Abstract
The in situ passivation is considered a feasible and effective remediation for moderately and lowly heavy-metal-polluted soil. Under natural precipitation, the continuous leaching characteristics of heavy metals with the immobilizers are unclear and require more study for practical applications. In this work, calcium superphosphate (CS) and activated carbon (AC) were added as stabilizers to passivate Cd in hydromorphic paddy (HP) and gray fluvo-aqvic (GF) soils. Simulated acid precipitation at different pH and salt concentrations were used as eluents. The leachate and soil were collected to analyze the stability and fraction changes of Cd. The results showed that with the eluents from 120–200 mL to 200–250 mL, the leached Cd increased and reached the highest concentration and then gradually decreased. Comparative analysis showed that the two passivators in GF soil had higher application values than those in HP soil, while AC showed 3–77 times the capacity of CS in multiple conditions. The addition of AC conversed the exchangeable and oxidized states of Cd to the residual and reduced states, while the addition of CS conversed the exchangeable and reduced states of Cd to the residual and oxidized states. The above results can provide important references for the immobilization of heavy metal cations in soil and the sustainable utilization of soil.
1. Introduction
Since the industrial revolution, mining, industrial production and the use of metals and metal compounds result in the accumulation of toxic metals in soil. Soil contamination with heavy metals and metalloids is a global environmental problem threatening human health and food safety. The heavy metal contamination of soil threatens a considerable number of countries, including China, India, Europe, and Japan [1,2,3]. The concentration of heavy metals and metalloids, arsenic, cadmium, chromium, mercury, lead, cobalt, copper, nickel, zinc, and selenium, are above the geographic baseline or regulatory level in some locations, which threatens the biosphere and water security [4,5].
Among various heavy metals, cadmium (Cd) is a great threat to the paddy soil environment [6]. It has caused the appalling “bone pain disease” because the enrichment of Cd in rice caused human poisoning. Heavy metal pollution in soil tends to accumulate in the rhizosphere of plants, and risk factors indicate a high potential ecological risk due to cadmium pollution [7]. The bioavailability, bioaccessibility, and accumulation of cadmium in soil–plant systems are the main drivers of its transfer to different trophic levels through different pathways. In the human system, the bioaccumulation of Cd can damage the antioxidant defense system because the production of reactive oxygen species can cause oxidative stress, which can further lead to different diseases [8].
The pollution and the remediation of the heavy metal in soil have attracted more and more attention [9,10]. Each method or strategy has advantages and disadvantages. Among various remediation methods, surface capping, encapsulation, electrokinetic extraction, soil flushing, and landfill can repair high-pollution sites in small areas, which have relatively strong effects on soil properties. This is not conductive to the sustainable use of soil. For most widely distributed but unevenly distributed heavy metal pollution in soil, immobilization (e.g., solidification, stabilization, and vitrification) is an effective and economical option [2,11,12].
Immobilization of heavy metals can be achieved by adsorption, precipitation, and complexation reactions, which can result in the redistribution of heavy metals from the solution phase to the solid phase, thereby reducing their bioavailability and environmental migration [13]. The addition of typical minerals, such as montmorillonite, to sandy soils can significantly slow soil water infiltration. Especially in heterogeneous soils, the addition reduces direct surface runoff and deep seepage, thereby reducing the migration of pollutants compared to homogeneous soil samples, making them an ideal structure for soil reconstruction in open pit coal mine dumps [14]. On the other hand, there are also studies showing that the binder composed of oxalic-acid-activated phosphate rock, monopotassium phosphate, and reactive magnesia reduced the concentrations of leached Pb and Zn below the regulatory limit of China MEP or USEPA [15,16].
Organic matter was also used for the remediation of heavy metal contaminated soils as promising materials. The addition of organic matter can not only improve the physical and chemical properties of the soil, but also often adsorb and passivate heavy metals in the soil [17,18,19]. The application of carbonaceous adsorbents (biochar) reduced bioavailable As and Pb by 40.42% and 64.21%, respectively, and soil bioactivity, soil enzyme activity, and the total number of microorganisms increased significantly [20]. With the xanthan-gum-based biopolymer, 20% and 90% of Cu was immobilized in the soil, and the immobilization rate increased with the biopolymer mixing ratios [21]. Activated carbon showed more than 50 mg/g adsorption amount of Cd in the solution [22], while it reduced 14.8–63% of copper in soil interstitial water [23].
Although many studies are concentrated on the passivation of heavy metals in soils, the knowledge about the state of heavy metals in real field conditions is limited. Weather, soil permeability, depth of contamination, and especially the potential deep leaching of chemicals need more consideration [24,25,26]. The long-term effect of passivating agents on the passivation of heavy metals in the soil and its stability under natural precipitation conditions is still questioned [27,28]. Many studies focus on the pH of precipitation [29,30], but few articles focus on the salinity of precipitation. Many studies focus on the leaching of heavy metals under precipitation conditions [6,28,29,30], while few articles focus on the dynamic persistence of this leaching change.
In this work, inorganic and organic stabilizers, calcium superphosphate (CS) and activated carbon (AC) were added to passivate Cd in hydromorphic paddy (HP) and gray fluvo-aqvic (GF) soils, respectively. The soils were washed with simulated acid precipitation at different pH and salt concentrations, and then, the leachates and the soils after leaching were collected and analyzed to obtain the effects of immobilizers in different soils on the leached Cd and the fraction of Cd in soils.
This study provides a simulated continuous long-term comparison of the two passivators with different precipitation conditions in the two soils. Based on the Cd leaching characteristics, it can provide practical guidance for the selection of passivators and the influence of soil differences in the treatment of heavy metal pollution in soil. According to the characteristics of regional precipitation, the stability and form of heavy metals in the soil can also be predicted, which provides a reference for the control of soil pollution and the sustainable use of soil resources.
2. Materials and Methods
2.1. Materials
2.1.1. Soils and Stabilizers
Hydromorphic paddy (HP) and gray fluvo-aqvic (GF) soils were collected from the Ap horizon (0–20 cm) of agricultural field, in Xiaonan District, Xiaogan, China. The particle size distribution, pH, organic matter, cation exchange capacity were measured with the pipette method [31], suspension of soil and water at ratio of 1:2.5, wet dichromate oxidation method [32], and ammonium acetate extraction [33], respectively (Table 1). The soils were air-dried and ground to pass through a 60-mesh sieve before use. The calcium superphosphate (CS) and commercial activated carbon produced according to HG/T 3491-1999 (2017) (AC, Chromatographic grade, through 30 mesh) were purchased from Sinopharm Chemical Company. Unless otherwise stated, all the reagents used were of analytical grade and supplied by Sinopharm Chemical Company (Shanghai, China), the water used was ultrapure water (Resistivity = 18.25 MΩ), and the experiments were performed at room temperature.

