Effect of Elevation Gradient on Carbon Pools in a Juniperus przewalskii Kom. Forest in Qinghai, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Site
2.2. Sample Plot Setting and Sampling Method
2.3. Data Processing
2.3.1. Establishment of Elevation Gradient
2.3.2. Biomass of Arboreal Layer of the Przewalski’s Juniper Forest
2.3.3. CD of Vegetation and Litter Layers in the Przewalski’s Juniper Forest
2.3.4. Soil Carbon Density
2.3.5. Ecosystem Carbon Density
3. Results
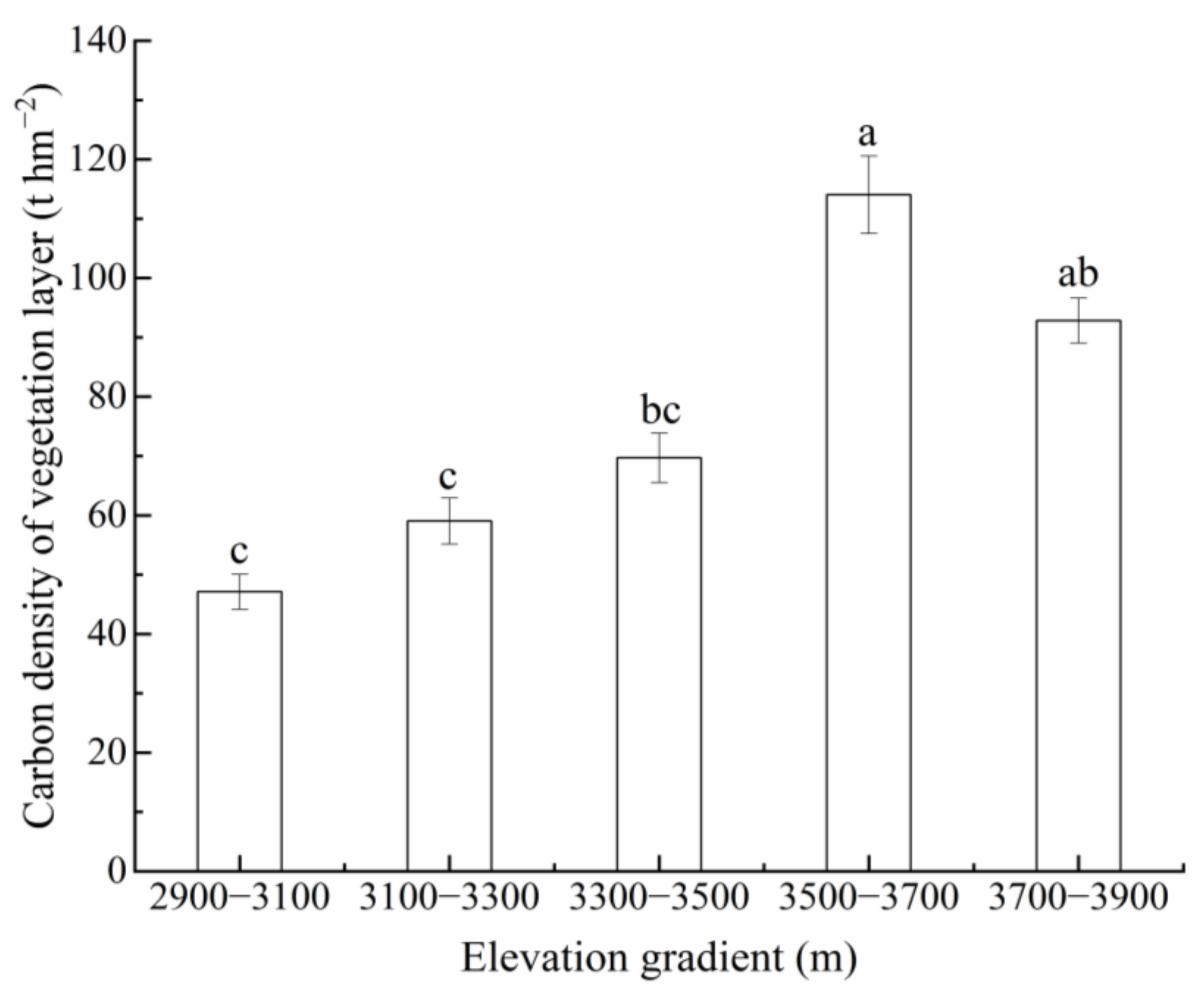
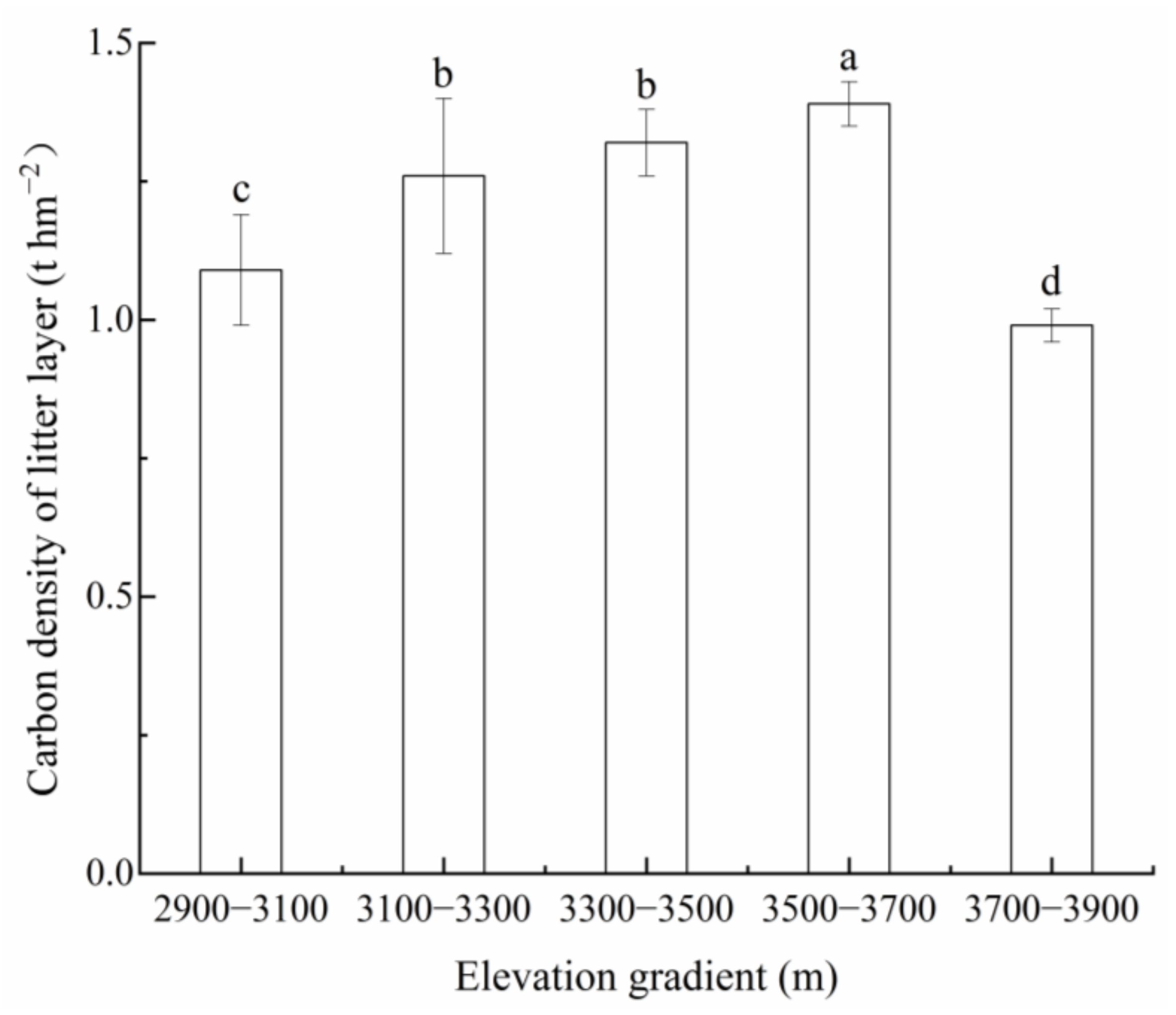
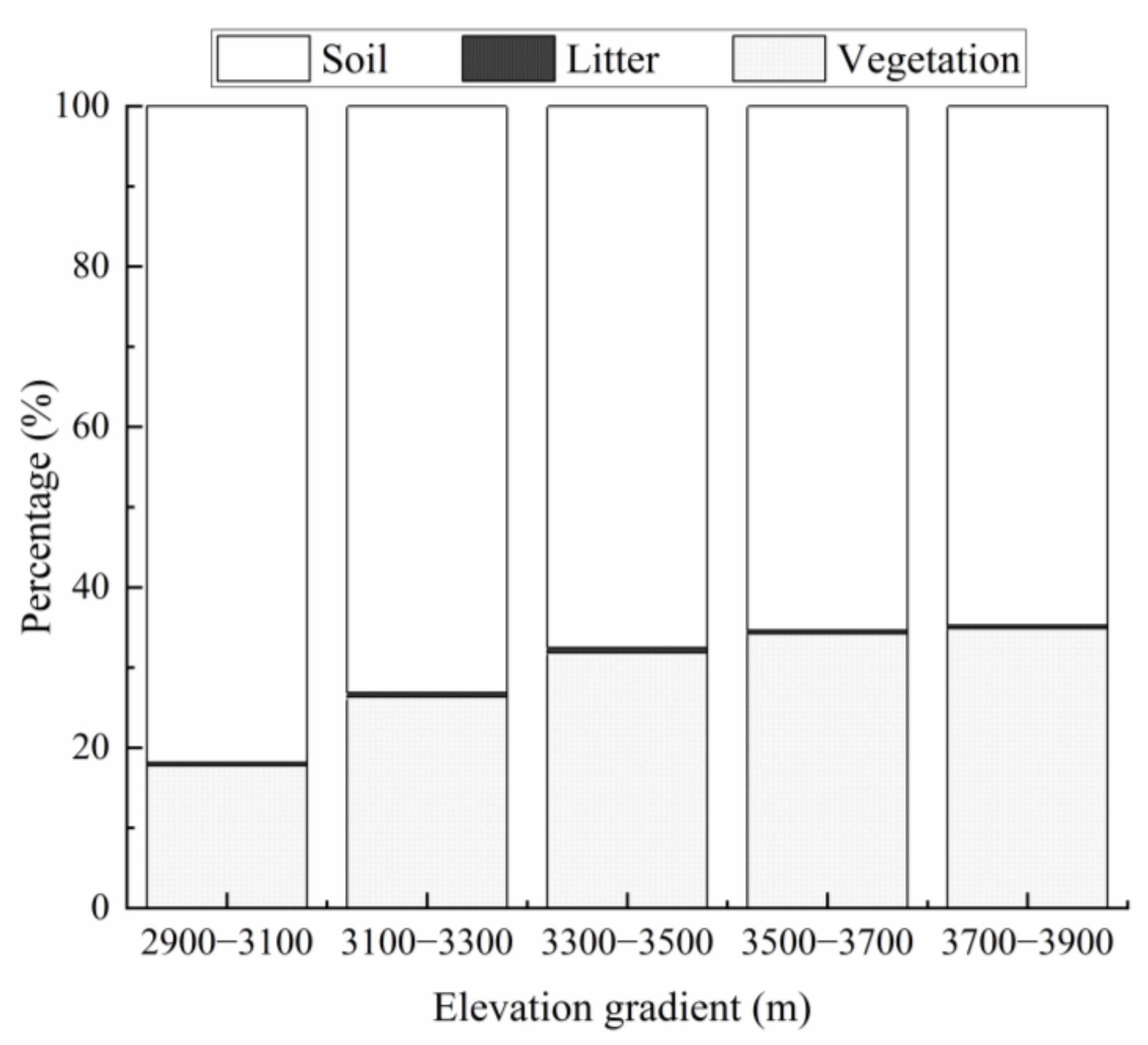
3.1. CD of Vegetation and Litter Layers
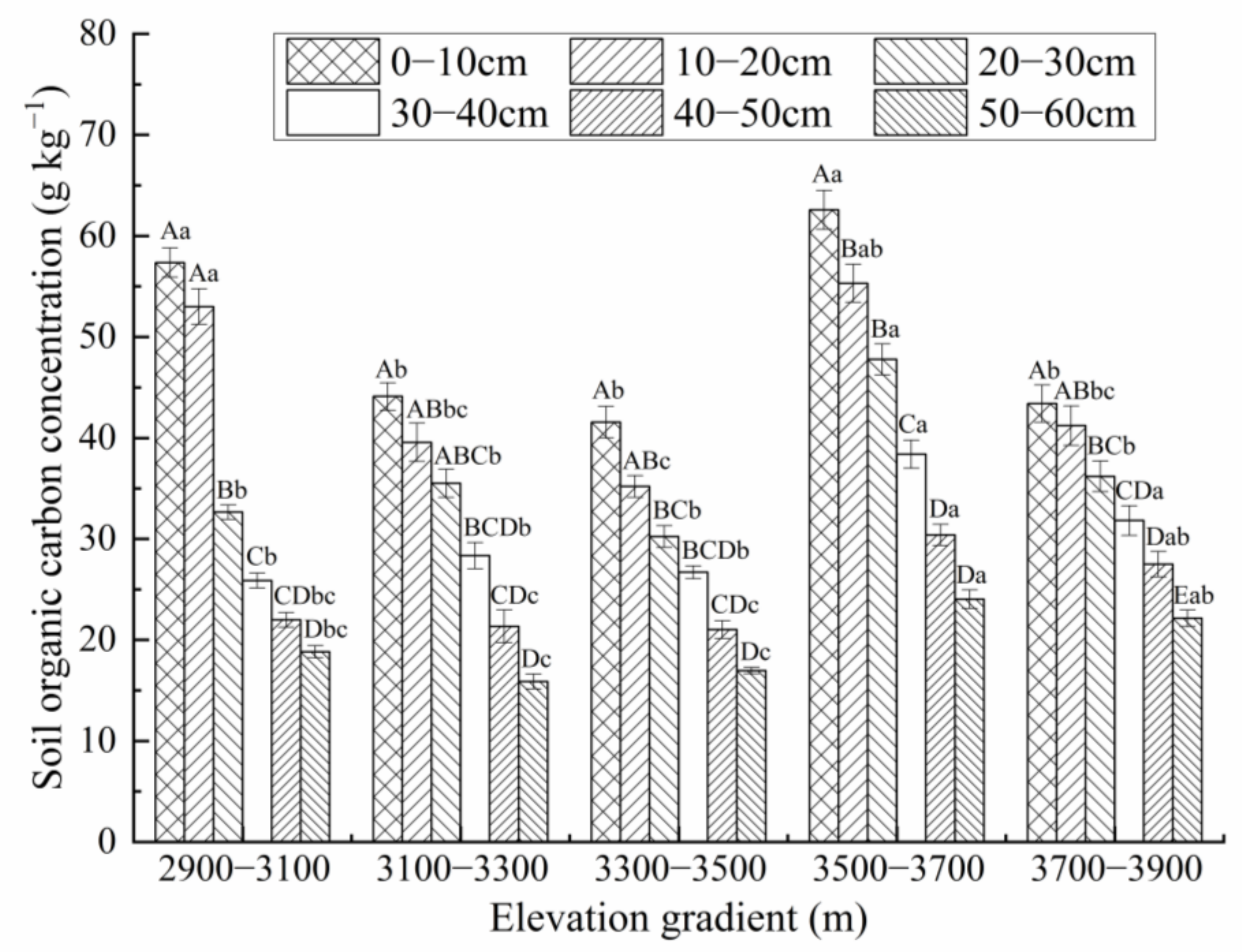
3.2. Distribution Characteristics of SOC Concentration along the Elevation Gradient
3.3. Distribution Characteristics of SCD along the Elevation Gradient
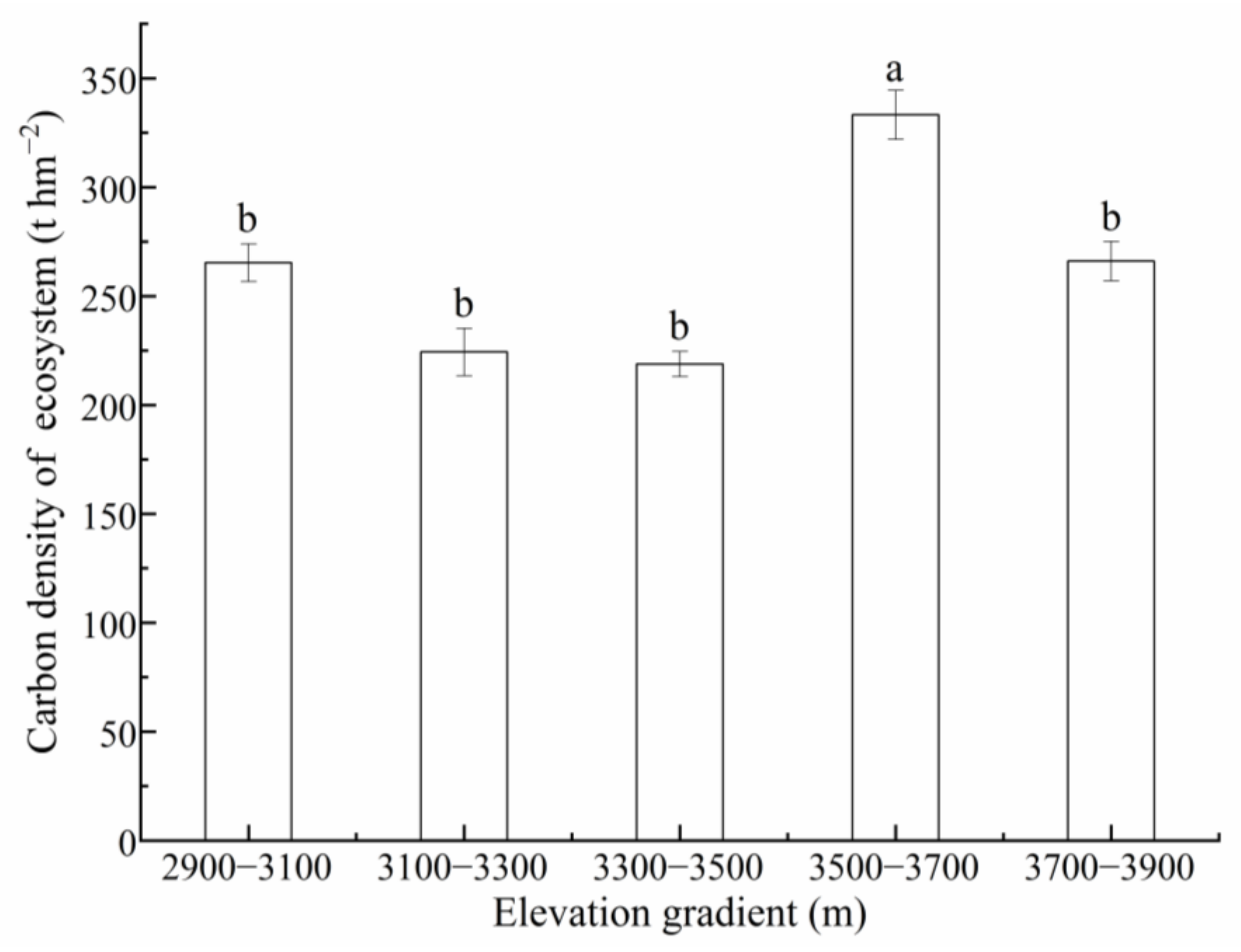
3.4. Distribution Characteristics of ECD along the Elevation Gradient
4. Discussion
4.1. Effect of Elevation on VCD in the Przewalski’s Juniper Forest
4.2. Effect of Elevation on SCD in the Przewalski’s Juniper Forest
4.3. ECD of the Przewalski’s Juniper Forest along the Elevation Gradient
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Global Forest Resources Assessment 2020, Main Report; FAO: Rome, Italy, 2020. [Google Scholar]
- Yin, Y.H.; Ma, D.Y.; Wu, S.H. Climate change risk to forests in China associated with warming. Sci. Rep. 2018, 8, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Ju, P.J.; Zhu, Q.A.; Xu, X.L.; Wu, N.; Gao, Y.H.; Feng, X.J.; Tian, J.Q.; Niu, S.L.; Zhang, Y.J.; et al. Carbon and nitrogen cycling on the Qinghai-Tibetan Plateau. Nat. Rev. Earth Env. 2022, 3, 701–716. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Andrade, A.B.; Araujo, M.A.; Lal, R. Soil organic carbon retention more affected by altitude than texture in a forested mountain range in Brazil. Soil Res. 2018, 56, 284–295. