Abstract
Nanoparticles are ubiquitous in nature and are also found in raw coal, which plays an irreplaceable role in the global economy. In this study, raw coal samples were obtained from Gaojiapu Coal Mine in Shanxi Province, China. The elementary composition of nanoparticles was determined using energy-dispersive X-ray spectroscopy. The structural and chemical characteristics of nanoparticles were analyzed using transmission electron microscopy. Results revealed that there were multiple types of nanoparticles in the raw coal, such as S-, Ca-, Ba-, Ni-, Cr-, Si-, Sr-, and V-bearing nanoparticles. These nanoparticles exhibited various sizes and complex, irregular shapes. Our findings revealed that elemental nanoparticles occur in raw coal. These nanoparticles include celestite and barite nanoparticles. In addition, nanoparticles with Ni, Cr, and V in composition are also included in raw coal. These nanoparticles, which contain heavy metal elements, have great potential to harm the human body. Meanwhile, compared with the characteristics of nanoparticles produced by coal combustion, the nanoparticles in raw coal may be an important potential source of the nanoparticles produced by coal combustion.
1. Introduction
China’s energy structure is dominated by coal, and the mainstream industries in China, both past and present, have been dependent on coal. At the end of the 20th century, coal accounted for 76.2% of China’s energy mix [1]. This highlights the importance of coal resources in China. Although coal plays an irreplaceable role in the global economy, it is impossible to deny that widespread coal combustion will produce large quantities of harmful substances, which will adversely impact both the natural environment and human health. In particular, coal combustion can produce a variety of nanoparticles that are deleterious to human health. For instance, coal generates large quantities of ultrafine-grained pulverized coal dust during combustion [2]. The nanoparticles can also cause DNA damage. Previous studies have shown substantial genomic DNA damage in peripheral blood mononuclear cells that contain Fe, Ni, Cu, and Cr nanoparticles [3]. Coal combustion also produces magnesium phases, which are toxic and can cause severe damage to human lungs [4]. In addition, coal combustion produces large amounts of silica nanoparticles, which can cause cancer; it has been known to increase the incidence rate of lung cancer in one place by nearly 20 times [5]. Because of the great harm nanoparticles can inflict on human health, it is of great importance to study nanoparticles.
While coal combustion produces nanoparticles that are harmful to human health, raw coal also contains several types of nanoparticles that are harmful to the environment and human health. Most previous studies have focused on nanoparticles produced during coal combustion, and research on nanoparticles in raw coal is scarce. A considerable number of nanoparticles on Earth are formed as a result of natural processes, such as microbial action [6], mineralization [7], volcanic eruptions [8], oxidation and reduction [9,10], and fault activity [11,12]. During the process of coal formation, coal is subjected to complex biochemical, geochemical, physical-chemical, and geological processes, which also produce large quantities of nanoparticles.
At the same time, due to the importance of nanoparticles in the process of coal formation and the importance of nanoparticles generated by coal combustion, many researchers have studied nanoparticles related to coal. For example, Hower et al. [13] studied the characteristics of nanoparticles containing Se, Pb, Co, Ti, Ba, and other elements produced by coal combustion in Kentucky, Ribeiro et al. [14] revealed the chemical composition and morphology of nanoparticles from coal-burning wastes in Portugal, and Silva and da Boit [15] have assessed the human health risks from coal bottom ash nanoparticles in Brazil.
The aim of this study is to investigate nanoparticles in raw coal and their adverse impacts on human health. These nanoparticles occur in a wide variety of types, as do the elements from which they are formed. The main components of nanoparticles in our study include Ni, Cr, Sr, S, Ba, and V, as well as other elements. These nanoparticles have the potential to severely harm human health. Transmission electron microscopy (TEM) was employed to study the morphology, structure, and chemical characteristics of the nanoparticles in the coal samples. The findings of this study are expected to serve as a reference for future research as well as a basis for deriving solutions to environmental and public health problems caused by such nanoparticles.
2. Materials and Methods
2.1. Sampling Location and Geological Background
Raw coal samples were obtained from Gaojiapu Coal Mine in Gaojiapu coalfield, Binchang mining area, Huanglong Jurassic coalfield, Shanxi Province (Figure 1).

Figure 1.
Topographic and geological map of the Gaojiabao mining area (left), the location of the Gaojiabao mining area (right).
The Binchang mining area is located in the Miaobin Sag on the northern edge of the Weibei Flexural Belt in the southern part of the Ordos Basin. The coal-bearing strata in the area belong to the Yan’an Formation, the lithology of which consists of dark-gray mudstones, sandy mudstones, siltstones and gray-white medium coarse-grained sandstones interbedded with carbonaceous mudstones, and coal seams. The estimated total reserves identified in Gaojiapu Coal Mine are 7.9604 × 108 t [16]. The coal seams in Gaojiapu Coal Mine are low metamorphic bituminous coal; the coals from all seams exhibit similar physical properties. The coal exhibits a black color, deep black streaks, an asphalt luster, a banded or uniform structure, horizontal bedding, and a jagged or conchoidal fracture. The average moisture content of raw coal in the coal seams ranges from 4.31 to 5.01%, the average Ad yield ranges from 14.71 to 15.98%, and the average Vdaf yield of the coal seams varies slightly, ranging from 31.65 to 32.29%, indicating that the coal is classified as medium- and high-volatile coal. The harmful components in coal from the Gaojiapu Coal Mine include S, Pd, Cl, F, As, and carbonate CO2 [17].
