Abstract
The increase in the atmospheric carbon dioxide concentration promotes its accumulation in trees by regulating the synthesis and transportation genes for endogenous hormones, such as IAA and GA, which are key factors in regulating various life activities, including growth rings. To explore the impact of changes in endogenous hormone levels such as IAA and GA on the growth of tree rings, and to provide a basis for improving the management of hybrid larch clonal forests, we investigated the effects of exogenous indole-3-acetic acid (IAA), gibberellic acid 3 (GA3), and their combination on tree-ring growth in hybrid larch. IAA, GA3, and a combination treatment were sprayed on the leaves of one clone of a hybrid larch seedling every three days. Small blocks were collected at the base stems for sequential anatomical observations. The phytohormone type, instead of the concentration, had a more significant effect on wood formation. Specifically, IAA treatment at 300 mg L−1 significantly increased latewood (LW) layers until 90 days after treatment (DAT). The 500 mg L−1 treatment significantly increased the wall radial thickness (WRT) of latewood (LW) cells. GA3 treatment at 100 mg L−1 significantly decreased the layers and width of total wood (TW), LW, and earlywood (EW). The 300 mg L−1 treatment significantly increased the WRT of EW. The IAA 100 mg L−1 + GA3 100 mg L−1 combination treatment significantly increased the layers and width of TW and LW by inducing cambium activity and increasing the rate of wood cell development. The WRT and lumen radial diameter (LRD) of EW or LW in this treatment were similar to those observed with the corresponding single phytohormone treatment. These results indicate that combination treatment at 100 mg L−1 + 100 mg L−1 was a better way to promote tree-ring growth. Our study suggests that changes in phytohormone levels and ratios are important factors that affect tree-ring formation. Hormone levels and ratios should be regarded as important indicators to guide the improvement of management practices in hybrid larch clonal plantations.
1. Introduction
The increasing CO2 (eCO2) in the atmosphere affects stomatal closure through the independent signal transduction pathway [1] or mediation by other phytohormone signaling transduction pathways [2]. Increasing CO2 can promote the accumulation of indole-3-acetic acid (IAA) and gibberellins (GAs), which regulate plant growth and development by promoting the expression of the auxin transporting gene LAX [3], IAA biosynthetic gene FZY [4], and GA biosynthetic gene GGPPS [5]. Specifically, eCO2 was shown to increase IAA content to 3 μg g−1 in Ginkgo biloba L. [6], which was higher than that in the control of 2 μg g−1. In Arabidopsis thaliana [7], the concentration of IAA and gibberellin A3 (GA3) increased from 31.27 ± 2.87 ng g−1 fresh weight (FW) and 211.05 ± 17.73 ng g−1 FW to 35.56 ± 2.97 and 327.96 ± 30.81 ng g−1 FW, respectively. In addition, in response to biotic and abiotic stresses, eCO2 promotes the accumulation of corresponding phytohormones such as ethylene [8], jasmonic acid, and salicylic acid [5,9,10,11]. Furthermore, it has been proven that IAA and GA are two of the most important phytohormones regulating tree-ring growth [12,13].
The most common auxin in most plants is indole-3-acetic acid (IAA). It affects many plant activities, including vascular development, cambium activity [14,15], and tree-ring growth [16,17]. IAA is the most studied plant hormone [18], and a clear pattern of its regulation of tree-ring growth has been established. In the early part of the growing season, the supply of IAA from young leaves and leaf primordia to the cambium is necessary for the production of xylem cells [19]. When the endogenous IAA level and the cambial growth rate reach the maximum, the xylem experiences its highest period of activity [20]. Endogenous IAA levels, after peaking when cambial and xylem differentiation are most active, decrease before latewood formation and then change only slightly until the end of the growing season and through cambial dormancy [20,21]. In gymnosperms, latewood formation is linked to a steeper IAA distribution gradient in the cambial and differentiating cells. Many experiments have also confirmed that IAA can induce cambium proliferation and xylem production. This pattern has been linked to a decrease in cell division and differentiation [20].
Previous studies have shown that gibberellins (gibberellic acids, GAs) also affect tree-ring growth [22,23,24,25,26]. GAs are mainly distributed in the differentiating xylem cells in the stem of trees [27]. Studies have shown that GAs, like IAA, also affect the cambium division in the cambial zone but do not affect xylogenesis, resulting in the apparent loss of an easily distinguished cambial meristem. Possibly the auxin deficiency in these stems results in de-differentiation and loss of meristem identity of the newly formed cells. GA3 application treatment also leads to wider xylem development and higher lignin content than controls [28,29]. These results indicate that GAs may mainly be involved in xylem differentiation and secondary cell wall (SCW) deposition in tree-ring formation. Furthermore, the distribution pattern has shown that IAA and GAs overlap in developing xylem cells during tree-ring growth [18]. In angiosperms, IAA with GA3 promotes more wood formation than either individually [30].
In conifers, few studies have reported the effects of auxin and GAs together on tree-ring growth (earlywood and latewood). Only Wang has reported that GA4/7 and IAA together, applied to the decapitated and defoliated current-year shoots of Pinus sylvestris increased shoot elongation, xylem, and phloem production, and IAA concentration in the early part of the growing season [31]. However, the effects of combination treatment with auxin and GAs on tree-ring growth and latewood formation are not completely clear yet in conifers. Larch is widely distributed in the northeast of Eurasia and is one of the four major afforestation species in China. Its wood can be used for construction and paper making. In this study, in order to investigate the effects of phytohormone levels such as IAA and GA3 together on tree-ring growth, especially in respect of latewood formation, we used a clone of 3-year-old hybrid larch as the material and conducted experimental treatments with exogenous IAA and GA3 to clarify the changes in tree-ring growth under single or combined hormone treatments. This study will provide a theoretical basis and application measures for improving the management model of larch plantations from the aspect of phytohormones.
2. Materials and Methods
2.1. Plant Material
Three-year-old hybrid larch trees (Larix Kaempferi 302 × Larix olgensis 23 8 –14) were grown in the field of Da Gu Jia Town, Liaoning Province, China, and were used in the present study. The growth medium was a sterile mixture of peat:perlite (2.5:1) in an adhesively bonded fabric planting bag of 20 cm × 25 cm (height × diameter).