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Ni, X.; Guo, W.; Li, X.; Li, S. Heterogeneity of Increases in Net Primary Production under Intensified Human Activity and Climate Variability on the Loess Plateau of China. Remote Sens. 2022, 14, 4706. [Google Scholar] [CrossRef]
- Wu, T.H.; Ma, W.S.; Wu, X.D.; Li, R.; Qiao, Y.P.; Li, X.F.; Yue, G.Y.; Zhu, X.F.; Ni, J. Weakening of carbon sink on the Qinghai-Tibet Plateau. Geoderma 2022, 412, 115707. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, H.; Zhu, Q.A.; Yang, Y.Z.; Li, M.X.; Peng, C.H.; Zhu, D.; He, Y.X. Assessment of frozen ground organic carbon pool on the Qinghai-Tibet Plateau. J. Soil. Sediment. 2019, 19, 128–139. [Google Scholar] [CrossRef]
- Wei, P.J.; Chen, S.Y.; Wu, M.H.; Jia, Y.L.; Xu, H.J.; Liu, D.M. Increased Ecosystem Carbon Storage between 2001 and 2019 in the Northeastern Margin of the Qinghai-Tibet Plateau. Remote Sens. 2021, 13, 3986. [Google Scholar] [CrossRef]
- Kučerík, J.; Tokarski, D.; Demyan, M.S.; Merbach, I.; Siewert, C. Linking soil organic matter thermal stability with contents of clay, bound water, organic carbon and nitrogen. Geoderma 2018, 316, 38–46. [Google Scholar] [CrossRef]
- Jia, Y.F.; Kuzyakov, Y.; Wang, G.A.; Tan, W.B.; Zhu, B.; Feng, X.J. Temperature sensitivity of decomposition of soil organic matter fractions increases with their turnover time. Land Degrad. Dev. 2020, 31, 632–645. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.Q.; Yang, L.C.; Xiong, F.; Li, C.B.; Fan, L.; Zhou, G.Y. Aboveground biomass of the alpine shrub ecosystems in Three-River Source Region of the Tibetan Plateau. J. Mt. Sci.-Engl. 2018, 15, 357–363. [Google Scholar] [CrossRef]
