainable Packaging Application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Luo, W.; Chen, G.; Xiao, N.; Xiao, G.; Liu, C. Development of Functional Hydroxyethyl Cellulose-Based Composite Films for Food Packaging Applications. Front. Bioeng. Biotechnol. 2022, 10, 989893. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Zhong, Y.; Zhao, Y.; Gao, P.; Tian, F.; Zhang, X.; Zhou, R.; Cullen, P.J. Emerging Food Packaging Applications of Cellulose Nanocomposites: A Review. Polymers 2022, 14, 4025. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent Trends and Applications in the Food Industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Zhang, X.; Ren, S.; Lei, T.; Li, W.; Xu, G.; Zhang, Q. Nanocellulose Films with Combined Cellulose Nanofibers and Nanocrystals: Tailored Thermal, Optical and Mechanical Properties. Cellulose 2018, 25, 1103–1115. [Google Scholar] [CrossRef]
- Shi, H.; Wu, L.; Luo, Y.; Yu, F.; Li, H. A Facile Method to Prepare Cellulose Fiber-Based Food Packaging Papers with Improved Mechanical Strength, Enhanced Barrier, and Antibacterial Properties. Food Biosci. 2022, 48, 101729. [Google Scholar] [CrossRef]
- Brodnjak, U.V. Microorganism Based Biopolymer Materials for Packaging Applications: A Review. J. Compos. Biodegrad. Polym. 2016, 4, 32–40. [Google Scholar] [CrossRef]
- Verdini, F.; Tabasso, S.; Mariatti, F.; Bosco, F.; Mollea, C.; Calcio Gaudino, E.; Cirio, A.; Cravotto, G. From Agri-Food Wastes to Polyhydroxyalkanoates through a Sustainable Process. Fermentation 2022, 8, 556. [Google Scholar] [CrossRef]
- Szacherska, K.; Moraczewski, K.; Czaplicki, S.; Oleskowicz-Popiel, P.; Mozejko-Ciesielska, J. Effect of Short- and Medium-Chain Fatty Acid Mixture on Polyhydroxyalkanoate Production by Pseudomonas Strains Grown under Different Culture Conditions. Front. Bioeng. Biotechnol. 2022, 10, 1583. [Google Scholar] [CrossRef]
- Bulantekin, Ö.; Alp, D.; Bulantekin, Ö.; Alp, D. Development of Food Packaging Films from Microorganism-Generated Polyhydroxyalkanoates; IntechOpen: London, UK, 2022; ISBN 978-1-80356-996-3. [Google Scholar]
- Koller, M. Poly(Hydroxyalkanoates) for Food Packaging: Application and Attempts towards Implementation. Appl. Food Technol. Biotechnol. 2014, 1, 3–15. [Google Scholar] [CrossRef]
- Reddy, V.U.N.; Ramanaiah, S.V.; Reddy, M.V.; Chang, Y.-C. Review of the Developments of Bacterial Medium-Chain-Length Polyhydroxyalkanoates (Mcl-PHAs). Bioengineering 2022, 9, 225. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Kumar, V.; Yadav, V.; Sarsaiya, S.; Awasthi, S.K.; Sindhu, R.; Binod, P.; Kumar, V.; Pandey, A.; Zhang, Z. Current State of the Art Biotechnological Strategies for Conversion of Watermelon Wastes Residues to Biopolymers Production: A Review. Chemosphere 2022, 290, 133310. [Google Scholar] [CrossRef]
- Pereira, J.R.; Araújo, D.; Freitas, P.; Marques, A.C.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Fortunato, E.; Reis, M.A.M.; Freitas, F. Production of Medium-Chain-Length Polyhydroxyalkanoates by Pseudomonas Chlororaphis Subsp. Aurantiaca: Cultivation on Fruit Pulp Waste and Polymer Characterization. Int. J. Biol. Macromol. 2021, 167, 85–92. [Google Scholar] [CrossRef]
- Angelini, S.; Cerruti, P.; Immirzi, B.; Scarinzi, G.; Malinconico, M. Acid-Insoluble Lignin and Holocellulose from a Lignocellulosic Biowaste: Bio-Fillers in Poly(3-Hydroxybutyrate). Eur. Polym. J. 2016, 76, 63–76. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M.; Holzer, A.; Stańczyk, D.; Sabura, E. Selected Fatty Acids Esters as Potential PHB-V Bioplasticizers: Effect on Mechanical Properties of the Polymer. J. Polym. Environ. 2021, 29, 38–53. [Google Scholar] [CrossRef]
- de Sousa Junior, R.R.; dos Santos, C.A.S.; Ito, N.M.; Suqueira, A.N.; Lackner, M.; dos Santos, D.J. PHB Processability and Property Improvement with Linear-Chain Polyester Oligomers Used as Plasticizers. Polymers 2022, 14, 4197. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.H.; Wainaina, S.; Taherzadeh, M.J.; Åkesson, D.; Ferreira, J.A. Production of Polyhydroxyalkanoates (PHAs) by Bacillus Megaterium Using Food Waste Acidogenic Fermentation-Derived Volatile Fatty Acids. Bioengineered 2021, 12, 2480–2498. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(Lactic Acid)—Mass Production, Processing, Industrial Applications, and End of Life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [Green Version]
- Muneer, F.; Nadeem, H.; Arif, A.; Zaheer, W. Bioplastics from Biopolymers: An Eco-Friendly and Sustainable Solution of Plastic Pollution. Polym. Sci. Ser. C 2021, 63, 47–63. [Google Scholar] [CrossRef]
- Nduko, J.M.; Taguchi, S. Microbial Production of Biodegradable Lactate-Based Polymers and Oligomeric Building Blocks From Renewable and Waste Resources. Front. Bioeng. Biotechnol. 2021, 8, 77. [Google Scholar] [CrossRef]
- Boey, J.Y.; Mohamad, L.; Khok, Y.S.; Tay, G.S.; Baidurah, S. A Review of the Applications and Biodegradation of Polyhydroxyalkanoates and Poly(Lactic Acid) and Its Composites. Polymers 2021, 13, 1544. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (Lactic Acid) and Poly (Glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Suaduang, N.; Ross, S.; Ross, G.M.; Wangsoub, S.; Mahasaranon, S. The Physical and Mechanical Properties of Biocomposite Films Composed of Poly(Lactic Acid) with Spent Coffee Grounds. Key Eng. Mater. 2019, 824, 87–93. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-Based Biodegradable Active Packaging and Its Application to Salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef]
- Huang, S.; Xue, Y.; Yu, B.; Wang, L.; Zhou, C.; Ma, Y. A Review of the Recent Developments in the Bioproduction of Polylactic Acid and Its Precursors Optically Pure Lactic Acids. Molecules 2021, 26, 6446. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent Advances in Chitin Based Materials Constructed via Physical Methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical Properties of Chitin and Chitosan Biopolymers with Application to Structural Color Analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Khaled, A. A Review on Natural Biodegradable Materials: Chitin and Chitosan. Chem. Adv. Mater. 2021, 6, 1–5. [Google Scholar]
- Pandharipande, S.; Bhagat, P.; Tech, B.; Semester, T. Synthesis of Chitin from Crab Shells and Its Utilization in Preparation of Nanostructured Film. Int. J. Sci. Eng. Technol. Res. 2016, 5, 2278–7798. [Google Scholar]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active Natural-Based Films for Food Packaging Applications: The Combined Effect of Chitosan and Nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef]
- Rubilar, J. Effect of Antioxidant and Optimal Antimicrobial Mixtures of Carvacrol, Grape Seed Extract and Chitosan on Different Spoilage Microorganisms and Their Application as Coatings on Different Food Matrices. Int. J. Food Stud. 2013, 2, 22–38. [Google Scholar] [CrossRef]
