Abstract
Dichlorodiphenyltrichloroethane (DDT) contamination in the Toce River in northern Italy was studied by collecting data from 2016 to 2021 upstream and downstream of a production factory which in the past had discharged technical DDT. Analysis of sediments and of bioaccumulation in different benthic invertebrate taxa (Gammaridae, Diptera, Ephemeroptera Baetidae and Heptageniidae) was carried out to assess the transfer of DDT from sediments to benthic invertebrates and the environmental risk of this legacy pollutant for the river ecosystem. DDT and its metabolites dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD), here called DDx, were analyzed by isotope dilution gas chromatography–mass spectrometry (GC-MS/MS). DDx values in sediments in upstream stations (1.14–2.25 ng g−1 1% Organic Carbon) were lower than downstream of the industrial site (5.60–7.60 ng g−1 1% Organic Carbon), often exceeding Sediment Quality Guidelines for total DDx. Peak levels derived from new inputs of parental DDT, as confirmed by fingerprint analysis. Bioaccumulation was higher at downstream sites (up to 5107 ng g−1 lipid weight), confirming the bioavailability of residual DDT as well as active metabolism, with the formation of DDD and DDE. The Biota-Sediment Accumulation Factor evidenced the highest values (over 4.2) for Diptera and Gammaridae, highlighting that invertebrates can transfer contamination from sediments to the trophic chain. Linear regression models were developed to estimate DDx concentrations in benthic invertebrates from DDx concentrations in sediments. However, determination coefficients R2 remained in the range of 0.36–0.51, highlighting the necessity of bioaccumulation analysis to fully estimate environmental risk. The results show that DDT contamination, even if residual, may still represent a risk due to its effective transfer to the trophic chain.
1. Introduction
In the past, the organochlorine pesticide DDT has been employed in Italy, as well as in many other countries in Europe and in the United States, as an insecticide for agricultural purposes such as controlling mosquitoes that spread malaria and as a remedy against epidemic typhus [1,2,3]. However, due to its persistence and toxicity, the use of DDT was banned in the 1970s (in 1978 in Italy), and the chemical was classified as a persistent organic pollutant under the Stockholm Convention in 2001 and as a priority substance under European Directive 2013/39/EU. Despite this, DDT is still used in many developing countries [4]. Due to its persistence and long-distance transport through the atmosphere, this chemical undergoes widespread distribution in the environment [5]. DDT shows a high bioaccumulation potential due to its lipophilicity and slow elimination rates, and this tendency is shared by its key metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE). These characteristics result in the biomagnification of DDT through the food chain, with peak concentrations at the top levels of the trophic chain, where secondary poisoning phenomena can occur [6,7,8].
DDT can reach freshwater ecosystems through many pathways, for instance, as runoff from contaminated soils, sewage effluents or atmospheric deposition [9]. Due to its hydrophobic characteristics, DDT and its metabolites tend to be absorbed onto suspended solids and particulate organic matter and to accumulate in bottom sediments, where they can be stored for decades [10]. From sediments, stored DDT can be released back into the water column [11,12] and can bioaccumulate in benthic organisms living in contact with the sediments, with potential biomagnification in the aquatic food web [13]. For these reasons, even if environmental concentrations may be residual, the ecological risk from DDT contamination may be significant and thus needs to be assessed.
Our study focused on the Toce River in northern Italy, which is characterized by documented legacy DDT contamination [14,15,16]. Here, an environmental risk assessment based on chemical, ecotoxicological and ecological analyses carried out in 2014 highlighted levels of DDT in sediments that were potentially toxic for aquatic invertebrates [17]. These concentrations were compared to the Sediment Quality Guidelines (SQGs), i.e., reference values considered to be protective for aquatic organisms, such as the consensus-based Threshold Effect Concentrations (cb-TECs) and Probable Effect Concentrations (cb-PECs) developed by [18]. Values in the Toce River exceeded the cb-TECs, i.e., the threshold below which adverse effects on aquatic organisms are not expected [17]. This approach provided an initial screening to evaluate environmental risk and pointed out reasons for environmental concern. However, the site-specific bioavailability of the contaminant, which depends on local chemical and physical factors hardly predictable in highly dynamic ecosystems such as rivers, was not assessed [19,20].
For this purpose, analysis of bioaccumulation in aquatic organisms can be carried out. Benthic invertebrates live in proximity to the bottom sediments of rivers, streams and lakes, they have small home ranges and limited dispersal abilities, and they are widely used as bioindicators of water quality [21]. Moreover, different taxa show specific ecological traits and functional feeding groups; therefore, they can be used to highlight different contamination transfer pathways, i.e., through passive diffusion across gills, dermal surfaces and digestive organs, from the water and/or sediment compartment or through predation. Despite these potentials, analysis of DDT in freshwater invertebrates has been scarcely carried out. For example, DDx levels were measured in dragonfly larvae (Odonata, Gomphidae) in the Olifants River Basin in South Africa [22], in other aquatic invertebrates in the Ga-Selati River in South Africa and in the Gudbrandsdalslågen and Rena Rivers in Norway [23]. These works emphasized significant DDT concentrations in some invertebrate taxa, showing the capacity of these organisms to transfer contamination from abiotic compartments (water, sediments) to the trophic chain. Invertebrates represent the basal level of the aquatic food chain; therefore, they may play a key role in the biomagnification potential of the contaminant.
DDT bioavailability can be quantified using the Biota Sediment Accumulation Factor (BSAF), i.e., the ratio between concentrations in biota and in sediments [24]. Usually this factor is calculated for organisms that live in contact with the sediment, for which a direct partitioning occurs between the sediment and the organism [25]. As regards DDT, this approach has been used to assess the bioaccumulation potential of this pollutant in fishes in the Cinca River in Spain to highlight the impact of a dicofol manufacturing factory [25], but also in mussels and fishes in the Oder River in Poland, where pollution was determined by intensive agriculture and big industrial–municipal agglomerations located in its catchment area, as well as significant loads of pollutants from industrialized areas in the Czech Republic, Germany, Upper Silesia and Lower Silesia [26]. The BSAF approach has also been applied in coastal areas, such as in Korea, where DDT was measured in Manila clams (Ruditapes philippinarum) to verify the safe consumption of this organism in this area [27].
In this study, both isomers of DDT and their metabolites DDD and DDE were analyzed in sediments and in four different benthic invertebrate taxa in the Toce River along a gradient of residual DDT contamination. Our aim was to quantify DDT bioavailability in this river ecosystem and to evaluate the potential role of invertebrates in contamination transfer from sediments to higher trophic levels through bioaccumulation analysis. Relations between concentrations in sediments and invertebrates were assessed using BSAF calculation and regression models.
2. Materials and Methods
2.1. Study Area
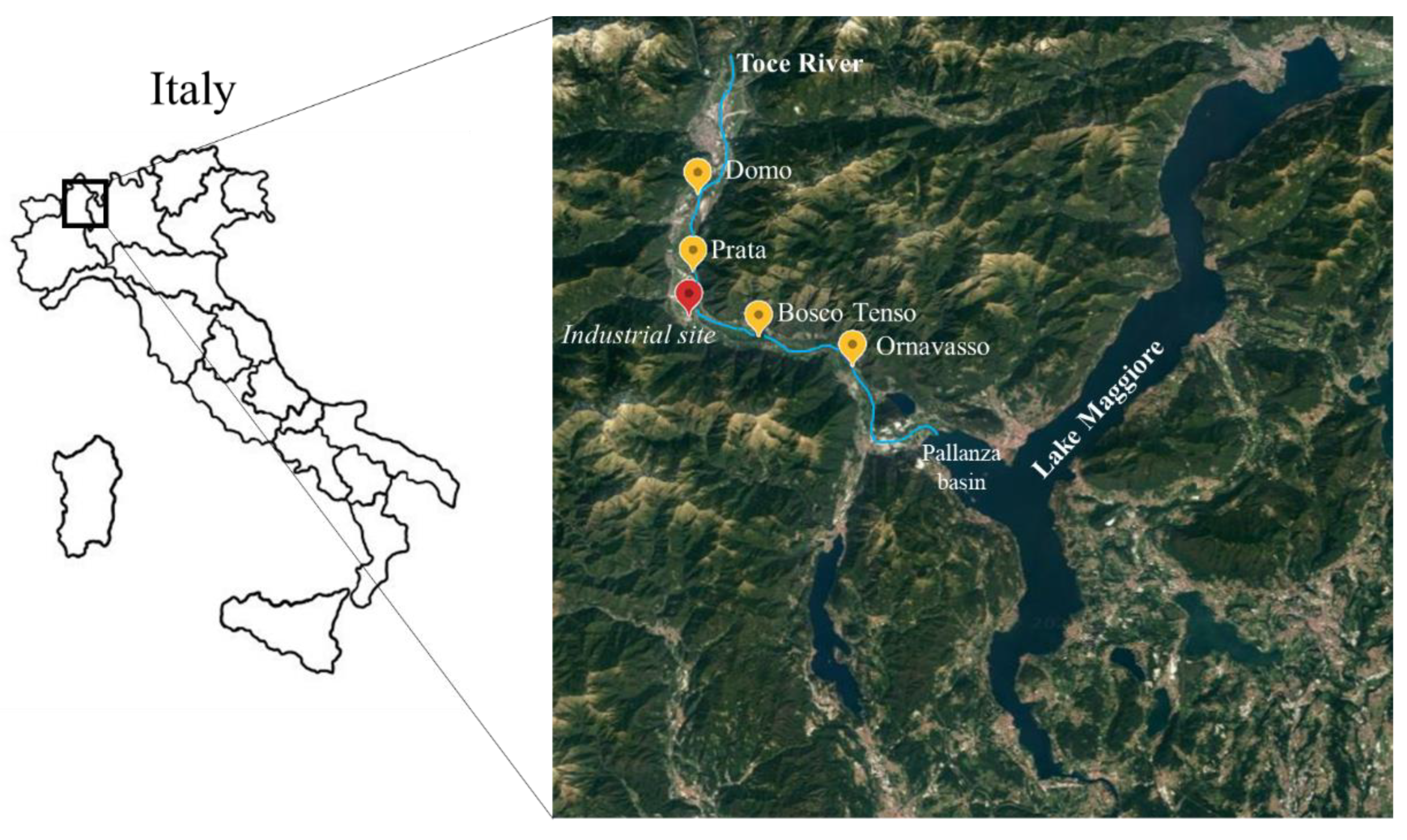
This study was carried out along the Toce River in the Ossola Valley, which is located in the Central–Western Alps, northern Italy. The Toce River originates in the upper Val Formazza by the confluence of some streams in the plain of Riale and is 84 km long. The basin is 1800 km2 wide, and land use is mainly natural (about 92% of the total surface) [28]. The Toce is one of the main tributaries of Lake Maggiore, flowing into the Pallanza Basin with an average annual flow of 62 m3 s−1 (Figure 1). A factory producing technical DDT, located at Pieve Vergonte (VB), about 20 km upstream from the river mouth, discharged wastewater into the river during the last century, causing heavy DDT pollution, as proven by the contaminant peaks of 13.000 ng g−1 in sediments in the 1960s and 1970s. DDT production was stopped definitively in 1996. DDT contamination was first pointed out by [29], which reported high concentrations of DDT in some fish species in Lake Maggiore, such as the twaite shad fish, above the fixed limit for human consumption of 1 mg kg−1 wet weight (w/w). Further analyses carried out on sediments and soils proved that the point source was the factory at Pieve Vergonte, where high concentrations of DDT can be still found in soils in the industrial area.