- Shi, S.L.; Liu, G.H.; Li, Z.S.; Ye, X. Elevation-dependent growth trends of forests as affected by climate warming in the southeastern Tibetan Plateau. Forest Ecol. Manag. 2021, 498, 119551. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Kumar, R.; Konsam, B.; Pala, N.A.; Bhat, J.A. Carbon stock potential in Pinus roxburghii forests of Indian Himalayan regions. Environ. Dev. Sustain. 2021, 23, 12463–12478. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Kumar, M.; Todaria, N.P.; Bhat, J.A.; Kumar, A.; Pandey, R. Contribution of Cedrus deodara forests for climate mitigation along altitudinal gradient in Garhwal Himalaya, India. Mitig. Adapt. Strat. Gl. 2021, 26, 5. [Google Scholar] [CrossRef]
- Poudel, A.; Sasaki, N.; Abe, I. Assessment of carbon stocks in oak forests along the altitudinal gradient: A case study in the Panchase Conservation Area in Nepal. Glob. Ecol. Conserv. 2020, 23, e1171. [Google Scholar] [CrossRef]
- Lu, S.B.; Xu, Y.; Fu, X.P.; Xiao, H.; Ding, W.; Zhang, Y.J. Patterns and drivers of soil respiration and vegetation at different altitudes in southern China. Appl. Ecol. Env. Res. 2019, 17, 3097–3106. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gou, X.H.; Alexander, M.R.; Xia, J.Q.; Wang, F.; Zhang, F.; Man, Z.H.; Pederson, N. Drought limits wood production of Juniperus przewalskii even as growing seasons lengthens in a cold and arid environment. Catena 2021, 196, 104936. [Google Scholar] [CrossRef]
- Sun, J.; Zhan, T.Y.; Liu, M.; Zhang, Z.C.; Wang, Y.; Liu, S.L.; Wu, G.L.; Liu, G.H.; Tsunekawa, A. Verification of the biomass transfer hypothesis under moderate grazing across the Tibetan plateau: A meta-analysis. Plant Soil 2021, 458, 139–150. [Google Scholar] [CrossRef]
- Qiu, J. China: The third pole. Nature 2008, 454, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Tao, J.; Zhang, Y.J.; Zhu, J.T.; Jiang, Y.B.; Zhang, X.Z.; Zhang, T.; Xi, Y. Elevation-dependent temperature change in the Qinghai–Xizang Plateau grassland during the past decade. Theor. Appl. Climatol. 2013, 117, 61–71. [Google Scholar] [CrossRef]