Table 1.
The properties of the soils for the leaching experiments.
2.1.2. Eluents
According to the precipitation components of typical acid rain in Southwest China [34], the eluent solution was prepared with CaCl2, NH4Cl, H2SO4, HNO3, and NaOH solutions. A stock solution (Salt 10) had ten times the salt concentration of acid rain in China, containing SO42−, NO3−, Cl−, NH4+, Ca2+, Mg2+, Na+, and K+ of 1000, 500, 300, 600, 800, 200, 200, and 200 umol/L, respectively, and the solution pH was adjusted to 4.68 with 0.01–1 mol/L HCl and NaOH. The stock solution was diluted to obtain 0.1, 0.2, 0.5, 1, 2, and 5 times salt concentration of the precipitation (Salt 0.1–5) for the study on the effect of salt concentration. On the other hand, the pH of the solution with typical salt concentration (Salt 1) was adjusted to pH 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6.0 for the study on the effect of pH. The eluents (pH 4.68) with 0.1–10 times the typical salt concentration of the precipitation and the eluents (typical salt concentration of precipitation) at pH 3.0–6.0, respectively, were marked as Salt 0.1–10 and pH 3.0–6.0.
2.2. Methods
2.2.1. Leaching and the Collection of Leachates
Cadmium nitrate solution was sprayed onto the flat grinded soils and the mixtures were turned over and mixed every day (total of 10 days) to make the concentration of Cd at 0.1 g per kg soil. The soils with Cd were aged for 30 days, and then, the superphosphate and activated carbon were added in the soils at the mass ratios of 5%. After mixing evenly, the immobilized soil samples were aged for another 30 days [35]. A batch of 330 mL inverted plastic bottles without bottom were filled with the above soils (500 g soils with 5% CS or AC), respectively. The mouths of the bottles were wrapped with filter paper and fixed with the caps.
A total of 120 mL eluent at the first time and then 5 mL each time were added in the bottoms of the inverted plastic bottle, respectively. From the mouth of the inverted bottle, each 5 mL leached solution was collected for the measurement. The leachate was diluted as needed, and the concentration was measured using an atomic absorption spectrophotometer (AA, TAS-990, Beijing Puxi General Apparatus Ltd., Co., Beijing, China). A standard of 5 mg/L Cd was added, and every 50 samples were measured to verify the accuracy of the measurement method, and the deviation was in the range of 99–102%. Each sample was measured 3 times on AA, and the deviation was in the range of 95–102%.
Taking 1000 mm as the median of the annual precipitation in subtropical, 370 mL in current work was approximately 47 days of continuous precipitation. The cross-section of the soil column was 28.23 cm2, so each 1 mL of eluent was equivalent to 0.35 mm of precipitation [2].
2.2.2. The Analysis of the Fraction of Cd in the Soils
After the leaching by eluents pH 5.0, HP and GF soils with or without immobilizers added, were air-dried and ground for Cd speciation analysis. Using the BCR sequential extraction method [36], after sequential extraction of a weakly acid-extracted state (exchange and carbonate bound state), reduced state (iron–manganese oxidation state), and oxidizable state (organically bound state). The residue was washed with deionized water, and the residue state of Cd was determined after digestion using HCl-HNO3-HClO4 (the initial concentration of Cd in soils was determined with these samples).
3. Results and Discussion
3.1. The Leached Cd from the Soils with Cd Immobilized by Activated Carbon
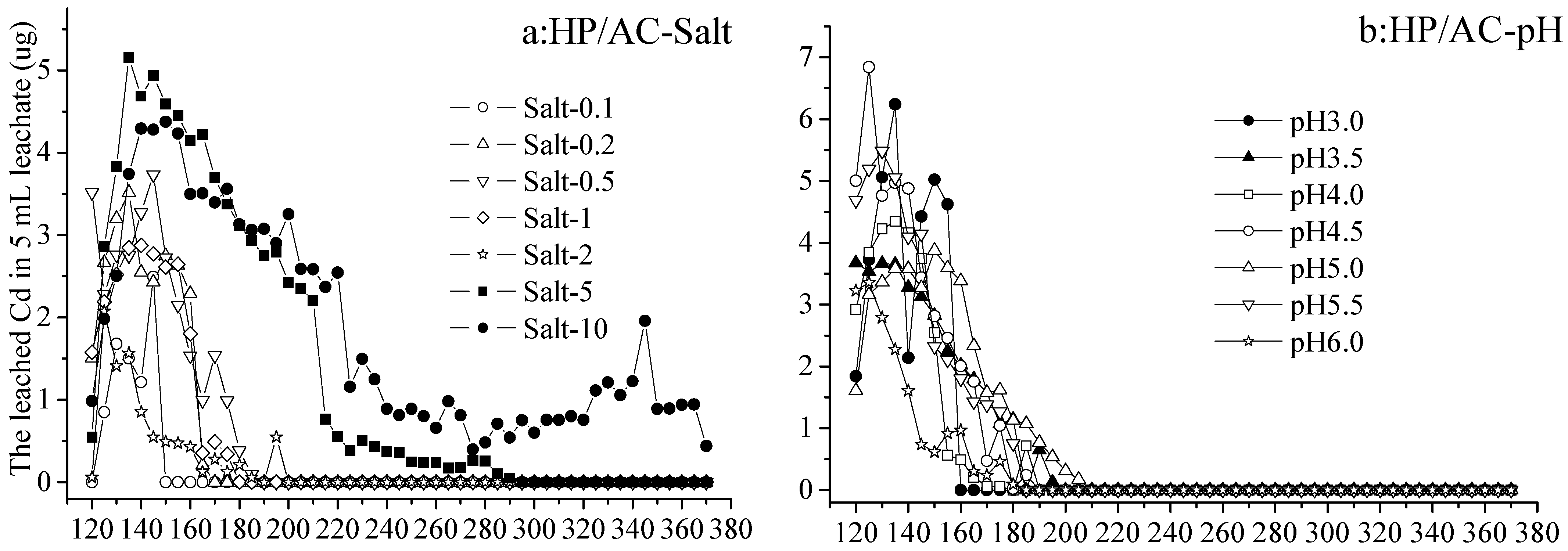
As the volume of eluents increased from 120 to 370 mL (no leachate with 0–120 mL eluents), the leached Cd in 5 mL leachate from Cd immobilized by activated carbon increased and then decreased in each treatment (Figure 1a–d). From activated-carbon-added hydromorphic paddy (HP/AC) soil, the Cd leached by Salt 0.1–2 increased from 0 to 3.76 µg and decreased to 0 µg (not detected) in 5 mL leachate with 120–150 mL and 150–370 mL eluents, respectively. While by eluents Salt 5 and 10, the leached Cd was greater than that of other treatments, increasing from 0.54 to 4.95 µg, and gradually decreasing to 0 or 1 µg with 120–150 mL and 150–370 mL eluents, respectively (Figure 1a). On the other hand, the Cd leached by eluents at various pH values (3.0–6.0) also increased from 1.58 to 6.82 µg and decreased to 0 µg (not detected) in 5 mL leachate with 120–210 mL and 210–370 mL eluents, respectively. The differences between treatments at various pH values were not significant and not enough to demonstrate that lower pH leached out more Cd (Figure 1b).