2.2. Experimental Design and Phytohormone Treatments
2.2.1. Experimental Design
Ten treatments were performed: IAA (100 mg L−1, 300 mg L−1, and 500 mg L−1), IAA + GA3 (100 mg L−1 + 100 mg L−1, 300 mg L−1 + 100 mg L−1, and 500 mg L−1 + 100 mg L−1), GA3 (100 mg L−1, 300 mg L−1, and 500 mg L−1), and distilled water as the control (CK). One hundred and twenty seedlings in total were divided into three replicates with forty seedlings per replicate. Four seedlings per treatment per replicate were treated, and all ten treatments were ordered randomly per replicate. All treated seedlings were kept in Da Gu Jia Town, Liaoning Province, China, with the same growing conditions of natural photoperiod and temperature.
2.2.2. Phytohormone Treatment
Indole-3-acetic acid (Genview, Beijing, China) and gibberellin A3 (Shanghai Ryon Biological Technology Co., Ltd., Shanghai, China) were dissolved in a small amount of 99% ethanol, then dissolved in distilled water with 0.05% Tween-20 (Sigma-Aldrich, Shanghai, China) to the corresponding concentration for every treatment. Seedlings were sprayed every 3 days with 4 mL of the mixture from 20 May to 21 September 2019. During the spraying treatments, the leaves of each seedling were covered with hormone solution to form droplets to ensure that each seedling could absorb the hormone solution in every treatment.
2.3. Collection of Samples
One block of about 0.3 cm3 was collected and fixed in 4% paraformaldehyde (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) from the base stem (2 cm above medium) of one seedling per treatment per replicate from 20 June (30 days after treatment: 30DAT) to 21 September, with an interval of 30 days. Additionally, one block was collected from each of three seedlings (i.e., three in total), on 20 May (day 0) to confirm the stage of tree-ring growth at the beginning of the experiment.
2.4. Anatomical Observations
For anatomical observations, the blocks were dehydrated through an ethanol series (ethanol:distilled water) with 75% (v/v), 90% (v/v), and pure ethanol, and then embedded into Spurr’s resin (Low Viscosity Embedding Media Spurr’s kit, Garfield Ave., West Chester, PA, USA) with ethanol in a series of 25% (v/v), 50% (v/v), and 75% (v/v). Each concentration of ethanol and resin was applied for two hours. Then the blocks were treated with pure resin three times for twelve hours at a time. Transverse sections about 4 μm thick were cut using a Leica microtome (LECICA EM UC7, Wetzlar, Germany), spread on a slide with fine tweezers, stained with 0.25% (w/v) toluidine blue O (Sigma, Shanghai, China) and observed under a microscope (Carl Zeiss Microimaging GmbH37081, Oberkochen, Germany).
2.5. Anatomical Measurements of Cell Morphology and Statistical Analysis
From the growth ring boundary formed in the last year toward the cambial zone, cells of three radial lines, selected randomly from the middle and both sides of one sample, for nine radial lines from three samples in total, were analyzed under every treatment. The layers of total wood (TW), earlywood (EW), latewood (LW), and cambium were collected on 20 May (0 days after treatment: 0 DAT), 27 June (30 DAT), 23 July (60 DAT), 24 August (90 DAT), and 21 September (120 DAT). The transverse sections of samples collected at 120 DAT were further measured and analyzed, including the width of the newly formed xylem (total wood), EW, and LW; the wall radial thickness (WRT); and the lumen radial diameter (LRD) of xylem cells using image analysis software (Image J; National Institutes of Health, Bethesda, MD, USA). Then, the ratio of LW to total wood (TW) was calculated. Each statistical analysis was performed using IBM SPSS Statistics 19.0 (IBM, Armonk, NY, USA). The data were analyzed using Ducan’s multiple-range test (p < 0.05) in triplicate. The wood cell development pattern was analyzed using the Gompertz function [32].
3. Results
3.1. ANOVA Analysis Revealed That IAA + GA3 Treatment Was a More Efficient Way to Promote Tree-Ring Growth
ANOVA analysis showed that the type of phytohormone significantly affected growth and tree-ring growth in hybrid larch seedlings. Indole-3-acetic acid (IAA) treatments only significantly decreased the earlywood layers (EWLs). Gibberellin A3 (GA3) treatments significantly increased the increment of height (IH) but significantly decreased the total wood layers (TWLs), EWLs, and latewood layers (LWLs). Combination treatments significantly increased the increment of base stem diameter (IBD), TWLs, LWLs, and cambium layers (CLs), but the survival rate (SR) was significantly decreased. The concentration of phytohormones significantly affected all of the phenotypes except TWL and LWL. A low concentration (100 mg L−1 in IAA or GA3 treatment and 100 mg L−1 + 100 mg L−1 in combination treatment) significantly increased IH and IBD, but significantly decreased EWLs. A medium concentration (300 mg L−1 in IAA or GA3 treatment and 300 mg L−1 + 100 mg L−1 in combination treatment) significantly increased IH and CLs but significantly decreased EWLs. A high concentration (500 mg L−1 in IAA or GA3 treatment and 500 mg L−1 + 100 mg L−1 in combination treatment) significantly increased CLs but significantly decreased the SR (Table 1).

Table 1.
ANOVA results for phenotypes under different phytohormone treatments.