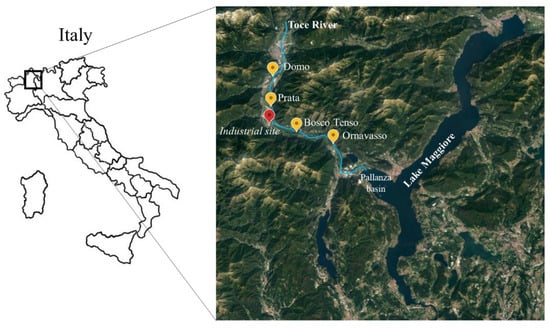
Figure 1.
Sampling locations along Toce River.
2.2. Sampling Sites
In this study, four sampling stations were considered, located along the last 30 km before the Toce mouth (Figure 1).
Two sites, Domo and Prata, are situated 8.6 and 3.4 km upstream of the factory at Pieve Vergonte, respectively, while Bosco Tenso and Ornavasso are located 3.7 and 13.1 km downstream, respectively. The geographical coordinates of the sampling sites are reported in Table 1.

Table 1.
Geographic coordinates of sampling sites.
This river stretch presents uniform hydromorphological characters, belonging to WFD intercalibration type R-A2 and to Italian river type 01SS4G, and it is divided into two different water bodies (according to WFD terminology): type G1 for the stretch upstream from Pieve Vergonte village and type G2 downstream, where the river discharge increases significantly due to the inflow of two large water channels [17]. The width of the riverbed is 40–60 m, the maximum depth of the water column is more than 1.5 m, and the mean slope of the studied stretch is 1.68 m km−1. The bottom substrate is largely composed of sand and gravel and hosts species-rich benthic invertebrate communities [17]. The river shows an Alpine hydrological regime, with maximum discharge in early summer due to precipitation and snow melting and minimum discharge in winter [30]. Concentrations of freely dissolved DDx in porewater and at the water–sediment interface were determined in 2014–2015 by using polyethylene passive samplers [16] that showed increasing contamination from Domo to Ornavasso and peak concentrations of 0.29 ng L−1 at the Ornavasso station.
2.3. Sediment and Benthic Invertebrates Sampling
From 2016 to 2021, sediment and benthic invertebrates were sampled at each station twice per year, in spring (March–April) and early autumn (September–October), i.e., in two periods of intense emergence for aquatic insects and of intermediate flow according to the river’s hydrological regime. Sampling was not performed in summer because, due to high discharge, most sampling sites were not wadable. In winter, benthic invertebrate biomass was lowest, so the sampling effort needed to obtain enough material for analysis was too high.
At each site, sampling was carried out in depositional areas characterized by the accumulation of the fine fraction of sediments where contaminants are adsorbed.
For sediments, different sub-samples were collected using a stainless-steel spoon and mixed to obtain a 2 L representative sample. Sediments were transported in acetone-washed amber glass bottles and kept in the dark at 4 °C until freeze-drying (Telstar LyoQuest). Dry samples were sieved to separate the fine fraction (<63 µm) for chemical analysis. The total number of data considering sampling station (4) × sampling (2) × year (6) was 48.
Benthic invertebrates were sampled by using hand nets following a qualitative sampling strategy. The most abundant invertebrate taxa were sorted on-site in different taxonomic groups until reaching a biomass suitable for chemical analysis and left in river water for at least four hours to allow gut purging. Organisms were then dried with absorbent paper and frozen at −18 °C until freeze-drying using the same procedure as for the sediments. Samples were then homogenized and preserved in dark glass bottles until analysis. The most abundant taxa, which were generally found at all sites, were Ephemeroptera Baetidae Baetis, Diptera Tipulidae, Tabanidae, and Limoniidae grouped together, and Ephemeroptera Heptageniidae Ecdyonurus and Crustacea Gammaridae Echinogammarus. A taxonomical identification of these taxa was performed in order to assess their main ecological traits and functional feeding group. Detailed information is reported in [31,32] and in Table S1 (Supplementary Material). Baetidae were considered grazers and Gammaridae shredders, while Diptera and Heptageniidae showed a mixed diet and were thus classified as gatherers–predators [31]. The total number of data per taxon (1) × sampling (2) × sampling station (4) × year (6) should be 48. However, considering that on some sampling dates the river was not wadable at the Prata station, and that in some cases biomass was not sufficient for analysis, the total number of data for Diptera and Heptageniidae was 43, for Baetidae 39 and for Gammaridae 38 (Table S2).
2.4. Chemical Analyses
Sediment and benthic invertebrates were analyzed for DDx by isotope dilution mass spectrometry, using the same method described in [16]. Samples of 0.5 g were spiked with 10 ng of internal standard solution. Samples were extracted using a Soxhlet apparatus (Buchi, Switzerland) using n-hexane/acetone (3:1, v/v) as the extraction solvent. Extracts were cleaned using a multi-layer column (1.5 × 20 cm) packed (bottom to top) with 1.5 g acidified silica gel 30% w/w sulphuric acid (Sigma-Aldrich, Steinheim Germany) and 1.5 g Florisil® (100–200 mesh, Sigma-Aldrich, Steinheim Germany). Extracts were concentrated under a gentle N2 flux to approximately 250 µL. Compound quantifications were performed by gas chromatography mass spectrometry (GC–MS/MS) following the conditions described in [16].
Instrument linearity was checked with a seven-point calibration curve, covering the range between 0.01 ng g−1 and 250 ng g−1. Sediment quality assurance was carried out by analyzing reference material IAEA-383, “Organochlorine compounds petroleum hydrocarbons and sterols in sediment sample”, while for DDx in biological samples the National Institute of Standard and Technology “NIST SRM 2974a” was used for method validation. Accuracy was calculated using certified materials, and all results were within the expected concentration range of ±30% of the certified values, while precision between replicates, calculated as the relative standard deviation, was below 20%. Internal standard recoveries were calculated for each sample, and analyses were repeated if this value was below 30%. The limit of detection (LOD) was fixed at 0.01 ng g−1 dry weight (d.w.) for each compound in the sediment and biological samples using a signal-to-noise ratio of 3:1. Procedural blanks were analyzed every eight samples to check for laboratory contamination. Procedural blanks were always <LOD for each analyzed compound.
For each sediment sample, the organic carbon (OC) content was determined in 0.5 g d.w. samples by back-titration after oxidation with potassium dichromate in the presence of sulphuric acid according to Walkley and Black [33].
For the organisms, the lipid content was determined gravimetrically by solvent extraction (n-hexane/acetone 3:1, v/v) using 0.5 g of the sample. The annual lipid content for each taxon was determined by pooling samples from spring and autumn, given the stability of the lipid content in the same year.
2.5. Data Analysis
The ratio between the metabolites DDE and DDD and the parental compound DDT in sediments was calculated as follows [34,35]:
where ƩDDE is the sum of the isomers 2,4′-DDE and 4,4′-DDE, ƩDDD is the sum of the isomers 2,4′-DDD and 4,4′-DDD, and ƩDDTs is the sum of the isomers 2,4′-DDT and 4,4′-DDT. This ratio can be used as an indicator of the time since the last DDT usage. If the ratio is >0.5, it suggests a long-term degradation of DDT, whereas a lower ratio indicates a recent DDT input in the environment.
(ƩDDE + ƩDDD)/ƩDDTs,
To highlight the mechanism driving the metabolic transformation of DDT in sediments, the ratio between DDT and DDE was calculated as follows [36]:
ƩDDD/ƩDDE,
Values of this ratio >1 indicate the anaerobic degradation of DDT, while ratios <1 indicate a predominance of aerobic degradation.
To assess the bioavailability of DDT to benthic organisms, BSAF was calculated as follows [37]:
where CB/fL represents the DDx concentration in organisms normalized to the correspondent lipid fraction and CS/fOC is the DDx concentration in sediments normalized to the respective organic carbon content.
BSAF = (CB/fL)/(CS/fOC),
Due to their hydrophobic nature, DDx compounds are adsorbed on sediment organic matter, so data normalization was performed according to this parameter to allow comparisons between sites [16]. Moreover, normalization was necessary to compare DDT concentrations with the Sediment Quality Guidelines in [18], which are set at 1% organic carbon.
As regards biota, the lipid content might significantly influence the bioaccumulation of lipophilic contaminants as DDx, and it is also related to the specific taxon, the tropic role of the organism and its life stage [38]. For these reasons, DDx data regarding benthic invertebrates are all expressed as lipid weight normalized (l.w.).
The non-parametric Kruskal–Wallis test followed by the Dunn post-hoc test with Bonferroni correction for multiple comparisons was performed to test differences between sites regarding total concentrations of DDx in sediment and in each taxon and the BSAFs of different taxa. The Mann–Whitney test was used to test differences between sampling periods in each station and between upstream and downstream sites. Prior to these analyses, normality of data and homogeneity of variance were assessed using the Shapiro–Wilk and Levene tests, respectively. In case of deviances, log10(x) transformation was carried out. Spearman’s rank correlation was employed to test for relation between variables.
Linear regression was calculated to develop predictive models to estimate lipid weight normalized DDx concentrations in benthic invertebrates from the organic carbon-normalized DDx concentrations in the respective sediments. Since normality was not met, log10(x) transformation of variables was carried out prior to analysis.
All statistical analyses were carried out using the R software package (4.2.0) and Past (4.03).
