Development of New Alkylated Carrageenan Derivatives: Physicochemical, Rheological, and Emulsification Properties Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
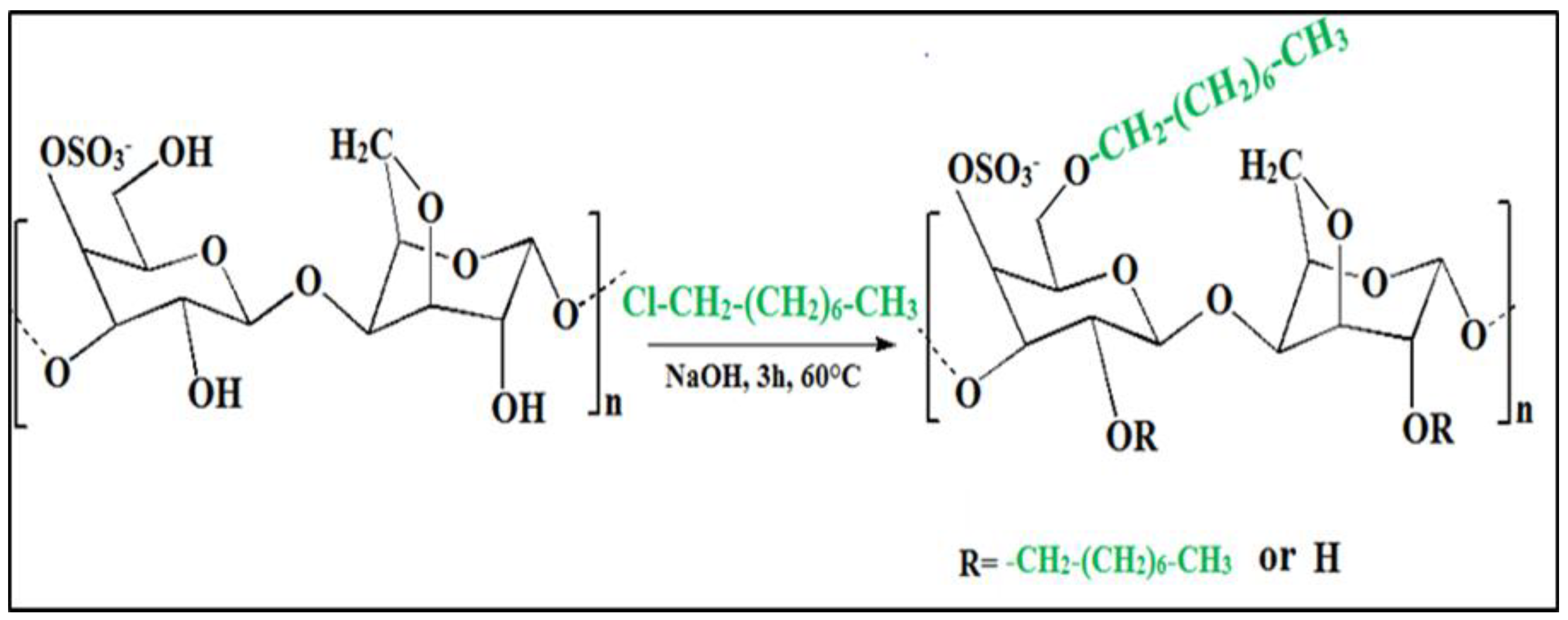
2.2. Hydrophobic Modification of Kappa Carrageenan (KC)
2.3. Physicochemical Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.2. 1H-NMR Analysis and Degree of Substitution (DS)
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. X-ray Diffraction Analysis
2.3.5. Viscosity Analysis
2.3.6. Critical Aggregation Concentration (CAC)
2.3.7. Emulsifying Study
2.3.8. Statistical Assessment
3. Results and Discussion
3.1. FTIR Analysis
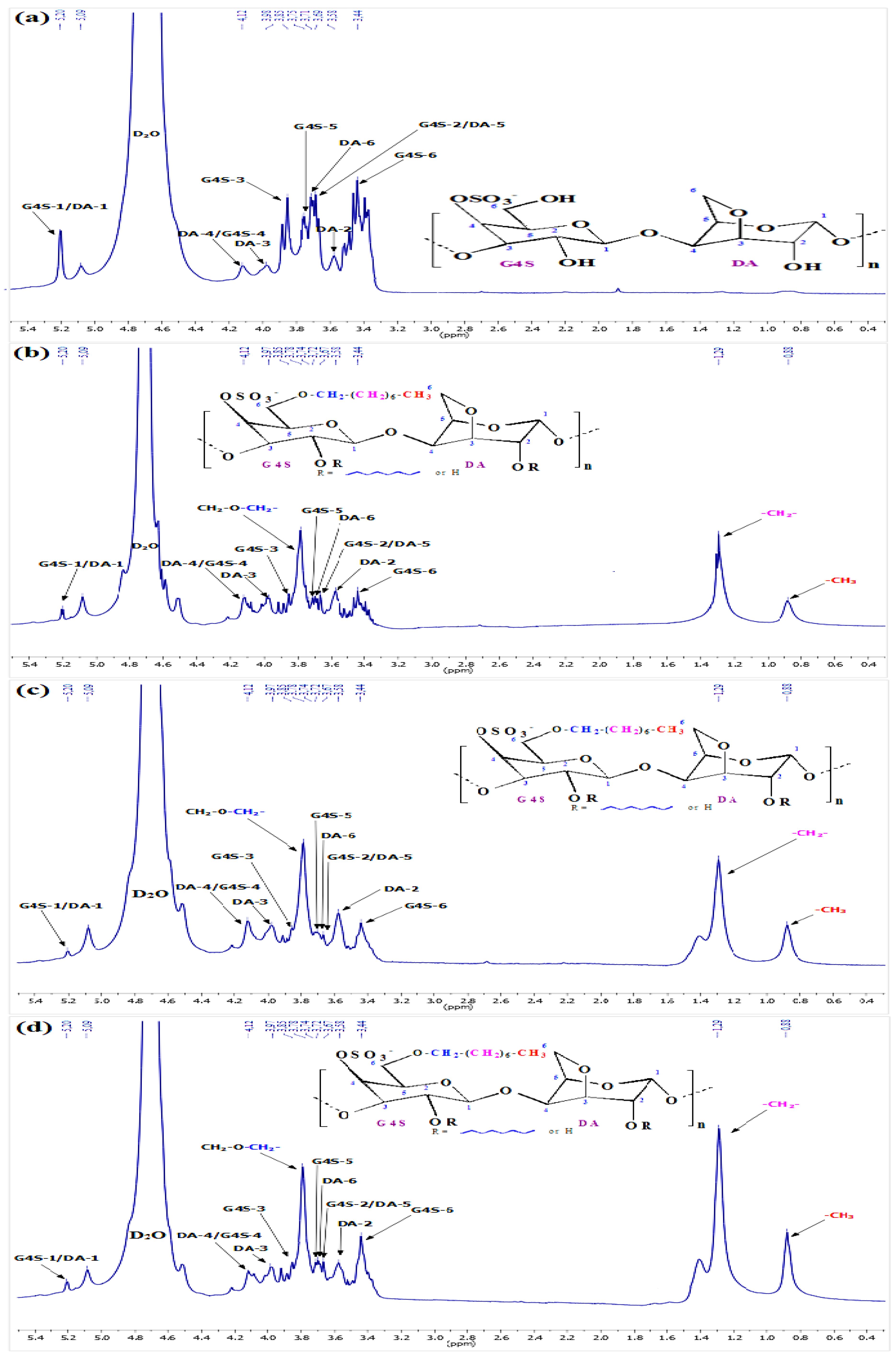
3.2. 1H-NMR Analysis
| Peaks | Chemical Shifts (δ ppm) | References |
|---|---|---|
| G4S-1 | 5.20 | [31,32] |
| G4S-2 | 3.69 | [31,32] |
| G4S-3 | 3.85 | [31,32] |
| G4S-4 | 4.12 | [31,32] |
| G4S-5 | 3.75 | [31,33] |
| G4S-6 | 3.44 | [31,33] |
| DA-1 | 5.20 | [22,31,32,33] |
| DA-2 | 3.58 | [22,31,32,33] |
| DA-3 | 3.98 | [22,31,32,33] |
| DA-4 | 4.12 | [22,31,32,33] |
| DA-5 | 3.69 | [22,31,32,33] |
| DA-6 | 3.71 | [22,31,32,33] |
3.3. Scanning Electron Microscopy (SEM)
3.4. X-ray Diffraction Analysis (XRD)
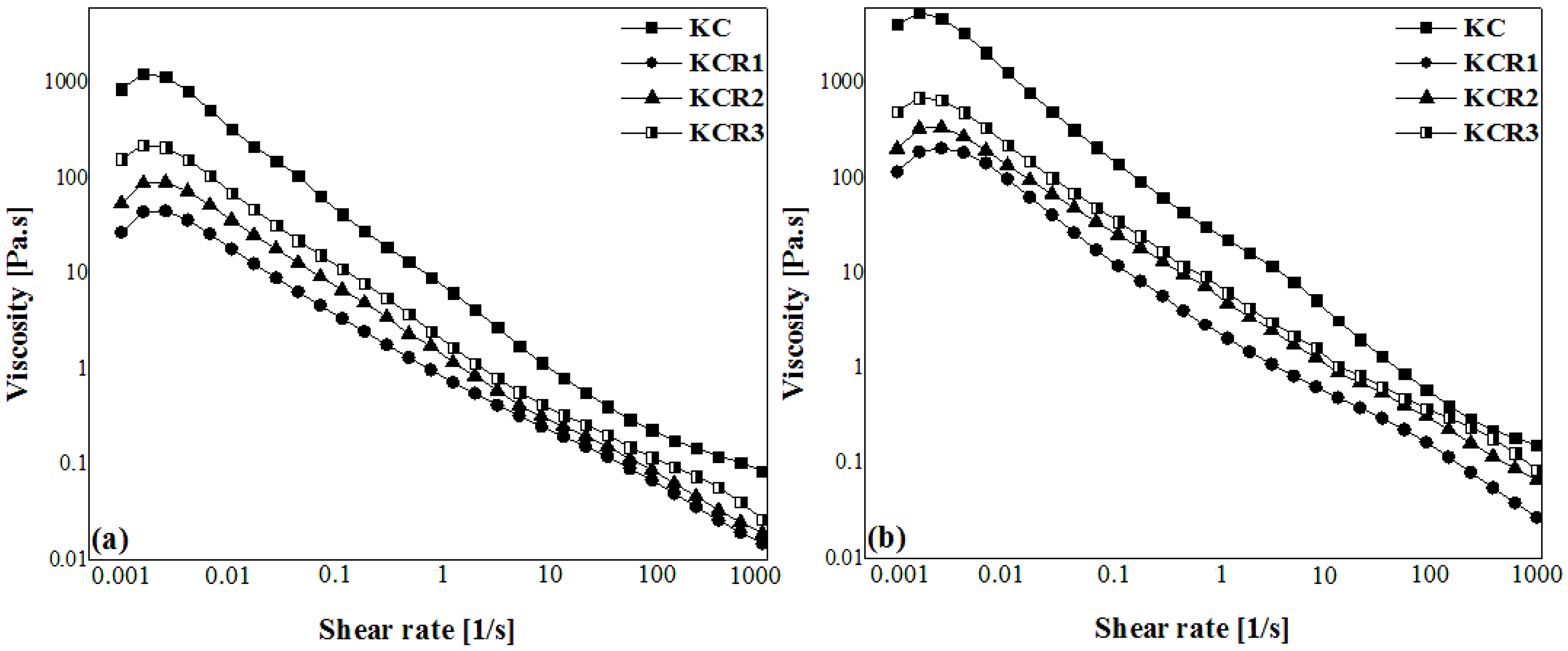
3.5. Viscosity Analysis
3.6. Critical Aggregation Concentration (CAC)
3.7. Emulsifying Properties
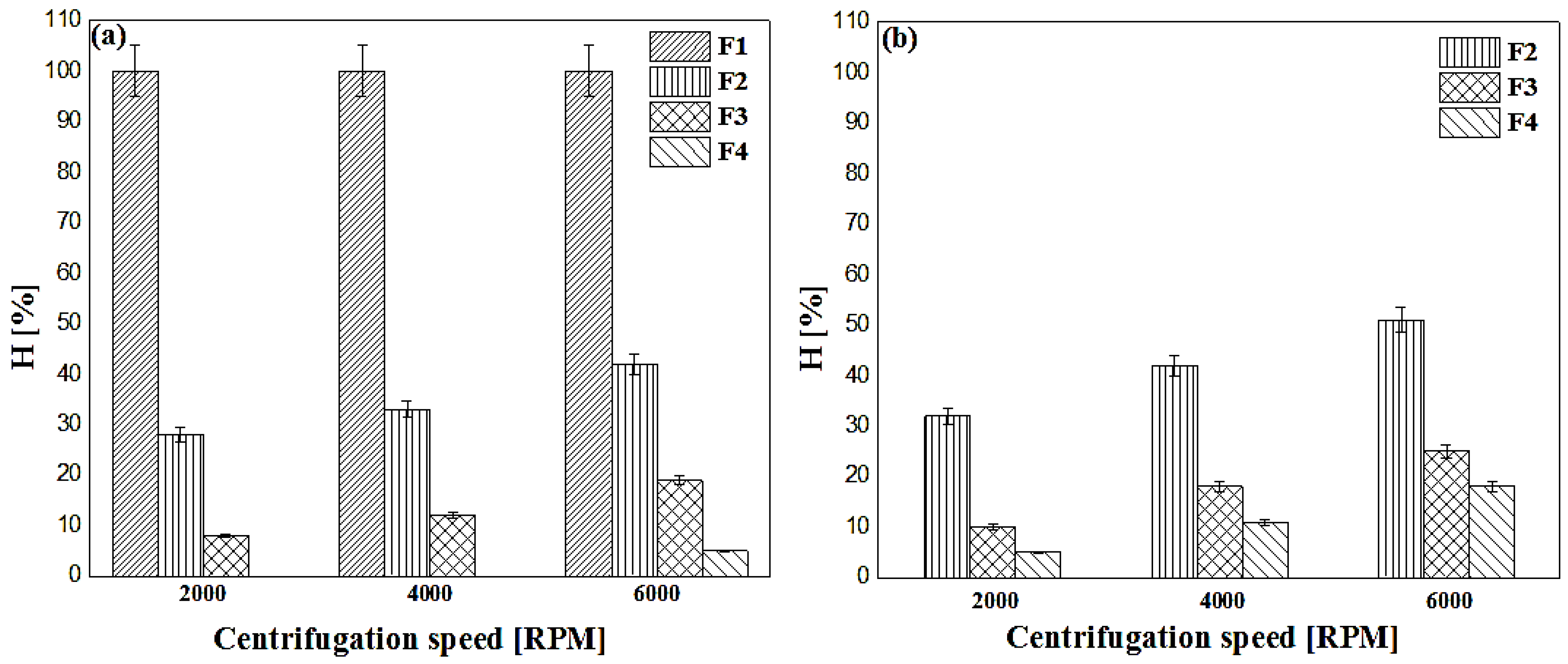
3.7.1. Accelerated and Long-Term Stability Testing (Creaming Index)
3.7.2. Microscopic Aspect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefnaoui, S.; Moulai-Mostefa, N. Investigation and Optimization of Formulation Factors of a Hydrogel Network Based on Kappa Carrageenan–Pregelatinized Starch Blend Using an Experimental Design. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 117–125. [Google Scholar] [CrossRef]