3.2. Tree-Ring Growth under Different Phytohormone Treatments
3.2.1. Development of Total Wood Layers
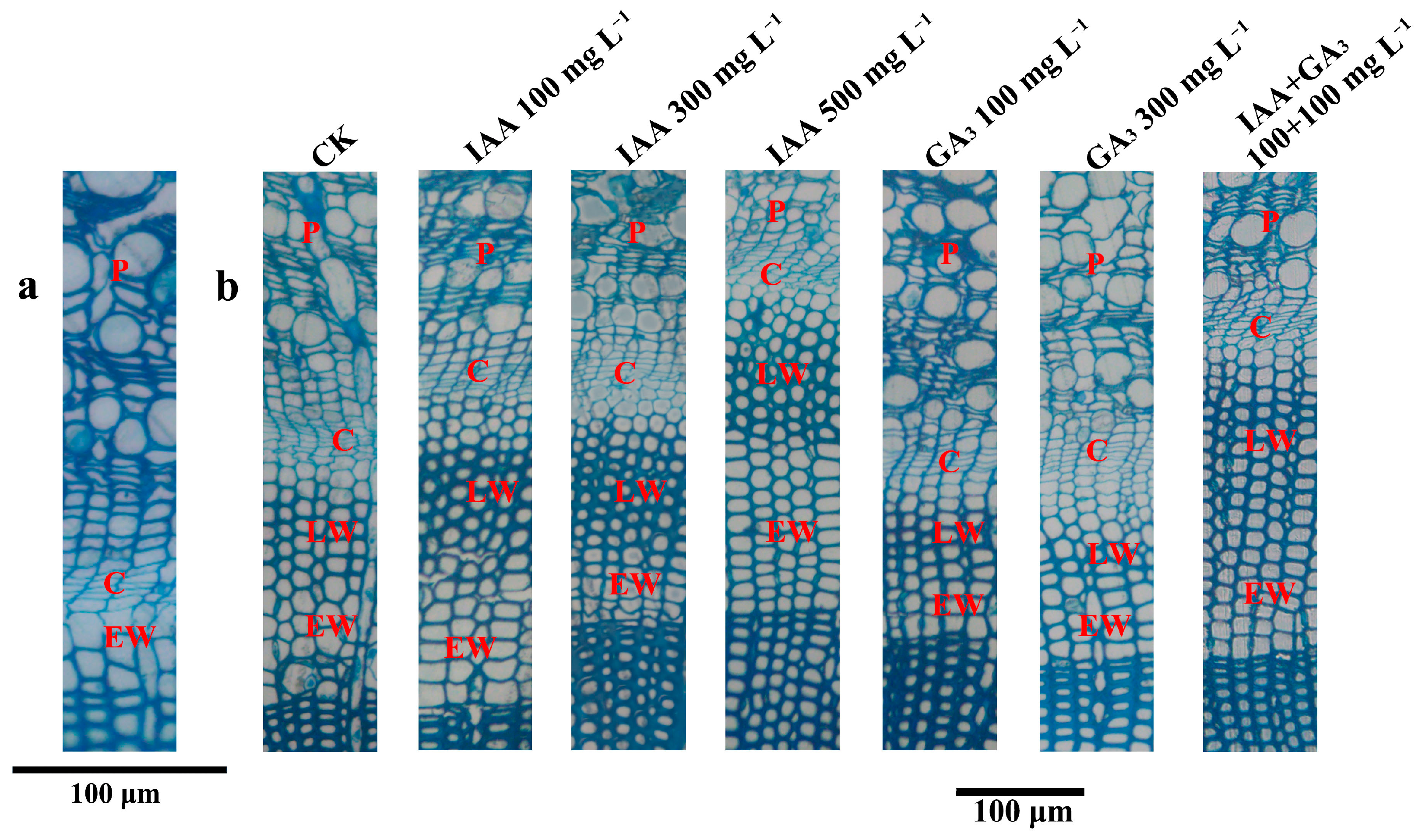
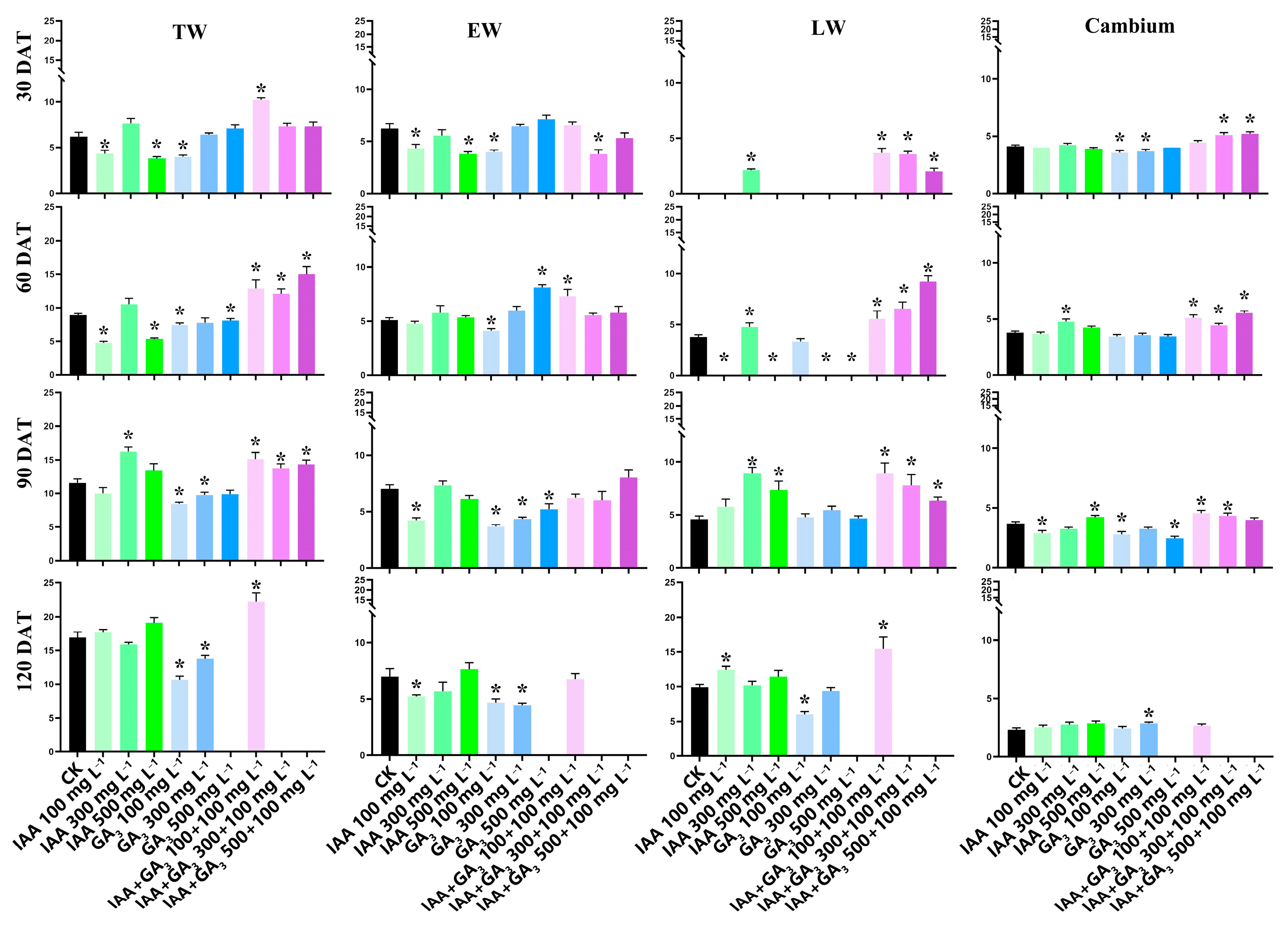
About three to four layers of newly formed xylem cells (total wood layers) and about five layers of cambium cells were detected at 0 days after treatment (DAT) (Figure 1a). At 30 DAT, IAA treatments with 100 mg L−1 and 500 mg L−1 significantly decreased the TWLs. The TWLs were also significantly decreased at GA3 treatment with 100 mg·L−1. Combination treatment at 100 mg L−1 + 100 mg L−1 significantly increased the TWLs. At 60 DAT, both IAA treatments and GA3 treatments at 100 mg L−1 and 500 mg L−1 significantly decreased the TWLs. All the combination treatments (100 mg L−1 + 100 mg L−1, 300 mg L−1 + 100 mg L−1, and 500 mg L−1 + 100 mg L−1) significantly increased the TWLs. At 90 DAT, IAA treatment at 300 mg L−1 significantly increased the TWLs. GA3 treatments at 100 mg L−1 and 300 mg L−1 significantly decreased the TWLs. All three combination treatments significantly increased the TWLs. At 120 DAT, IAA treatments had no effect on the TWLs. GA3 treatment at 100 mg L−1 and 300 mg L−1 significantly decreased the TWLs. The combination treatment at 100 mg L−1 + 100 mg L−1 significantly increased the TWLs.
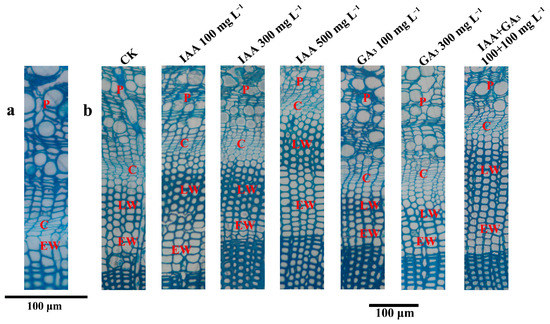
Figure 1.
Transverse sections of hybrid larch at 0 DAT and 120 DAT. (a) Transverse section at 0 DAT. (b) Transverse sections at 120 DAT. P: phloem; C: cambium; EW: earlywood; LW: latewood.
3.2.2. Development of Earlywood Layers
For EWLs, at 30 DAT, IAA treatments at 100 mg L−1 and 500 mg L−1 significantly decreased the EWLs. GA3 treatment at 100 mg L−1 significantly decreased the EWLs. Similarly, the EWLs were significantly decreased by combination treatment with 300 mg L−1 + 100 mg L−1. At 60 DAT, IAA treatments had no significant effect on the EWLs. The EWLs were significantly decreased by GA3 treatment at 100 mg L−1 but significantly increased at 500 mg L−1. The combination treatment at 100 mg L−1 + 100 mg L−1 significantly increased the EWLs. At 90 DAT, IAA treatment at 100 mg L−1 significantly decreased the EWLs. All three GA3 treatments significantly decreased the EWLs. The combination treatments had no significant effect on EWLs. At 120 DAT, IAA treatment at 100 mg L−1 significantly decreased the EWLs. GA3 treatments at 100 mg L−1 and 300 mg L−1 significantly decreased the EWLs. There was no significant difference between the combination treatment at 100 mg L−1 + 100 mg L−1 and CK.
