Evaluation of Physicochemical and Amphiphilic Properties of New Xanthan Gum Hydrophobically Functionalized Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Hydrophobic Modification of Xanthan Gum
2.3. Evaluation of BXGs Physicochemical Proprieties
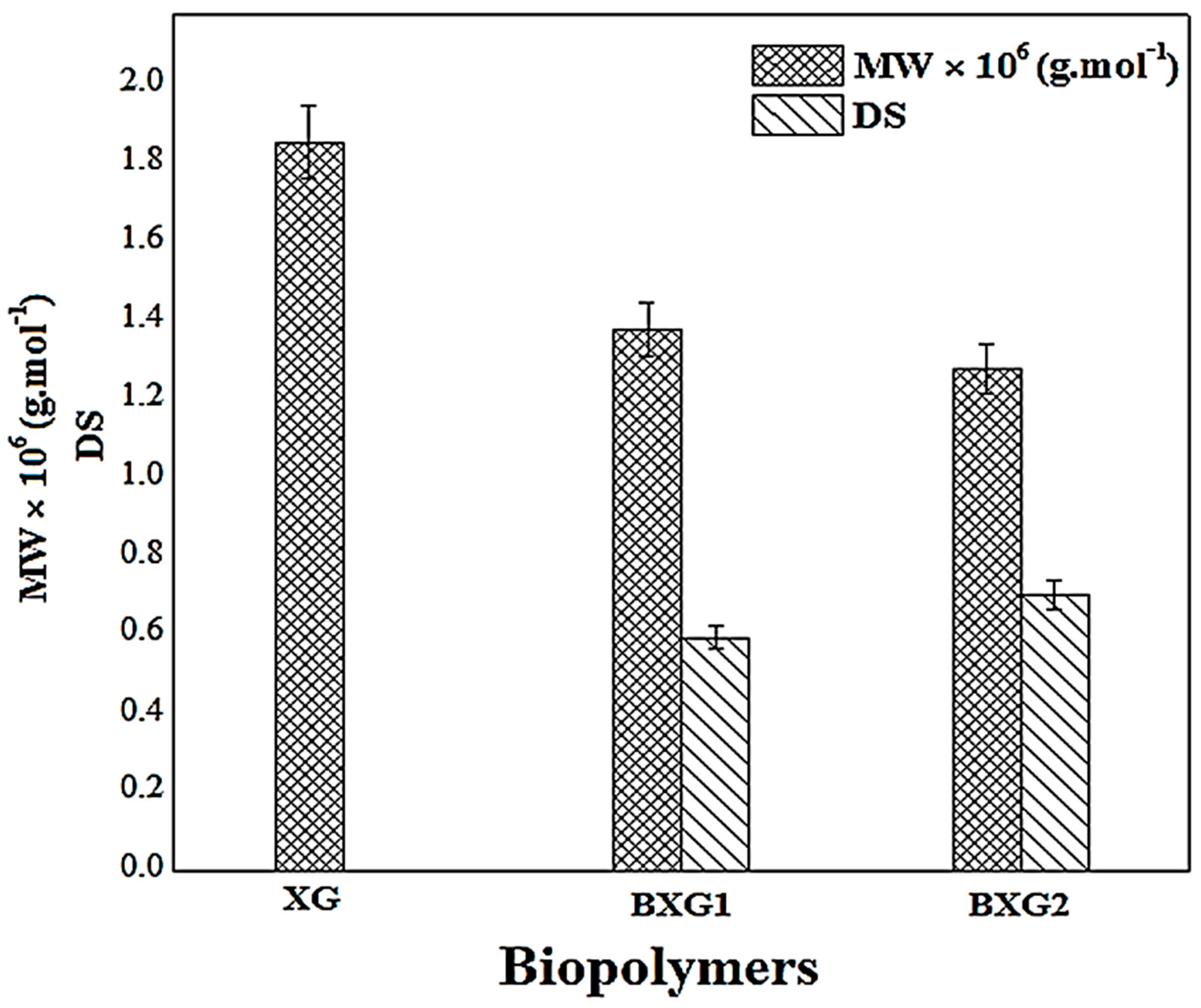
2.3.1. Fourier Transform Infrared Study (FTIR) and the Degree of Substitution (DS)
2.3.2. 1HNMR Analysis
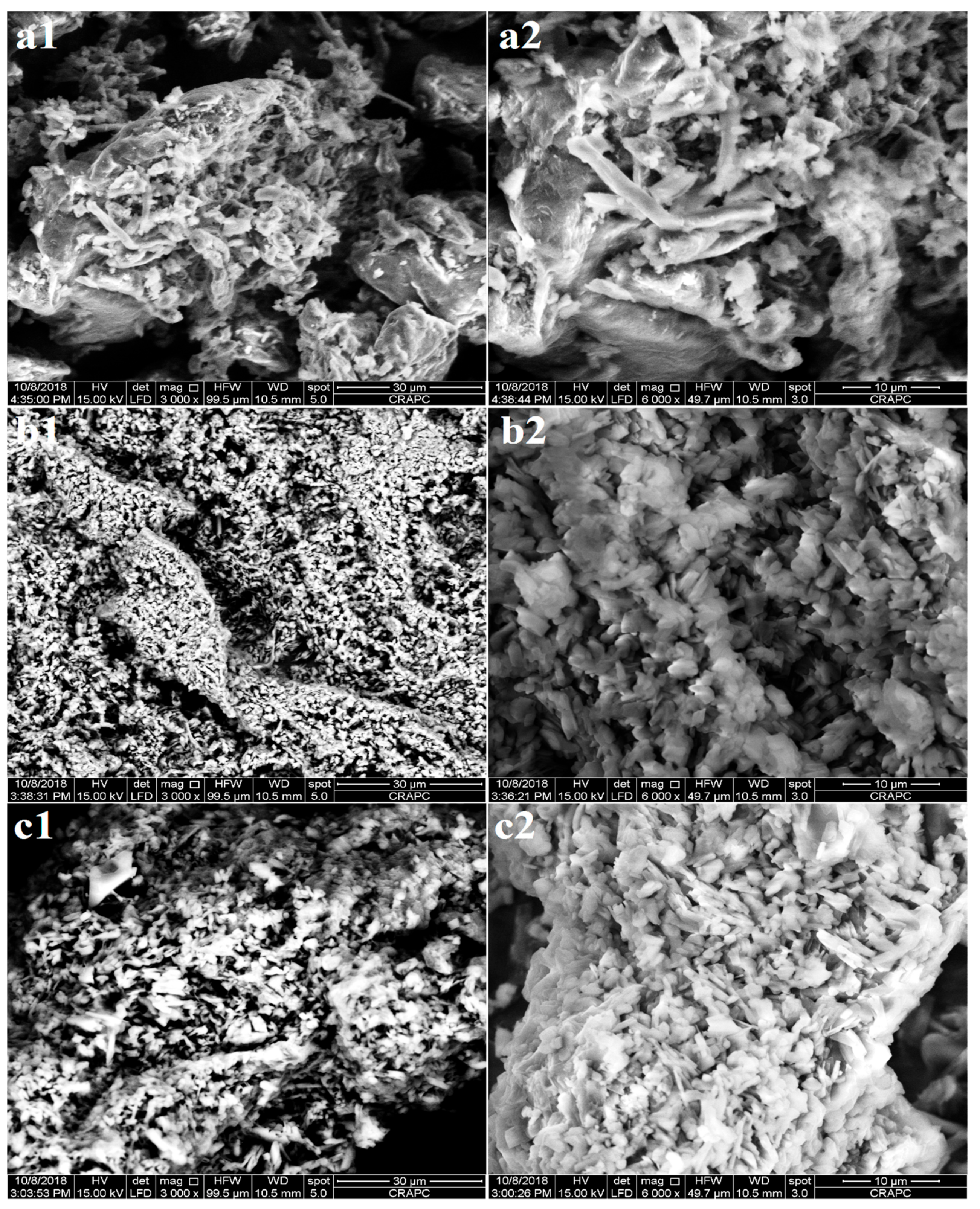
2.3.3. Morphological Analysis
2.3.4. X-ray Diffraction (XRD)
2.3.5. Rheological Study
2.3.6. Viscosity-Average Molecular Weight Determination
2.3.7. Critical Aggregation Concentration by Conductivity Measurements
2.3.8. Emulsifying Study
Emulsion Preparation
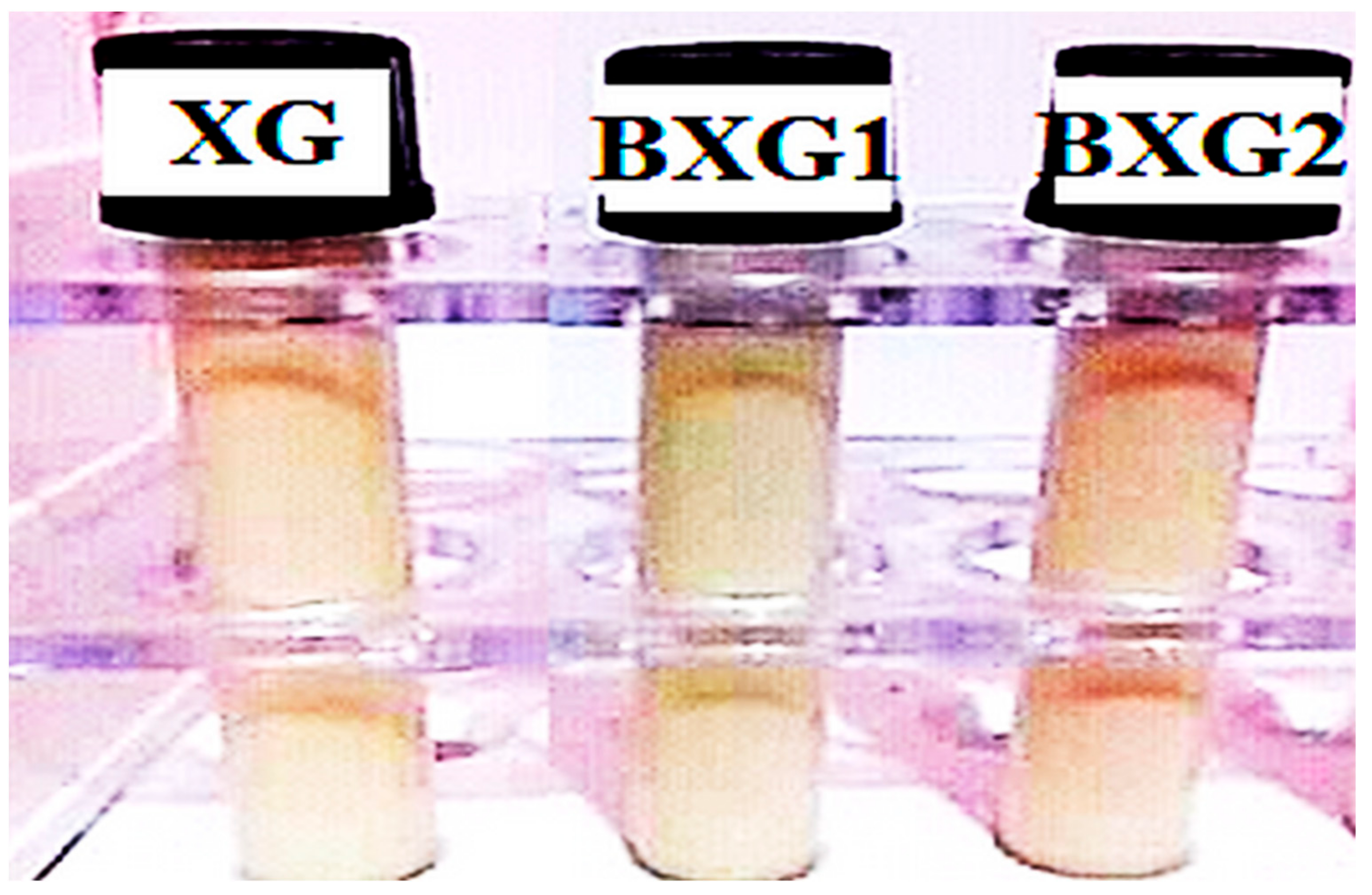
Macroscopic and Microscopic Assessments
2.3.9. Statistical Assessment
3. Results and Discussion
3.1. FTIR Analysis
3.2. 1HNMR Analysis
3.3. Morphological Analysis
3.4. X-ray Diffraction (XRD)
3.5. Rheological Study
3.6. Viscosity-Average Molecular Weight Determination
3.7. Critical Aggregation Concentration by Conductivity Measurements
3.8. Emulsifying Study
3.8.1. Macroscopic and Microscopic Aspects
3.8.2. Accelerated Stability Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Gong, H.; Dong, M.; Li, Y. Rheological Properties and Thickening Mechanism of Aqueous Diutan Gum Solution: Effects of Temperature and Salts. Carbohydr. Polym. 2015, 132, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Lefnaoui, S.; Moulai-Mostefa, N. Formulation and in Vitro Evaluation of Κ-carrageenan-pregelatinized Starch-based Mucoadhesive Gels Containing Miconazole. Starch-Stärke 2011, 63, 512–521. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, Y.; Chen, H.; Xu, D.; Lin, K.; Liu, W. Effect of Chitosan on the Enantioselective Bioavailability of the Herbicide Dichlorprop to Chlorella Pyrenoidosa. Environ. Sci. Technol. 2010, 44, 4981–4987. [Google Scholar] [CrossRef] [PubMed]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant Starch in Food: A Review. J. Sci. Food Agric. 