2.2. Analytical Methods
A powdered sample was collected from the coal seam, placed on the glass sheet for SEM analysis, and placed on the TEM copper net for TEM-EDS analysis. The elements were qualitatively analyzed using energy-dispersive X-ray spectroscopy (EDS). Scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) were used to view the particle morphology and element distributions (element-mapping) at the micrometer scale and to identify portions of interest in the samples. Nanoscale observations of samples were performed using high-resolution transmission electron microscopy (HRTEM). The samples were analyzed and tested using a Hitachi SU8220 instrument at 5 KV and 10 KV. The instrument is equipped with an energy dispersion spectrometer (EDS) and a STEM/HAADF detector with a TEM point resolution of 0.2 nm and a line resolution of 0.1 nm. Through high-resolution transmission electron microscopy, the energy spectrum map of coal nanoparticles, TEM and STEM micrographs, STEM HAADF high-resolution map, and SAED diffractive pattern could be obtained. After milling the samples with Au ions, the TEM foils, which were attached to Cu grids via Pt welding, were extracted and then thinned to 50–70 nm. Examination of the samples was carried out using a FEI Tecnai G2 F20 TEM operated at 200 kV and equipped with an EDS detector. All observations were conducted at Sinoma New Materials Research Institute (Guangzhou, China) Co., LTD.
3. Results
According to the morphological map of nanoparticles (Figure 2), these nanoparticles have different shapes and sizes (Figure S1). The samples contain granular and flakey nanoparticles, and most of these nanoparticles exist in the form of aggregates, with a few existing in the form of monomers. Among these nanoparticles, the smaller ones are only 40 × 70 nm in size, while the larger ones can reach 300 × 500 nm in size. Moreover, the elemental compositions of these nanoparticles vary, including Cr, Ni, O, S, Ba, Sr, and other elements. Among them, the representative nanoparticles are as follows:
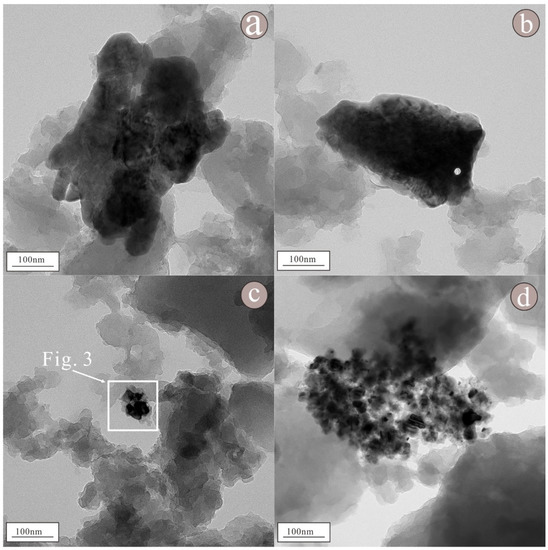
Figure 2.
Morphology of nanoparticles in raw coal. Among these nanoparticles, (a–d) are aggregates of granular nanoparticles, while (b) are aggregates of flake nanoparticles.
As shown in the figure of topography (Figure 3a), the nanoparticle consists of two granular nanoparticles clustered together; the two granular nanoparticles are not large, being approximately 20 × 40 nm and 30 × 35 nm in size. Moreover, its elemental composition is relatively simple. In the lower-left portion of the nanoparticle (Figure 3a), an obvious lattice fringe can be seen in the HRTEM image (Figure 3c), indicating that the nanoparticle is highly crystalline. Regularly spaced light spots can be seen in the selected area diffraction pattern (Figure 3b), indicating that the nanoparticle is a single crystal with a lattice spacing of approximately 0.20 nm (Figure 3d); the pattern is consistent with that of the (110) crystal plane of Cr. In the upper-right portion of the nanoparticle, lattice fringes can be clearly visible in the HRTEM image (Figure 4a), indicating that the nanoparticle is highly crystalline. Regularly spaced light spots can be seen in the selected area diffraction pattern (Figure 4b), indicating that the nanoparticle is a single crystal with a lattice spacing of approximately 0.20 nm (Figure 4c); the pattern is consistent with that of the (111) crystal plane of Ni. According to the EDS figure (Figure 5a) and EDS map (Figure 6a), the element in the lower-left portion of the nanoparticle is Cr (100%) (Table 1). According to the EDS figure (Figure 5b) and EDS map (Figure 6b), the element in the upper-right portion of the nanoparticle is Ni (100%) (Table 1). Therefore, we suggest that this nanoparticle is composed of two metal elements, Ni and Cr.