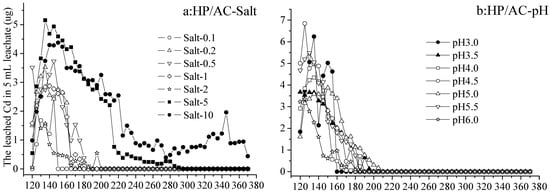
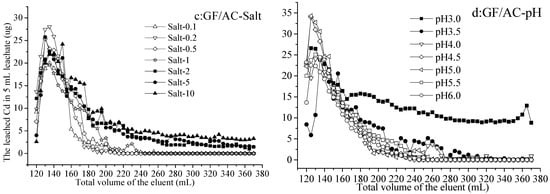
Figure 1.
The leached Cd from hydromorphic paddy (HP) and gray fluvo-aqvic (GF) soils with activated carbon (AC) added, respectively, by eluents of various pH values and salt concentrations (n = 3).
The release of heavy metal is not only related to the total concentration, occurrence state, and mobility of the heavy metal, but also affected by the pH value of soil solution [37]. He et al. [26] found that the highest leaching concentration of target heavy metal was observed under acidic conditions, and decreased with the increase in pH value. The present study also agreed with the conclusion that pH 2.0 had a great influence on the eluted Pb, and the leaching behavior of the soils differed little when the eluent was weakly acidic (pH~4.0) and neutral [29,30].
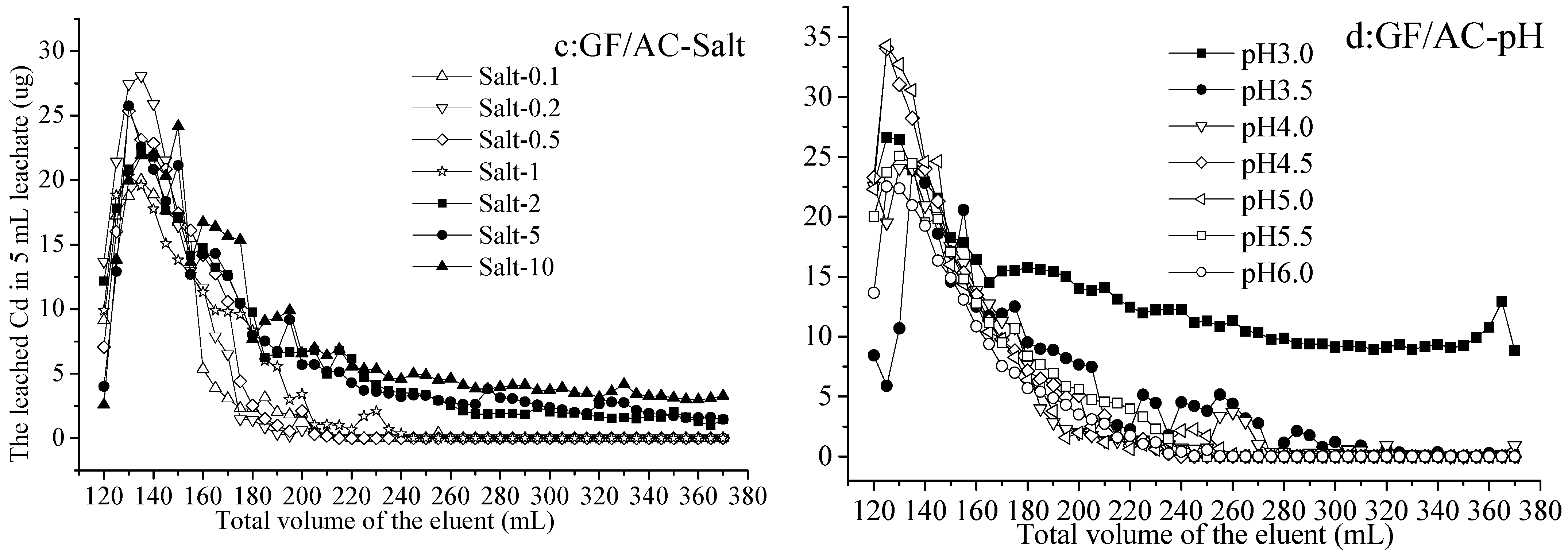
From activated-carbon-added gray fluvo-aqvic (GF/AC) soil, the Cd leached by Salt 0.1–10, increasing from 2.61 to 28.10 µg and decreased to 0–5.16 µg in 5 mL leachate with 120–230 mL and 230–370 mL eluents, respectively. The difference in the results of leaching by various concentrations of salt showed the Cd leached by Salt 0.1–1 was not detected, while that by Salt 2–10 was 1.37–5.09 µg with the 230–370 mL leaching (Figure 1c). On the other hand, the Cd leached by eluents at various pH values (3.0–6.0) increased from 8.55 to 34.31 µg and decreased to 0 (not detected) or 9.15 µg in 5 mL leachate with 120–240 mL and 210–370 mL eluents, respectively. It is worth noting that the 160–370 mL eluent at pH 3.0 leached Cd at 8.84–15.61 µg in each 5 mL leachate, was significantly higher than other treatments (Figure 1d).