2014, 95, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Lefnaoui, S.; Moulai-Mostefa, N. Investigation and Optimization of Formulation Factors of a Hydrogel Network Based on Kappa Carrageenan–Pregelatinized Starch Blend Using an Experimental Design. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 117–125. [Google Scholar] [CrossRef]
- Mafi, R.; Pelton, R.; Cui, Y.; Ketelson, H. Weak Gelation of Hydrophobic Guar by Albumin in Simulated Human Tear Solutions. Biomacromolecules 2014, 15, 4637–4642. [Google Scholar] [CrossRef]
- Lefnaoui, S.; Moulai-Mostefa, N.; Yahoum, M.M.; Gasmi, S.N. Design of Antihistaminic Transdermal Films Based on Alginate–Chitosan Polyelectrolyte Complexes: Characterization and Permeation Studies. Drug Dev. Ind. Pharm. 2018, 44, 432–443. [Google Scholar] [CrossRef]
- Alquraishi, A.A.; Alsewailem, F.D. Xanthan and Guar Polymer Solutions for Water Shut off in High Salinity Reservoirs. Carbohydr. Polym. 2012, 88, 859–863. [Google Scholar] [CrossRef]
- Lefnaoui, S.; Moulai-Mostefa, N. Synthesis and Evaluation of the Structural and Physicochemical Properties of Carboxymethyl Pregelatinized Starch as a Pharmaceutical Excipient. Saudi Pharm. J. 2015, 23, 698–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toumi, S.; Yahoum, M.M.; Lefnaoui, S.; Hadjsadok, A. Synthesis, Characterization and Potential Application of Hydrophobically Modified Carrageenan Derivatives as Pharmaceutical Excipients. Carbohydr. Polym. 2021, 251, 116997. [Google Scholar] [CrossRef]
- Yahoum, M.M.; Moulai-Mostefa, N.; Le Cerf, D. Synthesis, Physicochemical, Structural and Rheological Characterizations of Carboxymethyl Xanthan Derivatives. Carbohydr. Polym. 2016, 154, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.-E.; Kenne, L.; Lindberg, B. Structure of the Extracellular Polysaccharide from Xanthomonas Campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Katzbauer, B. Properties and Applications of Xanthan Gum. Polym. Degrad. Stab. 1998, 59, 81–84. [Google Scholar] [CrossRef]