3. Results and Discussion
3.1. DDx in Sediments
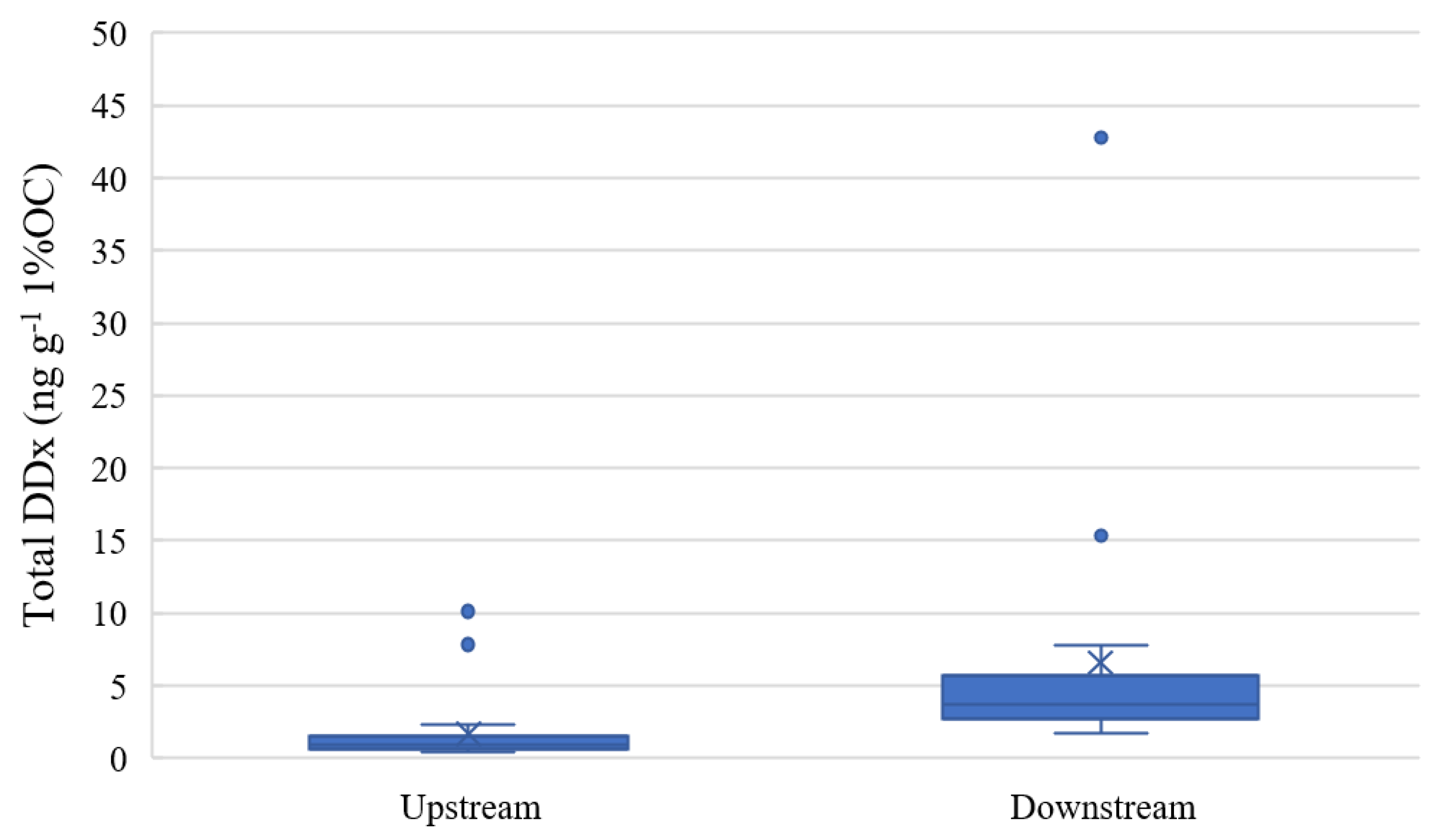
As expected, the correlation between total DDx d.w. and total organic carbon in the sediment samples was significant (ρ = 0.5135, p < 0.05) due to the hydrophobic nature of the compound. The lowest DDx concentrations were measured at Domo, with a mean concentration value of 1.14 ± 0.55 ng g−1 1%OC. Higher concentrations of DDx in sediments were measured depending on the proximity of the sampling site from the DDT factory at Pieve Vergonte, with mean concentrations of 2.25 ± 3.20 ng g−1 1%OC at Prata, 7.60 ± 11.71 ng g−1 1%OC at Bosco Tenso and 5.60 ± 3.83 ng g−1 1%OC at Ornavasso. Low concentrations at Domo and Prata could have been caused by the atmospheric deposition of pollutants coming from Pieve Vergonte, as already highlighted in previous research [16,17]. Mean concentrations at Domo were almost stable during the years 2016–2021, while peaks of total DDx concentrations at the downstream sites were registered in 2017, possibly because some reclamation activities on course in the contaminated industrial area may have caused a remobilization of the contaminant via soil leaching or atmospheric transport [39]. Since differences in sediment concentrations between Domo and Prata and between Bosco Tenso and Ornavasso were not significant (p > 0.05), the stations were grouped into upstream and downstream sites (Figure 2).
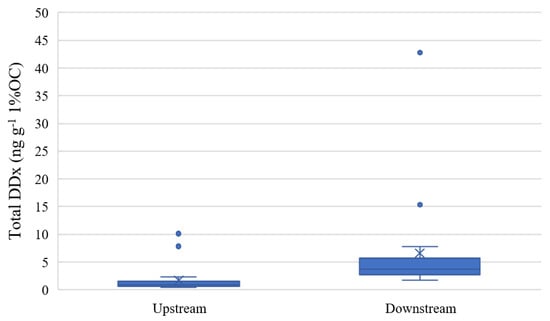
Figure 2.
Boxplots of total DDx concentration at upstream (Domo and Prata, n = 12) and downstream (Bosco Tenso and Ornavasso, n = 12) sites (ng g−1 1%OC).
The Mann–Whitney test (p < 0.05) revealed statistical differences between the two groups, confirming the impact of the industrial area located at Pieve Vergonte on the DDT pollution of the Toce River sediments. Concentrations at Domo and Prata (0.15–21.37 ng g−1 d.w.) were in the range of values reported in other case studies (Table 2), such as in rivers in Belgium [40], Congo [41], China [42], Spain [43], South Africa [44] and Romania [45] which are close to heavily urbanized and densely populated areas or where the historical use of DDT is documented, confirming that these levels should be considered the baseline level nowadays in legacy-contaminated areas. In contrast, DDx levels measured downstream of Pieve Vergonte (1.28–97.97 ng g−1 d.w.) are comparable to those detected in other polluted sites, such as on the Cinca River in Spain [25], which are downstream of an industry which uses DDT as an intermediate in the production of dicofol, or such as the Fuchun reservoir of Qiantang River in China [46], where the high-level use of DDT pesticides is documented before the dam’s construction, or as in other rivers located in Moldova (e.g., Bîc River, Rǎut River) or Ukraine [47] which cross several densely populated cities [9].

Table 2.
Mean sediment concentrations (ng g−1) of DDx in Toce River compared to other studies.
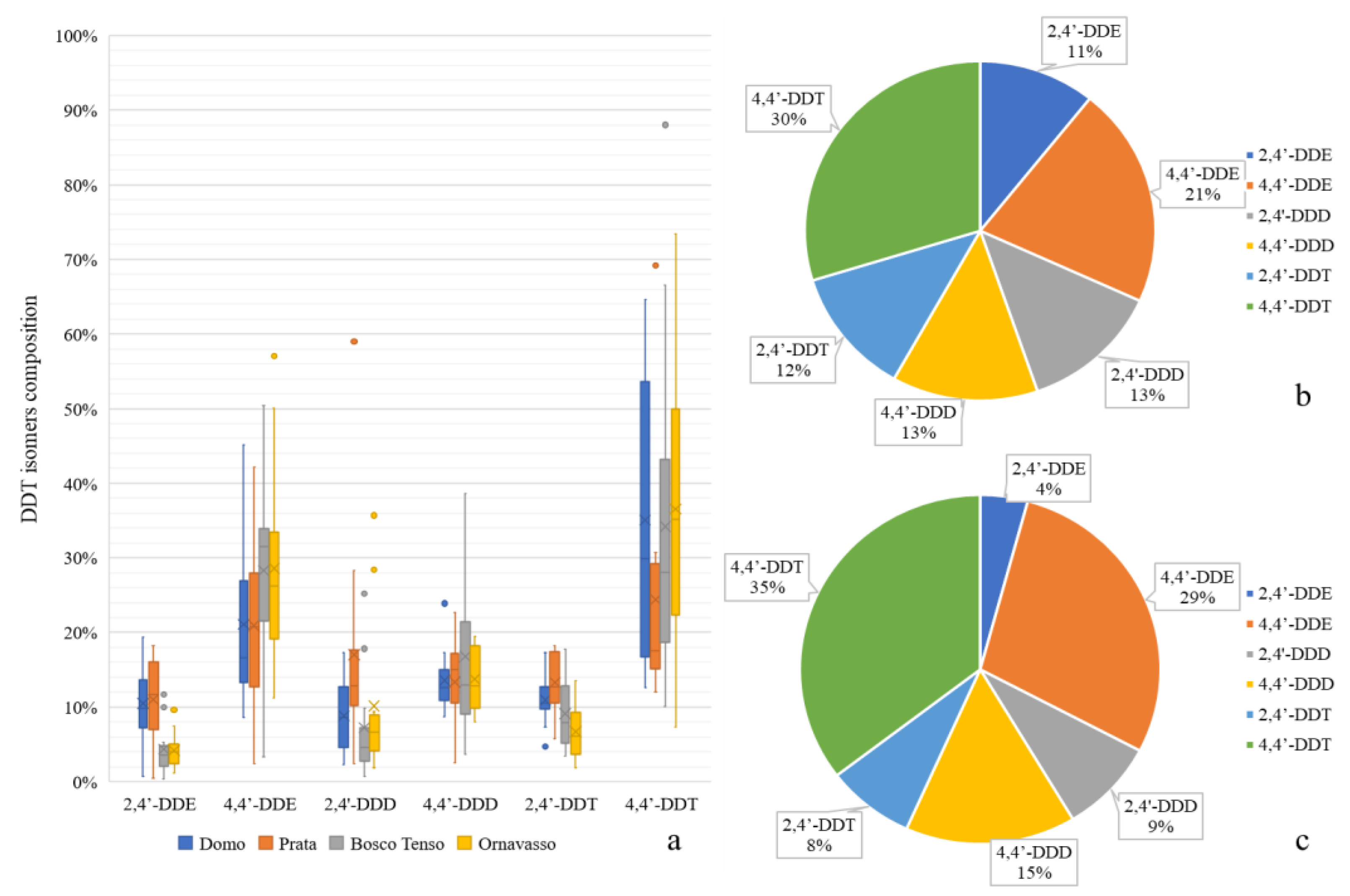
Regarding the relative presence of DDT metabolites and isomers, the composition of DDT transformation products in sediments is shown in Figure 3.
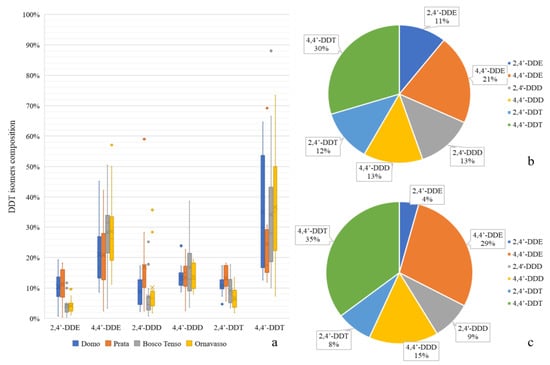
Figure 3.
Box-plot showing the composition of DDT-related compounds in sediments of the four considered stations (a). Mean DDT-related compounds in sediments of upstream (b) and downstream (c) stations.
In general, the number of 4,4′-isomers was higher than that of 2,4′-isomers at all stations, which is in line with the technical composition of DDT, with 4,4′-DDT being the main detected compound and 4,4′-DDE the main metabolite, as reported for other rivers [9]. Regarding isomer composition, no differences were measured between sites, except in the case of 2,4′-DDE, which was less represented at the downstream sites (p < 0.05). However, this compound represented less than 10% of the total DDx in the sediment samples. The most highly represented compounds were the metabolites DDE and DDD, which accounted for >50% at the upstream sites, while their contribution decreased to 43% at Bosco Tenso and Ornavasso. This could be related to new inputs of parental DDT coming from the area of the Pieve Vergonte factory. In fact, a strong correlation was found between the sum of DDD + DDE and the total DDx in Toce River sediments (ρ = 0.92, p < 0.05). Similarly, sediments with higher total DDx concentrations showed a higher proportion of ƩDDT (ρ = 0.899, p < 0.05), and even the correlation between ƩDDE and ƩDDD was significant (ρ = 0.67, p < 0.05). As suggested by [48], these correlations indicate that measured levels of DDx in the sediment samples were related to the input of DDT and its degradation products. These observations prove that, even if DDT production stopped in 1996 and soils and groundwater in the industrial area have been stabilized, new inputs of the contaminant into the river ecosystems are still present. Technical DDT contains high fractions of parental compounds in comparison to its metabolites: 77% 4,4′-DDT, 15% 2,4′-DDT, 4% 4,4′-DDE and <5% 4,4′-DDD according to [49]. The ratio between DDE and DDD and the parental compound DDT at all the sampling sites was generally above 0.5, indicating long-term degradation. These results are in line with other works that analyzed the isomer composition of DDT in river sediments, such as in Spain [50], in Moldova [9] or in South Africa [22]. In these studies, the ratio resulting from Equation (1) was always higher than 0.5, indicating that sediment contamination was not due to recent DDT input but rather to its past use and incomplete degradation. However, some samples at Bosco Tenso and Ornavasso in 2017 and 2020 showed ratios <0.5, in correspondence with peaks in DDx levels, indicating that new inputs of parental DDT had reached the river sediments. DDT can be degraded via aerobic and anaerobic pathways depending on its in situ redox state. In the present study, most samples had a ratio resulting from Equation (2) < 1, indicating that metabolic products were formed mainly under aerobic conditions, which can thus be considered the main transformation pathway [36].