The release of heavy metal could be divided into two distinct stages, with rapid changes in the early stage, slow and steady changes in the late stage, and a slight increase in the middle [38]. In addition, the leaching efficiency of Cd, Zn, and Mn was about 1.5 times higher at pH 2.9 than at pH 6.5 [38], which is similar to the results of this experiment. Even if the initial Cd concentration in the HP (0.268 mg/kg, Table 1) was higher than that in the GF (0.102 mg/kg, Table 1), there were more Cd leached from GF than HP under the same elution conditions. From the HP soil, it seemed that leaching with salt at different concentrations produced differences in the leached Cd than acid leaching at different concentrations, while from the GF soil, various acid concentrations of eluents led to different amounts of leached Cd.
Both Figure 1 and Figure 2 showed that the Cd amount of leachate in the initial stage was small, and the peak amount of leaching was reached only after continuous leaching [39]. The literature also points out that the leaching of heavy metals was related to the destruction of organic matter, which might be the reason for this phenomenon. The release of heavy metals from soil was closely related to their composition and soil properties, such as pH, soil texture, and organic matter [29].
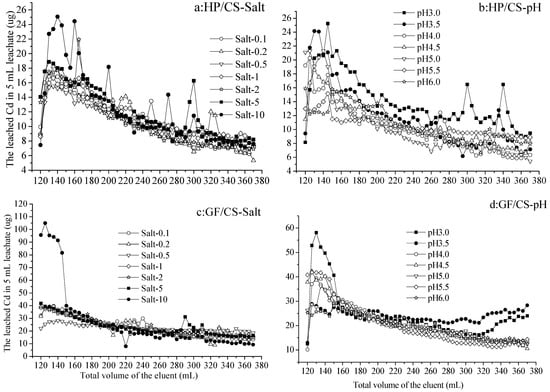
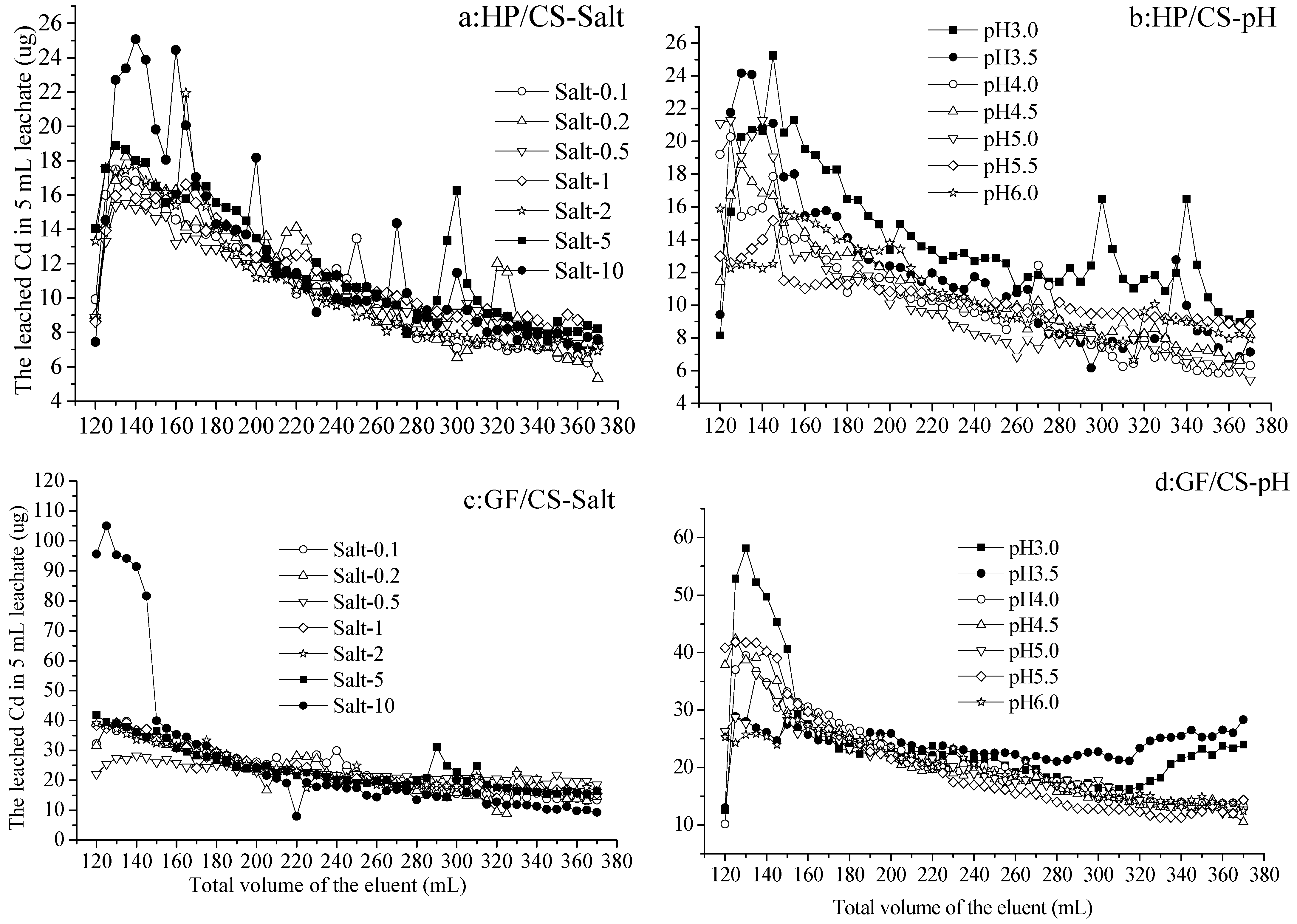
Figure 2.
The leached Cd from the hydromorphic paddy (HP) and gray fluvo-aqvic (GF) soils with calcium superphosphate (CS) added, respectively, with eluents of various pH values and salt concentrations (n = 3).
3.2. The Leached Cd from the Soil with Cd Immobilized by Calcium Superphosphate
As the volume of eluents increased from 120 to 370 mL (no leachate with 0–120 mL eluents), the leached Cd in 5 mL leachate Cd from calcium superphosphate (CS)-added soil increased and then decreased with each treatment, and could be continuously detected (Figure 2a–d). From calcium-superphosphate-added hydromorphic paddy (HP/CS) soil, the Cd leached by Salt 0.1–5, increasing from 7.36 to 18.92 µg and decreased to 6.15 µg in 5 mL leachate with 120–130 mL and 130–370 mL eluents, respectively. Occasionally, the amount of Cd leached by Salt-5 and Salt-10 was significantly higher than that of other treatments, and reached 25.16 and 24.53 µg with 140 and 160 mL Salt-10 eluent, respectively (Figure 2a). On the other hand, the Cd leached by eluents at various pH values (3.0–6.0) also increased from 8.14 to 25.37 µg and decreased to 5.44 µg in the 5 mL leachate with 120–145 mL and 145–370 mL eluents, respectively. The Cd leached by eluent pH 3.0 was higher than or equal to that by other treatments with different eluents volumes (Figure 2b).