3.2.3. Development of Latewood Layers
For LWLs, at 30 DAT, IAA treatment at 300 mg L−1 significantly increased LW layers (LWLs). No LW was found in GA3 treatments. The LWLs were significantly increased by all three combination treatments. At 60 DAT, IAA treatment at 300 mg L−1 significantly increased the LWLs, while no LW was found in the other two IAA treatments. There was no significant difference between GA3 treatment at 100 mg L−1 and CK. All three combination treatments significantly increased the LWLs. At 90 DAT, IAA treatments at 300 mg L−1 and 500 mg L−1 significantly increased the LWLs. GA3 treatments had no effect on LWLs. All three combination treatments significantly increased LWLs. At 120 DAT, IAA treatment at 100 mg L−1 significantly increased the LWLs. GA3 treatment at 100 mg L−1 significantly decreased the LWLs. Combination treatment at 100 mg L−1 + 100 mg L−1 significantly increased the LWLs.
3.2.4. Development of Cambium Layers
At 30 DAT, the cambium layers (CLs) were similar at all three concentrations of IAA treatment. The CLs were significantly decreased by GA3 treatments at 100 mg L−1 and 300 mg L−1. Combination treatments with 300 mg L−1 + 100 mg L−1 and 500 mg L−1 + 100 mg L−1 significantly increased the CLs. At 60 DAT, IAA treatment at 300 mg L−1 significantly increased the CLs. There was no significant difference between GA3 treatments and CK. All three combination treatments significantly increased the CLs. At 90 DAT, IAA treatments at a low concentration (100 mg L−1) significantly decreased the CLs, while a high concentration (500 mg L−1) significantly increased the CLs. GA3 treatments at 100 mg L−1 and 500 mg L−1 significantly decreased the CLs. Combination treatments at 100 mg L−1 + 100 mg L−1 and 300 mg L−1 + 100 mg L−1 significantly increased the CLs. At 120 DAT, only 2–3 cambium layers were detected in all of the treatments and CK. Combination treatments and IAA treatments had no effect on the CLs, but GA3 treatment at 300 mg L−1 significantly increased the CLs (Figure 1b and Figure 2).