- Kuang, X.; Jiao, J.J. Review on climate change on the Tibetan Plateau during the last half century. J. Geophys. Res-Atmos. 2016, 121, 3979–4007. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Liu, B.Y.; You, Y.; Li, W.P.; Liu, M.; Shang, H.; He, J.S. Solar radiation regulates the leaf nitrogen and phosphorus stoichiometry across alpine meadows of the Tibetan Plateau. Agr. Forest Meteorol. 2019, 271, 92–101. [Google Scholar] [CrossRef]
- Schurman, J.S.; Babst, F.; Bjorklund, J.; Rydval, M.; Bace, R.; Cada, V.; Janda, P.; Mikolas, M.; Saulnier, M.; Trotsiuk, V.; et al. The climatic drivers of primary Picea forest growth along the Carpathian arc are changing under rising temperatures. Glob. Chang. Biol. 2019, 25, 3136–3150. [Google Scholar] [CrossRef]
- Zhou, T.C.; Liu, M.; Sun, J.; Li, Y.R.; Shi, P.; Tsunekawa, A.; Zhou, H.K.; Yi, S.H.; Xue, X. The patterns and mechanisms of precipitation use efficiency in alpine grasslands on the Tibetan Plateau. Agric. Ecosyst. Environ. 2020, 292, 106833. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Q.M.; Gou, X.H.; Du, M.M.; Wang, F.; Zhang, J.Z. Climatic control on the growth and regeneration of Juniperus przewalskii at alpine treeline in the eastern Qilian Mountains, northwest China. Trees 2021, 35, 1085–1097. [Google Scholar] [CrossRef]
- Yang, B.; Sonechkin, D.M.; Datsenko, N.M.; Liu, J.J.; Qin, C. Establishment of a 4650-year-long eigenvalue chronology based on tree-ring cores from Qilian junipers (Juniperus przewalskii Kom.) in Western China. Dendrochronologia 2017, 46, 56–66. [Google Scholar] [CrossRef]