- Garcıa-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gómez, E. Xanthan Gum: Production, Recovery, and Properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Zheng, M.; Lian, F.; Zhu, Y.; Liu, B.; Chen, Z.; Zhang, Y.; Zheng, B.; Zhang, L. Modified Xanthan Gum for Crystal Violet Uptake: Kinetic, Isotherm, and Thermodynamic Behaviors. Water Sci. Technol. 2019, 79, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, M.; Kumar, A.; Singh, K. Synthesis, Characterization and in Vitro Release Behavior of Carboxymethyl Xanthan. Int. J. Biol. Macromol. 2012, 51, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Makhado, E.; Pandey, S.; Nomngongo, P.N.; Ramontja, J. Fast Microwave-Assisted Green Synthesis of Xanthan Gum Grafted Acrylic Acid for Enhanced Methylene Blue Dye Removal from Aqueous Solution. Carbohydr. Polym. 2017, 176, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lian, F.; Xiong, Y.; Liu, B.; Zhu, Y.; Miao, S.; Zhang, L.; Zheng, B. The Synthesis and Characterization of a Xanthan Gum-Acrylamide-Trimethylolpropane Triglycidyl Ether Hydrogel. Food Chem. 2019, 272, 574–579. [Google Scholar] [CrossRef]
- Hassani, L.N.; Hendra, F.; Bouchemal, K. Auto-Associative Amphiphilic Polysaccharides as Drug Delivery Systems. Drug Discov. Today 2012, 17, 608–614. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Jiang, B.; Chen, J. A Review of the Enzymatic, Physical, and Chemical Modification Techniques of Xanthan Gum. Int. J. Biol. Macromol. 2021, 186, 472–489. [Google Scholar] [CrossRef]
- Fantou, C.; Roy, A.N.; Dé, E.; Comesse, S.; Grisel, M.; Renou, F. Chemical Modification of Xanthan in the Ordered and Disordered States: An Open Route for Tuning the Physico-Chemical Properties. Carbohydr. Polym. 2017, 178, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Hu, Y.; Huang, Z.; Wenmeng, D. Preparation and Property Evaluation of a Hydrophobically Modified Xanthan Gum XG-C16. J. Dispers. Sci. Technol. 2019, 41, 656–666. [Google Scholar] [CrossRef]