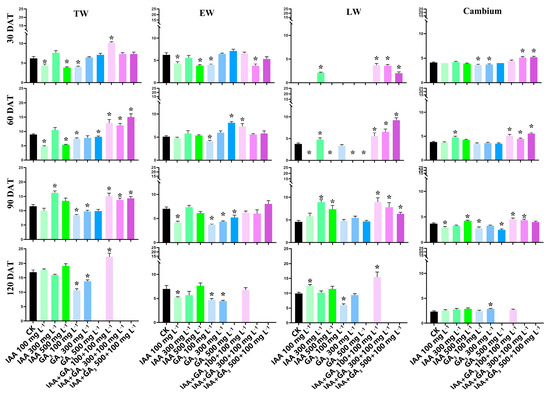
Figure 2.
Calculation of total wood layers, earlywood layers, latewood layers, and cambium layers during the experiment. The T-test was used, and the least significant range analysis at 5% significance is shown with * at the same stage for the control and treatments. All data were calculated in triplicate.
3.3. Calculation of Wood Cell Morphology under Different Phytohormone Treatments
3.3.1. Calculation of Newly Formed Wood Width, including Latewood and Earlywood
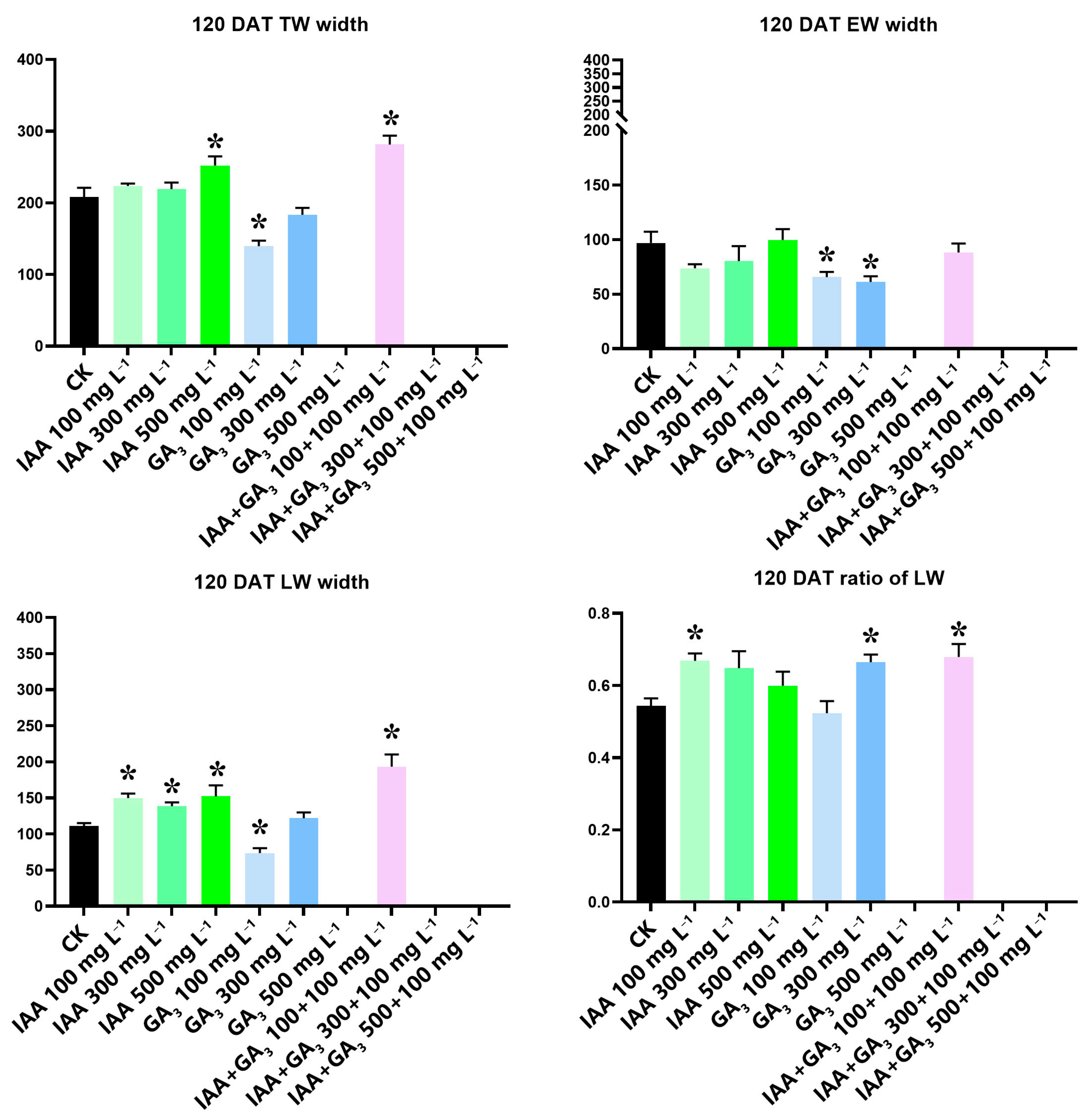
We measured the total wood width (TWW), earlywood width (EWW), and latewood width (LWW) in 2019 for the present study at 120 DAT. Then, the ratio of LWW to TWW was calculated. The IAA treatment at 500 mg L−1 significantly increased the TWW. GA3 treatment at 100 mg L−1 significantly decreased TWW. Combination treatment at 100 mg L−1 + 100 mg L−1 significantly increased TWW. IAA treatments had no significant effect on earlywood width (EWW). GA3 treatments at 100 mg L−1 and 300 mg L−1 significantly decreased EWW. Combination treatment had no significant effect on EWW. All three IAA treatments significantly increased LWW. GA3 treatment at 100 mg L−1 significantly decreased LWW. Combination treatment at 100 mg L−1 + 100 mg L−1 significantly increased LWW. The ratio of LWW to TWW was significantly increased by IAA treatment at 100 mg L−1, GA3 treatment at 300 mg L−1, and combination treatment at 100 mg L−1 + 100 mg L−1 (Figure 3).