- Wan, A.; Xu, Q.; Li, H. Antioxidant Activity of High Molecular Weight Chitosan and N,O-Quaternized Chitosans. J. Agric. Food Chem. 2013, 61. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Gelatin-Chitosan Edible Film Activated with Boldo Extract for Improving Microbiological and Antioxidant Stability of Sliced Prato Cheese. Int. J. Food Sci. Technol. 2019, 54, 1617–1624. [Google Scholar] [CrossRef]
- Kasai, D.R.; Radhika, D.; Chalannavar, R.K.; Chougale, R.B.; Mudigoudar, B.; Kasai, D.R.; Radhika, D.; Chalannavar, R.K.; Chougale, R.B.; Mudigoudar, B. A Study on Edible Polymer Films for Food Packaging Industry: Current Scenario and Advancements; IntechOpen: London, UK, 2022; ISBN 978-1-83768-226-3. [Google Scholar]
- Rhim, J.W.; Shellhammer, T.H. Lipid-Based Edible Films and Coatings. In Innovations in Food Packaging; Han, J.H., Ed.; Food Science and Technology; Elsevier: London, UK, 2005; pp. 362–383. ISBN 978-0-12-311632-1. [Google Scholar]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in Biodegradable Active Films for Food Packaging: Effects of Nano/Microcapsule Incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, K.; Ayadi, M.; Allouche, N.; Chemtob, A. Renewable Photopolymer Films Derived from Low-Grade Lampante and Pomace Olive Oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1700003. [Google Scholar] [CrossRef] [Green Version]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of Edible Films and Coatings Formulated with Cassava Starch, Glycerol, Carnauba Wax and Stearic Acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Caceres, C.A.; Ribeiro, H.L.; de Abreu, R.F.A.; Cunha, A.P.; Azeredo, H.M.C. Influence of Cassava Starch and Carnauba Wax on Physical Properties of Cashew Tree Gum-Based Films. Food Hydrocoll. 2014, 38, 147–151. [Google Scholar] [CrossRef]
- Mali, S. Biodegradable Foams in the Development of Food Packaging. In Polymers for Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 329–345. ISBN 978-3-319-94624-5/978-3-319-94625-2. [Google Scholar]
- Araque, L.M.; Alvarez, V.A.; Gutiérrez, T.J. Composite Foams Made from Biodegradable Polymers for Food Packaging Applications. In Polymers for Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 347–355. ISBN 978-3-319-94624-5/978-3-319-94625-2. [Google Scholar]
- Engel, J.B.; Ambrosi, A.; Tessaro, I.C. Development of Biodegradable Starch-Based Foams Incorporated with Grape Stalks for Food Packaging. Carbohydr. Polym. 2019, 225, 115234. [Google Scholar] [CrossRef]
- Sohn, J.; Kim, H.; Cha, S. Bio-Based Foamed Cushioning Materials Using Polypropylene and Wheat Bran. Sustainability 2019, 11, 1670. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho, F.A.; Bilck, A.P.; Yamashita, F.; Mali, S. Baked Foams Based on Cassava Starch Coated with Polyvinyl Alcohol with a Higher Degree of Hydrolysis. J. Polym. Environ. 2018, 26, 1445–1452. [Google Scholar] [CrossRef]
- Rodrigues, N.H.P.; de Souza, J.T.; Rodrigues, R.L.; Canteri, M.H.G.; Tramontin, S.M.K.; de Francisco, A.C. Starch-Based Foam Packaging Developed from a By-Product of Potato Industrialization (Solanum tuberosum L.). Appl. Sci. 2020, 10, 2235. [Google Scholar] [CrossRef] [Green Version]
- Donati, N.; Spada, J.C.; Tessaro, I.C. Recycling Rice Husk Ash as a Filler on Biodegradable Cassava Starch-Based Foams. Polym. Bull. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Dassanayake, R.S.; Fernando, N.M.L.; Wanninayaka, D.B.; Rajapaksha, S.M.; Manamperi, A.; Gangoda, M.; Manchanda, A.; et al. Preparation and Characterization of Dual-Modified Cassava Starch-Based Biodegradable Foams for Sustainable Packaging Applications. ACS Omega 2022, 7, 19579–19590. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Barros Ferreira, R.S.; Lizárraga, E.; Tapia-Blácido, D.R.; Silva, N.C.C.; Angelats-Silva, L.; Siche, R. Bioactive Andean Sweet Potato Starch-Based Foam Incorporated with Oregano or Thyme Essential Oil. Food Packag. Shelf Life 2020, 23, 100457. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Soares, N.F.F.; Stringheta, P.C. Development of Colorimetric Altered Intelligent Packaging Incorporated with Anthocyanins: A Critical Review. Braz. J. Food Technol. 2021, 24, 2021033. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [Green Version]
- Aman Mohammadi, M.; Dakhili, S.; Mirza Alizadeh, A.; Kooki, S.; Hassanzadazar, H.; Alizadeh-Sani, M.; McClements, D.J. New Perspectives on Electrospun Nanofiber Applications in Smart and Active Food Packaging Materials. Crit. Rev. Food Sci. Nutr. 2022, 19, 1–17. [Google Scholar] [CrossRef]
- Siemann, U. Solvent Cast Technology—A Versatile Tool for Thin Film Production. In Progress in Colloid & Polymer Science: Scattering Methods and the Properties of Polymer Materials; Kremer, F., Richtering, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; p. 175. ISBN 9783540253235. [Google Scholar]
- Abdul Khalil, H.P.S.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Rhim, J.W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the Processing Methods on the Performance of Polylactide Films: Thermocompression versus Solvent Casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Lin, Y.; Kang, K.; Chen, F.; Zhang, L.; Lavernia, E.J. Gradient Metal Matrix Composites. In Comprehensive Composite Materials II; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 4, pp. 331–346. ISBN 9780081005330. [Google Scholar]
- Scheibe, A.S.; De Moraes, J.O.; Laurindo, J.B. Production and Characterization of Bags from Biocomposite Films of Starch-Vegetal Fibers Prepared by Tape Casting. J. Food Process Eng. 2014, 37, 482–492. [Google Scholar] [CrossRef]
- De Moraes, J.O.; Scheibe, A.S.; Sereno, A.; Laurindo, J.B. Scale-up of the Production of Cassava Starch Based Films Using Tape-Casting. J. Food Eng. 2013, 119, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Rao, H.G.R.; Thejaswini, M.L. Extrusion Technology: A Novel Method Of Food Processing. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 358–369. [Google Scholar]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Extruded Films of Blended Chitosan, Low Density Polyethylene and Ethylene Acrylic Acid. Carbohydr. Polym. 2013, 91, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Filli, K.B.; Jideani, A.I.O.; Jideani, V.A. Extrusion Bolsters Food Security in Africa. Food Technol. 2014, 68, 46–52. [Google Scholar]
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Llamazares, S.R. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 14, 136–177. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The Potential of Proteins for Producing Food Packaging Materials: A Review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Packaging Europe Thermoforming With Biobased Plastics for Greater Sustainability. Available online: https://packagingeurope.com/thermoforming-with-biobased-plastics-for-greater-sustainability/1447.article (accessed on 15 December 2022).