- Gao, L.L.; Gou, X.H.; Deng, Y.; Yang, M.X.; Zhao, Z.Q.; Cao, Z.Y. Dendroclimatic response of Picea crassifolia along an altitudinal gradient in the eastern Qilian mountains, northwest China. Arct. Antarct. Alp. Res. 2013, 45, 491–499. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gou, X.H.; Manzanedo, R.D.; Zhang, F.; Pederson, N. Cambial phenology and xylogenesis of Juniperus przewalskii over a climatic gradient is influenced by both temperature and drought. Agric. For. Meteorol. 2018, 260–261, 165–175. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; You, C.M.; Cao, R.; Tan, B.; Li, H.; Jiang, Y.R.; Yang, W.Q. The three-dimension zonal pattern of soil organic carbon density in China’s forests. Catena 2021, 196, 104950. [Google Scholar] [CrossRef]
- Hou, L.; Hou, S.J. How long should the fully hillside-closed forest protection be implemented on the Loess Plateau, Shaanxi, China? PeerJ 2017, 5, e3764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Y.; Ju, K.J.; Fu, H.E.; Chang, X.X.; Song, C.F.; He, H.Y. Study on biomass of water conservation forest on north slope of Qilian Mountains. J. Fujian Coll. For. 1997, 18, 319–323. [Google Scholar] [CrossRef]
- Johnson, W.C.; Sharpe, D.M. The ratio of total to merchantable forest biomass and its application to the global carbon budget. Can. J. Forest Res. 1983, 13, 372–383. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, G.S. Carbon storage of forest vegetation in China and its relationship with climatic factors. Clim. Change 2006, 74, 175–189. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, X.; Bai, Y.F.; Tang, Z.Y.; Wang, W.T.; Zhao, Y.C.; Wan, H.W.; Xie, Z.Q.; Shi, X.Z.; Wu, B.F.; et al. Carbon pools in China’s terrestrial ecosystems: New estimates based on an intensive field survey. Proc. Natl. Acad. Sci. USA 2018, 115, 4021–4026. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.Y.; Chen, A.P.; Peng, C.H.; Zhao, S.Q.; Ci, L.J. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef]
