Predicting the Potential Distribution of the Alien Invasive Alligator Gar Atractosteus spatula in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution Data of A. Spatula
2.2. Selecting Environmental Variables
2.3. Modeling Species Distribution
3. Results
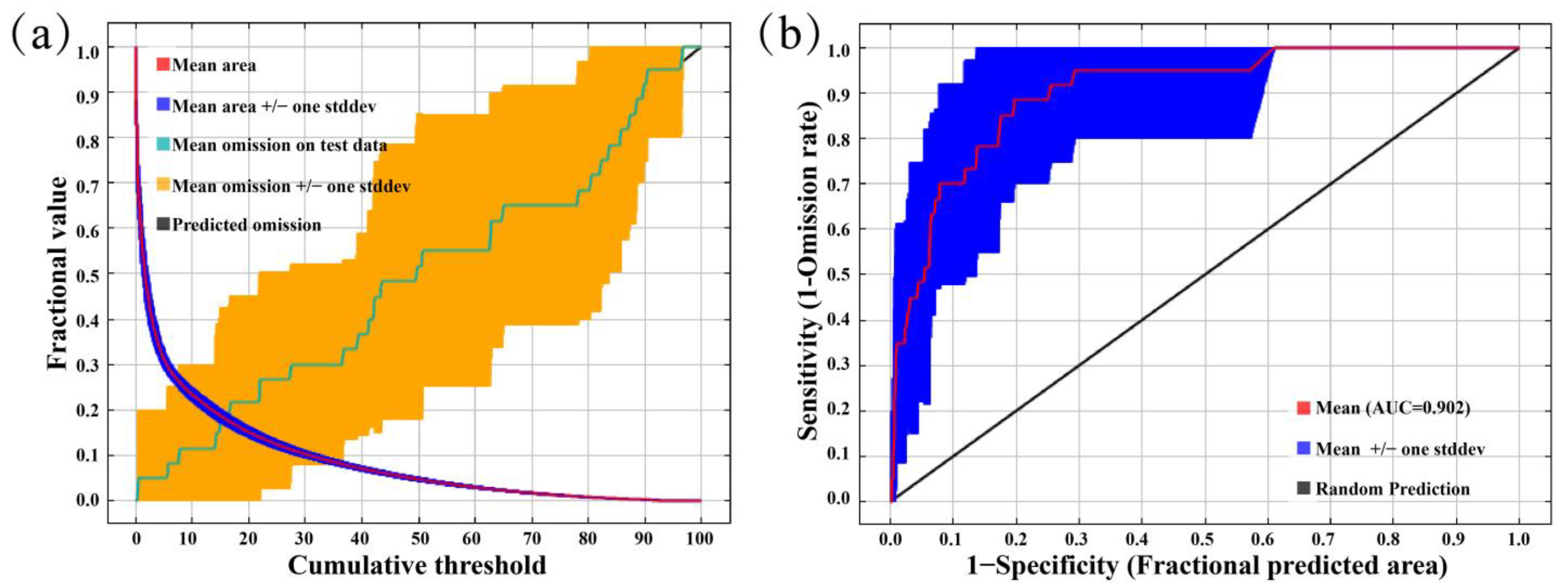
3.1. Validating Modeling Results
3.2. Assessing Contributions of Environmental Factors
3.3. Predicting Distribution Pattern
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.-A.; Hemming, D.J.; Roberts, A.; Diaz-Soltero, H. The threat of invasive species to IUCN-listed critically endangered species: A systematic review. Glob. Ecol. Conserv. 2021, 26, e01476. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Kumar Rai, P.; Singh, J.S. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indicators 2020, 111, 106020. [Google Scholar] [CrossRef]
- Teem, J.L.; Alphey, L.; Descamps, S.; Edgington, M.P.; Edwards, O.; Gemmell, N.; Harvey-Samuel, T.; Melnick, R.L.; Oh, K.P.; Piaggio, A.J.; et al. Genetic Biocontrol for Invasive Species. Front. Bioeng. Biotech. 2020, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.G.; Zimmerman, K.; Schardt, J.D.; Stone, J.; Lukens, R.R.; Reichard, S.; Randall, J.; Cangelosi, A.A.; Cooper, D.; Thompson, J.P. Invasive species defined in a policy context: Recommendations from the federal invasive species advisory committee. Invas. Plant Sci. Mana. 2008, 1, 414–421. [Google Scholar] [CrossRef]
- Fantle-Lepczyk, J.E.; Haubrock, P.J.; Kramer, A.M.; Cuthbert, R.N.; Turbelin, A.J.; Crystal-Ornelas, R.; Diagne, C.; Courchamp, F. Economic costs of biological invasions in the United States. Sci. Total Environ. 2022, 806, 151318. [Google Scholar] [CrossRef]
- Marsh, A.S.; Hayes, D.C.; Klein, P.N.; Zimmerman, N.; Dalsimer, A.; Burkett, D.A.; Huebner, C.D.; Rabaglia, R.; Meyerson, L.A.; Harper-Lore, B.L.; et al. Sectoral impacts of invasive species in the United States and approaches to management. In Invasive Species in Forests and Rangelands of the United States: A Comprehensive Science Synthesis for the United States Forest Sector; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 203–229. [Google Scholar]