From calcium-superphosphate-added gray fluvo-aqvic (GF/CS) soil, the Cd leached by Salt 0.1–5 decreased from 21.97–41.94 µg to 9.14–14.46 µg in 5 mL leachate with 120–370 mL eluents, respectively. By the 120–145 mL eluent of Salt-10, there was higher Cd leached of 80.96–104.37 µg than other treatments in the 5 mL leachate (Figure 2c). On the other hand, the Cd leached by eluents at various pH values (3.0–6.0) increased from 10.13 to 25.64–58.19 µg and decreased to 11.02–28.27 µg in 5 mL leachate by 120–130 mL and 130–370 mL eluents, respectively. The Cd leached by eluent pH 3.0 and 3.5 were higher than or equal to that by other treatments with 270–370 mL of eluents (Figure 2d).
Similar to the results of the previous section, there was more Cd leached from GF than HP soil under the same elution conditions with the addition of calcium superphosphate. From the HP and GF soils, leaching with eluents Salt-10 and pH 3.0 was more likely to produce differences in the leached Cd than other treatments. Cd was always detected in the 370 mL leaching, which was different from that from the soil with activated carbon (Figure 1), indicating that the immobilization by activated carbon was stronger than that by calcium superphosphate.
3.3. Comparison of Total Leached Cd
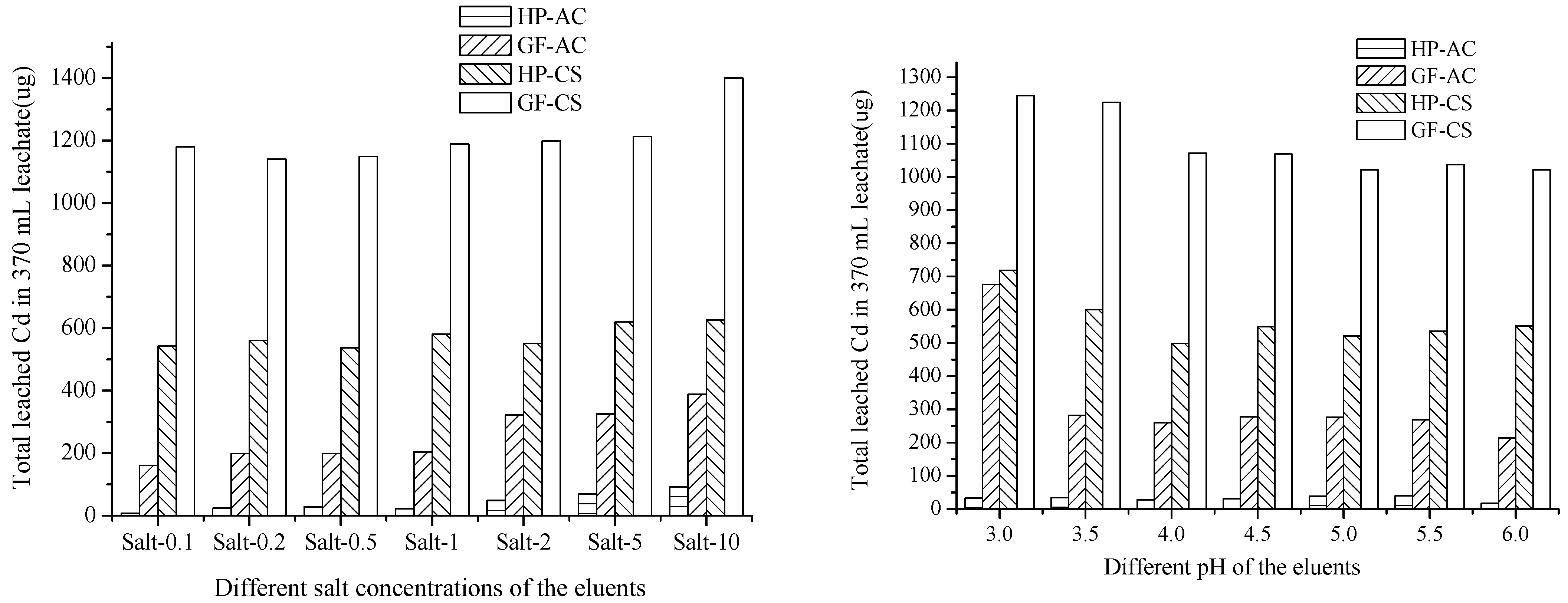
The total amount of leached Cd from the HP and GF soils without the addition of immobilizer was 4088 and 9179 µg, respectively. By the leaching of eluent Salt-0.1 to Salt 10, the Cd immobilized ratio was 66–96% in the GF soil (161–388 and 1140–1399 µg Cd leached, compared to the 4088 µg), which was lower than 93–100% in the HP soil (7–93 and 537–625 µg Cd leached, compared to the 9179 µg). As shown in Table 1, the initial pH of the HP and GF soils were 5.8 and 7.6, respectively (Figure 3). The leaching mechanisms of heavy metals were controlled by diffusion, which was caused by the destruction of soil components or responsible structures [39]. The greater change of pH might lead to greater destruction and more activation of Cd, which may be the cause for a greater amount of leached Cd from the GF soil.
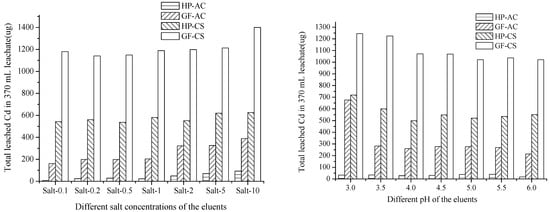
Figure 3.
Total leached Cd with eluents of different pH values (Cd leached from the control soil, 4088 and 9179 µg, had been subtracted).