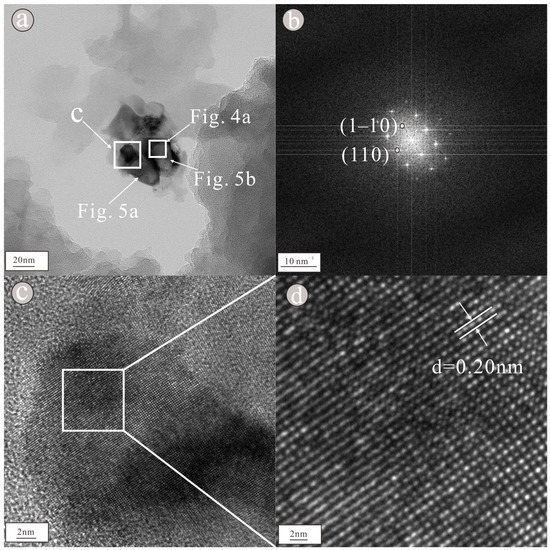
Figure 3.
Morphology (a) showed that the nanoparticles were composed of a granular assemblage, selected area diffraction pattern (b) showed regular light spots, HRTEM image (c) showed obvious lattice stripes and lattice spacing (d) showed that the lattice spacing is 0.20 nm of nanoparticles containing Cr.
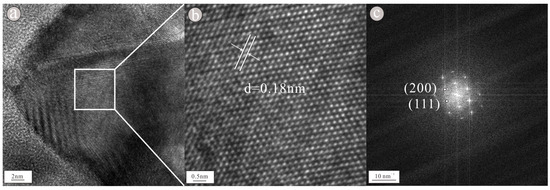
Figure 4.
HRTEM image (a) showed obvious lattice stripes, lattice spacing (b) showed that the lattice spacing is 0.18 nm and selection area diffraction pattern (c) showed regular light spots of nanoparticles containing Ni.

Figure 5.
EDS figure of Cr (a) and Ni (b) nanoparticles.

Figure 6.
EDS scanning map of Cr (a) and Ni (b) nanoparticles.
As shown in the figure of topography (Figure 7a), the nanoparticle occurs as a semi-autologous sheet, and it is approximately 200 × 400 nm in size. According to the EDS figure (Figure 8) and EDS map (Figure 9), the nanoparticles mainly consist of Ba (50.5%), S (9.84%), and O (39.6%) (Table 1). Moreover, the HRTEM image (Figure 7c) exhibits obvious lattice fringes, indicating that the nanoparticles have high crystallinity. The selected area diffraction image (Figure 7b) shows regularly spaced light spots, indicating that the nanoparticle is a single crystal with a lattice spacing of 0.31 nm (Figure 7d), corresponding to the (021) face of barite. The nanoparticle is believed to be barite.

Figure 7.
Topography (a) showed that the nanoparticles were in sheets, selected area diffraction pattern (b) showed regular light spots, HRTEM image (c) showed obvious lattice stripes and lattice spacing (d) showed that the lattice spacing is 0.31 nm of barite nanoparticles.

Figure 8.
EDS figure of barite nanoparticles shows that it is composed of O, S, and Ba.
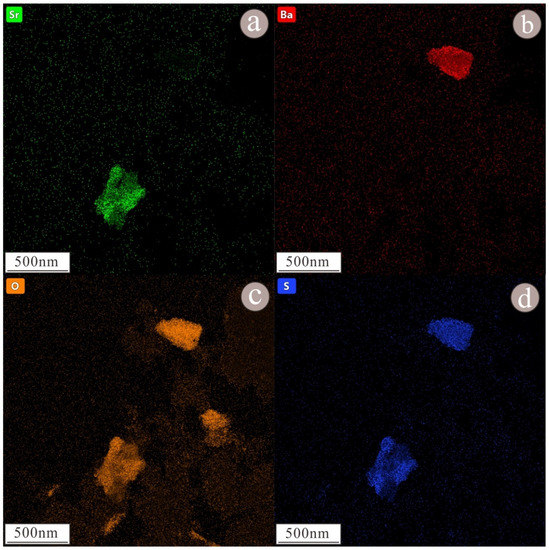
Figure 9.
EDS scanning map of barite and celestite nanoparticles, the displayed element is Sr (a), Ba (b), O (c), S (d). Nanoparticles of barite at the upper right, whose main components are Ba, S, and O. According to the EDS scanning map, nanoparticles of celestite at the upper left and, according to the EDS scanning map, its major elements are Sr, S, and O.