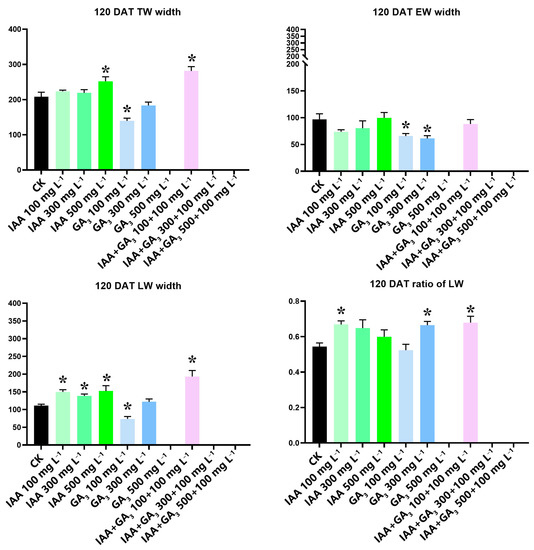
Figure 3.
Measurement and calculation of newly formed wood width, earlywood width, and the ratio of latewood width to total wood width at 120 DAT. The T-test was used, and the least significant range analysis at 5% significance is shown at the same stage with * for the control and treatments. All data were calculated in triplicate.
3.3.2. Measurement of Wall Radial Thickness and Lumen Radial Diameter in Latewood and Earlywood
The wall radial thickness (WRT) and lumen radial diameter (LRD) of EW and LW were measured, respectively, and the ratio of WRT to LRD was calculated (Table 2). Only GA3 treatment at 300 mg L−1 significantly increased the WRT of EW. The three IAA treatments and the combination treatment at 100 mg L−1 + 100 mg L−1 had no significant effect on the WRT of EW. The LRD of EW was significantly decreased by IAA treatments at 100 mg L−1 and 500 mg L−1. GA3 treatment at 100 mg L−1 significantly decreased the LRD of EW but significantly increased it at 300 mg L−1. Combination treatment at 100 mg L−1 + 100 mg L−1 significantly decreased the LRD of EW. The ratio of WRT to LRD in EW was not significantly affected in any of the treatments. The WRT of LW was significantly decreased by IAA treatments at low and middle concentrations (100 mg L−1 and 300 mg L−1) but was significantly increased by a high concentration (500 mg L−1). GA3 treatments at 100 mg L−1 and 300 mg L−1 significantly decreased the WRT of LW. Similarly, combination treatment at 100 mg L−1 + 100 mg L−1 significantly decreased the WRT of LW. The LRD of LW was significantly decreased by IAA treatments and GA3 treatments at low and middle concentrations (100 mg L−1 and 300 mg L−1). Combination treatment at 100 mg L−1 + 100 mg L−1 significantly decreased the LRD of LW. The ratio of WRT to LRD in LW was significantly increased under combination treatment, GA3 treatments, and IAA treatments (Table 2).

Table 2.
Measurement of wood cell morphology at 120 DAT.
3.4. The Pattern of Tree-Ring Growth
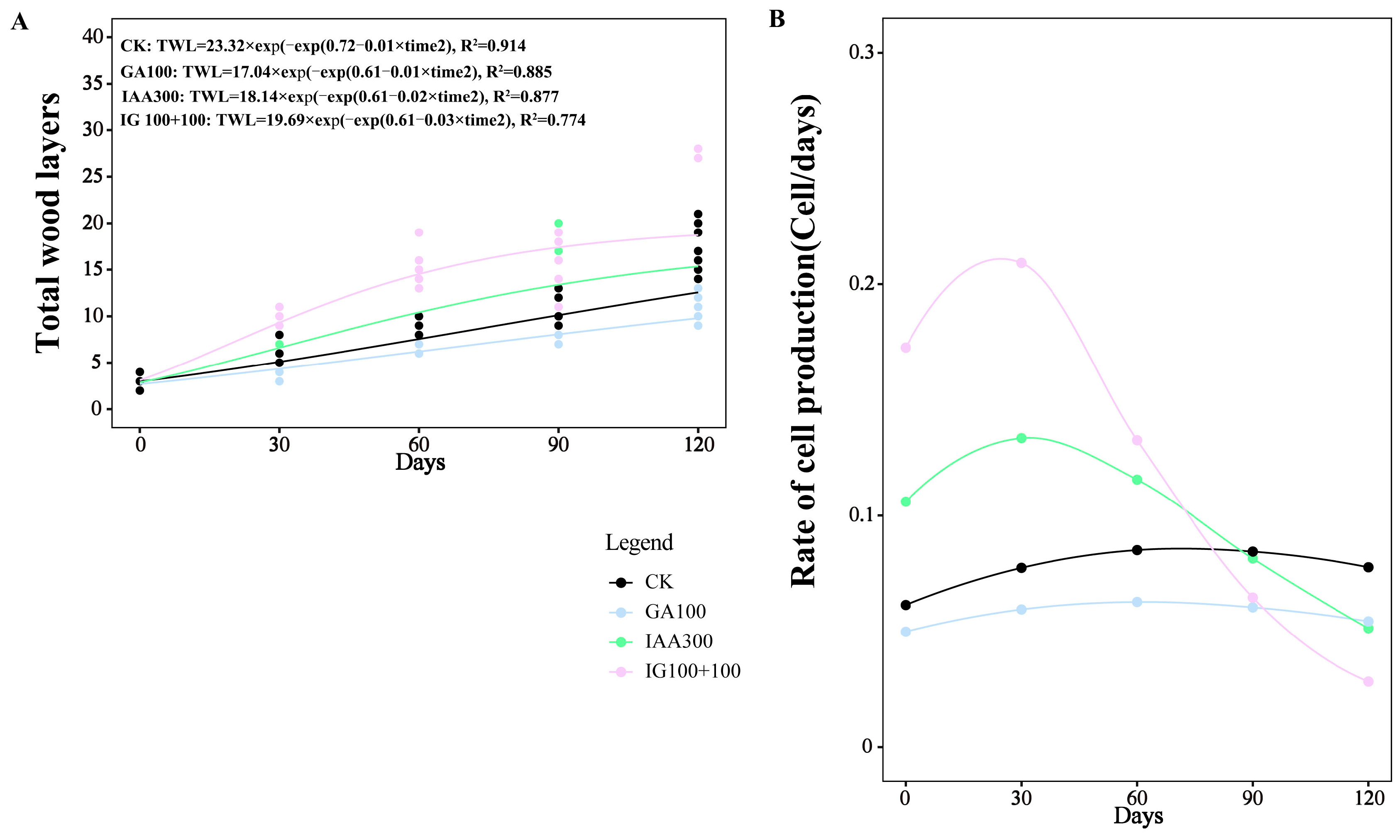
To obtain the tree-ring growth pattern, we used the Gompertz function to model the increase in the wood cell number (TWLs) for the three most representative treatments, including the treatment that most significantly promoted tree-ring growth (100 mg L−1 + 100 mg L−1 combination treatment), the treatment significantly that most inhibited the tree-ring growth (100 mg L−1 GA3 treatment), the treatment that had no significant effect on tree-ring growth (300 mg L−1 IAA treatment), and CK in the present study. The dynamics of TWLs fitted the Gompertz function, with R2 = 0.914, R2 = 0.855, R2 = 0.877, and R2 = 0.774 for CK, GA3 treatment at 100 mg L−1, IAA treatment at 300 mg L−1, and combination treatment at 100 mg L−1 + 100 mg L−1, respectively (Figure 4).