- Bai, L.; Liu, F.; Xu, X.; Huan, S.; Gu, J.; McClements, D.J. Impact of Polysaccharide Molecular Characteristics on Viscosity Enhancement and Depletion Flocculation. J. Food Eng. 2017, 207, 35–45. [Google Scholar] [CrossRef]
- Stephen, A.M.; Phillips, G.O. Food Polysaccharides and Their Applications; CRC Press: Boca Raton, FL, USA, 2016; ISBN 0-429-11616-0. [Google Scholar]
- Krylova, N.V.; Kravchenko, A.O.; Iunikhina, O.V.; Pott, A.B.; Likhatskaya, G.N.; Volod’ko, A.V.; Zaporozhets, T.S.; Shchelkanov, M.Y.; Yermak, I.M. Influence of the Structural Features of Carrageenans from Red Algae of the Far Eastern Seas on Their Antiviral Properties. Mar. Drugs 2022, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa-carrageenan Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- Jiang, J.-L.; Zhang, W.-Z.; Ni, W.-X.; Shao, J.-W. Insight on Structure-Property Relationships of Carrageenan and Its Chemically Modified Derivatives: A Review. Carbohydr. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and Its Applications in Drug Delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Lefnaoui, S.; Moulai-Mostefa, N. Polyelectrolyte Complex Based on Carboxymethyl-Kappa-Carrageenan and Eudragit RL 30D as Prospective Carriers for Sustained Drug Delivery. Chem. Eng. Res. Des. 2015, 97, 165–174. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I. The Effect of Nanoparticles on Gastrointestinal Release from Modified κ-Carrageenan Nanocomposite Hydrogels. Carbohydr. Polym. 2012, 89, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Falshaw, R.; Bixler, H.J.; Johndro, K. Structure and Performance of Commercial Kappa-2 Carrageenan Extracts: I. Structure Analysis. Food Hydrocoll. 2001, 15, 441–452. [Google Scholar] [CrossRef]
- Meena, R.; Lehnen, R.; Schmitt, U.; Saake, B. Effect of Oat Spelt and Beech Xylan on the Gelling Properties of Kappa-Carrageenan Hydrogels. Carbohydr. Polym. 2011, 85, 529–540. [Google Scholar] [CrossRef]