- Huang, T.; Hao, J.T.; Du, Y.C.; Tian, C.C.; Zhang, J.; Wang, H.; Hou, L. Regeneration characteristics of three natural Juniperus forests in the Three-River Headwater Region of Qinghai Province, China. Chin. J. Appl. Ecol. 2022, 33, 297–303. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gou, X.H.; Pederson, N.; Zhang, F.; Niu, H.G.; Zhao, S.D.; Wang, F. Cambial phenology in Juniperus przewalskii along different altitudinal gradients in a cold and arid region. Tree Physiol. 2018, 38, 840–852. [Google Scholar] [CrossRef] [Green Version]
- Ren, P.; Rossi, S.; Gricar, J.; Liang, E.; Cufar, K. Is precipitation a trigger for the onset of xylogenesis in Juniperus przewalskii on the north-eastern Tibetan Plateau? Ann. Bot.-Lond. 2015, 115, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.Q.; Lu, H.Y.; Wang, J.N.; Wang, X.F.; Sun, J. Dual influence of climate change and anthropogenic activities on the spatiotemporal vegetation dynamics over the Qinghai-Tibetan Plateau from 1981 to 2015. Earths Future 2022, 10, e2021EF002566. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Gou, X.H.; Zhang, Y.X.; Lu, M.; Xu, X.Y.; Zhang, F.; Liu, W.H.; Gao, L.L. Forward modeling analyses of Qilian Juniper (Sabina przewalskii) growth in response to climate factors in different regions of the Qilian Mountains, northwestern China. Trees 2016, 30, 175–188. [Google Scholar] [CrossRef]
- Ma, Y.J.; Xie, T.; Li, X.Y. Spatial variation of soil organic carbon in the Qinghai Lake watershed, northeast Qinghai-Tibet Plateau. Catena 2022, 213, 106187. [Google Scholar] [CrossRef]
- Liu, X.; Cong, J.; Lu, H.; Xue, Y.D.; Wang, X.L.; Li, D.Q.; Zhang, Y.G. Community structure and elevational distribution pattern of soil Actinobacteria in alpine grasslands. Acta Ecol. Sin. 2017, 37, 213–218. [Google Scholar] [CrossRef]
- Davidson, E.A.; Trumbore, S.E.; Amundson, R. Soil warming and organic carbon content. Nature 2000, 408, 789–790. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Yan, Y.J.; Dai, Q.H.; He, Z.J.; Yi, X.S. Latitudinal and altitudinal patterns and influencing factors of soil humus carbon in the low-latitude plateau regions. Forests 2023, 14, 344. [Google Scholar] [CrossRef]