Analysis between sediment samples collected in spring and autumn at each site proved no significant differences in DDT concentrations (p > 0.05) between sampling periods, as was reported in previous studies carried out on the Toce River [16]. In fact, river sediments are affected by continuous mixing caused by the river flow, especially during flooding events, due also to the abundant sandy fractions of the Toce sediments.
To assess potential risks related to DDx levels in Toce sediments, concentrations were compared with the SQGs by [18]. All samples resulted in being below the cb-PEC, i.e., the threshold above which harmful effects are likely to be observed, fixed for total DDx at 572 ng g−1 d.w. 1%OC, but also for ∑DDE (31.3 ng g−1 d.w. 1%OC), ∑DDD (28 ng g−1 d.w. 1%OC) and ∑DDT (62.9 ng g−1 d.w. 1%OC). Considering cb-TEC, i.e., the threshold below which adverse effects on benthic organisms are not expected, mean values upstream of the Pieve Vergonte factory were always below the value fixed for total DDx (5.28 ng g−1 d.w. 1%OC), apart from Prata sediments during Autumn 2016 and Spring 2017. At Bosco Tenso and Ornavasso, values exceeded the cb-TEC for Total DDx in some years (from 7.76 to 42.78 ng g−1 1%OC and from 10.40 to 15.66 ng g−1 d.w. 1%OC, respectively). This was mainly due to the contribution of ∑DDT exceeding the cb-TEC value in the same years, evidencing parental DDT as the predominant compound in those periods. Values in the Toce above the cb-TEC showed the need to further assess potential risks for aquatic organisms. Furthermore, Ref. [51] measured DDT levels in sediments higher than the cb-TEC in marine and adjacent riverine areas of North Bohai Sea, China, and the authors concluded that adverse effects on benthic invertebrates were likely to be expected. In the Haihe Plain, India, DDx in sediments was classified as potentially toxic for aquatic organisms, showing concentrations higher than the cb-TEC. SQGs by [18] are commonly applied to freshwater sediments to evaluate potential toxicity for benthic organisms, not only because they are derived from field and laboratory-based studies but because they have been validated by subsequent toxicity studies. For example, Ref. [52] carried out a battery of sediment-contact ecotoxicological tests by using different organisms, such as higher plants (Myriophyllum aquaticum), nematodes (Caenorhabditis elegans), oligochaetes (Lumbriculus variegatus), zebrafish embryos (Danio rerio) and bacteria (Arthrobacter globiformis), representing various trophic levels and exposure pathways, and compared the toxicity classification obtained with that proposed by [18]. The author-derived classification generally agreed well with consensus-based sediment quality guidelines (SQGs), especially regarding sediments with high toxic potential, thus confirming its applicability. However, the interpretation of potential risks for benthic communities based on the SQGs can be considered a first screening approach concerning the evaluation of adverse effects on aquatic organisms, since it does not consider factors such as the bioavailability of contaminants, the mixture’s effects between contaminants and, in the case of DDT, its bioaccumulation and biomagnification potential [20].
3.2. DDx Bioaccumulation in Benthic Invertebrates
To assess the bioavailability of the contaminant for aquatic organisms, bioaccumulation in native invertebrates belonging to different taxonomic and functional feeding groups was analyzed. Different species-specific characteristics can influence bioaccumulation, such as the preferential habitat of the organism, its diet and other environmental parameters [53,54,55]. In our study, the analysis focused on four taxa of benthic invertebrates generally present and abundant at all the stations considered (Supplementary Material, Table S2).
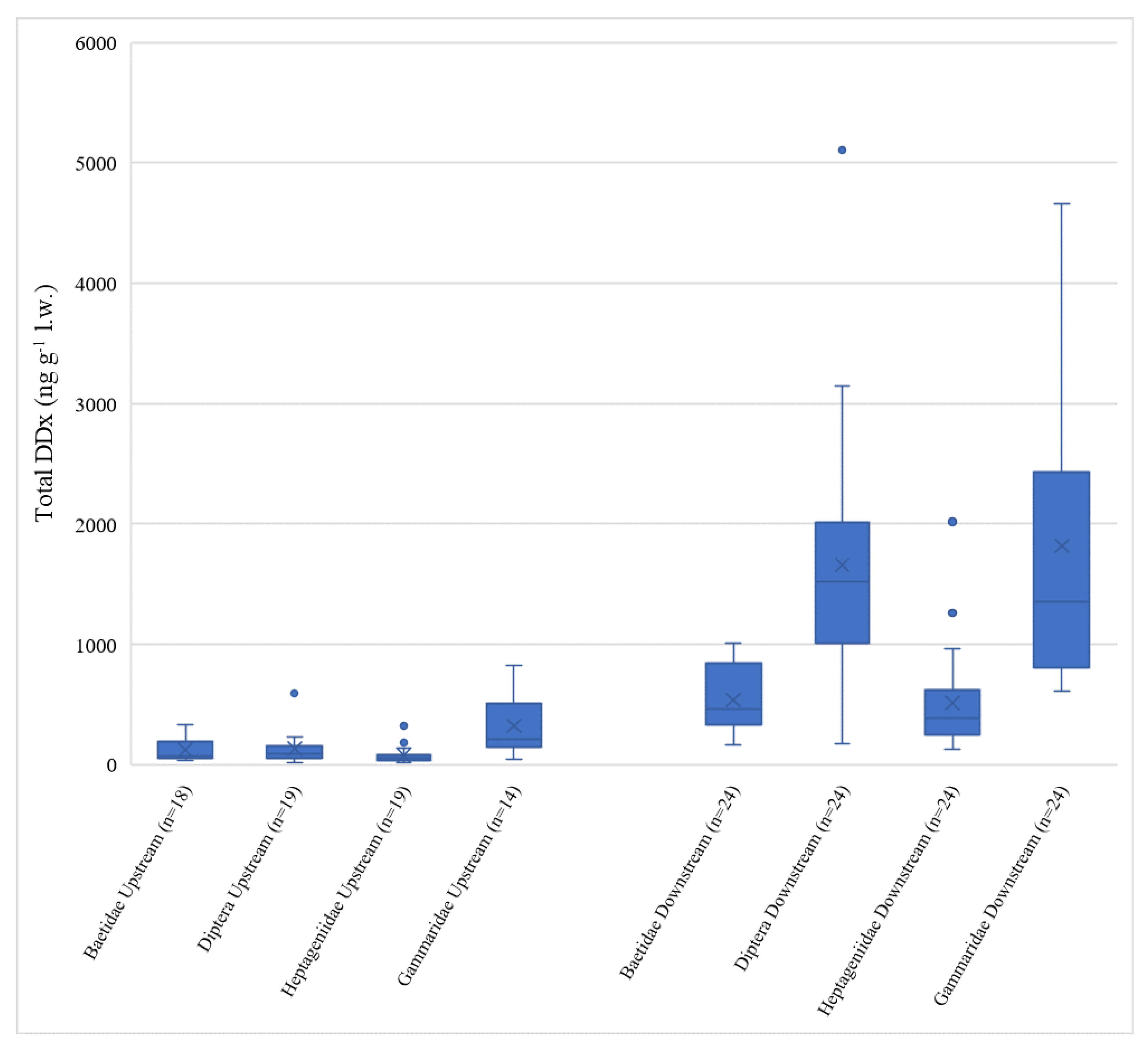
Total DDx levels measured in invertebrates followed the same pattern among sites described for sediments, with Domo and Prata showing concentrations consistently lower than Bosco Tenso and Ornavasso (Kruskal–Wallis test, p < 0.05) (Figure 4).
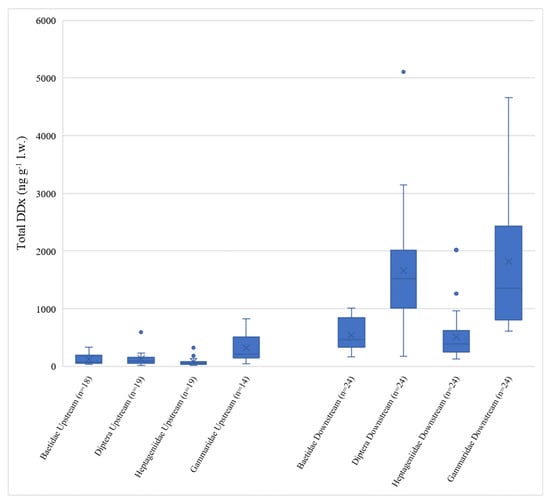
Figure 4.
Boxplots of total DDx concentration (ng g−1 l.w.) in the four invertebrate taxa considered. Data are grouped in upstream sites (Domo + Prata) on the left and downstream sites (Bosco Tenso + Ornavasso) on the right. n = number of data per each group.
Total DDx concentrations at upstream sites ranged from <LOD to 829 ng g−1 l.w. (mean values of 110 ± 133 ng g−1 l.w. at Domo and 198 ± 184 ng g−1 l.w. at Prata), while at downstream sites the range was between 129 ng g−1 l.w. and 5107 ng g−1 l.w (mean values of 1150 ± 956 ng g−1 l.w. at Bosco Tenso and 1130 ± 1113 ng g−1 l.w. at Ornavasso), evidencing clear enrichment deriving from the industrial area of Pieve Vergonte. DDx levels like those measured at Domo and Prata were reported for dragonfly larvae (Odonata, Gomphidae) of the Olifants River Basin (145 ng g−1 l.w.), South Africa [22], where relevant anthropogenic pressures are not present. On the contrary, values detected downstream of Pieve Vergonte (109 ± 81 ng g−1 d.w. at Bosco Tenso and 113 ± 103 ng g−1 d.w at Ornavasso) were comparable to those measured in dragonfly larvae in another South African river (133 ng g−1 w.w.) located in a basin where the use of DDT in towns is still very common to prevent malaria [23].