In Figure 3 (left), in the HP and GF soils, AC (7–93 and 161–388 µg Cd leached) exhibited 3–77 times more passivation ability than CS toward the salt leaching (537–625 and 1140–1399 µg Cd leached). This may be due to the large adsorption capacity of AC for the Cd cation. Using Salt 0.1–10, the leached Cd values were 542, 560, 537, 580, 551, 619, 625 and 1179, 1140, 1149, 1188, 1198, 1212, 1399 µg from the CS-added HP and GF soils, 7, 23, 28, 23, 29, 70, 92 and 161, 199, 198, 203, 322, 325, 388 µg from the AC-added HP and GF soil, respectively. With the increase in the concentration of salt in the eluents, the leached Cd from soils with added AC increased rapidly, while it increased slowly with the increase in the concentration of salt from soils with added CS. That indicated that the application of CS for Cd immobilization could adapt a wider range of salt concentration changes.
In Figure 3 (right), as immobilizers in the HP and GF soils, AC (17–39 and 214–676 µg Cd leached) exhibited better passivation properties than CS (499–719 and 1012–1244 µg Cd leached). By eluent pH 3.0–6.0, the leached Cd values were 719, 600, 499, 548, 521, 535, 550 and 1244, 1224, 1071, 1069, 1012, 1037, 1021 µg from the CS-added HP and GF soils, respectively. By contrast, Cd leached were 33, 34, 27, 30, 38, 39, 17 and 676, 281, 259, 277, 275, 268, 214 µg from the AC-added HP and GF soils, respectively.
In all series of leaching experiments, the total amount of Cd in leachates followed the sequence, GF/CS (1012–1399 µg) > HP/CS (499–719 µg) > GF/AC (161–676 µg) > HP/AC (8–70 µg). This indicated that AC has a stronger ability to passivate Cd than CS, and HP soil could fix heavy metals better than GF soil. This might be due to the higher initial pH (7.5) and lower organic matter content (19.8 g/kg) of GF. The effect of the pH of the eluents was not obvious in the leachate from the HP-AC; however, from the GF-AC, HP-CS and GF-CS, the eluents at pH 3.0 leached more Cd than other treatments. The effect of the salt concentration of the eluents was obvious in the leachate from the HP-AC, GF-AC, HP-CH, and GF-CH. The eluents Salt 10 leached more Cd than other treatments.
Overall, the differences in leaching caused by different acidities were greater than those caused by different salt concentrations. Under the conditions of continuous acid and salt leaching, the passivation effect of HP on Cd was better than that of GF, and the passivation ability of AC on Cd was stronger than that of CS.
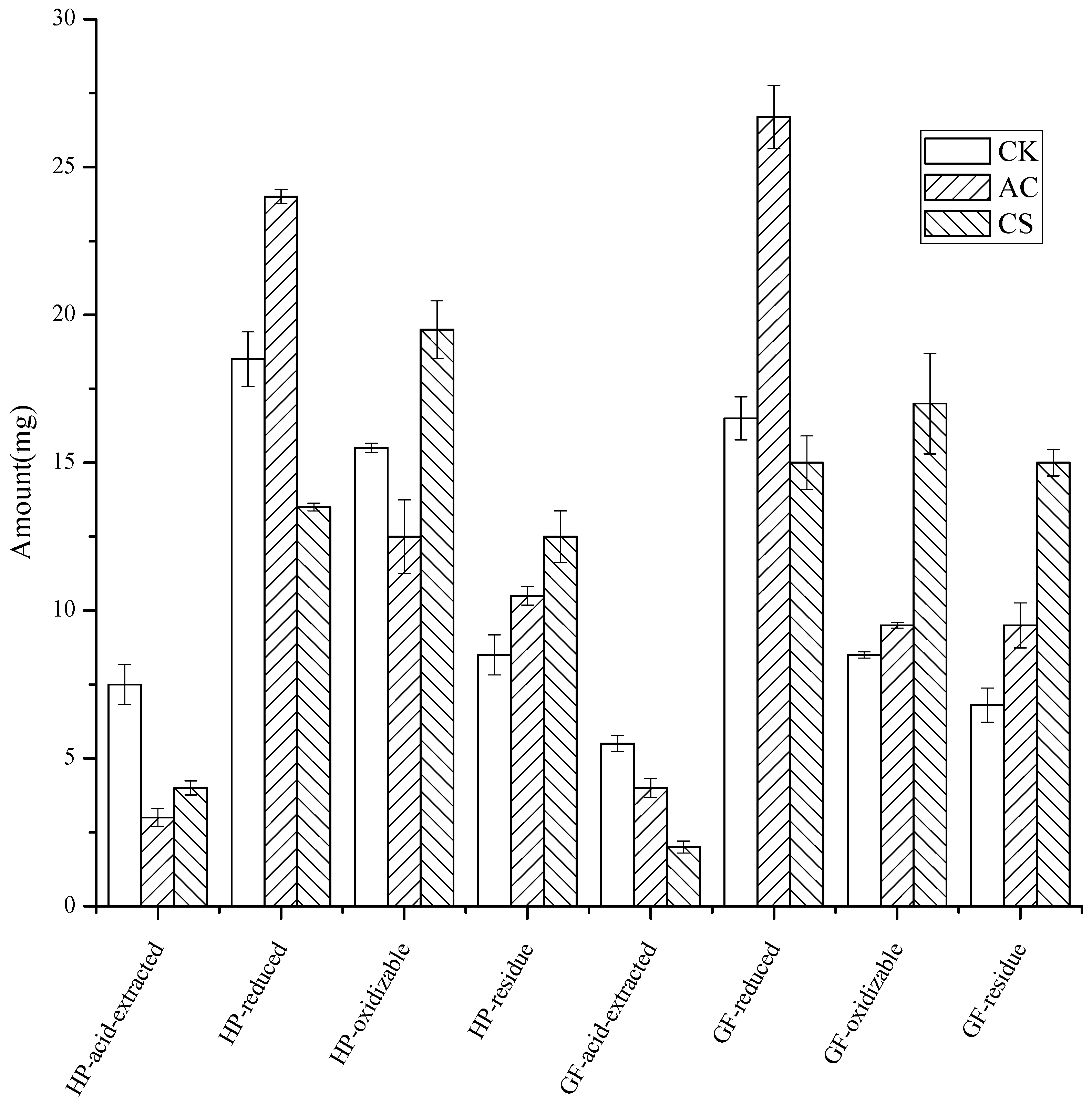
3.4. The Fraction of Cd in the Soils after the Leaching
As shown in Figure 4, for the HP soil leached by eluent pH 5.0 with standard salt concentration, compared with the control soil, AC addition resulted in a 60% and 19% reduction in the acid-extracted and oxidizable states, while the reduced and residue states increased by 30% and 24%, respectively. CS addition resulted in a 47% and 27% decrease in both the acid-extracted and reduced states, and a 26% and 47% increase in the oxidizable and residue states, respectively.