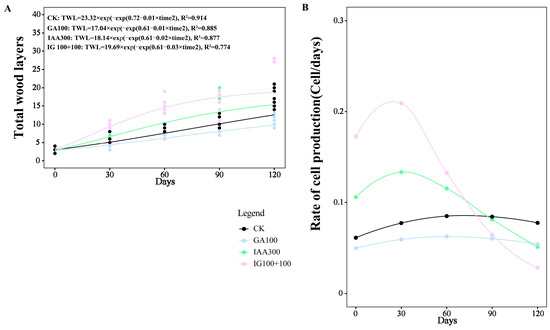
Figure 4.
Dynamics and rate of wood cell development with different phytohormones treatments. (A) Development of the total wood layers. (B) Rate of cell production.
The pattern in the rate of tree-ring growth was significantly changed by combination treatment AT 100 mg L−1 + 100 mg L−1 compared to CK (Figure 4). The time when the maximum rate (TMR) appeared was earliest in combination treatment at 100 mg L−1 + 100 mg L−1, at 21.40 DAT, followed by IAA treatment at 300 mg L−1, at 25.40 DAT. The TMR in GA3 treatment with 100 mg L−1 was close to that of CK (53.90 DAT and 54.40 DAT, respectively). The maximum rate (MR) in combination treatment at 100 mg L−1 + 100 mg L−1 was about twice as much as in the CK (0.21 and 0.11, respectively), followed by IAA treatment at 300 mg L−1 (0.16) and GA3 treatment at 100 mg L−1 (0.07). Similar to the trend of MR, the average rate (AR) was highest in combination treatment at 100 mg L−1 + 100 mg L−1, followed by IAA treatment at 300 mg L−1, CK, and GA3 treatment at 100 mg L−1 (0.13, 0.10, 0.07, and 0.04, respectively) (Table 3).

Table 3.
Parameter estimates of the model for the total wood layers produced during treatments.
4. Discussion
4.1. IAA and GA3 (1:1) Combination Treatment Best Induced Tree-Ring Growth
Increased CO2 would promote the accumulation of phytohormones in plants. In previous studies, endogenous phytohormones such as IAA and GA3 reached levels of about 300 μg g−1 FW [6,7], or much lower at about 10 ng g−1 [33]. Choosing the concentrations of exogenous phytohormone to apply in treatment based on endogenous phytohormone concentrations is not appropriate. Concentrations in studies on exogenous phytohormones application in plants have typically been much higher than endogenous phytohormones, at about 200 mg L−1 [33,34]. Previous studies have shown that the concentration of exogenous IAA and GAs that promotes wood formation is 100 mg L−1 for foliar application [12,30]. The 1:1 proportion of combination treatment with IAA and GAs may better promote tree-ring growth [30,31,35] than IAA and GA3 treatment alone. However, previous research has also shown that high IAA/low GA combination treatment favors xylem formation in Populous robusta [30]. To obtain the best proportion for combination treatment in terms of wood formation, particularly inducing latewood formation, in this study the combination treatment was designed as IAA+GA3 at 100 mg L−1 + 100 mg L−1 (1:1), 300 mg L−1 + 100 mg L−1 (3:1), and 500 mg L−1 + 100 mg L−1 (5:1), and corresponding single phytohormone treatments were also considered. The appropriate proportion of IAA+ GA3 combination treatment was 1:1 (100 mg L−1 + 100 mg L−1). It is well known that the secondary xylem (wood) is derived from the vascular cambium [36]. Combination treatment at 100 mg L−1 + 100 mg L−1 accelerated the rate of wood cell production and caused the MR to appear earlier through increasing cambium division so that tree-ring growth was promoted. Combination treatment at 100 mg L−1 + 100 mg L−1 significantly promoted LW formation but not EW in this treatment because the experiment started too late to affect EW formation. In previous studies, it has been reported that auxin and GA induce the division of the cambium [37]. Results from Eucalyptus grandis and Populus tremula L. × tremuloides Michx showed that the application of GA would stimulate polar auxin transport [28,38]. These reports indicate that GA3 may strengthen the function of IAA in inducing cambium activity in the present study. Similar results were also found in Populous robusta [30] and conifers [31,35] after the application of GA and IAA together, but the dose of each phytohormone was different between different species. The tree-ring growth was inhibited by GA3 treatments in the present study, especially at 100 mg L−1, because the cambium activity was inhibited during the experiment. These results are similar to the findings in Populus simonii × P. nigra [12] and carrots [39]. However, the mechanism remains unclear.