- Bonnaud, M.; Weiss, J.; McClements, D.J. Interaction of a Food-Grade Cationic Surfactant (Lauric Arginate) with Food-Grade Biopolymers (Pectin, Carrageenan, Xanthan, Alginate, Dextran, and Chitosan). J. Agric. Food Chem. 2010, 58, 9770–9777. [Google Scholar] [CrossRef] [PubMed]
- Madruga, L.Y.; Sabino, R.M.; Santos, E.C.; Popat, K.C.; Balaban, R.D.C.; Kipper, M.J. Carboxymethyl-Kappa-Carrageenan: A Study of Biocompatibility, Antioxidant and Antibacterial Activities. Int. J. Biol. Macromol. 2020, 152, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, W.; Li, X.; Lü, X.; Li, N.; Gao, X.; Song, J. Preparation and in Vitro Antioxidant Activity of κ-Carrageenan Oligosaccharides and Their Oversulfated, Acetylated, and Phosphorylated Derivatives. Carbohydr. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef]
- Suchaoin, W.; Bonengel, S.; Hussain, S.; Huck, C.W.; Ma, B.N.; Bernkop-Schnürch, A. Synthesis and in Vitro Evaluation of Thiolated Carrageenan. J. Pharm. Sci. 2015, 104, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Opoku, G.; Qiu, X.; Doctor, V. Effect of Oversulfation on the Chemical and Biological Properties of Kappa Carrageenan. Carbohydr. Polym. 2006, 65, 134–138. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, L.; Lyu, Y.; Zi, Y.; Li, X.; Li, D.; Mu, C. Preparation, Characterization and Antibacterial Activity of Oxidized κ-Carrageenan. Carbohydr. Polym. 2017, 174, 1051–1058. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Pujol, C.A.; Damonte, E.B.; Stortz, C.A. Partial and Total C-6 Oxidation of Gelling Carrageenans. Modulation of the Antiviral Activity with the Anionic Character. Carbohydr. Polym. 2015, 128, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Barahona, T.; Prado, H.J.; Bonelli, P.R.; Cukierman, A.L.; Fissore, E.L.; Gerschenson, L.N.; Matulewicz, M.C. Cationization of Kappa-and Iota-Carrageenan–Characterization and Properties of Amphoteric Polysaccharides. Carbohydr. Polym. 2015, 126, 70–77. [Google Scholar] [CrossRef]