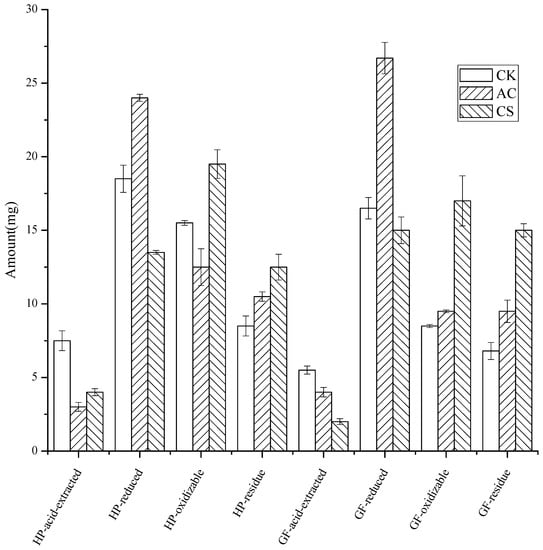
Figure 4.
The four states of Cd in soils after leaching (the error bars represented for standard error, n = 3).
On the other hand, for the GF, the addition of AC resulted in a 27% decrease in the form of an acid-extracted state, while the reduced, oxidizable, and residue states increased by 62%, 12%, and 39%, respectively. The addition of CS caused the acid-extracted and reduced states to decrease by 64% and 9%, while the oxidizable and residue states increased by 100% and 121%, respectively.
AC addition tended to convert acid-extracted and oxidizable states to reduced and residue states (slightly increased oxidizable states in GF soil), while CS addition converted acid-extracted and reduced states to oxidizable and residue states. Both AC and CS realized the transformation of the acid-extracted form into a stable form, which is consistent with the previous reports that the reduction in the carbonate-bound state [40,41] and reduced state [25], and the increase in the residue state [41] were the characteristics of heavy metal passivation [25,40]. Whether the immobilizer was AC or CS, more residue states were formed in GF, indicating that the two passivators will have better application in GF soil rather than in HP for the long-term effect.
4. Conclusions
This study found that the immobilized Cd was released with the precipitation of 150–250 mL (equivalent to 53–88 mm precipitation), and the leached Cd gradually reduced with more leaching. The activated-carbon-immobilized Cd was hardly leached during 200–250 mL. Under the same leaching conditions, the hydromorphic paddy soil could immobilize more Cd than the gray fluvo-aqvic soil, and more Cd in the gray fluvo-aquic soil was converted to a soluble form than in the hydromorphic paddy soil. Moreover, lower pH and higher salt concentration had a stronger dissolution effect on passivated Cd.
Activated carbon and calcium superphosphate in the immobilization of Cd in hydromorphic paddy and gray fluvo-aqvic soils behaved differently; activated carbon converted Cd to acid-extracted and oxidizable states, and calcium superphosphate converted Cd to oxidizable and residue states, respectively. Both immobilizers had better immobilization ability in the GF soil than in HP.
This study also provides the continuous leaching characteristics of passivated Cd in soil under continuous leaching, and compares the effects of salt and acid in precipitation on passivated Cd, which can provide a reference for subsequent research and application practice.
Author Contributions
Conceptualization, Y.D.; methodology, K.C. and C.L.; software, Y.D.; validation, Y.T.; resources, Y.D. and H.H.; data curation, C.H. and Y.T.; writing—original draft preparation, C.H.; writing—review and editing, C.H. and H.H.; supervision, C.H.; project administration, H.H.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Natural Science Foundation of Hubei Province (2020CFB855) and Outstanding young and middle-aged science and technology innovation team project in colleges and universities of Hubei Province (T2022030) in China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their appreciation to the Natural Science Foundation and Outstanding young and middle-aged science and technology innovation team project in colleges and universities of the Hubei Province in China, for funding this research work through project no. (2020CFB855) and (T2022030), respectively.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Bhattacharyya, R.; Ghosh, B.N.; Mishra, P.K.; Mandal, B.; Rao, C.S.; Sarkar, D.; Das, K.; Anil, K.S.; Lalitha, M.; Hati, K.M.; et al. Soil degradation in India: Challenges and potential solutions. Sustainability 2015, 7, 3528–3570. [Google Scholar] [CrossRef]
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Y.; Zhang, S.; Han, Y.; Bate, B.; Ke, H.; Chen, Y. Soil heavy metal pollution of industrial legacies in China and health risk assessment. Sci. Total Environ. 2022, 816, 151632. [Google Scholar] [CrossRef]
- Ren, S.; Song, C.; Ye, S.; Cheng, C.; Gao, P. The spatiotemporal variation in heavy metals in China’s farmland soil over the past 20 years: A meta-analysis. Sci. Total Environ. 2022, 806, 150322. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, R.; Chen, W.; Peng, C.; Markert, B. Effects of urbanization on heavy metal accumulation in surface soils, Beijing. J. Environ. Sci. 2018, 64, 328–334. [Google Scholar] [CrossRef]
- Sun, R.; Gao, Y.; Yang, Y. Leaching of heavy metals from lead-zinc mine tailings and the subsequent migration and transformation characteristics in paddy soil. Chemosphere 2022, 291, 132792. [Google Scholar] [CrossRef]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Bioaccumulation of potentially toxic elements in three mangrove species and human health risk due to their ethnobotanical uses. Environ. Sci. Pollut. Res. 2021, 28, 33042–33059. [Google Scholar] [CrossRef]
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of cadmium pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Yang, B.; Cao, Y.; Ren, J.; Wang, M.; Luo, H.; Li, F. Water incubation-induced fluctuating release of heavy metals in two smelter-contaminated soils. J. Environ. Sci. 2019, 82, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Cai, C.; Zhao, M.; Yu, Z.; Rong, H.; Zhang, C. Utilization of nanomaterials for in-situ remediation of heavy metal(loid) contaminated sediments: A review. Sci. Total Environ. 2019, 662, 205–217. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Yaseen, M.; Hussain, B.; Zehra, A.; Aziz, M.; He, Z.; Yang, X. Comparative efficacy of organic and inorganic amendments for cadmium and lead immobilization in contaminated soil under rice-wheat cropping system. Chemosphere 2019, 214, 259–268. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Jia, J.; Zhang, X. Soil Reclamation Models by Soil Water Infiltration for Refuse Dumps in Opencast Mining Area of Northern China. Sustainability 2022, 14, 15929. [Google Scholar] [CrossRef]