As shown in the figure of topography (Figure 10a), such particles occur as granular aggregates, with a size of approximately 200 × 400 nm. According to the EDS figure (Figure 11) and EDS map (Figure 9), the nanoparticles mainly consist of Sr (42.4%), S (14.0%), and O (42.2%), followed by Ba (1.40%) (Table 1). The HRTEM image (Figure 10c) indicates that the nanoparticle exhibits obvious lattice fringes and high crystallinity. The selected area diffraction pattern (Figure 10b) shows regularly spaced light spots, indicating that the nanoparticle is a single crystal with a lattice spacing of 0.21 nm (Figure 10d), corresponding to the (122) face of celestite. The nanoparticle is presumed to be celestite. We found that celestite in raw coal consists not only of highly crystalline (Figure S2) nanoparticles, but also of some amorphous celestite nanoparticles (Figure S4). The EDS diagram of these nanoparticles showed that Sr, S, and O were the main elements, followed by Ba (Figures S3 and S5).
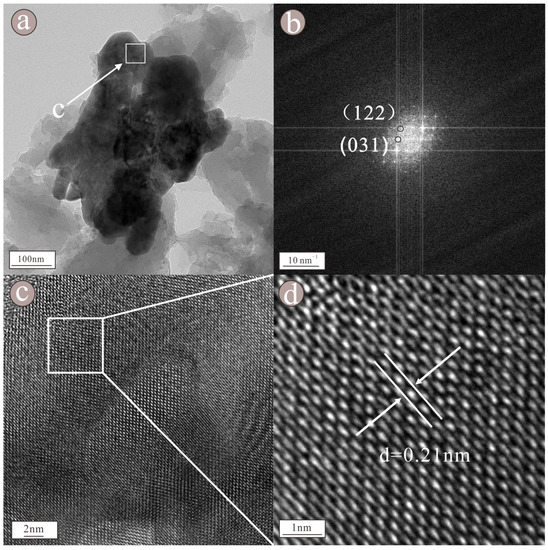
Figure 10.
Topography (a) showed that the nanoparticles were composed of a granular assemblage, selected area diffraction pattern (b) showed regular light spots, HRTEM image (c) showed obvious lattice stripes and lattice spacing (d) showed that the lattice spacing is 0.21 nm of barite nanoparticles.
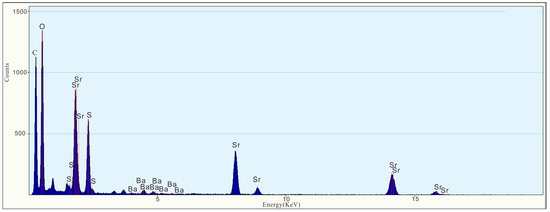
Figure 11.
EDS figure of celestite nanoparticles shows that the major elements are O, S, Sr, and Ba.
As shown in the figure of topography (Figure 12a), the nanoparticle occurs as a large granular aggregate with size of approximately 300 × 500 nm. According to the EDS figure (Figure 13) and EDS map (Figure 14), the main components of the nanoparticle are Ni (55.2%) and V (44.8%) (Table 1). Lattice fringes can be clearly observed in the HRTEM image (Figure 12c), indicating that the nanoparticle has high crystallinity. Regularly spaced light spots can be seen in the selected area diffraction pattern (Figure 12b), indicating that the nanoparticle is a single crystal with a lattice spacing of approximately 0.21 nm (Figure 12d).
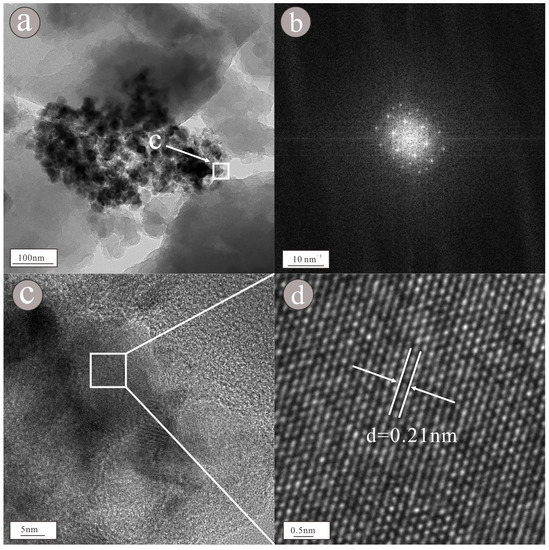
Figure 12.
Morphology (a) showed that the nanoparticles were composed of a granular assemblage, selected area diffraction pattern (b) showed regular light spots, HRTEM image (c) showed obvious lattice stripes and lattice spacing (d) showed that the lattice spacing is 0.21 nm of Ni-V nanoparticles.
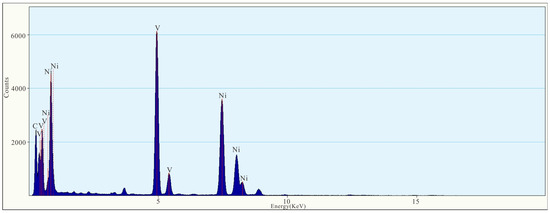
Figure 13.
EDS figure of Ni-V nanoparticles.