- Sara, H.; Yahoum, M.M.; Lefnaoui, S.; Abdelkader, H.; Moulai-Mostefa, N. New Alkylated Xanthan Gum as Amphiphilic Derivatives: Synthesis, Physicochemical and Rheological Studies. J. Mol. Struct. 2020, 1207, 127768. [Google Scholar] [CrossRef]
- Wang, X.; Xin, H.; Zhu, Y.; Chen, W.; Tang, E.; Zhang, J.; Tan, Y. Synthesis and Characterization of Modified Xanthan Gum Using Poly (Maleic Anhydride/1-Octadecene). Colloid Polym. Sci. 2016, 294, 1333–1341. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhao, X.; Ye, F.; Zhao, G. Hydrophobically Modified Polysaccharides and Their Self-Assembled Systems: A Review on Structures and Food Applications. Carbohydr. Polym. 2022, 284, 119182. [Google Scholar] [CrossRef]
- Wong, T.; Brault, L.; Gasparotto, E.; Vallée, R.; Morvan, P.-Y.; Ferrières, V.; Nugier-Chauvin, C. Formation of Amphiphilic Molecules from the Most Common Marine Polysaccharides, toward a Sustainable Alternative? Molecules 2021, 26, 4445. [Google Scholar] [CrossRef] [PubMed]
- Desbrières, J.; Petit, C.; Reynaud, S. Microwave-Assisted Modifications of Polysaccharides. Pure Appl. Chem. 2014, 86, 1695–1706. [Google Scholar] [CrossRef] [Green Version]
- García-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from Macroalgae: Recent Advances, Innovative Technologies and Challenges in Extraction and Purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Zheng, G.-W.; Zong, M.-H.; Li, N.; Lou, W.-Y. Recent Progress on Deep Eutectic Solvents in Biocatalysis. Bioresour. Bioprocess. 2017, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Abou Dib, M.; Hucher, N.; Gore, E.; Grisel, M. Original Tools for Xanthan Hydrophobization in Green Media: Synthesis and Characterization of Surface Activity. Carbohydr. Polym. 2022, 291, 119548. [Google Scholar] [CrossRef]
- Abou Dib, M.; Gore, E.; Grisel, M. Intrinsic and Rheological Properties of Hydrophobically Modified Xanthan Synthesized under Green Conditions. Food Hydrocoll. 2023, 138, 108461. [Google Scholar] [CrossRef]