- Ellis, A.L.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. The Hydrophobic Modification of Kappa Carrageenan Microgel Particles for the Stabilisation of Foams. J. Colloid Interface Sci. 2019, 538, 165–173. [Google Scholar] [CrossRef]
- Toumi, S.; Yahoum, M.M.; Lefnaoui, S.; Hadjsadok, A. Synthesis, Characterization and Potential Application of Hydrophobically Modified Carrageenan Derivatives as Pharmaceutical Excipients. Carbohydr. Polym. 2021, 251, 116997. [Google Scholar] [CrossRef] [PubMed]
- Shahin, M.; Hady, S.A.; Hammad, M.; Mortada, N. Development of Stable O/W Emulsions of Three Different Oils. Int. J. Pharm. Stud. Res. 2011, 2, 45–51. [Google Scholar]
- Fan, L.; Peng, K.; Li, M.; Wang, L.; Wang, T. Preparation and Properties of Carboxymethyl κ-Carrageenan/Alginate Blend Fibers. J. Biomater. Sci. Polym. Ed. 2013, 24, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Tranquilan-Aranilla, C.; Nagasawa, N.; Bayquen, A.; Rosa, A.D. Synthesis and Characterization of Carboxymethyl Derivatives of Kappa-Carrageenan. Carbohydr. Polym. 2012, 87, 1810–1816. [Google Scholar] [CrossRef]
- Fang, J.M.; Fowler, P.A.; Sayers, C.; Williams, P.A. The Chemical Modification of a Range of Starches under Aqueous Reaction Conditions. Carbohydr. Polym. 2004, 55, 283–289. [Google Scholar] [CrossRef]
- Han, L.; Ratcliffe, I.; Williams, P.A. Synthesis, Characterisation and Physicochemical Properties of Hydrophobically Modified Inulin Using Long-Chain Fatty Acyl Chlorides. Carbohydr. Polym. 2017, 178, 141–146. [Google Scholar] [CrossRef]