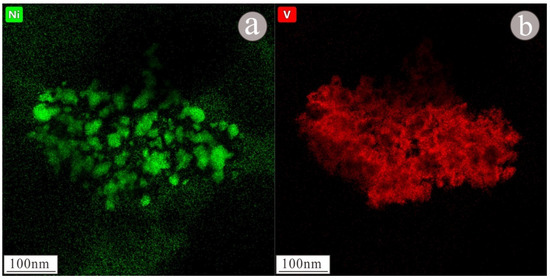
Figure 14.
EDS scanning map of Ni (a)-V (b) nanoparticles.

Table 1.
Elemental compositions of nanoparticles occurring in raw coal.
4. Discussion
4.1. A New Form of Elements in Coal
The elemental composition of coal is relatively complex. Its major elements are C, H, N, O, Na, Mg, Al, Si, S, K, Ca, Fe, and other elements [18]; it also includes Se, Cr, U, V, Mo, Na, K, Mg, Sr, Ba, Ni, Cr, and other elements in trace amounts [19]. The main elements identified in the nanoparticles in this study include Ni, Cr, V, Sr, Ba, and other elements, which are very important trace elements in coal. Previous studies on the occurrence of these elements have also been relatively conclusive. Cr occurs in mineral and organic forms in coal [20]. Ba mainly occurs in coal in minerals such as barite. In coal with relatively low metamorphism, elemental barium is typically bound or combined with organic matter [21]. Sr mainly occurs in carbonate and sulfate minerals [22]. V primarily occurs in clay minerals or in combination with organic matter [23]. Ni occurs in coal mainly in the form of sulfide and aluminosilicates, followed by carbonates.
However, our results reveal that raw coal contains a large number of nanoparticles, and that these nanoparticles mainly consist of Ni, Cr, V, Sr, Ba, S, O, and other elements. We also identified nanoparticles of barite and celestite, which are in the form of nano-mineral particles. Most of these nanoparticles have a high degree of crystallization, but amorphous nanoparticles are also included (Figure S4). We also found nanoparticles mainly composed of Ni and Cr and nanoparticles composed of Ni and V. The discovery of nanoparticles containing various elements in raw coal indicates that raw coal contains a large number of nanoparticles. In addition, these results provide strong evidence that elements can occur in coal not only in the aforementioned forms, but also in the form of nanoparticles, which may be an important transportation form in raw coal.
4.2. The Potential Harm of Nanoparticles in Coal to Human Health
In 1980, the PECH Group of the National Geochemical Commission of the United States classified the elements in coal into six categories according to their degree of harmfulness. Cr, Ni, and V, contained in the nanoparticles found in raw coal, were placed in the second category of elements requiring attention, and Ba and Sr were placed in the third category of elements requiring attention [24]. Cr interferes with the functions of the reproductive system. In addition, it causes great harm to human skin, respiratory tracts, eyes, ears, and gastrointestinal tracts [25]. Ni is a carcinogenic element, and coal combustion is the main source of atmospheric Ni [26]. Ni is also the most common sensitizing metal, and approximately 20% of the global population is allergic to Ni. Once induced, Ni allergy can persist in the human body and cause inflammation, cancer, neurasthenic syndrome, and nervous system disorders [27]. V can cause damage to the skin, which may include both primary irritation and sensitization. It can also cause respiratory irritation, abnormally small airways, and fibrosis in the lung interstitial tissue. Acute V poisoning can cause irritation of the eyes and respiratory mucosa. In severe cases, V poisoning impairs the functions of the heart, kidney, gastrointestinal tract, and central nervous system [28].
These elements, which are extremely harmful to human health, are found in the nanoparticles in raw coal, and may enter the human body during coal mining or use, causing great harm. Compared to larger particles with the same chemical composition, nanoparticles can provide a larger surface area for the binding of chemicals (including heterogeneous organisms and transition metals) [29,30,31,32]. Moreover, because they are much smaller than biological cells, they can easily enter cells and are more environmentally active in terms of biological absorption and additional health risks [33,34]. This means that nanoparticles could directly affect the natural defense systems of the human body and other species, then enter cells and impair their function. Thus, these harmful elements can not only enter the human body in the form of more macroscopic particles, but can also easily enter the human body in the form of nanoparticles, thus causing great harm. Nanoparticles found in raw coal samples contain heavy metal elements such as Ni, Cr, and V, which are very harmful to the human body, and there is a huge risk of these nanoparticles entering into the human body and causing significant harm to human health. The nanoparticles that we found in coal therefore have a huge potential to affect human health, and the study of nanoparticles in raw coal is very important. During the process of coal mining, a mechanism for the evaluation of nanoparticles in coal should be established to prevent the migration of nanoparticles from coal to the surrounding environment. This should help tackle the problem at its source. Studying nanoparticles in coal is important for us to address future environmental challenges and prevent harm to human health.