- Panzavolta, T.; Bracalini, M.; Benigno, A.; Moricca, S. Alien invasive pathogens and pests harming trees, forests, and plantations: Pathways, global consequences and management. Forests 2021, 12, 1364. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, Y.-L.; Leskey, T.C. A review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia-Pacif. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Kettunen, M.; Genovesi, P.; Gollasch, S.; Pagad, S.; Starfinger, U.; Ten Brink, P.; Shine, C. Technical Support to EU Strategy on Invasive Species (IAS)-Assessment of the Impacts of IAS in Europe and the EU (Final Module Report for the European Commission); 2008. Available online: https://ieep.eu/wp-content/uploads/2009/11/ias_assessments.pdf (accessed on 2 March 2023).
- Xu, H.; Ding, H.; Li, M.; Qiang, S.; Guo, J.; Han, Z.; Huang, Z.; Sun, H.; He, S.; Wu, H.; et al. The distribution and economic losses of alien species invasion to China. Biol. Invasions 2006, 8, 1495–1500. [Google Scholar] [CrossRef]
- Weerasena, L.; Hunt, N.; Bandara, D.; McKnight, M. Spatially explicit multi-objective mathematical model for invasive species management. Biol. Invasions 2022, 24, 1839–1862. [Google Scholar] [CrossRef]
- Salnikov, V.B. First finding of gar Atractosteus sp. (Actinopterygii, Lepisosteiformes, Lepisosteidae) in the Caspian Sea near the coast of Turkmenistan. Russ. J. Biol. Invasions 2010, 1, 17–20. [Google Scholar] [CrossRef]
- Nur, M.; Ulayya, N.; Azis, M.; Maryanto, A.; Andayani, N. Methods to maximize environmental DNA (eDNA) for detection the presence of Alligator Gar (Atractosteus spatula). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changchun, China, 21–23 August 2020; p. 012018. [Google Scholar] [CrossRef]
- Yang, W.; Gludovatz, B.; Zimmermann, E.A.; Bale, H.A.; Ritchie, R.O.; Meyers, M.A. Structure and fracture resistance of alligator gar (Atractosteus spatula) armored fish scales. Acta Biomater. 2013, 9, 5876–5889. [Google Scholar] [CrossRef]
- Han, Y. The invasion of the alien species alligator gar all over china. Int. J. Mol. Ecol. Conserv. 2022, 12, 1–6. [Google Scholar] [CrossRef]
- Hasan, V.; Widodo, M.S.; Islamy, R.A.; Pebriani, D.A. New records of alligator gar, Atractosteus spatula (Actinopterygii: Lepisosteiformes: Lepisosteidae) from Bali and Java, Indonesia. Acta Ichthyol. Pisc. 2020, 50, 233–236. [Google Scholar] [CrossRef]
- Mills, M.D.; Rader, R.B.; Belk, M.C. Complex interactions between native and invasive fish: The simultaneous effects of multiple negative interactions. Oecologia 2004, 141, 713–721. [Google Scholar] [CrossRef]
- Yang, J. Boy Bitten by Landscape Pond, Expert: Alligator Gar has Fierce Habits and are Forbidden to be Released. Available online: http://www.ctdsb.net/c1476_202208/1475962.html (accessed on 2 October 2022).