- Badía-Villas, D.; Girona-García, A. Soil humus changes with elevation in Scots pine stands of the Moncayo Massif (NE Spain). Appl. Soil Ecol. 2018, 123, 617–621. [Google Scholar] [CrossRef]
- Hou, L.; Sun, S.; Liang, L.Y.; Liang, G.; Jiang, L.X. Effects of selective thinning and residue removal on ground layer structure and diversity in a mixed pine-oak stand of the Qinling Mountains, China. Aust. J. Bot. 2017, 65, 485. [Google Scholar] [CrossRef]
- Zhou, Y.R.; Yu, Z.L.; Zhao, S.D. Carbon storage and budget of major Chinese forest types. Acta Phytoecol. Sin. 2000, 24, 518–522. [Google Scholar]
- Guan, J.H.; Deng, L.; Zhang, J.G.; He, Q.Y.; Shi, W.Y.; Li, G.Q.; Du, S. Soil organic carbon density and its driving factors in forest ecosystems across a northwestern province in China. Geoderma 2019, 352, 1–12. [Google Scholar] [CrossRef]
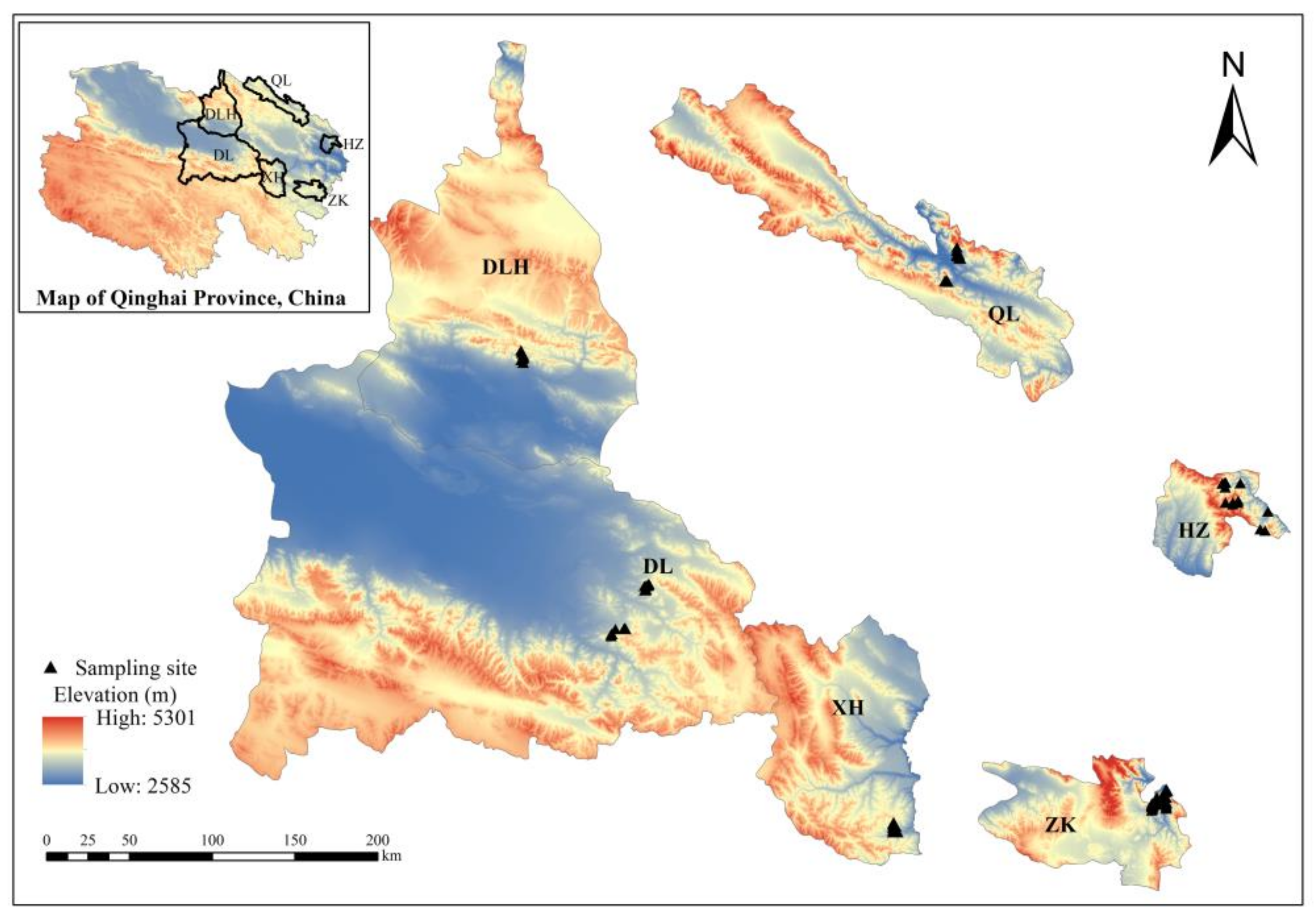

| Sampling Sites | Number of Plots | Elevation (m) | Slope (°) | Canopy Density | Average Tree Height (m) | Average DBH (cm) | AAT (℃) | AAP (mm) |
|---|---|---|---|---|---|---|---|---|
| ZK | 33 | 2915–3535 | 32 | 0.6 | 7.12 | 20.32 | −1.33 | 483.4 |
| QL | 10 | 3170–3216 | 27 | 0.5 | 10.56 | 22.73 | 1.00 | 420.0 |
| XH | 22 | 3170–3851 | 35 | 0.7 | 9.72 | 39.25 | 1.71 | 377.9 |
| DL | 18 | 3730–3780 | 30 | 0.5 | 7.41 | 39.20 | 3.41 | 179.1 |
| DLH | 7 | 3520–3800 | 36 | 0.6 | 7.67 | 23.98 | 1.00 | 420.0 |
| HZ | 13 | 2940–3060 | 28 | 0.6 | 7.23 | 19.70 | 4.01 | 502.5 |
| Biomass (t hm−2) | Elevation Gradient (m) | ||||
|---|---|---|---|---|---|
| 2900–3100 | 3100–3300 | 3300–3500 | 3500–3700 | 3700–3900 | |
| Arboreal | 90.58 ± 2.65 b | 118.16 ± 3.58 b | 143.20 ± 4.90 b | 243.00 ± 8.32 a | 202.41 ± 6.78 a |