- Averous, L.; Fringant, C.; Moro, L. Starch-Based Biodegradable Materials Suitable for Thermoforming Packaging. Starch 2001, 53, 368. [Google Scholar] [CrossRef]
- Tatara, R.A. Compression Molding. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 291–320. ISBN 9780323390408. [Google Scholar]
- Soffarina, M. Methodology of Press System of Compression Moulding. 2016. Available online: https://www.researchgate.net/publication/301754897_Methodology_of_Press_System_of_Compression_Moulding?channel=doi&linkId=5725f2db08aee491cb3ef741&showFulltext=true (accessed on 12 March 2023). [CrossRef]
- Zubeldía, F.; Ansorena, M.R.; Marcovich, N.E. Material Characterisation Wheat Gluten Films Obtained by Compression Molding. Polym. Test. 2015, 43, 68–77. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of Yerba Mate Extract on the Performance of Starch Films Obtained by Extrusion and Compression Molding as Active and Smart Packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Finazzi, M.; Tavazzi, S.; Piergiovanni, L. Tunable Green Oxygen Barrier through Layer-by-Layer Self-Assembly of Chitosan and Cellulose Nanocrystals. Carbohydr. Polym. 2013, 92, 2128–2134. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef] [Green Version]
- Goksen, G.; Nisha, P.; Ibrahim Ekiz, H. Electrospinning Technology: Its Process Conditions and Food Packaging Applications. In Food Engineering Series; Režek Jambrak, A., Ed.; Springer: Cham, Switzerland, 2022; pp. 447–468. [Google Scholar]
- Leidy, R.; Maria Ximena, Q.-C. Use of Electrospinning Technique to Produce Nanofibres for Food Industries: A Perspective from Regulations to Characterisations. Trends Food Sci. Technol. 2019, 85, 92–106. [Google Scholar] [CrossRef]
- Dhiman, A.; Suhag, R.; Singh, A.; Prabhakar, P.K. Mechanistic Understanding and Potential Application of Electrospraying in Food Processing: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8288–8306. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; Jiang, S.; He, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L. Biorefinery as a Promising Approach to Promote Microalgae Industry: An Innovative Framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Senthil Kumar, P.; Varjani, S. Valorization of Agro-Industrial Wastes for Biorefinery Process and Circular Bioeconomy: A Critical Review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar] [CrossRef]
- Demirbas, A.; Fatih Demirbas, M. Importance of Algae Oil as a Source of Biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Jõgi, K.; Bhat, R. Valorization of Food Processing Wastes and By-Products for Bioplastic Production. Sustain. Chem. Pharm. 2020, 18, 100326. [Google Scholar] [CrossRef]
- Redondo-Gómez, C.; Quesada, M.R.; Astúa, S.V.; Zamora, J.P.M.; Lopretti, M.; Vega-Baudrit, J.R. Biorefinery of Biomass of Agro-Industrial Banana Waste to Obtain High-Value Biopolymers. Molecules 2020, 25, 3829. [Google Scholar] [CrossRef]
- Panyasiri, P.; Yingkamhaeng, N.; Lam, N.T.; Sukyai, P. Extraction of Cellulose Nanofibrils from Amylase-Treated Cassava Bagasse Using High-Pressure Homogenization. Cellulose 2018, 25, 1757–1768. [Google Scholar] [CrossRef]
- Saelee, K.; Yingkamhaeng, N.; Nimchua, T.; Sukyai, P. An Environmentally Friendly Xylanase-Assisted Pretreatment for Cellulose Nanofibrils Isolation from Sugarcane Bagasse by High-Pressure Homogenization. Ind. Crops Prod. 2016, 82, 149–160. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Production of Lignocellulose Nanofibers from Wheat Straw by Different Fibrillation Methods. Comparison of Its Viability in Cardboard Recycling Process. J. Clean. Prod. 2019, 239, 118083. [Google Scholar] [CrossRef]
- Hideno, A.; Abe, K.; Yano, H. Preparation Using Pectinase and Characterization of Nanofibers from Orange Peel Waste in Juice Factories. J. Food Sci. 2014, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, X.; Lin, L.; Zhao, L.; Liu, A.; Wei, C. Properties of Starch from Root Tuber of Stephania Epigaea in Comparison with Potato and Maize Starches. Int. J. Food Prop. 2017, 20, 1740–1750. [Google Scholar] [CrossRef] [Green Version]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Garofulić, I.E.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica Dioica l.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef]
- Ngoc, H.N.; Mair, L.; Nghiem, D.T.; Le Thien, K.; Gostner, J.M.; Stuppner, H.; Ganzera, M. Phenolic Compounds from the Stems of Fissistigma Polyanthoides and Their Anti-Oxidant Activities. Fitoterapia 2019, 137, 104252. [Google Scholar] [CrossRef]
- Lima, A.R.; Cristofoli, N.L.; Rosa, A.M. Comparative Study of the Production of Cellulose Nanofibers from Agro-Industrial Waste Streams of Salicornia Ramosissima by Acid and Enzymatic Treatment. Food Bioprod. Process. 2023, 137, 214–225. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An Overview of the Recent Trends on the Waste Valorization Techniques for Food Wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, P. Life Cycle Assessment: Blazing a Trail for Bioresources Management. Energy Convers. Manag. X 2020, 10, 100063. [Google Scholar] [CrossRef]
- Naranjo, J.M.; Cardona, C.A.; Higuita, J.C. Use of Residual Banana for Polyhydroxybutyrate (PHB) Production: Case of Study in an Integrated Biorefinery. Waste Manag. 2014, 34, 2634–2640. [Google Scholar] [CrossRef]
- Ioannidou, S.M.; Pateraki, C.; Ladakis, D.; Papapostolou, H.; Tsakona, M.; Vlysidis, A.; Kookos, I.K.; Koutinas, A. Sustainable Production of Bio-Based Chemicals and Polymers via Integrated Biomass Refining and Bioprocessing in a Circular Bioeconomy Context. Bioresour. Technol. 2020, 307, 123093. [Google Scholar] [CrossRef]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A Review on Circular Economy: The Expected Transition to a Balanced Interplay of Environmental and Economic Systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Thi, N.B.D.; Kumar, G.; Lin, C.Y. An Overview of Food Waste Management in Developing Countries: Current Status and Future Perspective. J. Environ. Manag. 2015, 157, 220–229. [Google Scholar] [CrossRef]
- Simon, F. EU Official: Further Efforts Needed to Address ‘Ecological Limits’ of Biomass–EURACTIV.Com. Available online: https://www.euractiv.com/section/biomass/interview/eu-official-further-efforts-needed-to-address-ecological-limits-of-biomass/ (accessed on 23 March 2023).