- Abdullah, A.H.J.; Abdullah, S.A.; Yaseen, A.T. A composition and abundance of alien fish species in inland waters, southern Iraq. Iraqi. J. Agric. Sci. 2021, 62, 373–386. [Google Scholar] [CrossRef]
- Atique, U.; An, K.-G. Potential risky exotic fish species, their ecological impacts and potential reasons for invasion in Korean aquatic ecosystems. J. Ecol. Environ. 2022, 46, 41–53. [Google Scholar] [CrossRef]
- Saba, A.O.; Ismail, A.; Zulkifli, S.Z.; Halim, M.R.A.; Wahid, N.A.A.; Amal, M.N.A. Species composition and invasion risks of alien ornamental freshwater fishes from pet stores in Klang Valley, Malaysia. Sci. Rep. 2020, 10, 17205. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Work Programme to Further Strengthen the Prevention and Control of Invasive Alien Species. Available online: http://www.kjs.moa.gov.cn/hbny/202102/t20210204_6361148.htm (accessed on 26 October 2022).
- Ferrara, A.M. Life-History Strategy of Lepisosteidae: Implications for the Conservation and Management of Alligator Gar; Auburn University: Auburn, AL, USA, 2001. [Google Scholar]
- Hundessa, S.; Li, S.; Liu, D.L.; Guo, J.; Guo, Y.; Zhang, W.; Williams, G. Projecting environmental suitable areas for malaria transmission in China under climate change scenarios. Environ. Res. 2018, 162, 203–210. [Google Scholar] [CrossRef]
- Puchałka, R.; Dyderski, M.K.; Vítková, M.; Sádlo, J.; Klisz, M.; Netsvetov, M.; Prokopuk, Y.; Matisons, R.; Mionskowski, M.; Wojda, T. Black locust (Robinia pseudoacacia L.) range contraction and expansion in Europe under changing climate. Global. Change. Biol. 2021, 27, 1587–1600. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions Version 3.4.1; American Museum of Natural History: New York, NY, USA, 2021. [Google Scholar]
- QGIS. QGIS Geographic Information System (Version 3.20); Open Source Geospatial Foundation Project; QGIS Association: Switzerland, 2021; Available online: https://www.qgis.org/en/site/ (accessed on 15 October 2022).
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. MaxEnt modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef]
- Gebrewahid, Y.; Abrehe, S.; Meresa, E.; Eyasu, G.; Abay, K.; Gebreab, G.; Kidanemariam, K.; Adissu, G.; Abreha, G.; Darcha, G. Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Process. 2020, 9, 6. [Google Scholar] [CrossRef]
- Karlsson, R.; Obst, M.; Berggren, M. Analysis of potential distribution and impacts for two species of alien crabs in Northern Europe. Biol. Invasions 2019, 21, 3109–3119. [Google Scholar] [CrossRef]
- Venne, S.; Currie, D.J. Can habitat suitability estimated from MaxEnt predict colonizations and extinctions? Divers. Distrib. 2021, 27, 873–886. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.; Wu, Y.; Liu, H.; Li, Y.; Chen, H.; Zhang, Y.; Zhao, Y.; et al. Integrating Maxent model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, 106472. [Google Scholar] [CrossRef]
- Xie, C.; Li, M.; Jim, C.Y.; Liu, D. Spatio-temporal patterns of an invasive species Mimosa bimucronata (DC.) Kuntze under different climate scenarios in China. Front. For. Glob. Chang. 2023, 6, 1144829. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; Group, N.P.S.D.W. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Paź-Dyderska, S.; Jagodziński, A.M.; Dyderski, M.K. Possible changes in spatial distribution of walnut (Juglans regia L.) in Europe under warming climate. Reg. Environ. Chang. 2021, 21, 18. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquacult. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.R. Energetic Responses of Salmon to Temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerkd). Am. Zool. 1971, 11, 99–113. [Google Scholar] [CrossRef]