4.3. Important Potential Sources of Nanoparticles during Coal Combustion
Previous studies have shown that a large number of nanoparticles can be produced during coal combustion, and the elemental composition of these nanoparticles includes Ti, V, Cr, Mn, Fe, Ba, Co, Ni, Cu, Zn, As, Sr, Ca, Si, S, and other elements (Table 2). These nanoparticles typically occur as spherical or single diamond particles and spherical micellar nanoparticles. A single nanoparticle usually has a smooth surface and is generally small, usually between 20 and 50 nm in size. Aggregates have no definite shape or fixed number of nanoparticles, and their diameters can be more than 500 nm (Table 2). Previous studies have mainly focused on nanoparticles formed by the conversion of inorganic minerals during coal combustion [35]. However, a large number of nanoparticles are also found in raw coal. The nanoparticles in raw coal consist of Sr, Ba, S, Ca, Ni, Cr, and other elements; these elements are also present in nanoparticles produced by coal combustion, and the morphology of the two sets of nanoparticles is essentially identical (Table 2).
This similarity between the nanoparticles produced by coal combustion and those found in raw coal suggests a link between the two sets of nanoparticles. When coal is burned at high temperatures in a furnace, a series of solid by-products is produced, including coal ash, slag, flue gas, desulfurized gypsum, and fine particulate matter that is discharged from the chimney [36]; the nanoparticles existing in raw coal can also be released from the coal through this process. Therefore, we speculate that the nanoparticles released during coal combustion are not all produced during the process of coal combustion, but may also include a large number of nanoparticles originally occurring in raw coal. Thus, nanoparticles originally occurring in coal may be an important potential source of nanoparticles produced during the process of coal combustion.

Table 2.
Comparison of the characteristics of nanoparticles produced by coal combustion with those of nanoparticles occurring in raw coal.
Table 2.
Comparison of the characteristics of nanoparticles produced by coal combustion with those of nanoparticles occurring in raw coal.
| Nanoparticles from Coal Combustion | Nanoparticles Occurred in Raw Coal | |
|---|---|---|
| Elemental composition | Ti, V, Cr, Mn, Fe, Ba, Co, Ni, Cu, Zn, As, Sr, Ca, Si, S etc. [37,38]. | Sr, Ba, S, Ni, S, Cr etc. |
| Morphological characteristics | Spherical or single diamond particles and Nanoparticle aggregates [39]. | Spherical or single diamond particles and Nanoparticle aggregates |
| The size of granule | Spherical or single diamond particles: 20–50 nm Nanoparticle aggregates: <500 nm [39]. | Spherical or single diamond particles: 40–70 nm Nanoparticle aggregates: 200–500 nm |
5. Conclusions and Prospection
Our results revealed that raw coal contains naturally formed nanoparticles, which are of various sizes and exhibit complex and irregular shapes. Their constituent elements include Cr, Ni, Si, Ca, V, Ba, S, and Sr. Our findings also reveal a new mode of element occurrence in coal, namely that various elements exist in raw coal in the form of nanoparticles. Based on current practices employed during coal mining and combustion, we infer that the nanoparticles contained in the coal studied in this work can migrate to the natural environment during the aforementioned processes, causing harm to the environment and to human health. Furthermore, our findings highlight a new potential source for the nanoparticles produced by coal combustion, as they indicate that the nanoparticles produced during coal combustion may be the natural nanoparticles contained in the raw coal, which are released from the raw coal through the process of coal combustion. The discovery of nanoparticles contained in coal and the study of their harmful effects are of great significance to meet future environmental challenges and prevent harm to human health.
As there is a widespread existence of nanoparticles in coal seams, the significance of these nanoparticles cannot be ignored any longer. This study is just a beginning for revealing the characteristics of elements in raw coal at a nanoscale. Therefore, there are many phenomena and mechanisms that need to be explained, such as the formation environment of these nanoparticles, the relationship between nanoparticles and coal seams, and how to restrict the nanoparticles in raw coal from entering into the coal combustion, etc. With the further development of nanogeoscience, the study of nanoparticles in coal seams may provide many new ideas and methods for studying coal deposits.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15076276/s1, Figure S1: Morphology map of nanoparticles in raw coal, these nanoparticles are mostly granular, less layered and flake, mostly exist in the form of aggregate, with different sizes. Figure S2: For the nanoparticles present in raw coal, topography (a) shows that the shape of the nanoparticles is granular, with a size of about 70 × 100 nm; HRTEM image (c) shows obvious lattice fringe of the nanoparticles, which proves that the nanoparticles have high crystallinity; selective diffraction pattern (b) shows regular light spots, which proves that the nanoparticles are single crystals. The lattice spacing (d) is 0.35 nm, corresponding to the (200) crystal plane of celestite. Combined with Figure S3, the composition elements of the nanoparticle are mainly Sr, S and O, followed by Ba, and it is speculated that the nanoparticle is celestite. Figure S3: The EDS image of celestite nanoparticles shows that Sr, S and O are the main components, followed by Ba. Figure S4: For the nanoparticles present in raw coal, the topography (a) shows that the nanoparticles are granular with a size of about 150 × 300 nm, and the selection diffraction pattern (b) shows that the nanoparticles are amorphous without diffraction spots, combined with the EDS diagram (Figure S5), the main components of the nanoparticle are Sr, S and O, followed by Ba, it is speculated that the nanoparticle is amorphous celestite nanoparticle. Figure S5: The EDS image of celestite nanoparticles shows that Sr, S and O are the main components, followed by Ba.