- European Commission. Biorefineries Distribution in the EU; Publications Office of the European Union: Brussels, Belgium, 2018. [Google Scholar]
- Yadav, V.; Sarker, A.; Yadav, A.; Miftah, A.O.; Bilal, M.; Iqbal, H.M.N. Integrated Biorefinery Approach to Valorize Citrus Waste: A Sustainable Solution for Resource Recovery and Environmental Management. Chemosphere 2022, 293, 133459. [Google Scholar] [CrossRef]
- Manhongo, T.T.; Chimphango, A.; Thornley, P.; Röder, M. Techno-Economic and Environmental Evaluation of Integrated Mango Waste Biorefineries. J. Clean. Prod. 2021, 325, 129335. [Google Scholar] [CrossRef]
- Manhongo, T.T.; Chimphango, A.; Thornley, P.; Röder, M. An Economic Viability and Environmental Impact Assessment of Mango Processing Waste-Based Biorefineries for Co-Producing Bioenergy and Bioactive Compounds. Renew. Sustain. Energy Rev. 2021, 148, 111216. [Google Scholar] [CrossRef]
- Demichelis, F.; Fiore, S.; Pleissner, D.; Venus, J. Technical and Economic Assessment of Food Waste Valorization through a Biorefinery Chain. Renew. Sustain. Energy Rev. 2018, 94, 38–48. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral Use of Orange Peel Waste through the Biorefinery Concept: An Experimental, Technical, Energy, and Economic Assessment. Biomass Convers. Biorefinery 2021, 11, 645–659. [Google Scholar] [CrossRef]
- Caldeira, C.; Vlysidis, A.; Fiore, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of Food Waste Biorefinery: A Review on Valorisation Pathways, Techno-Economic Constraints, and Environmental Assessment. Bioresour. Technol. 2020, 312, 123575. [Google Scholar] [CrossRef]
- Giller, C.; Malkani, B.; Parasar, J. Coffee to Biofuels; Penn Libraries: Philadelphia, PA, USA, 2017. [Google Scholar]
- Tokimoto, T.; Kawasaki, N.; Nakamura, T.; Akutagawa, J.; Tanada, S. Removal of Lead Ions in Drinking Water by Coffee Grounds as Vegetable Biomass. J. Colloid Interface Sci. 2005, 281, 56–61. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential Co-Production of Biodiesel and Bioethanol with Spent Coffee Grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of Oil Extracted from Spent Coffee Grounds for Sustainable Production of Polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- IEA Bioenergy. Bio-Based Chemicals: Value Added Products from Biorefineries; IEA Bioenergy: Wageningen, The Netherlands, 2012. [Google Scholar]
- Tock, J.Y.; Lai, C.L.; Lee, K.T.; Tan, K.T.; Bhatia, S. Banana Biomass as Potential Renewable Energy Resource: A Malaysian Case Study. Renew. Sustain. Energy Rev. 2010, 14, 798–805. [Google Scholar] [CrossRef]
- Harish, K.; Srijana, M.; Madhusudhan, R.; Gopal, R. Coculture Fermentation of Banana Agro-Waste to Ethanol by Cellulolytic Thermophilic Clostridium Thermocellum CT2. Afr. J. Biotechnol. 2012, 9, 1926–1934. [Google Scholar] [CrossRef] [Green Version]
- Duque, S.H.; Cardona, C.A.; Moncada, J. Techno-Economic and Environmental Analysis of Ethanol Production from 10 Agroindustrial Residues in Colombia. Energy Fuels 2015, 29, 775–783. [Google Scholar] [CrossRef]
- Guerrero, A.B.; Ballesteros, I.; Ballesteros, M. The Potential of Agricultural Banana Waste for Bioethanol Production. Fuel 2018, 213, 176–185. [Google Scholar] [CrossRef]
- Tarrés, Q.; Espinosa, E.; Domínguez-Robles, J.; Rodríguez, A.; Mutjé, P.; Delgado-Aguilar, M. The Suitability of Banana Leaf Residue as Raw Material for the Production of High Lignin Content Micro/Nano Fibers: From Residue to Value-Added Products. Ind. Crops Prod. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Getachew, A.; Woldesenbet, F. Production of Biodegradable Plastic by Polyhydroxybutyrate (PHB) Accumulating Bacteria Using Low Cost Agricultural Waste Material. BMC Res. Notes 2016, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Bonilla, P.; Salas-Arias, J.; Esquivel, M.; Vega-Baudrit, J.R. Optimization of Microwave-Assisted and Conventional Heating Comparative Synthesis of Poly(Lactic Acid) by Direct Melt Polycondensation from Agroindustrial Banana (Musa AAA Cavendish) and Pineapple (Ananas Comosus) Fermented Wastes. J. Polym. Environ. 2014, 22, 393–397. [Google Scholar] [CrossRef]
- Vijay, R.; Tarika, K. Banana Peel as an Inexpensive Carbon Source for Microbial Polyhydroxyalkanoate (PHA) Production. Int. Res. J. Environ. Aciences 2018, 7, 28–36. [Google Scholar]
- European Bioplastics Bioplastic Market Data. Available online: https://www.european-bioplastics.org/market/ (accessed on 4 January 2023).