- van de Velde, F.; Rollema, H.S.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Tromp, R.H. Coil–Helix Transition of Ι-carrageenan as a Function of Chain Regularity. Biopolym. Orig. Res. Biomol. 2002, 65, 299–312. [Google Scholar] [CrossRef]
- Sara, H.; Yahoum, M.M.; Lefnaoui, S.; Abdelkader, H.; Moulai-Mostefa, N. New Alkylated Xanthan Gum as Amphiphilic Derivatives: Synthesis, Physicochemical and Rheological Studies. J. Mol. Struct. 2020, 1207, 127768. [Google Scholar] [CrossRef]
- Yahoum, M.M.; Moulai-Mostefa, N.; Le Cerf, D. Synthesis, Physicochemical, Structural and Rheological Characterizations of Carboxymethyl Xanthan Derivatives. Carbohydr. Polym. 2016, 154, 267–275. [Google Scholar] [CrossRef]
- Toumi, S.; Yahoum, M.M.; Lefnaoui, S.; Hadjsadok, A. Synthesis and Physicochemical Evaluation of Octenylsuccinated Kappa-Carrageenan: Conventional versus Microwave Heating. Carbohydr. Polym. 2022, 286, 119310. [Google Scholar] [CrossRef]
- Liew, J.W.Y.; Loh, K.S.; Ahmad, A.; Lim, K.L.; Wan Daud, W.R. Synthesis and Characterization of Modified κ-Carrageenan for Enhanced Proton Conductivity as Polymer Electrolyte Membrane. PLoS ONE 2017, 12, e0185313. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-Assisted Extraction and Structural Characterization by NMR of Alginates and Carrageenans from Seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.A.; Weese, E.; Nakamatsu, J.; Torres, F. Development of Biopolymer Nanocomposites Based on Polysaccharides Obtained from Red Algae Chondracanthus Chamissoi Reinforced with Chitin Whiskers and Montmorillonite. Polym.-Plast. Technol. Eng. 2016, 55, 1557–1564. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; German, J.B.; Mortazavian, A.M.; Mohammadi, A.; Shojaee-Aliabadi, S.; Khaksar, R. Preparation and Characterization of Alginate and Alginate-Resistant Starch Microparticles Containing Nisin. Carbohydr. Polym. 