- Polêto, M.D.; Rusu, V.H.; Grisci, B.I.; Dorn, M.; Lins, R.D.; Verli, H. Aromatic Rings Commonly Used in Medicinal Chemistry: Force Fields Comparison and Interactions with Water toward the Design of New Chemical Entities. Front. Pharmacol. 2018, 9, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selley, R.C.; Sonnenberg, S.A.; Sonnenberg, R. The Physical and Chemical Properties of Petroleum. In Elements of Petroleum Geology; Academic Press: Cambridge, MA, USA, 2015; pp. 13–39. [Google Scholar]
- Skender, A.; Hadj-Ziane-Zafour, A.; Flahaut, E. Chemical Functionalization of Xanthan Gum for the Dispersion of Double-Walled Carbon Nanotubes in Water. Carbon 2013, 62, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-F.; Sun, S.-N.; Xu, F.; Sun, R. Benzylation and Characterization of Cold NaOH/Urea Pre-Swelled Bamboo. BioResources 2012, 7, 1876–1890. [Google Scholar] [CrossRef] [Green Version]
- Masuelli, M.A. Mark-Houwink Parameters for Aqueous-Soluble Polymers and Biopolymers at Various Temperatures. J. Polym. Biopolym. Phys. Chem. 2014, 2, 37–43. [Google Scholar]
- Maity, S.; Sa, B. Ca-Carboxymethyl Xanthan Gum Mini-Matrices: Swelling, Erosion and Their Impact on Drug Release Mechanism. Int. J. Biol. Macromol. 2014, 68, 78–85. [Google Scholar] [CrossRef]
- Tinland, B.; Rinaudo, M. Dependence of the Stiffness of the Xanthan Chain on the External Salt Concentration. Macromolecules 1989, 22, 1863–1865. [Google Scholar] [CrossRef]
- Shahin, M.; Hady, S.A.; Hammad, M.; Mortada, N. Development of Stable O/W Emulsions of Three Different Oils. Int. J. Pharm. Stud. Res. 2011, 2, 45–51. [Google Scholar]
- Toumi, S.; Yahoum, M.M.; Lefnaoui, S.; Hadjsadok, A. Synthesis and Physicochemical Evaluation of Octenylsuccinated Kappa-Carrageenan: Conventional versus Microwave Heating. Carbohydr. Polym. 2022, 286, 119310. [Google Scholar] [CrossRef]