- Grand View Research. Food Packaging Market Size, Share & Growth Report, 2030; Grand View Research: San Francisco, CA, USA, 2022. [Google Scholar]
- Grand View Research. Global Industrial Starch Market Size Report, 2020–2028; Grand View Research: San Francisco, CA, USA, 2021. [Google Scholar]
- Facts & Factors. Global Cellulose Fiber Market Size Worth; Facts & Factors: Pune, India, 2022. [Google Scholar]
- Fact.MR. Pigments Market Size, Share Industry Growth 2031; Fact.MR: Dublin, Ireland, 2021. [Google Scholar]
- New Food. Study Shows Growth in the Polysaccharides and Oligosaccharides Market; New Food: Kent, UK, 2019. [Google Scholar]
- Grand View Research. Global Antimicrobial Coatings Market Size Report, 2030; Grand View Research: San Francisco, CA, USA, 2021. [Google Scholar]
- Grand View Research. Chitosan Market Size-Global Industry Analysis Report, 2020–2027; Grand View Research: San Francisco, CA, USA, 2021. [Google Scholar]
- Fortune Business Insights. Antioxidants Market Size, Share-Global Report [2021–2028]; Fortune Business Insights: Pune, India, 2021. [Google Scholar]
- Straits Research. Pectin Market Trend, Growth to 2022–2030; Straits Research: Pune, India, 2022. [Google Scholar]
- Fortune Business Insights. Polylactic Acid Market Size & Share-Global Report [2021–2028]; Fortune Business Insights: Pune, India, 2021. [Google Scholar]
- Fortune Business Insights. Nanocellulose Market Size & Growth-Global Report [2020–2027]; Fortune Business Insights: Pune, India, 2021. [Google Scholar]
- Credence Research. Polyhydroxybutyrate (PHB) Market Size, Trends & Share-2028; Credence Research: Pune, India, 2021. [Google Scholar]
- Global Market Insights. Polyhydroxyalkanoate Market Size-Industry Report, 2022–2030; Global Market Insights: Selbyville, DE, USA, 2022. [Google Scholar]
- CelluForce About CelluForce. Available online: https://celluforce.com/about-celluforce/ (accessed on 24 January 2023).
- VTT Cellulose Films and Coatings. Available online: https://www.vttresearch.com/en/ourservices/cellulose-films-and-coatings (accessed on 24 January 2023).
- Tejayadi, S.; Cheryan, M. Lactic Acid from Cheese Whey Permeate. Productivity and Economics of a Continuous Membrane Bioreactor. Appl. Microbiol. Biotechnol. 1995, 43, 242–248. [Google Scholar] [CrossRef]
- Pharmacompass Lactic Acid-Price Per Kg. Available online: https://www.pharmacompass.com/price/lactic-acid (accessed on 14 December 2022).
- Biddy, M.J.; Scarlata, C.; Kinchin, C. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential; Alliance for Sustainable Energy, LLC: Golden, CO, USA, 2016. [Google Scholar]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and Opportunities in Lactic Acid Bioprocess Design—From Economic to Production Aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Grand View Research. Global Polylactic Acid Market Size Report, 2022–2030; Grand View Research: San Francisco, CA, USA, 2022. [Google Scholar]
- Galactic Galactic Group PLA Production Unit Was Launched in China. Available online: https://www.lactic.com/en/news/galactic-group-pla-production-unit-was-launched-china (accessed on 19 December 2022).
- Futerro Futerro Aims to Set-up a New Fully Integrated PLA Biorefinery in Normandy, France. Available online: https://www.futerro.com/news-media/futerro-aims-set-new-fully-integrated-pla-biorefinery-normandy-france (accessed on 19 December 2022).
- Corbion Alternative Feedstock-Sustainable Resource for Bioplastics. Available online: https://www.corbion.com/en/Innovation/Alternative-feedstock (accessed on 21 December 2022).
- NatureWorks How Ingeo Is Made. Available online: https://www.natureworksllc.com/What-is-Ingeo/How-Ingeo-is-Made (accessed on 20 December 2022).
- Lopez-Arenas, T.; González-Contreras, M.; Anaya-Reza, O.; Sales-Cruz, M. Analysis of the Fermentation Strategy and Its Impact on the Economics of the Production Process of PHB (Polyhydroxybutyrate). Comput. Chem. Eng. 2017, 107, 140–150. [Google Scholar] [CrossRef]
- Bio-on Turn off Pollution. Available online: http://www.bio-on.it/?lin=portoghese (accessed on 21 December 2022).
- Full Cycle Produce PHA Biopolymers. Available online: https://fullcyclebio.com/solutions/ (accessed on 4 January 2023).
- Genecis Waste into High Value Materials. Available online: https://genecis.co/ (accessed on 3 January 2023).
- European Bioplastics Market Drivers and Development. Available online: https://www.european-bioplastics.org/market/market-drivers/ (accessed on 5 January 2023).
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of Bioplastic through Food Waste Valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- FoodPrint The Environmental Impact of Food Packaging. Available online: https://foodprint.org/issues/the-environmental-impact-of-food-packaging/#easy-footnote-bottom-1-1295 (accessed on 17 November 2022).
- Shin, J.; Selke, S.E.M. Food Packaging. In Food Processing: Principles and Applications; Clark, S., Jung, S., Lamsal, B., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 249–268. ISBN 978-0-470-67114-6. [Google Scholar]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Licciardello, F. Packaging, Blessing in Disguise. Review on Its Diverse Contribution to Food Sustainability. Trends Food Sci. Technol. 2017, 65, 32–39. [Google Scholar] [CrossRef]
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of Bioplastics for Food Packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- EPA Reducing Wasted Food & Packaging: A Guide for Food Services and Restaurants. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/reducing_wasted_food_pkg_tool.pdf (accessed on 14 January 2023).
- Eurostat Packaging Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Packaging_waste_statistics (accessed on 10 December 2022).
- European Comission. Regulation of the European Parliament and of the Council on Packaging and Packaging Waste, Amending Regulation (EU) 2019/1020 and Directive (EU) 2019/904, and Repealing Directive 94/62/EC; European Comission: Brussels, Belgium, 2020. [Google Scholar]
- Dörnyei, K.R.; Bauer, A.; Krauter, V.; Herbes, C. (Not) Communicating the Environmental Friendliness of Food Packaging to Consumers—An Attribute- and Cue-Based Concept and Its Application. Foods 2022, 11, 1371. [Google Scholar] [CrossRef]
- Herbes, C.; Beuthner, C.; Ramme, I. How Green Is Your Packaging—A Comparative International Study of Cues Consumers Use to Recognize Environmentally Friendly Packaging. Int. J. Consum. Stud. 2020, 29, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Testa, F.; Iovino, R.; Iraldo, F. The Circular Economy and Consumer Behaviour: The Mediating Role of Information Seeking in Buying Circular Packaging. Bus. Strategy Environ. 2020, 29, 3435–3448. [Google Scholar] [CrossRef]
- United Nations Development Programme. The Sustainable Development Goals; United Nations Development Programme (UNDP): New York, NY, USA, 2015. [Google Scholar]
- ASTM:D6400-22; Standard Specification for Labeling of Plastics Designed to Be Aerobically Composted in Municipal or Industrial Facilities. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM:D5388-15; Biodegradation Test-Composting. ASTM: West Conshohocken, PA, USA, 2021.