2014, 103, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Pradhan, M.; Tripathi, D.K. Synthesis of Graft Copolymer of Kappa-Carrageenan Using Microwave Energy and Studies of Swelling Capacity, Flocculation Properties, and Preliminary Acute Toxicity. Turk. J. Chem. 2016, 40, 283–295. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Eskandari, M.H.; Van der Meeren, P.; Hosseini, S.M.H. Study on Hydrophobic Modification of Basil Seed Gum-Based (BSG) Films by Octenyl Succinate Anhydride (OSA). Carbohydr. Polym. 2019, 219, 155–161. [Google Scholar] [CrossRef]
- Jacquier, J.-C.; MacArtain, P.; Dawson, K.A. Hydrophobically Modified Calcium-Induced κ-Carrageenan Gels. Trends Colloid Interface Sci. XV 2001, 118, 127–131. [Google Scholar]
- Chen, Y.; Hao, Y.; Li, S.; Luo, Z.; Gao, Q. Preparation of Hydroxybutyl Starch with a High Degree of Substitution and Its Application in Temperature-Sensitive Hydrogels. Food Chem. 2021, 355, 129472. [Google Scholar] [CrossRef]
- Sav, A.K.; Fule, R.A.; Ali, M.T.; Amin, P. Synthesis and Evaluation of Octenyl Succinate Anhydride Derivative of Fenugreek Gum as Extended Release Polymer. J. Pharm. Investig. 2013, 43, 417–429. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Yao, W.; Pang, X.; Yin, D.; Gao, X. Carboxymethylation of a Polysaccharide Extracted from Ganoderma Lucidum Enhances Its Antioxidant Activities in Vitro. Carbohydr. Polym. 2009, 78, 227–234. [Google Scholar] [CrossRef]
- Yang, S.R.; Jeong, J.H.; Park, K.; Kim, J.-D. Self-Aggregates of Hydrophobically Modified Poly (2-Hydroxyethyl Aspartamide) in Aqueous Solution. Colloid Polym. Sci. 2003, 281, 852–861. [Google Scholar] [CrossRef]
- Muhammad, M.T.; Khan, M.N. Study of Electrolytic Effect on the Interaction between Anionic Surfactant and Methylene Blue Using Spectrophotometric and Conductivity Methods. J. Mol. Liq. 2017, 234, 309–314. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.-Q. Fabrication and Characterization of Pickering Emulsions Stabilized by Octenyl Succinic Anhydride-Modified Gliadin Nanoparticle. Food Hydrocoll. 2019, 90, 19–27. [Google Scholar] [CrossRef]