As observed for sediments, no difference between sampling seasons was highlighted at all sites for each taxon (p > 0.05), and thus data collected in the two different periods were pooled together for further analysis. Comparing different taxa, Gammaridae and Diptera registered the highest DDx values (Figure S1), but differences between the Baetidae and Heptageniidae taxa were statistically significant only at the stations located downstream of the Pieve Vergonte factory, Bosco Tenso and Ornavasso (p < 0.05). All taxa showed their maxima at Bosco Tenso, as was also observed for sediment contamination. Gammaridae proved to have the highest DDT bioaccumulation potential, especially considering the lipid-normalized concentrations, probably because the thick external cuticle of chitin in these Crustacea may bind the contaminant or may facilitate the entry of DDT into the animal’s body by selectively concentrating the compound by adsorption phenomena [56,57]. In fact, starting from this evidence, many authors supported this theory by carrying out studies which used chitin as a natural material to remove pollutants as pesticides from water due to the high adsorption capacity of this polymer [58,59]. The high DDx concentrations detected in Diptera may instead be linked to their feeding habits, which are partly detritivorous and partly predatory [31]. The lowest values were found for Heptageniidae and were in line with those of Baetidae (p > 0.05 between taxa). To our knowledge, data regarding DDT bioaccumulation in freshwater benthic invertebrates are very scarce in the literature. However, our results can be compared to those of [21], who used different taxa of invertebrates to assess the distribution and sources of persistent organic pollutants in different rivers in South Wales, measuring values of total DDx up to 17.4 ng g−1 w.w. They found different bioaccumulation levels of organochlorine compounds in different taxa sampled in the same location, with Heptageniidae and Baetidae having the lowest concentrations due, according to the authors, to their similar feeding behavior as grazers. Since recent studies highlighted that Heptageniidae may behave as omnivores, we classified this taxon as collectors–predators [31]. However, we also observed comparable DDT values in Heptageniidae and Baetidae (grazers), so further investigation would be necessary to understand which taxon-specific characteristics may explain this result.
Significant differences between concentrations at upstream and downstream sites (p < 0.05) were highlighted for all taxa (Figure 4). This finding supports the concerns deriving from sediment analysis, also demonstrating the increased bioavailability of the contaminant downstream of the industrial site. Moreover, the results show the key role of invertebrates in transferring contamination from sediments to the biotic compartment, with a consequent potential toxicity risk for higher trophic levels such as fish, given the biomagnification capacity of this contaminant [60,61]. In fact, concerning fishes in the Toce River, DDx concentrations above 30.000 ng g−1 l.w. and above 10.000 ng g−1 l.w. were recently determined [62] in specimens of chub and brown trout, respectively. However, analysis of the biomass of each taxon in the benthic community at the studied sites would be necessary to understand their effective role in the transfer of DDT contamination to the highest trophic levels.
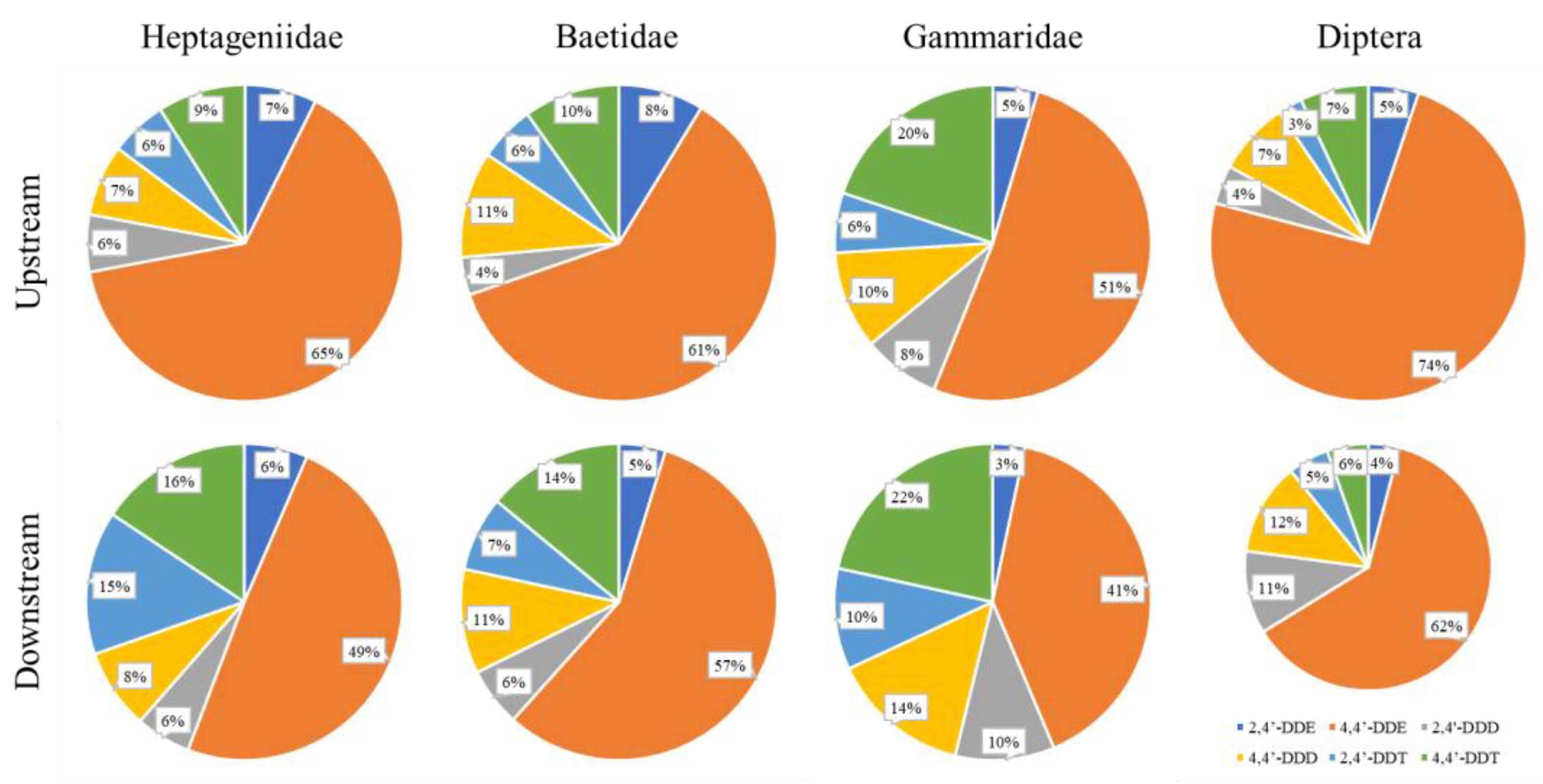
Regarding DDx composition in biota, 4,4′-DDE was the main compound accumulated in all taxa, with a mean contribution from 44 ± 16% in Gammaridae to 67 ± 17% in Diptera of the total DDx (Figure 5).
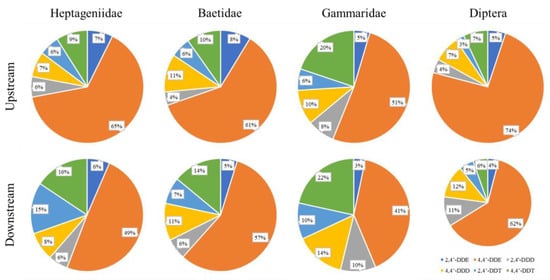
Figure 5.
Pie charts of DDx composition (%) measured in four taxa of benthic invertebrates sampled in upstream (first row) and downstream (second row) stations.
This finding agrees with the metabolism of DDT in DDE already reported for different aquatic organisms belonging to different trophic levels such as phytoplankton, macroinvertebrates and fishes [38,41]. For example, Ref. [63] analyzed 4,4′-DDE in larvae of the damselfly Ischnura elegans (Odonata, Coenagrionidae) from different ponds in Flanders, Belgium, finding 4,4′-DDE levels of up to 3.30 ng g−1 w.w. in some of the studied ponds. In addition, between all DDT metabolites, DDE shows the highest stability and the fastest bioaccumulation capacity in lipid tissues [64].
Comparisons between sites revealed a significant enrichment of parental DDT between Domo, the reference site, and Bosco Tenso, the site closest to Pieve Vergonte (p < 0.05), confirming that new inputs of parental DDT deriving from the industrial area are driven into the fluvial ecosystem. This was also confirmed by the comparison between upstream and downstream sites for single taxa (Figure 5), which showed a significant enrichment in parental DDT isomers and the corresponding reduction of the metabolite 4,4′-DDE (p < 0.05). Besides this, concerning Diptera, a significative increase in 2,4′-DDD was also noticed.
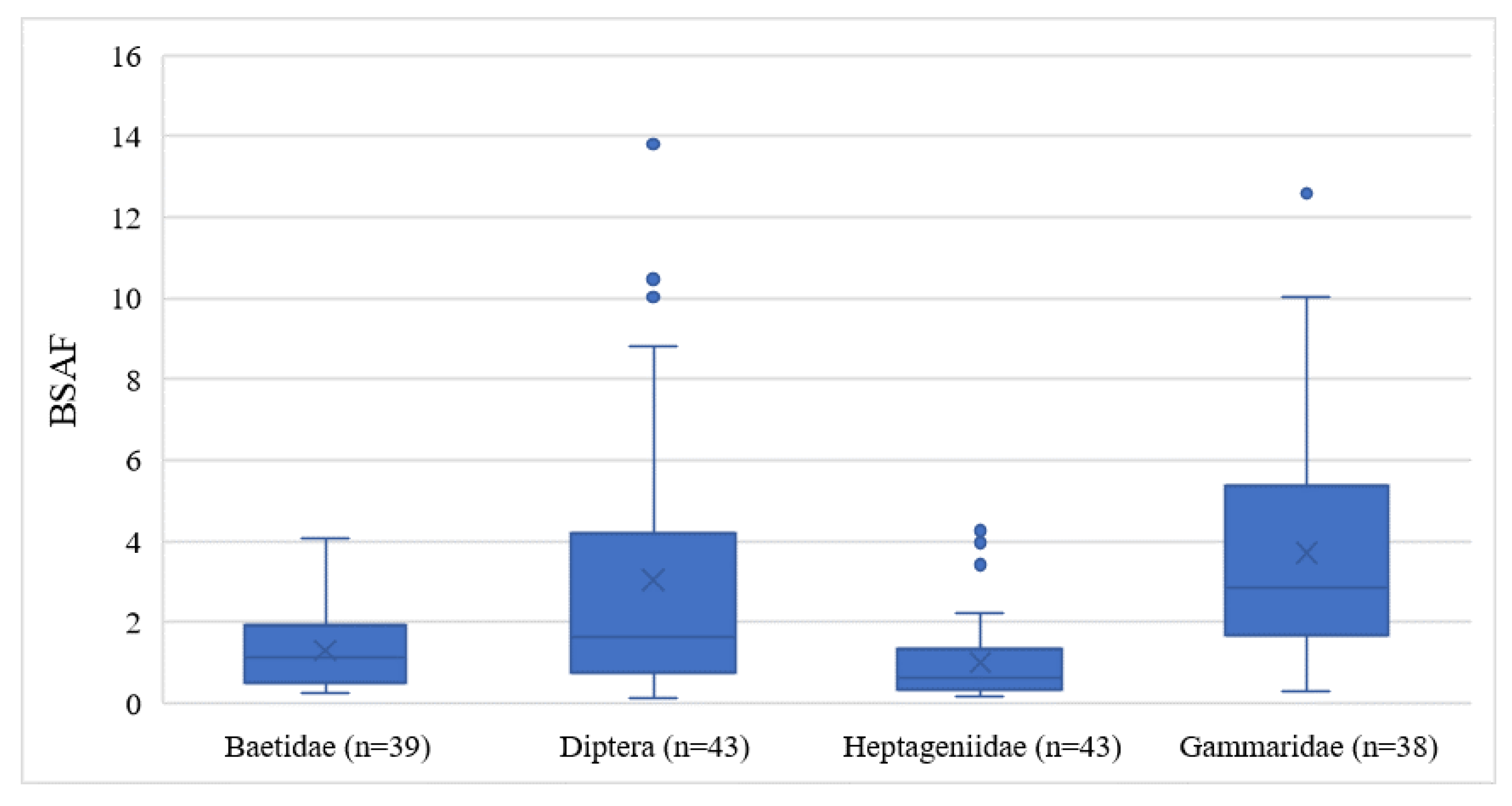
To estimate the bioavailability of DDx in the Toce River, the BSAF model was considered. BSAF is based on contaminant equilibrium partitioning between the lipidic content of biotic tissues and sediment organic carbon. BSAF values are theoretically independent of sediment type and can be employed over a wide variety of environmental conditions to perform a first-level screening of bioaccumulation potential under real field conditions [24]. Figure 6 shows the distribution of BSAF values for each taxon.