- Ge, X.; Wang, L.; Zhang, W.; Putnis, C.V. Molecular Understanding of Humic Acid-Limited Phosphate Precipitation and Transformation. Environ. Sci. Technol. 2020, 54, 207–215. [Google Scholar] [CrossRef]
- Huang, G.; Gao, R.; You, J.; Zhu, J.; Fu, Q.; Hu, H. Oxalic acid activated phosphate rock and bone meal to immobilize Cu and Pb in mine soils. Ecotoxicol. Environ. Saf. 2019, 174, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wei, M.; Chen, J.; Liu, H.; Kou, M. Comparative study of the adsorption /immobilization of Cu by turmeric residues after microbial and chemical extraction. Sci. Total Environ. 2019, 691, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef]
- Malik, K.M.; Khan, K.S.; Rukh, S.; Khan, A.; Akbar, S.; Billah, M.; Bashir, S.; Danish, S.; Alwahibi, M.S.; Elshika, M.S.; et al. Immobilization of Cd, Pb and Zn through organic amendments in wastewater irrigated soils. Sustainability 2021, 13, 2392. [Google Scholar] [CrossRef]
- Wang, G.; Tariq, M.; Liang, W.; Wan, J.; Peng, C.; Zhang, W.; Cao, X.; Lou, Z. A comparative and modeled approach for three biochar materials in simultaneously preventing the migration and reducing the bioaccessibility of heavy metals in soil: Revealing immobilization mechanisms. Environ. Pollut. 2022, 309, 119792. [Google Scholar] [CrossRef]
- Ko, M.; Jeon, Y.; Kim, K. Novel application of xanthan gum-based biopolymer for heavy metal immobilization in soil. J. Environ. Chem. Eng. 2022, 10, 108240. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef] [PubMed]
- Que, W.; Zhou, Y.; Liu, Y.; Wen, J.; Tan, X.; Liu, S.; Jiang, L. Appraising the effect of in-situ remediation of heavy metal contaminated sediment by biochar and activated carbon on Cu immobilization and microbial community. Ecol. Eng. 2019, 127, 519–526. [Google Scholar] [CrossRef]
- Derakhshan-Nejad, Z.; Rezania, S.; Jung, M.C.; Al-Ghamdi, A.A.; Mustafa, A.E.Z.M.A.; Elshikh, M.S. Effects of fine fractions of soil organic, semi-organic, and inorganic amendments on the mitigation of heavy metal(loid)s leaching and bioavailability in a post-mining area. Chemosphere 2021, 271, 129538. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, P.; Guo, G.; Hu, H.; Zhu, J.; Fu, Q. Efficiency of several leaching reagents on removal of Cu, Pb, Cd, and Zn from highly contaminated paddy soil. Environ. Sci. Pollut. Res. 2016, 23, 23271–23280. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhang, S.; Zhang, X.; Zhang, Z.; Zhao, Y.; Ding, H. Copper slag: The leaching behavior of heavy metals and its applicability as a supplementary cementitious material. J. Environ. Chem. Eng. 2021, 9, 105132. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, X.; Li, B.; Wang, Y.; Guo, J. Distribution of Cd and Cu fractions in Chinese soils and their relationships with soil pH: A meta-analysis. Sustainability 2019, 11, 337. [Google Scholar] [CrossRef]
- Li, J.; Jia, C.; Lu, Y.; Tang, S.; Shim, H. Multivariate analysis of heavy metal leaching from urban soils following simulated acid rain. Microchem. J. 2015, 122, 89–95. [Google Scholar] [CrossRef]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Liu, Z.P.; Jin, F. Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil. J. Hazard. Mater. 2014, 271, 131–140. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods; Klute, A., Ed.; ASA/SSSA: Madison, WI, USA, 1986. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter: Laboratory methods. In Methods of Soil Analysis, Part 3, Chemical Methods; Sparks, D.L., Ed.; ASA/SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation and exchange capacity and exchange coefficients. In Methods of Soil Analysis, Part 3, Chemical Methods; Sparks, D.L., Ed.; ASA/SSSA: Madison, WI, USA, 1996; pp. 1201–1231. [Google Scholar]
- Zhai, P.; Zhang, X.; Wan, H.; Pan, X. Trends in total precipitation and frequency of daily precipitation extremes over China. J. Clim. 2005, 18, 1096–1108. [Google Scholar] [CrossRef]
- Borthakur, A.; Chhour, K.L.; Gayle, H.L.; Prehn, S.R.; Stenstrom, M.K.; Mohanty, S.K. Natural aging of expanded shale, clay, and slate (ESCS) amendment with heavy metals in stormwater increases its antibacterial properties: Implications on biofilter design. J. Hazard. Mater. 2022, 429, 128309. [Google Scholar] [CrossRef]
- Quevauviller, P.; Rauret, G.; López-Sánchez, R.J.F.R.; Muntau, A.U.H. Certification of trace metal extractable contents in a sediment reference material (CRM 601) following a three-step sequential extraction procedure. Sci. Total Environ. 1997, 205, 223–234. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, H.; Peng, T.; Ding, W.; Liu, B.; Liu, Q. Comprehensive evaluation of environmental availability, pollution level and leaching heavy metals behavior in non-ferrous metal tailings. J. Environ. Manag. 2021, 290, 112639. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Jiang, Y.; Bai, Y.; Cheng, L. The evolution of different dissolved organic matter components and release characteristics of heavy metals in leaching process from sewage sludge under simulated rain. Environ. Sci. Pollut. Res. 2022, 29, 86651–86664. [Google Scholar] [CrossRef]
- Wei, M.; Li, Y.; Yu, B.; Liu, L.; Xue, Q.; Du, Y. Assessment of semi-dynamic leaching characteristics of lead and zinc from stabilized contaminated soil using sustainable phosphate-based binder after carbonation. J. Clean. Prod. 2022, 332, 130126. [Google Scholar] [CrossRef]
- Cui, J.; Luo, C.; Tang, C.; Chan, T.; Li, X. Speciation and leaching of trace metal contaminants from e-waste contaminated soils. J. Hazard. Mater. 2017, 329, 150–158. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Matsufuru, H.; Sato, T. Attenuation of lead leachability in shooting range soils using poultry waste amendments in combination with indigenous plant species. Chemosphere 2008, 73, 643–649. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).