| Composition (%) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| BSO | 20 | 20 | 20 | 20 |
| KC | 1 | - | - | - |
| KCR1 | - | 1 | - | - |
| KCR2 | - | - | 1 | - |
| KCR3 | - | - | - | 1 |
| Water | 79 | 79 | 79 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toumi, S.; Yahoum, M.M.; Lefnaoui, S.; Hadjsadok, A.; Sid, A.N.E.H.; Hassein-Bey, A.H.; Amrane, A.; Zhang, J.; Assadi, A.A.; Mouni, L. Development of New Alkylated Carrageenan Derivatives: Physicochemical, Rheological, and Emulsification Properties Assessment. Sustainability 2023, 15, 6473. https://doi.org/10.3390/su15086473
Toumi S, Yahoum MM, Lefnaoui S, Hadjsadok A, Sid ANEH, Hassein-Bey AH, Amrane A, Zhang J, Assadi AA, Mouni L. Development of New Alkylated Carrageenan Derivatives: Physicochemical, Rheological, and Emulsification Properties Assessment. Sustainability. 2023; 15(8):6473. https://doi.org/10.3390/su15086473
Chicago/Turabian StyleToumi, Selma, Madiha Melha Yahoum, Sonia Lefnaoui, Abdelkader Hadjsadok, Asma Nour El Houda Sid, Amel Hind Hassein-Bey, Abdeltif Amrane, Jie Zhang, Amin Aymen Assadi, and Lotfi Mouni. 2023. "Development of New Alkylated Carrageenan Derivatives: Physicochemical, Rheological, and Emulsification Properties Assessment" Sustainability 15, no. 8: 6473. https://doi.org/10.3390/su15086473
APA StyleToumi, S., Yahoum, M. M., Lefnaoui, S., Hadjsadok, A., Sid, A. N. E. H., Hassein-Bey, A. H., Amrane, A., Zhang, J., Assadi, A. A., & Mouni, L. (2023). Development of New Alkylated Carrageenan Derivatives: Physicochemical, Rheological, and Emulsification Properties Assessment. Sustainability, 15(8), 6473. https://doi.org/10.3390/su15086473