- López-ibáñez, S.; Beiras, R. Science of the Total Environment Is a Compostable Plastic Biodegradable in the Sea ? A Rapid Standard Protocol to Test Mineralization in Marine Conditions. 2022, 831, 154860. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Bartkiene, E.; Florença, S.G.; Djekić, I.; Bizjak, M.Č.; Tarcea, M.; Leal, M.; Ferreira, V.; Rumbak, I.; Orfanos, P.; et al. Environmental Issues as Drivers for Food Choice: Study from a Multinational Framework. Sustainability 2021, 13, 2869. [Google Scholar] [CrossRef]
- Macena, M.W.; Carvalho, R.; Cruz-Lopes, L.P.; Guiné, R.P.F. Plastic Food Packaging: Perceptions and Attitudes of Portuguese Consumers about Environmental Impact and Recycling. Sustainability 2021, 13, 9953. [Google Scholar] [CrossRef]
- Ipsos Consumers Want Brands to Help Them Reduce Their Waste. Available online: https://www.ipsos.com/en-us/news-polls/Consumers-want-brands-to-help-them-reduce-their-waste (accessed on 19 November 2022).
- United Nations Development Programme. Peoples’ Climate Vote; United Nations Development Programme (UNDP): New York, NY, USA, 2021. [Google Scholar]
- The Coca-Cola Company Sprite Switching from Green to Clear PET Bottles in Southeast Asia. Available online: https://www.coca-colacompany.com/press-releases/sprite-switching-to-clear-pet-bottles-in-southeast-asia (accessed on 18 November 2022).
- Packaging Europe On-the-Go Cup Recycling Scheme Launched by McDonald’s and Costa. Available online: https://packagingeurope.com/news/on-the-go-cup-recycling-scheme-launched-by-mcdonalds-and-costa/8492.article (accessed on 18 November 2022).
- Danone, S.A. Ellen MacArthur Foundation. Available online: https://ellenmacarthurfoundation.org/global-commitment-2021/signatory-reports/ppu/danone-sa (accessed on 30 November 2022).
- PepsiCo Europe PepsiCo Europe Sets Ambition to Eliminate Virgin Fossil-Based Plastic in all of Its Crisp and Chip Bags by the End of the Decade. Available online: https://www.pepsico.com/our-stories/press-release/pepsico-europe-sets-ambition-to-eliminate-virgin-fossil-based-plastic-in-all-of-its-crisp-and-chip-bags-by-the-end-of-the-decade (accessed on 18 November 2022).
- Unilever We’re Introducing Paper Tubs for Our Carte D’or Ice Cream. Available online: https://www.unilever.com/news/news-search/2022/were-introducing-paper-tubs-for-our-carte-dor-ice-cream/ (accessed on 18 November 2022).
- The Kraft Heinz Company. Developing and Testing Recyclable Fiber-Based Microwavable Cup. Available online: https://news.kraftheinzcompany.com/press-releases-details/2021/Kraft-Mac--Cheese-Developing-and-Testing-Its-First-Recyclable-Fiber-Based-Microwavable-Cup/default.aspx (accessed on 18 November 2022).
- The Kraft Heinz Company. Shake ‘ N Bake to Save 900, 000 Pounds of Plastic Waste Annually with Brand ’s First-Ever Packaging Update. Available online: https://news.kraftheinzcompany.com/press-releases-details/2022/Shake-N-Bake-to-Save-900000-Pounds-of-Plastic-Waste-Annually-with-Brands-First-Ever-Packaging-Update/default.aspx (accessed on 18 November 2022).
- Mondelez International Our Commitment To 100% Recyclable Packaging. Available online: https://www.mondelezinternational.com/News/100-Recyclable-Packaging (accessed on 18 November 2022).
- News, F.B. Food Business News. Available online: https://www.foodbusinessnews.net/articles/15229-nestle-investing-2-billion-in-sustainable-packaging-innovation (accessed on 30 November 2022).
- Tesco PLC Tesco Engages Suppliers to Accelerate Plans to Tackle Plastic Waste. Available online: https://www.tescoplc.com/news/2022/tesco-engages-suppliers-to-accelerate-plans-to-tackle-plastic-waste/ (accessed on 18 November 2022).
- Starbucks Starbucks to Eliminate Plastic Straws Globally by 2020. Available online: https://news.starbucks.com/press-releases/starbucks-to-eliminate-plastic-straws-globally-by-2020 (accessed on 18 November 2022).
- Bacardi Limited Bacardi Cuts Plastic in Packaging. Available online: https://www.bacardilimited.com/media/news-archive/bacardi-cuts-plastic-in-packaging/ (accessed on 18 November 2022).
- G.A. Circular Accelerating the Circular Economy for Post-Consumer PET Bottles in Southeast Asia. Available online: https://www.gacircular.com/full-circle/ (accessed on 24 November 2022).
- Ligthart, T.N.; Ansems, T.A.M.M. Modelling of Recycling in LCA. Post-Consum. Waste Recycl. Optim. Prod. 2012, 185–210. [Google Scholar] [CrossRef] [Green Version]
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Genève, Switzerland, 2006.
- Pauer, E.; Wohner, B.; Heinrich, V.; Tacker, M. Assessing the Environmental Sustainability of Food Packaging: An Extended Life Cycle Assessment Including Packaging-Related Food Losses and Waste and Circularity Assessment. Sustainability 2019, 11, 925. [Google Scholar] [CrossRef] [Green Version]
- Siracusa, V.; Ingrao, C.; Lo Giudice, A.; Mbohwa, C.; Dalla Rosa, M. Environmental Assessment of a Multilayer Polymer Bag for Food Packaging and Preservation: An LCA Approach. Food Res. Int. 2014, 62, 151–161. [Google Scholar] [CrossRef]
- Toniolo, S.; Mazzi, A.; Niero, M.; Zuliani, F.; Scipioni, A. Comparative LCA to Evaluate How Much Recycling Is Environmentally Favourable for Food Packaging. Resour. Conserv. Recycl. 2013, 77, 61–68. [Google Scholar] [CrossRef]
- Wender, B.A.; Foley, R.W.; Prado-Lopez, V.; Ravikumar, D.; Eisenberg, D.A.; Hottle, T.A.; Sadowski, J.; Flanagan, W.P.; Fisher, A.; Laurin, L.; et al. Illustrating Anticipatory Life Cycle Assessment for Emerging Photovoltaic Technologies. Environ. Sci. Technol. 2014, 48, 10531–10538. [Google Scholar] [CrossRef]