- Bosmans, J.; Wanders, N.; Bierkens, M.F.P.; Huijbregts, M.A.J.; Schipper, A.M.; Barbarossa, V. FutureStreams, a global dataset of future streamflow and water temperature. Sci. Data 2022, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, O.; Stefan, H.G. Stream temperature/air temperature relationship: A physical interpretation. J. Hydrol. 1999, 218, 128–141. [Google Scholar] [CrossRef]
- Stefan, H.G.; Preud’homme, E.B. Stream temperature estimation from air temperature1. J. Am. Water Resour. As. 1993, 29, 27–45. [Google Scholar] [CrossRef]
- Beitinger, T.L.; Bennett, W.A.; McCauley, R.W. Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fishes 2000, 58, 237–275. [Google Scholar] [CrossRef]
- Matvienko, N.; Levchenko, A.; Danchuk, O.; Kvach, Y. Assessment of the occurrence of microorganisms and other fish parasites in the freshwater aquaculture of Ukraine in relation to the ambient temperature. Acta Ichthyol. Pisc. 2020, 50, 333–348. [Google Scholar] [CrossRef]
- Long, J.M.; Snow, R.A.; Porta, M.J. Effects of temperature on hatching rate and early development of alligator gar and spotted gar in a laboratory setting. N. Am. J. Fish. Manage. 2020, 40, 661–668. [Google Scholar] [CrossRef]
- Bartnicki, J.; Snow, R.A.; Taylor, A.T.; Butler, C.J. Critical thermal minima of alligator gar (Atractosteus spatula, [Lacépède, 1803]) during early life stages. J. Appl. Ichthyol. 2021, 37, 572–577. [Google Scholar] [CrossRef]
- Sun, F. Chinese climate and vernacular dwellings. Buildings 2013, 3, 143–172. [Google Scholar] [CrossRef]
- Yang, X.; Ma, H. Natural environment suitability of china and its relationship with population distributions. Int. J. Env. Res. Public Health 2009, 6, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Xue, G.; Zhao, J.; Ma, W.; Qian, Y.; Wu, M.; Zhang, Z.; Gao, P.; Su, C.; et al. Key technologies and equipment for contaminated surface/groundwater environment in the rural river network area of China: Integrated remediation. Environ. Sci. Eur. 2021, 33, 5. [Google Scholar] [CrossRef]


| Bioclimatic Variable | Description | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Bio6 | Minimum temperature of the coldest month | 48.9 | 34.4 |
| Bio10 | Mean temperature of the warmest quarter | 33.5 | 47.6 |
| Bio2 | Mean diurnal temperature range | 8.7 | 2.4 |
| Bio19 | Precipitation of the coldest quarter | 5.8 | 8.7 |
| Bio14 | Precipitation of the driest month | 1.3 | 1 |
| Bio3 | Isothermality (Bio2/Bio7) (×100) | 1.1 | 0.5 |
| Bio18 | Precipitation of the warmest quarter | 0.6 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Xie, C.; Jim, C.Y.; Liu, Y.; Hou, S. Predicting the Potential Distribution of the Alien Invasive Alligator Gar Atractosteus spatula in China. Sustainability 2023, 15, 6419. https://doi.org/10.3390/su15086419
Liu D, Xie C, Jim CY, Liu Y, Hou S. Predicting the Potential Distribution of the Alien Invasive Alligator Gar Atractosteus spatula in China. Sustainability. 2023; 15(8):6419. https://doi.org/10.3390/su15086419
Chicago/Turabian StyleLiu, Dawei, Chunping Xie, Chi Yung Jim, Yanjun Liu, and Senlin Hou. 2023. "Predicting the Potential Distribution of the Alien Invasive Alligator Gar Atractosteus spatula in China" Sustainability 15, no. 8: 6419. https://doi.org/10.3390/su15086419
APA StyleLiu, D., Xie, C., Jim, C. Y., Liu, Y., & Hou, S. (2023). Predicting the Potential Distribution of the Alien Invasive Alligator Gar Atractosteus spatula in China. Sustainability, 15(8), 6419. https://doi.org/10.3390/su15086419