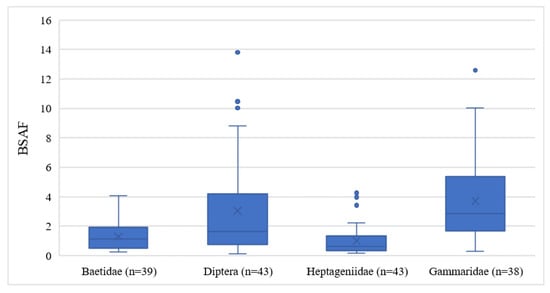
Figure 6.
Boxplots of Biota–Sediment Accumulation Factors (BSAFs) measured in the four benthic invertebrate taxa of the Toce River.
Gammaridae (ranging from 0.30 to 12.59) and Diptera (0.11 to 13.80) were the organisms with the highest BSAFs, while Heptageniidae (0.15 to 4.26) and Baetidae (0.24 to 4.07) expressed lower BSAFs, reflecting the DDT concentrations observed in the biological samples. Gammaridae showed higher BSAFs (p < 0.001) than Heptageniidae and Baetidae, probably due to the DDx adsorption mechanisms favored by chitin, while Diptera expressed higher BSAFs only in comparison to Baetidae. Even considering only the downstream sites, two different groups were highlighted: Baetidae and Heptageniidae with lower BSAFs (mean values 1.38 ± 1.03 and 1.20 ± 0.98, respectively) and Diptera and Gammaridae with higher BSAFs (mean values 4.47 ± 3.64 and 4.25 ± 3.06, respectively). Different studies indicate a theoretical BSAF of 1.7 as the value from which a substance may bioaccumulate [41,62,63,64]. A BSAF value < 1.7 reveals less partitioning of an organic compound into lipids, and a BSAF value > 1.7 reveals a greater uptake of the compound. Diptera and Gammaridae are therefore subject to DDx bioaccumulation, with the consequent maximum potential of transferring contamination to higher trophic levels.
As regards different DDx metabolites and isomers measured at all stations, the highest BSAF values were calculated for DDE and the second-highest for DDD in all taxa (Figure S2). This result was expected since benthic organisms can metabolize parental DDT into DDE or DDD. Because the DDT metabolite 4,4′-DDE constituted the largest fraction in all invertebrates, the BSAF values for 4,4′-DDE could have affected the overall BSAF distribution. If excluding the data of this metabolite, as in [24], only Gammaridae has significantly higher BSAF values than the other taxa (p = 0.01), while Diptera shows values comparable with the other taxa. The metabolite 4,4′-DDE therefore appears to influence Diptera to an extent.
An inverse relation between BSAF and DDx in sediment was highlighted for all taxa (Figure S3). This result was expected based on Equation (3), but it is reported here to highlight that the bioaccumulation capacity was higher at lower environmental concentrations. This evidence was already highlighted by [65] for organic chemicals and by [31,66] for metals, proving that physiology, the decreased availability of pollutants with increasing loads in sediments and different levels of pollutant partitioning among sediment particle fractions with aging may each influence bioaccumulation rates [31,65]. This consideration gains relevance in ecosystems where contamination levels are decreasing with time, as the case of the Toce River, where DDT sediment contamination levels are lowering, even if at slower rate in comparison with those of other contaminants [31]. Therefore, even if concentrations in sediments will decrease, it is expected that the response of benthic communities in terms of bioaccumulation, and in terms of the entire food web, will be slower.
3.3. Relations between DDx Bioaccumulation in Benthic Invertebrates and Sediment Concentrations
The best fit between concentrations in sediments and in biota was obtained using log–log regressions (Tables S3 and S4). In fact, log transformation can be used to reduce the leverage of extreme points and can help to normalize data distribution. Moreover, this model has been widely used to explore relations between concentrations in sediments and invertebrates and is easier to use within an environmental management framework than non-linear regressions [24,67,68,69].
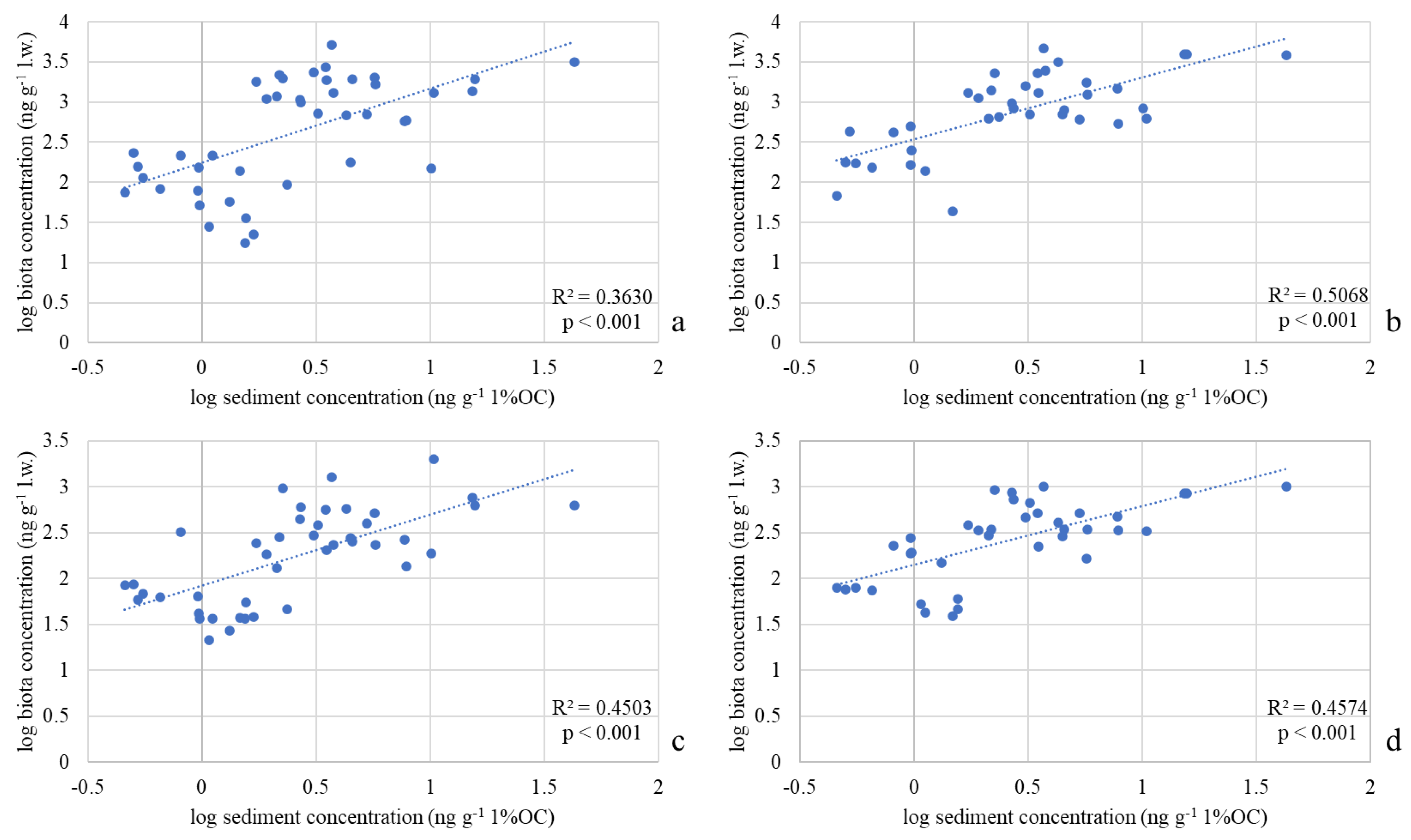
Significant correlations were found between OC-normalized DDx concentrations in sediments and lipid weight-normalized concentrations in Diptera (ρ = 0.60, p < 0.001), Gammaridae (ρ = 0.71, p < 0.001), Heptageniidae (ρ = 0.67, p < 0.001) and Baetidae (ρ = 0.67, p < 0.001), confirming that sediment concentration can be considered a predictor of DDx accumulation in all the analyzed organisms. In Figure 7 the resulting log–log linear regressions between OC-normalized DDx concentrations in sediments and lipid weight-normalized concentrations in the four taxa are shown in Figure 7.
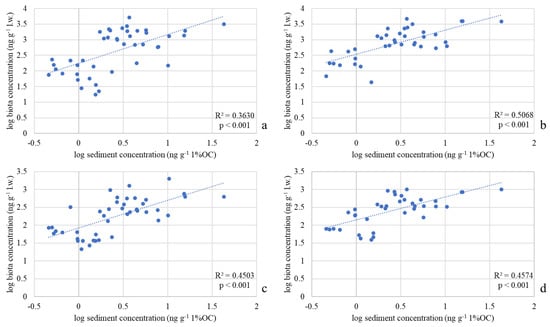
Figure 7.
Log-log regressions between DDx concentrations in the four invertebrate taxa collected in River Toce (ng g−1 l.w.) and the respective concentrations in sediments (ng g−1 1%OC): (a) Diptera; (b) Gammaridae; (c) Heptageniidae; (d) Baetidae. Coefficient of determination (R2) and p values are also reported.
The linear regression equations obtained for each taxon are:
where Log10[DDx]Diptera, Log10[DDx]Gammaridae, Log10[DDx]Heptageniidae and Log10[DDx]Baetidae are the log-transformed DDx concentrations in Diptera, Gammaridae, Heptageniidae and Baetidae (lipid weight normalized), respectively, and log10[DDx]Sed is the log-transformed organic carbon normalized DDx concentration measured in sediments.
Log10[DDx]Diptera = (0.924 ± 0.19) × log10[DDx]Sed + (2.243 ± 0.11),
Log10[DDx]Gammaridae = (0.769 ± 0.12) × log10[DDx]Sed + (2.540 ± 0.08),
Log10[DDx]Heptageniidae = (0.773 ± 0.133) × log10[DDx]Sed + (1.926 ± 0.080),
Log10[DDx]Baetidae = (0.641 ± 0.116) × log10[DDx]Sed + (2.146 ± 0.070),
According to the R2 values (Figure 7), Gammaridae is the taxon that best fits the model and therefore represents in this study the best indicator of DDT concentration in the Toce. However, the moderate R2 value suggests that the model should be improved for a more precise estimation of bioaccumulation. First of all, it must be considered that the point-measure of DDT in sediments may be not always representative of the exposure conditions experienced by the organisms during the previous months. Furthermore, the inclusion in the models of other environmental factors could enable the regression model to better explain the relationship between the two variables considered in the model itself. In fact, other variables could significantly affect DDT bioaccumulation, such as the hydrological regime of the river, conductivity, pH, the redox state at the water–sediment interface, the clay content in sediments, suspended material, the chemical–physical changes at the water–sediment interface, or the metabolic rate or life cycle of the organisms [22,23,31,40].