- Bezergianni, S.; Chrysikou, L.P. Application of Life-Cycle Assessment in Biorefineries. In Waste Biorefinery: Integrating Biorefineries for Waste Valorisation; Bhaskar, T., Rene, E.R., Pandey, A., Tsang, D.C.w., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; pp. 455–480. ISBN 9780128182284. [Google Scholar]
- Elginoz, N.; Khatami, K.; Owusu-Agyeman, I.; Cetecioglu, Z. Life Cycle Assessment of an Innovative Food Waste Management System. Front. Sustain. Food Syst. 2020, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Leceta, I.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Bio-Based Films Prepared with by-Products and Wastes: Environmental Assessment. J. Clean. Prod. 2014, 64, 218–227. [Google Scholar] [CrossRef]
- Günkaya, Z.; Banar, M. An Environmental Comparison of Biocomposite Film Based on Orange Peel-Derived Pectin Jelly-Corn Starch and LDPE Film: LCA and Biodegradability. Int. J. Life Cycle Assess. 2016, 21, 465–475. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Papadaki, S.; Krokida, M. Life Cycle Analysis of β-Carotene Extraction Techniques. J. Food Eng. 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Papadaki, S.; Kyriakopoulou, K.; Tzovenis, I.; Krokida, M. Environmental Impact of Phycocyanin Recovery from Spirulina Platensis Cyanobacterium. Innov. Food Sci. Emerg. Technol. 2017, 44, 217–223. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae Based Biorefinery Promoting Circular Bioeconomy-Techno Economic and Life-Cycle Analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lane, J.L.; Grant, T.; Pratt, S.; Lant, P.A.; Laycock, B. Environmental Impact of Biodegradable Food Packaging When Considering Food Waste. J. Clean. Prod. 2018, 180, 325–334. [Google Scholar] [CrossRef]
- Lam, C.; Yu, I.K.M.; Hsu, S.; Tsang, D.C.W. Life-Cycle Assessment on Food Waste Valorisation to Value-Added Products. J. Clean. Prod. 2018, 199, 840–848. [Google Scholar] [CrossRef]
- Croxatto Vega, G.; Sohn, J.; Voogt, J.; Birkved, M.; Olsen, S.I.; Nilsson, A.E. Insights from Combining Techno-Economic and Life Cycle Assessment—A Case Study of Polyphenol Extraction from Red Wine Pomace. Resour. Conserv. Recycl. 2021, 167, 105318. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Dávila, I.; Labidi, J.; Gonzalez-Garcia, S. Comparative environmental Life Cycle Assessment of integral revalorization of vine shoots from a biorefinery perspective. Sci. Total Environ. 2018, 624, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Garcia, S.; Gullón, B.; Moreira, M.T. Environmental Assessment of Biorefinery Processes for the Valorization of Lignocellulosic Wastes into Oligosaccharides. J. Clean. Prod. 2018, 172, 4066–4073. [Google Scholar] [CrossRef] [Green Version]
- Santiago, B.; Arias Calvo, A.; Gullón, B.; Feijoo, G.; Moreira, M.T.; González-García, S. Production of Flavonol Quercetin and Fructooligosaccharides from Onion (Allium Cepa L.) Waste: An Environmental Life Cycle Approach. Chem. Eng. J. 2020, 392, 123772. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Castro-Puyana, M.; Börjesson, P.; Mendiola, J.A.; Turner, C.; Ibáñez, E. Life Cycle Assessment of Green Pilot-Scale Extraction Processes to Obtain Potent Antioxidants from Rosemary Leaves. J. Supercrit. Fluids 2012, 72, 205–212. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Life Cycle Assessment of a New Technology to Extract, Functionalize and Orient Cellulose Nanofibers from Food Waste. ACS Sustain. Chem. Eng. 2015, 3, 1047–1055. [Google Scholar] [CrossRef]
- do Nascimento, D.M.; Dias, A.F.; de Araújo Junior, C.P.; de Freitas Rosa, M.; Morais, J.P.S.; de Figueirêdo, M.C.B. A Comprehensive Approach for Obtaining Cellulose Nanocrystal from Coconut Fiber. Part II: Environmental Assessment of Technological Pathways. Ind. Crops Prod. 2016, 93, 58–65. [Google Scholar] [CrossRef]
- Vauchel, P.; Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of Ultrasound-Assisted Extraction of Polyphenols from Chicory Grounds under Different Operational Conditions. J. Clean. Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]
- Frascari, D.; Molina Bacca, A.E.; Wardenaar, T.; Oertlé, E.; Pinelli, D. Continuous Flow Adsorption of Phenolic Compounds from Olive Mill Wastewater with Resin XAD16N: Life Cycle Assessment, Cost–Benefit Analysis and Process Optimization. J. Chem. Technol. Biotechnol. 2019, 94, 1968–1981. [Google Scholar] [CrossRef]
- Garcia-Garcia, G.; Rahimifard, S.; Matharu, A.S.; Dugmore, T.I.J. Life-Cycle Assessment of Microwave-Assisted Pectin Extraction at Pilot Scale. ACS Sustain. Chem. Eng. 2019, 7, 5167–5175. [Google Scholar] [CrossRef] [Green Version]
- Kothari, R.; Pandey, A.; Ahmad, S.; Kumar, A.; Pathak, V.V.; Tyagi, V.V. Microalgal Cultivation for Value-Added Products: A Critical Enviro-Economical Assessment. 3 Biotech 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Ingrao, C.; Gigli, M.; Siracusa, V. An Attributional Life Cycle Assessment Application Experience to Highlight Environmental Hotspots in the Production of Foamy Polylactic Acid Trays for Fresh-Food Packaging Usage. J. Clean. Prod. 2017, 150, 93–103. [Google Scholar] [CrossRef]
- Tetra Pak LCA Examples Investigating Environmental Impact of Food Packaging. Available online: https://www.tetrapak.com/sustainability/planet/environmental-impact/a-value-chain-approach/life-cycle-assessment/lca-examples (accessed on 4 December 2022).
- Billerud How to Perform a Life Cycle Assessment of Packaging. Available online: https://www.billerudkorsnas.com/managed-packaging/knowledge-center/articles/how-to-perform-a-life-cycle-assessment-of-packaging (accessed on 18 December 2022).