Author Contributions
Writing—original draft, P.Z.; Investigation, J.L.; Data curation, L.Z.; Supervision, Y.W.; Writing—review & editing, R.L.; Formal analysis, D.T.; Visualization, Z.C.; Data curation, G.T.; Software, K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the National Natural Science Foundation of China (Grant No. 42102076), project ZR2021QD037 supported by the Shandong Provincial Natural Science Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Some or all data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We especially thank the anonymous reviewers and Huaizhi Shao in Shandong University of Technology for their valuable and constructive comments.
Conflicts of Interest
The work described herein is original research that has not been published previously and is not under consideration for publication elsewhere, in whole or in part. No conflict of interest exists in the submission of this manuscript, and the manuscript has been approved by all authors for publication.
References
- Gao, H. Analysis on the Present Situation and Development Prospect of Coal Chemical Industry. Mod. Chem. Res. 2020, 17, 10–11. [Google Scholar]
- Xiong, L.L.; Wu, T.S.; Tang, M. Research on advance of health effects of nanoparticles on air pollution in China. Chin. J. Prev. Med. 2015, 49, 835–839. [Google Scholar]
- Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Ali, A.Y.S.; Musarrat, J. Characterization of coal fly ash nanoparticles and induced oxidative DNA damage in human peripheral blood mononuclear cells. Sci. Total Environ. 2012, 437, 331–338. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, B.; Hower, J.; Schindler, M.; Winkler, C.; Brandt, J.; Giulio, R.D.; Ge, J.; Liu, M.; Fu, Y. Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal-burning. Nat. Commun. 2017, 8, 194. [Google Scholar] [CrossRef]
- Li, G.J.; Huang, Y.C.; Liu, Y.J.; Guo, L.; Zhou, Y.C. In Vitro Toxicity of Naturally Occurring Silica Nanoparticles in C1 Coal in Bronchial Epithelial Cells. Chin. J. Lung Cancer 2012, 15, 561–568. [Google Scholar]
- Lengke, M.; Southam, G. Bioaccumulation of gold by sulfate-reducing bacteria cultured in the presence of gold (I)-thiosulfate complex. Geochim. Cosmochim. Acta 2006, 70, 3646–3661. [Google Scholar] [CrossRef]
- Palenik, C.S.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Wang, L.; Ewing, R.C. “Invisible” gold revealed: Direct imaging of gold nanoparticles in a Carlin-type deposit. Am. Miner. 2004, 89, 1359–1366. [Google Scholar] [CrossRef]
- Lähde, A.; Gudmundsdottir, S.S.; Joutsensaari, J.; Tapperd, U.; Ruusunen, J.; Ihalainen, M.; Karhunen, T.; Torvela, T.; Jokiniemi, J.; Järvinen, K.; et al. In vitro evaluation of pulmonary deposition of airborne volcanic ash. Atmos. Environ. 2013, 70, 18–27. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P.; Hitchen, G.J.; Hart, R.; Reddy, S.M.; Saunders, M.; Clode, P.; Vaughan, D.; Lowe, J.; Gray, D.J.; et al. Naturally occurring gold nanoparticles and nanoplates. Geology 2008, 36, 571–574. [Google Scholar] [CrossRef]
- Griffin, S.; Masood, M.I.; Nasim, M.J.; Sarfraz, M.; Jacob, C. Natural nanoparticles: A particular matter inspired by nature. Antioxidants 2018, 7, 3. [Google Scholar] [CrossRef]
- Wilson, B.; Dewers, T.; Reches, Z.; Brune, J. Particle size and energetics of gouge from earthquake rupture zones. Nature 2005, 434, 749–752. [Google Scholar] [CrossRef]
- Hu, G.; Cao, J. Occurrence and significance of natural ore-related Ag nanoparticles in groundwater systems. Chem. Geol. 2019, 515, 9–21. [Google Scholar] [CrossRef]
- Hower, J.C.; Graham, U.M.; Dozier, A.; Tseng, M.T.; Khatri, R.A. Association of the sites of heavy metals with nanoscale carbon in a Kentucky electrostatic precipitator fly ash. Environ. Sci. Technol. 2008, 42, 8471–8477. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Flores, D.; Ward, C.R.; Silva, L.F. Identification of nanominerals and nanoparticles in burning coal waste piles from Portugal. Sci. Total Environ. 2010, 408, 6032–6041. [Google Scholar] [CrossRef]
- Silva, L.F.; da Boit, K.M. Nanominerals and nanoparticles in feed coal and bottom ash: Implications for human health effects. Environ. Monit. Assess. 2011, 174, 187–197. [Google Scholar] [CrossRef]
- Chen, B.G. Study on Controlling Effect of Sedimentary Microfacies to Rock Burst in Gaojiapu Coal Mine; China University of Mining and Technology: Beijing, China, 2022. [Google Scholar]