4. Conclusions
Our study illustrates how the legacy hydrophobic contaminant DDT shows strong persistence in aquatic environments, even several years after its ban. Peak DDT levels and isomer composition at the sites located downstream of the DDT production factory showed new inputs from dismissed industrial areas and the re-mobilization of contaminated sediments. Comparisons with SQGs, which could provide a screening assessment for this pollutant, evidenced exceedances with respect to the threshold values considered to be protective for aquatic ecosystems. The possible consequent transfer and metabolic transformation in aquatic organisms living in close association with sediments can be assessed through bioaccumulation analysis as in this study. Our results showed that, between the analyzed taxa, Gammaridae and Diptera were the organisms with the highest DDT concentrations and BSAF values especially for DDE. Integrating analysis of biological samples with sediment analysis can thus elucidate distribution, quantity and the potential ecological risk from organic pollutants in river ecosystems, together with their transfer into the biota compartment. Benthic invertebrates are suitable for this kind of evaluation, since they live in close association with sediments, perform different ecological functions, are prey for higher trophic levels and show high taxonomic richness. Therefore, analysis of different taxa together with analysis of the biomass of each studied taxon can provide an overview of pollutant distribution and transfer pathways to understand their relative contribution to the transfer of the pollutant to higher trophic levels. Finally, integrating data from analysis of sediment and benthic invertebrates may allow for the development of predictive models useful to fix remediation goals. After the selection of environmental variables that could affect the transfer of pollutants from the abiotic to the biotic compartment, such models may allow us to obtain the concentration of DDx in benthic macroinvertebrates starting from environmental variables which are easily measured, such as DDT concentrations in sediments. However, direct evaluation of bioaccumulation in aquatic organisms provides the most realistic evaluation of the risk at the basis of the riverine food webs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15086493/s1, Table S1: ecological information on the four invertebrate taxa analyzed; Table S2: Number of samples of each benthic invertebrate taxon at each station and sampling year analysed for DDT bioaccumulation; Table S3: Results of different non-linear fitting models between concentrations in sediments and biota (raw data); Table S4: Results of linear and non-linear fitting models be-tween concentrations in sediments and biota (log10-transfomed data); Figure S1: Boxplots of total DDx concentration of each taxon in every sampling site; Figure S2: Boxplots of BSAF values calculated on each DDx compounds in the considered invertebrates’ taxa; Figure S3: Relation be-tween DDx concentrations in sediments and BSAF values for different invertebrate taxa collected in River Toce; Figure S4: Relation between DDx concentrations in sediments and in the different invertebrate taxa collected in River Toce (raw data). References [31,32] are cited in Supplementary file.
Author Contributions
Conceptualization, S.T. and L.M.; data curation, S.T. and C.R.; investigation, C.R. and L.M.; formal analysis, S.T.; writing—original draft preparation, S.T.; writing—review and editing, C.R., L.M. and L.G.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the International Commission for the Protection of Italian-Swiss Waters (CIPAIS), Research Programs 2016–2018, 2019–2021; www.cipais.org (accessed on 1 December 2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the reports of the International Commission for the Protection of Italian-Swiss Waters (CIPAIS) regarding hazardous substances in Lake Maggiore (years 2016–2021), which are published and downloadable at www.cipais.org (accessed 13 January 2023).
Acknowledgments
We thank Susan Kastner for the revision of the english text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jaga, K.; Dharmani, C. Global Surveillance of DDT and DDE Levels in Human Tissues. Int. J. Occup. Med. Environ. Health 2003, 16, 7–20. [Google Scholar] [PubMed]
- Blanton, F.S. The Control of Epidemic Typhus. J. N. Y. Entomol. Soc. 1952, 60, 153–156. [Google Scholar]
- Li, Y.F.; Zhulidov, A.V.; Robarts, R.D.; Korotova, L.G.; Zhulidov, D.A.; Gurtovaya, T.Y.; Ge, L.P. Dichlorodiphenyltrichloroethane Usage in the Former Soviet Union. Sci. Total Environ. 2006, 357, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Omlin, F.X.; Carlson, J.C.; Ogbunugafor, C.B.; Hassanali, A. Anopheles Gambiae Exploits the Treehole Ecosystem in Western Kenya: A New Urban Malaria Risk? American Society of Tropical Medicine and Hygiene: Arlington, VA, USA, 2007. [Google Scholar]
- Beard, J. DDT and Human Health. Sci. Total Environ. 2006, 355, 78–89. [Google Scholar] [CrossRef]
- Jongbloed, R.H.; Traas, T.P.; Luttik, R. A Probabilistic Model for Deriving Soil Quality Criteria Based on Secondary Poisoning of Top Predators: II. Calculations for Dichlorodiphenyltrichloroethane (DDT) and Cadmium. Ecotoxicol. Environ. Saf. 1996, 34, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Nendza, M.; Herbst, T.; Kussatz, C.; Gies, A. Potential for Secondary Poisoning and Biomagnification in Marine Organisms. Chemosphere 1997, 35, 1875–1885. [Google Scholar] [CrossRef]
- Holm, L.; Blomqvist, A.; Brandt, I.; Brunström, B.; Ridderstråle, Y.; Berg, C. Embryonic Exposure to o,P′-DDT Causes Eggshell Thinning and Altered Shell Gland Carbonic Anhydrase Expression in the Domestic Hen. Environ. Toxicol. Chem. 2006, 25, 2787–2793. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Wiberg, K.; Ahrens, L.; Zubcov, E.; Dahlberg, A.-K. Spatial Distribution of Legacy Pesticides in River Sediment from the Republic of Moldova. Chemosphere 2021, 279, 130923. [Google Scholar] [CrossRef] [PubMed]
- Karickhoff, S.W.; Brown, D.S.; Scott, T.A. Sorption of Hydrophobic Pollutants on Natural Sediments. Water Res. 1979, 13, 241–248. [Google Scholar] [CrossRef]
- Liber, Y.; Mourier, B.; Marchand, P.; Bichon, E.; Perrodin, Y.; Bedell, J.-P. Past and Recent State of Sediment Contamination by Persistent Organic Pollutants (POPs) in the Rhône River: Overview of Ecotoxicological Implications. Sci. Total Environ. 2019, 646, 1037–1046. [Google Scholar] [CrossRef]
- Zoumis, T.; Schmidt, A.; Grigorova, L.; Calmano, W. Contaminants in Sediments: Remobilisation and Demobilisation. Sci. Total Environ. 2001, 266, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Nfon, E.; Cousins, I.T.; Broman, D. Biomagnification of Organic Pollutants in Benthic and Pelagic Marine Food Chains from the Baltic Sea. Sci. Total Environ. 2008, 397, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Binelli, A.; Provini, A. DDT Is Still a Problem in Developed Countries: The Heavy Pollution of Lake Maggiore. Chemosphere 2003, 52, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Bettinetti, R.; Croce, V.; Galassi, S. Ecological Risk Assessment for the Recent Case of DDT Pollution in Lake Maggiore (Northern Italy). Water Air Soil Pollut. 2005, 162, 385–399. [Google Scholar] [CrossRef]
- Pisanello, F.; Marziali, L.; Rosignoli, F.; Poma, G.; Roscioli, C.; Pozzoni, F.; Guzzella, L. In Situ Bioavailability of DDT and Hg in Sediments of the Toce River (Lake Maggiore Basin, Northern Italy): Accumulation in Benthic Invertebrates and Passive Samplers. Environ. Sci. Pollut. Res. 2016, 23, 10542–10555. [Google Scholar] [CrossRef] [PubMed]
- Marziali, L.; Rosignoli, F.; Drago, A.; Pascariello, S.; Valsecchi, L.; Rossaro, B.; Guzzella, L. Toxicity Risk Assessment of Mercury, DDT and Arsenic Legacy Pollution in Sediments: A Triad Approach under Low Concentration Conditions. Sci. Total Environ. 2017, 593–594, 809–821. [Google Scholar] [CrossRef]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef]
- Xu, Y.; Spurlock, F.; Wang, Z.; Gan, J. Comparison of Five Methods for Measuring Sediment Toxicity of Hydrophobic Contaminants. Environ. Sci. Technol. 2007, 41, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Maruya, K.A.; Lao, W.; Tsukada, D.; Diehl, D.W. A Passive Sampler Based on Solid Phase Microextraction (SPME) for Sediment-Associated Organic Pollutants: Comparing Freely-Dissolved Concentration with Bioaccumulation. Chemosphere 2015, 137, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Pereira, M.G.; Tyler, C.R.; Ormerod, S.J. River Organisms as Indicators of the Distribution and Sources of Persistent Organic Pollutants in Contrasting Catchments. Environ. Pollut. 2019, 255, 113144. [Google Scholar] [CrossRef]
- Verhaert, V.; Newmark, N.; D’Hollander, W.; Covaci, A.; Vlok, W.; Wepener, V.; Addo-Bediako, A.; Jooste, A.; Teuchies, J.; Blust, R.; et al. Persistent Organic Pollutants in the Olifants River Basin, South Africa: Bioaccumulation and Trophic Transfer through a Subtropical Aquatic Food Web. Sci. Total Environ. 2017, 586, 792–806. [Google Scholar] [CrossRef]
- Govaerts, A.; Verhaert, V.; Covaci, A.; Jaspers, V.L.; Berg, O.K.; Addo-Bediako, A.; Jooste, A.; Bervoets, L. Distribution and Bioaccumulation of POPs and Mercury in the Ga-Selati River (South Africa) and the Rivers Gudbrandsdalslågen and Rena (Norway). Environ. Int. 2018, 121, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.S.; Capel, P.D.; Nowell, L.H. National-Scale, Field-Based Evaluation of the Biota−Sediment Accumulation Factor Model. Environ. Sci. Technol. 2001, 35, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- de la Cal, A.; Eljarrat, E.; Raldúa, D.; Durán, C.; Barceló, D. Spatial Variation of DDT and Its Metabolites in Fish and Sediment from Cinca River, a Tributary of Ebro River (Spain). Chemosphere 2008, 70, 1182–1189. [Google Scholar] [CrossRef]
- Tomza-Marciniak, A.; Witczak, A. Bioaccumulation of DDT and Its Metabolites in the Międzyodrze Ecosystem, Poland. Pol. J. Environ. Stud. 2009, 18, 467–474. [Google Scholar]
- Choi, J.Y.; Yang, D.B.; Hong, G.H.; Shin, K.H. Distribution and Bioaccumulation of Polychlorinated Biphenyls and Organochlorine Pesticides Residues in Sediments and Manila Clams (Ruditapes Philippinarum) from along the Mid-Western Coast of Korea. Mar. Pollut. Bull. 2014, 85, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Marziali, L.; Guzzella, L.; Salerno, F.; Marchetto, A.; Valsecchi, L.; Tasselli, S.; Roscioli, C.; Schiavon, A. Twenty-Year Sediment Contamination Trends in Some Tributaries of Lake Maggiore (Northern Italy): Relation with Anthropogenic Factors. Environ. Sci. Pollut. Res. 2021, 28, 38193–38208. [Google Scholar] [CrossRef] [PubMed]
- Ceschi, M.; De Rossa, M.; Jäggli, M. Contaminanti Organici, Inorganici e Radionuclidi Nell’ittiofauna Dei Laghi Ceresio e Verbano (Bacini Svizzeri). Mitt. Geb. Lebensm. Hyg. 1996, 87, 189–211. [Google Scholar]
- Ravazzani, G.; Dalla Valle, F.; Gaudard, L.; Mendlik, T.; Gobiet, A.; Mancini, M. Assessing Climate Impacts on Hydropower Production: The Case of the Toce River Basin. Climate 2016, 4, 16. [Google Scholar] [CrossRef]
- Marziali, L.; Roscioli, C.; Valsecchi, L. Mercury Bioaccumulation in Benthic Invertebrates: From Riverine Sediments to Higher Trophic Levels. Toxics 2021, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Gerner, N.V.; Koné, M.; Ross, M.S.; Pereira, A.; Ulrich, A.C.; Martin, J.W.; Liess, M. Stream Invertebrate Community Structure at Canadian Oil Sands Development Is Linked to Concentration of Bitumen-Derived Contaminants. Sci. Total Environ. 2017, 575, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; U.S. Environmental Protection Agency: Washington, DC, USA, 2002. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=63371 (accessed on 17 March 2023).