- Coffigniez, F.; Matar, C.; Gaucel, S.; Gontard, N.; Guilbert, S.; Guillard, V. The Use of Modeling Tools to Better Evaluate the Packaging Benefice on Our Environment. Front. Sustain. Food Syst. 2021, 5, 38. [Google Scholar] [CrossRef]
- Corrado, S.; Ardente, F.; Sala, S.; Saouter, E. Modelling of Food Loss within Life Cycle Assessment: From Current Practice towards a Systematisation. J. Clean. Prod. 2017, 140, 847–859. [Google Scholar] [CrossRef]
- Omolayo, Y.; Feingold, B.J.; Neff, R.A.; Romeiko, X.X. Life Cycle Assessment of Food Loss and Waste in the Food Supply Chain. Resour. Conserv. Recycl. 2021, 164, 105119. [Google Scholar] [CrossRef]


| Name | Food Packaging Applications | Reference |
|---|---|---|
| Sugarcane bagasse | Disposable cups, plates, and carton boxes Polylactic acid (PLA) Polyhydroxyalkanoate (PHA) Polyurethanes Bio-polyethylene Starch-based nano-cellulosic bioplastics Carboxymethyl cellulose (CMC) biofilm Coating films | [26] [27] [28] |
| Rice straw | Disposable cups, plates, and carton boxes | [26] |
| Rice husk | CMC biofilm | [27] |
| Cocoa pod husk | Cellulose bioplastic film | [29] |
| Wheat straw | Polyhydroxy-co-3-butyrate-co-3-valerate (PHBV)/wheat straw fibers composite films | [30] |
| Corn waste | Biomaterials (paper and cardboard) | [31] |
| Cassava peels | Starch-based bioplastics Cellulose-based bioplastics PLA Poly hydroxybutyrate (PHB) | [32] |
| Banana peels | Starch-based bioplastics Cellulose-based bioplastics PLA PHB | [32] |
| Tomato peels | Cutin-based edible films Active bio-composites | [33] [34] |
| Apricot, cherry, and grape pomace | PHA | [35] |
| Crustacean shells waste | Chitin-based bioplastic Nanostructured film | [32] |
| Pomegranate peels | Films | [36] |
| Avocado seeds | Starch-based biofilms | [37] |
| Fish skin | Active films Gelatin | [38] |
| Spent coffee grounds | Phenolic compound PHA/PHB | [39] |
| Olive pomace | Gelling agent | [40] |
| Olive leaves and pomace | Active film | [41] |
| Grape pomace and olive leaf | Antioxidant film | [42] |
| Bio-Based Material | Market Size (USD) (Year) | Reference |
|---|---|---|
| Starch fiber | 97.85 Bn (2020) | [197] |
| Cellulose fiber | 35.20 Bn (2021) | [198] |
| Pigment | 34 Bn (2020) | [199] |
| Polysaccharide | 12.2 Bn (2018) | [200] |
| Antimicrobial coating | 9 Bn (2021) | [201] |
| Chitosan | 6.8 Bn (2019) | [202] |
| Antioxidant | 3.92 Bn (2020) | [203] |
| Pectin | 944.45 Mn (2021) | [204] |
| Polylactic Acid (PLA) | 698.200 Mn (2020) | [205] |
| Nanocellulose | 291.53 Mn (2019) | [206] |
| Poly-3-Hydroxybutyrate (PHB) | 102.4 Mn (2021) | [207] |
| Polyhydroxyalkanoates (PHA) | 85 Mn (2021) | [208] |
| Company | Packaging Strategy | Sustainable Action | Reference |
|---|---|---|---|
| Coca-Cola | Clear PET | Transition from green to clear polyethylene terephthalate (PET) | [245] |
| McDonald’s and Costa Coffee | Paper cups | On-the-go cup recycling scheme | [246] |
| Danone | PET and rPET cups | Replace the packaging from PS (polystyrene) to PET | [247] |
| PepsiCo | 100% recycled or renewable plastic | Eliminate the use of virgin fossil-based plastics in the crisp packets | [248] |
| Unilever (Carte D’Or) | Paper tubs and lids | Transition of the ice cream packaging from plastic to paper tubs and lids | [249] |
| Kraft Heinz (Kraft Mac & Cheese) | Recyclable fiber-based microwavable cup | Replace non-recyclable plastic cups | [250] |
| Kraft Heinz (Shake ‘N Bake) | Reusable container | Removal of the plastic “shaker” bag from its products | [251] |
| Mondelez | Recyclable packaging | Replace all non-recyclable packaging to packaging from 100% recyclable material. | [252] |
| Nestlé | Sustainable packaging solutions | Accelerate the development of sustainable packaging solutions | [253] |
| Tesco | Reusable and refillable packaging | Tesco’s 4Rs packaging strategy (Remove, Reduce, Reuse, Recycle) | [254] |
| Starbucks | Recyclable strawless lid and paper or compostable plastic straw | Eliminate single-use plastic straws and develop alternative-material straw | [255] |
| Bacardi | Recyclable plastic | Replacing Non-Refillable Fitment (NRF) plastic commonplace throughout the spirits industry with | [256] |
| Raw Material | Bioproduct | LCA | Reference |
|---|---|---|---|
| Agro-industrial by-products and marine residues | Polymers | LCA of bio-based films. Identifying the most pollutant phases of the life cycle for biofilms from different resources | [266] |
| Orange peel-derived pectin jelly and corn starch | Pectin | LCA as a cradle-to-gate model. Biodegradation performance compared to a LDPE film | [267] |
| Dunaliella salina microalga and carrot (Daucus carota) | β-carotene | LCA of extraction methods (solvent, microwave, and ultrasound) | [268] |
| Spirulina platensis | Phycocyanin | LCA of extraction methods (solvent extraction and ultrasound) | [269] |
| Microalgae | Pigments, biodiesel | LCA and TEA * of three biorefinery routes | [270] |
| Packaging | Starch and PHA | LCA of biodegradable and conventional plastic packaging | [271] |
| Food waste valorization (bread, rice, and fruit waste) | Hydroxymethylfurfural (HMF) | LCA of different solvents to evaluate the environmental performance | [272] |
| Red wine pomace | Polyphenol | TEA and LCA of solvent extraction and pressurized liquid extraction | [273] |
| Vine shoots | Oligosaccharides | LCA to identify the most sustainable biorefining route | [274] |
| Sugar beet pulp | Oligosaccharides | LCA to analyze different extraction | [275] |
| Onion waste | Quercetin and frutooligosaccharides | LCA of solvent extraction | [276] |
| Rosemary leaves | Antioxidants | LCA of supercritical extraction and water extraction, particle formation on-line process (WEPO) and pressurized hot water extraction | [277] |
| Carrot waste | Cellulose nanofiber | LCA to evaluate production process | [278] |
| Coconut waste | Cellulose nanocrystal | LCA of extraction methods | [279] |
| Chicory grounds | Polyphenol | LCA of extraction methods | [280] |
| Olive mill wastewater | Phenolic compounds | LCA and CBA ** of process | [281] |
| Citrus waste | Pectin | LCA of extraction methods (solvent and microwave) | [282] |
| Microalgal cultivation | Value-added products | Enviro-economical assessment of microalgal production | [283] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristofoli, N.L.; Lima, A.R.; Tchonkouang, R.D.N.; Quintino, A.C.; Vieira, M.C. Advances in the Food Packaging Production from Agri-Food Waste and By-Products: Market Trends for a Sustainable Development. Sustainability 2023, 15, 6153. https://doi.org/10.3390/su15076153
Cristofoli NL, Lima AR, Tchonkouang RDN, Quintino AC, Vieira MC. Advances in the Food Packaging Production from Agri-Food Waste and By-Products: Market Trends for a Sustainable Development. Sustainability. 2023; 15(7):6153. https://doi.org/10.3390/su15076153
Chicago/Turabian StyleCristofoli, Nathana L., Alexandre R. Lima, Rose D. N. Tchonkouang, Andreia C. Quintino, and Margarida C. Vieira. 2023. "Advances in the Food Packaging Production from Agri-Food Waste and By-Products: Market Trends for a Sustainable Development" Sustainability 15, no. 7: 6153. https://doi.org/10.3390/su15076153
APA StyleCristofoli, N. L., Lima, A. R., Tchonkouang, R. D. N., Quintino, A. C., & Vieira, M. C. (2023). Advances in the Food Packaging Production from Agri-Food Waste and By-Products: Market Trends for a Sustainable Development. Sustainability, 15(7), 6153. https://doi.org/10.3390/su15076153