| Shrub | 10.40 ± 0.74 a | 8.84 ± 0.64 b | 7.05 ± 0.55 c | 5.44 ± 1.14 d | 0.84 ± 0.08 e |
| Herbaceous | 3.68 ± 0.20 c | 4.20 ± 0.29 b | 4.63 ± 0.14 a | 4.94 ± 0.15 a | 2.99 ± 1.06 d |
| Vegetation | 104.66 ± 6.25 c | 131.19 ± 8.16 c | 154.86 ± 9.71 bc | 253.38 ± 14.33 a | 206.24 ± 8.78 ab |
| Litter | 2.43 ± 0.10 c | 2.81 ± 0.14 b | 2.94 ± 0.06 b | 3.10 ± 0.04 a | 2.21 ± 0.03 d |
| Elevation Gradient (m) | Soil Layer (cm) | Total | |||||
|---|---|---|---|---|---|---|---|
| 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | ||
| 2900–3100 | 57.43 ± 1.38 a | 53.87 ± 1.66 a | 34.07 ± 0.83 ab | 27.46 ± 0.62 ab | 23.81 ± 0.72 ab | 20.51 ± 0.68 a | 217.15 ± 8.23 a |
| 3100–3300 | 35.20 ± 1.62 c | 36.50 ± 1.71 bc | 32.55 ± 1.77 ab | 25.88 ± 0.70 b | 19.44 ± 0.94 b | 14.48 ± 0.48 c | 164.05 ± 4.67 b |
| 3300–3500 | 33.92 ± 1.76 c | 29.59 ± 1.20 c | 26.01 ± 0.78 b | 23.62 ± 0.81 b | 19.15 ± 0.81 b | 15.50 ± 0.74 bc | 147.79 ± 7.18 b |
| 3500–3700 | 47.87 ± 1.07 b | 45.32 ± 1.69 ab | 40.36 ± 1.56 a | 34.16 ± 1.24 a | 27.35 ± 0.96 a | 22.77 ± 0.91 a | 217.84 ± 9.76 a |
| 3700–3900 | 34.66 ± 1.70 c | 34.46 ± 1.17 c | 31.12 ± 1.44 b | 27.80 ± 1.30 ab | 24.22 ± 0.72 ab | 20.00 ± 0.81 ab | 172.26 ± 11.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Hou, L.; Ruan, Q. Effect of Elevation Gradient on Carbon Pools in a Juniperus przewalskii Kom. Forest in Qinghai, China. Sustainability 2023, 15, 6163. https://doi.org/10.3390/su15076163
Dong Z, Hou L, Ruan Q. Effect of Elevation Gradient on Carbon Pools in a Juniperus przewalskii Kom. Forest in Qinghai, China. Sustainability. 2023; 15(7):6163. https://doi.org/10.3390/su15076163
Chicago/Turabian StyleDong, Zhenjie, Lin Hou, and Qi Ruan. 2023. "Effect of Elevation Gradient on Carbon Pools in a Juniperus przewalskii Kom. Forest in Qinghai, China" Sustainability 15, no. 7: 6163. https://doi.org/10.3390/su15076163