- Pérez-Maldonado, I.N.; Trejo, A.; Ruepert, C.; del Carmen Jovel, R.; Méndez, M.P.; Ferrari, M.; Saballos-Sobalvarro, E.; Alexander, C.; Yáñez-Estrada, L.; Lopez, D.; et al. Assessment of DDT Levels in Selected Environmental Media and Biological Samples from Mexico and Central America. Chemosphere 2010, 78, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Sudaryanto, A.; Isobe, T.; Takahashi, S.; Tanabe, S. Assessment of Persistent Organic Pollutants in Sediments from Lower Mekong River Basin. Chemosphere 2011, 82, 679–686. [Google Scholar] [CrossRef]
- Hitch, R.K.; Day, H.R. Unusual Persistence of DDT in Some Western USA Soils. Bull. Environ. Contam. Toxicol. 1992, 48, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, L. Estimation of Biota Sediment Accumulation Factor (BSAF) from Paired Observations of Chemical Concentrations in Biota and Sediment; U.S. Environmental Protection Agency, Ecological Risk Assessment Support Center: Cincinnati, OH, USA, 2006. [Google Scholar]
- Bizzotto, E.C.; Villa, S.; Vighi, M. POP Bioaccumulation in Macroinvertebrates of Alpine Freshwater Systems. Environ. Pollut. 2009, 157, 3192–3198. [Google Scholar] [CrossRef]
- International Commission for the Protection of the Italian-Swiss Waters (CIPAIS) Indagini Sulle Sostanze Pericolose Nell’ecosistema del Lago Maggiore; Programma 2016–2018; Rapporto Annuale 2018; CIPAIS: Verbania, Italy. 2018. Available online: https://www.cipais.org/ (accessed on 18 January 2023).
- Van Ael, E.; Covaci, A.; Blust, R.; Bervoets, L. Persistent Organic Pollutants in the Scheldt Estuary: Environmental Distribution and Bioaccumulation. Environ. Int. 2012, 48, 17–27. [Google Scholar] [CrossRef]
- Verhaert, V.; Covaci, A.; Bouillon, S.; Abrantes, K.; Musibono, D.; Bervoets, L.; Verheyen, E.; Blust, R. Baseline Levels and Trophic Transfer of Persistent Organic Pollutants in Sediments and Biota from the Congo River Basin (DR Congo). Environ. Int. 2013, 59, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Yu, G.; Hong, H. Occurrence of PAHs, PCBs and Organochlorine Pesticides in the Tonghui River of Beijing, China. Environ. Pollut. 2004, 130, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.A.; Alonso, C.; González, M.J.; Hernández, L.M. Occurrence of Organochlorine Insecticides, PCBs and PCB Congeners in Waters and Sediments of the Ebro River (Spain). Chemosphere 1999, 38, 33–43. [Google Scholar] [CrossRef]
- Quinn, L.; Pieters, R.; Nieuwoudt, C.; Borgen, A.R.; Kylin, H.; Bouwman, H. Distribution Profiles of Selected Organic Pollutants in Soils and Sediments of Industrial, Residential and Agricultural Areas of South Africa. J. Environ. Monit. 2009, 11, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Neamtu, M.; Ciumasu, I.M.; Costica, N.; Costica, M.; Bobu, M.; Nicoara, M.N.; Catrinescu, C.; van Slooten, K.B.; De Alencastro, L.F. Chemical, Biological, and Ecotoxicological Assessment of Pesticides and Persistent Organic Pollutants in the Bahlui River, Romania. Environ. Sci. Pollut. Res. 2009, 16, 76–85. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L.; Kong, Q. Persistent Chlorinated Pesticides in Fish Species from Qiantang River in East China. Chemosphere 2007, 68, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.M.; Konovets, I.M.; Kipnis, L.S.; Lyashenko, A.V.; Grintsov, V.A.; Petrov, A.N.; Terletskaya, A.V.; Milyukin, M.V.; Povolotskii, M.I.; Demchenko, V.Y.; et al. Distribution, Magnitude and Characterization of the Toxicity of Ukrainian Estuarine Sediments. Mar. Pollut. Bull. 2011, 62, 2442–2462. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Hu, Z.; Zhang, G.; Li, X.; Xu, W.; Tang, J.; Li, J. Levels and Mass Burden of DDTs in Sediments from Fishing Harbors: The Importance of DDT-Containing Antifouling Paint to the Coastal Environment of China. Environ. Sci. Technol. 2009, 43, 8033–8038. [Google Scholar] [CrossRef]
- DDT and Its Derivatives: Environmental Aspects; International Programme on Chemical Safety, UNEP, Weltgesundheitsorganisation, Internationale Arbeitsorganisation; Environmental Health Criteria; World Health Organization: Geneva, Switzerland, 1989; ISBN 978-92-4-154283-8.
- Lacorte, S.; Raldúa, D.; Martínez, E.; Navarro, A.; Diez, S.; Bayona, J.M.; Barceló, D. Pilot Survey of a Broad Range of Priority Pollutants in Sediment and Fish from the Ebro River Basin (NE Spain). Environ. Pollut. 2006, 140, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, T.; Khim, J.S.; Luo, W.; Jiao, W.; Lu, Y.; Naile, J.E.; Chen, C.; Zhang, X.; Giesy, J.P. HCH and DDT in Sediments from Marine and Adjacent Riverine Areas of North Bohai Sea, China. Arch. Environ. Contam. Toxicol. 2010, 59, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Feiler, U.; Höss, S.; Ahlf, W.; Gilberg, D.; Hammers-Wirtz, M.; Hollert, H.; Meller, M.; Neumann-Hensel, H.; Ottermanns, R.; Seiler, T.-B.; et al. Sediment Contact Tests as a Tool for the Assessment of Sediment Quality in German Waters. Environ. Toxicol. Chem. 2013, 32, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D. Food Chain Concentration of Chlorinated Hydrocarbon Pesticides in Invertebrate Communities: A Re-Evaluation. Quaest. Entomol. 1975, 11, 97–110. [Google Scholar]
- Leppänen, M. The Role of Feeding Behaviour in Bioaccumulation of Organic Chemicals in Benthic Organisms. Ann. Zool. Fenn. 1995, 32, 247–255. [Google Scholar]
- Sidney, L.A.; Diepens, N.J.; Guo, X.; Koelmans, A.A. Trait-Based Modelling of Bioaccumulation by Freshwater Benthic Invertebrates. Aquat. Toxicol. 2016, 176, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.G.; Cutkomp, L.K. Correlation between the Possession of a Chitinous Cuticle and Sensitivity to DDT. Biol. Bull. 1946, 90, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Lord, K.A. The Sorption of DDT and Its Analogues by Chitin. Biochem. J. 1948, 43, 72–78. [Google Scholar] [CrossRef]
- Tran, V.S.; Ngo, H.H.; Guo, W.; Zhang, J.; Liang, S.; Ton-That, C.; Zhang, X. Typical Low Cost Biosorbents for Adsorptive Removal of Specific Organic Pollutants from Water. Bioresour. Technol. 2015, 182, 353–363. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhan, J.; Costa, N. Use of Shell Chitin Extracted from Seafood Processing Waste in Recycling of Industrial Wastewater. In Proceedings of the Environmentally Conscious Manufacturing, Boston, MA, USA, 6–8 November 2000; SPIE: Washington, DC, USA, 2001; Volume 4193, pp. 403–412. [Google Scholar]
- Wang, Y.; He, W.; Qin, N.; He, Q.-S.; Kong, X.-Z.; Tao, S.; Xu, F.-L. Distributions, Sources, and Ecological Risks of DDT-Related Contaminants in Water, Suspended Particulate Matter, and Sediments from Haihe Plain, Northern China. Environ. Monit. Assess. 2013, 185, 1777–1790. [Google Scholar] [CrossRef]
- Deribe, E.; Rosseland, B.O.; Borgstrøm, R.; Salbu, B.; Gebremariam, Z.; Dadebo, E.; Skipperud, L.; Eklo, O.M. Biomagnification of DDT and Its Metabolites in Four Fish Species of a Tropical Lake. Ecotoxicol. Environ. Saf. 2013, 95, 10–18. [Google Scholar] [CrossRef] [PubMed]
- CIPAIS. International Commission for the Protection of the Italian-Swiss Waters (CIPAIS) Indagini Sulle Sostanze Pericolose Nell’ecosistema del Lago Maggiore; Programma 2022–2024; Rapporto Annuale 2022; CIPAIS: Verbania, Italy, 2022; Available online: https://www.cipais.org/ (accessed on 18 January 2023).
- Van Praet, N.; Covaci, A.; Teuchies, J.; De Bruyn, L.; Van Gossum, H.; Stoks, R.; Bervoets, L. Levels of Persistent Organic Pollutants in Larvae of the Damselfly Ischnura Elegans (Odonata, Coenagrionidae) from Different Ponds in Flanders, Belgium. Sci. Total Environ. 2012, 423, 162–167. [Google Scholar] [CrossRef]
- Norén, K.; Meironyté, D. Certain Organochlorine and Organobromine Contaminants in Swedish Human Milk in Perspective of Past 20–30 Years. Chemosphere 2000, 40, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Voets, J.; Covaci, A.; Chu, S.; Qadah, D.; Smolders, R.; Schepens, P.; Blust, R. Use of Transplanted Zebra Mussels (Dreissena Polymorpha) to Assess the Bioavailability of Microcontaminants in Flemish Surface Waters. Environ. Sci. Technol. 2005, 39, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- McGeer, J.C.; Brix, K.V.; Skeaff, J.M.; DeForest, D.K.; Brigham, S.I.; Adams, W.J.; Green, A. Inverse Relationship between Bioconcentration Factor and Exposure Concentration for Metals: Implications for Hazard Assessment of Metals in the Aquatic Environment. Environ. Toxicol. Chem. 2003, 22, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.; Silke, R.; Carr, J. Biota-Sediment Accumulation Factors (BSAF) for Radionuclides and Sediment Associated Biota of the Ottawa River. AECL Nucl. Rev. 2013, 2, 3–15. [Google Scholar] [CrossRef]
- Landrum, P.F.; Robinson, S.D.; Gossiaux, D.C.; You, J.; Lydy, M.J.; Mitra, S.; ten Hulscher, T.E.M. Predicting Bioavailability of Sediment-Associated Organic Contaminants for Diporeia spp. and Oligochaetes. Environ. Sci. Technol. 2007, 41, 6442–6447. [Google Scholar] [CrossRef] [PubMed]
- Melwani, A.R.; Greenfield, B.K.; Byron, E.R. Empirical Estimation of Biota Exposure Range for Calculation of Bioaccumulation Parameters. Integr. Environ. Assess. Manag. 2009, 5, 138–149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).