- Najafi, P.; Penchah, H.R.; Ghaemi, A. Synthesis and Characterization of Benzyl Chloride-Based Hypercrosslinked Polymers and Its Amine-Modification as an Adsorbent for CO2 Capture. Environ. Technol. Innov. 2021, 23, 101746. [Google Scholar] [CrossRef]
- Faria, S.; de Oliveira Petkowicz, C.L.; De Morais, S.A.L.; Terrones, M.G.H.; De Resende, M.M.; de Franca, F.P.; Cardoso, V.L. Characterization of Xanthan Gum Produced from Sugar Cane Broth. Carbohydr. Polym. 2011, 86, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Liebert, T.; Hänsch, C.; Heinze, T. Click Chemistry with Polysaccharides. Macromol. Rapid Commun. 2006, 27, 208–213. [Google Scholar] [CrossRef]
- Hartman, J.; Albertsson, A.-C.; Sjöberg, J. Surface-and Bulk-Modified Galactoglucomannan Hemicellulose Films and Film Laminates for Versatile Oxygen Barriers. Biomacromolecules 2006, 7, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3, ISBN 0-08-093369-6. [Google Scholar]
- Akiyama, E.; Morimoto, N.; Kujawa, P.; Ozawa, Y.; Winnik, F.M.; Akiyoshi, K. Self-Assembled Nanogels of Cholesteryl-Modified Polysaccharides: Effect of the Polysaccharide Structure on Their Association Characteristics in the Dilute and Semidilute Regimes. Biomacromolecules 2007, 8, 2366–2373. [Google Scholar] [CrossRef]
- Wu, M.; Qu, J.; Shen, Y.; Dai, X.; Wei, W.; Shi, Z.; Li, G.; Ma, T. Gel Properties of Xanthan Containing a Single Repeating Unit with Saturated Pyruvate Produced by an Engineered Xanthomonas Campestris CGMCC 15155. Food Hydrocoll. 2019, 87, 747–757. [Google Scholar] [CrossRef]
- Khouryieh, H.A.; Herald, T.J.; Aramouni, F.; Bean, S.; Alavi, S. Influence of Deacetylation on the Rheological Properties of Xanthan–Guar Interactions in Dilute Aqueous Solutions. J. Food Sci. 2007, 72, C173–C181. [Google Scholar] [CrossRef]
- Li, Y.J.; Ha, Y.M.; Wang, F.; Li, Y.F. Effect of Irradiation on the Molecular Weight, Structure and Apparent Viscosity of Xanthan Gum in Aqueous Solution. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2011; Volume 239, pp. 2632–2637. [Google Scholar]