- Lin, L. Study on the Law of Sandstone Sedimentation Control in Luohe Group in Gaojiabao Coal Mine; Xi’an University of Science and Technology: Xi’an, China, 2020. [Google Scholar]
- Dai, S.F.; Ren, D.Y.; Tang, Y.G. Modes of occurrence of major elements in coal and their study significance. Coal Geol. Explor. 2005, 33, 1–5. [Google Scholar]
- Zhao, F.H. Study on the Mechanism of Distributions and Occurrences of Hazardous Minor and Trace Elements in Coal and Leaching Experiments of Coal Combustion Residues; China University of Mining and Technology: Beijing, China, 1997. [Google Scholar]
- Vassilev, S.V.; Eskenazy, G.M.; Vassileva, C.G. Contents, modes of occurrence and origin of chlorine and bromine in coal. Fuel 2000, 79, 903–921. [Google Scholar] [CrossRef]
- Zhang, Y. Fundamental Research on Propagation Law of Ultra-Wideband Radar Wave in Coal and Localiztion; Xi’an University of Science and Technology: Xi’an, China, 2018. [Google Scholar]
- Zhao, S.H. The Coal Quality and Its Controlling Factors in the Main Coalfields of Junggar and Yili Basins, Northern Xinjiang Province; China University of Geosciences: Wuhan, China, 2019. [Google Scholar]
- Liu, H.W. The Dynamic Differentiation Characteristics and Mechanisms of Stress-Sensitive Elements and Minerals in Tectonically Deformed Coals; China University of Mining and Technology: Beijing, China, 2020. [Google Scholar]
- Guo, J.F. Geochemical Characteristics and Geological Significance of Trace Elements in Coals from the Taiping Coal Mine in Panzhihua, Sichuan Province; Anhui University of Science and Technology: Huainan, China, 2018. [Google Scholar]
- Wang, Y.M. Pollution and harm of chromium. Hanzhong Sci. Technol. 2011, 6, 2. [Google Scholar]
- Ren, D.Y.; Zhao, F.H.; Dai, S.F.; Zhang, J.Y.; Luo, K.L. Trace Element Geochemistry of Coal; Science Press: Beijing, China, 2006. [Google Scholar]
- Wei, Y.H.; Huang, Q.C.; Su, X.F. Review on the Toxicological Effect and the Mechanism of Nickel to the Human Health. Environ. Sci. Manag. 2008, 33, 45–48. [Google Scholar]
- Lin, J. Occupational hazards of vanadium compounds. Ind. Health Occup. Dis. 1998, 24, 253–256. [Google Scholar]
- Donaldson, K.; Stone, V.; Borm, P.J.A.; Jimenez, L.A. William MacNee, Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic. Biol. Med. 2003, 34, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, M.R.; Vallyathan, V. Nanoparticles: Health effects—Pros and cons. Environ. Health Perspect. 2006, 114, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Duffin, R.; Mills, N.L.; Donaldson, K. Nanoparticles—A thoracic toxicology perspective. Yonsei Med. J. 2007, 48, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Saquib, Q.; Al-Khedhairy, A.A.; Siddiqui, M.A.; Abou-Tarboush, F.M.; Azam, A.; Musarrat, J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol. Vitr. 2012, 26, 351–361. [Google Scholar] [CrossRef]
- Gilmour, M.I.; O’Connor, S.; Dick, C.A.J.; Miller, C.A.; Linak, W.P. Differential pulmonary inflammation and in vitro cytotoxicity of size-fractionated fly ash particles from pulverized coal combustion. J. Air Waste Manag. Assoc. 2004, 54, 286–295. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Persp. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Ji, M.J. Properties and Control of Fine Particulate Matter During the Combustion of High Ash Fusion Temperature Coal; Anhui University of Science and Technology: Huainan, China, 2013. [Google Scholar]
- Wu, J.Y. Distribution and Environmental Impact of Metal Containing Nanoparticles in Coal Fly Ashes from Coal Fired Power Plants; East China Normal University: Shanghai, China, 2021. [Google Scholar]
- Wu, J.; Tou, F.; Guo, X.; Liu, C.; Sun, Y.; Xu, M.; Liu, M.; Yang, Y. Vast emission of Fe-and Ti-containing nanoparticles from representative coal-fired power plants in China and environmental implications. Sci. Total Environ. 2022, 838, 156070. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Pinto, D.; Lima, B.D. Implications of iron nanoparticles in spontaneous coal combustion and the effects on climatic variables. Chemosphere 2020, 254, 126814. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xu, M.; Yao, H.; Liu, X.; Zhou, K.; Wen, C.; Li, L. Physicochemical properties and potential health effects of nanoparticles from pulverized coal combustion. Chin. Sci. Bull. 2009, 54, 1243–1250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).