- Sawada, S.; Yukawa, H.; Takeda, S.; Sasaki, Y.; Akiyoshi, K. Self-Assembled Nanogel of Cholesterol-Bearing Xyloglucan as a Drug Delivery Nanocarrier. J. Biomater. Sci. Polym. Ed. 2017, 28, 1183–1198. [Google Scholar] [CrossRef]
- Fantou, C.; Comesse, S.; Renou, F.; Grisel, M. Hydrophobically Modified Xanthan: Thickening and Surface-Active Agent for Highly Stable Oil in Water Emulsions. Carbohydr. Polym. 2019, 205, 362–370. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.-Q. Fabrication and Characterization of Pickering Emulsions Stabilized by Octenyl Succinic Anhydride-Modified Gliadin Nanoparticle. Food Hydrocoll. 2019, 90, 19–27. [Google Scholar] [CrossRef]








| Composition | F1 | F2 | F3 |
|---|---|---|---|
| BSO (%) | 20 | 20 | 20 |
| XG (%) | 1 | - | - |
| BXG1 (%) | - | 1 | - |
| BXG2 (%) | - | - | 1 |
| Water (%) | 79 | 79 | 79 |
| Emulsions | Time (min) | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | 30 | |
| F1 | + | + | + | + | − | − |
| F2 | + | + | + | + | + | − |
| F3 | + | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Hadjsadok, A.; Amrane, A.; Kebir, M.; Zhang, J.; Assadi, A.A.; Mouni, L. Evaluation of Physicochemical and Amphiphilic Properties of New Xanthan Gum Hydrophobically Functionalized Derivatives. Sustainability 2023, 15, 6345. https://doi.org/10.3390/su15086345
Yahoum MM, Toumi S, Tahraoui H, Lefnaoui S, Hadjsadok A, Amrane A, Kebir M, Zhang J, Assadi AA, Mouni L. Evaluation of Physicochemical and Amphiphilic Properties of New Xanthan Gum Hydrophobically Functionalized Derivatives. Sustainability. 2023; 15(8):6345. https://doi.org/10.3390/su15086345
Chicago/Turabian StyleYahoum, Madiha Melha, Selma Toumi, Hichem Tahraoui, Sonia Lefnaoui, Abdelkader Hadjsadok, Abdeltif Amrane, Mohammed Kebir, Jie Zhang, Aymen Amine Assadi, and Lotfi Mouni. 2023. "Evaluation of Physicochemical and Amphiphilic Properties of New Xanthan Gum Hydrophobically Functionalized Derivatives" Sustainability 15, no. 8: 6345. https://doi.org/10.3390/su15086345