4.2. Effect of Phytohormone Application on Wood Cell Morphology
IAA treatment at 500 mg L−1 significantly increased the WRT of LW in the present study, similar to findings in maize (Zea mays L.) [40]. Auxin treatment was also able to induce the deposition of the latewood cell wall in conifers in this study. Similarly, studies have shown that IAA is one of the most important plant hormones in the process of secondary cell wall deposition [16]. In this study, the enhanced deposition of the cell wall in LW resulted in a significantly higher TWW in the IAA treatment at 500 mg L−1. These results confirm the function of IAA in wood formation [14,17] and indicate that IAA participates in inducing the differentiation of latewood. The WRT of EW was significantly increased by GA3 treatment at 300 mg L−1, which was similar to reports that cell wall thickness and deposition are enhanced by GAs [24,41]. This could have been caused by the induced expression of secondary wall biosynthesis-related genes [42,43]. The wood cell morphology in the combination treatment was similar to the corresponding IAA treatment alone or GA3 treatment alone. Only one of the two phytohormones in the combination treatment was related to wood cell morphology, instead of requiring crosstalk between them.
4.3. High IAA/low GA3 Combination Treatment and High GA3 Treatment Led to the Death of Treated Seedlings
Finally, only the combination treatment at 1:1 promoted tree-ring growth and kept the treated seedlings alive. High IAA/low GA combination treatment at 3:1 and 5:1 in the present study and GA3 treatment at 500 mg L−1 resulted in the death of treated seedlings. In the present study, via the high GA3 treatment and combination treatment with high IAA/low GA3, we aimed to find the threshold of concentration and proportion and then to find the best proportion for the combination treatment. These results indicate that the treatments mentioned above cannot be used in the future. Previous research has shown that IAA treatment significantly increases abscisic acid (ABA) content [44]. The application of GAs could promote the accumulation of IAA [38]. Hence, it can be assumed that combination treatments with high IAA/low GA3 cause a higher endogenous ABA content, increasing to a lethal dose, through inducing IAA accumulation with GAs help than corresponding IAA treatment alone. Even though there was a negative effect between GAs and ABA [45,46], the GA content may not have been enough to inhibit the lethal effect in high IAA/low GA3 treatment (3:1 and 5:1) in the present study. For the 1:1 combination treatment, the increased ABA content was not high enough to cause the death of seedlings because of the relatively low IAA and inhibition of GA3 toward ABA. The death of the treated seedlings in the GA3 treatment at 500 mg L−1 could have been caused by the crosstalk between GA3 and IAA, leading to lethal ABA accumulation, or simply have been caused by the high GA3 itself. All the hypotheses mentioned above require further study in the future.
5. Conclusions and Implications
This study is the first to demonstrate the synergistic effect of IAA and GA3 on tree-ring growth of hybrid larch, especially latewood formation, and the optimal hormone ratio conducive to its growth is obtained.
Exogenous IAA and GA3 were applied together to investigate the effects of combination treatment with these two phytohormones on tree-ring growth of hybrid larch in the study. We found that the IAA + GA3 combination treatment at 100 mg L−1 +100 mg L−1 (1:1) was the most appropriate to promote tree-ring growth in hybrid larch seedlings. Specifically, it increased the rate of wood cell production, especially the LW, by inducing cambium activity to promote tree-ring growth. The effect of a single phytohormone treatment alone with IAA on wood formation was not steady and even inhibited it at 100 mg L−1 GA3 treatment. These results indicate that the wood formation of hybrid larch may be affected by a complex phytohormone network. In conclusion, the application of IAA and GA3 together promoted tree-ring growth, and the content and proportion of endogenous phytohormones should be regarded as important indicators in improving the management of hybrid larch clonal plantations.
Under future climate change, the impact of elevated atmospheric CO2 (eCO2) on plants is to disturb tree growth through plant hormone channels [1,2,3,6]. Therefore, an in-depth understanding of the synergistic mechanism of hormones in tree growth can help to understand the response of trees to future climate change and is of great significance for sustainable forest development. In addition, this progress also provides a reference for high-quality seedling cultivation of trees. Specifically, during the seedling stage of hybrid larch, the combined application of IAA and GA3 (1:1) can promote the growth of hybrid seedlings and shorten the seedling period to meet the afforestation requirements as soon as possible. These results are of great significance in theory and practice for improving the management model of larch plantations.
Author Contributions
Conceptualization, Y.L. and Y.X.; methodology, Y.L. and Y.X.; software, Y.L.; validation, Y.L., Y.X. and X.S.; formal analysis, Y.L.; investigation, Y.L.; resources, Y.L.; data curation, Y.L. and Y.X; writing—original draft preparation, Y.L.; writing—review and editing, Y.X. and X.S.; visualization, Y.L.; supervision, Y.X. and X.S.; project administration, Y.X., X.S. and S.Z.; funding acquisition, X.S. and S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The National Key Research and Development Program of China (2022YFD2200103), General Program of National Natural Science Foundation of China (31971652), and The Special Funds of Research Institute of Forestry (LYSZX202002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Po-Kai, H.; Yohei, T.; Shintaro, M.; Ebe, M.; Kristiina, L.; Rainer, W.; Dianne, P.; Hannes, K.; Schroeder, J.I. Abscisic acid-independent Stomatal CO2 Signal Transduction Pathway and Convergence of CO2 and ABA Signaling Downstream of OST1 Kinase. Proc. Natl. Acad. Sci. USA 2018, 115, 9971–9980. [Google Scholar] [CrossRef]
- Chater, C.; Peng, K.; Movahedi, M.; Dunn, J.; Walker, H.; Liang, Y.; Mclachlan, D.; Casson, S.; Isner, J.; Wilson, I. Elevated CO2-Induced Responses in Stomata Require ABA and ABA Signaling. Curr. Biol. 2015, 25, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gou, J.; Yordanov, Y.; Zhang, H.; Thakur, R.; Jones, W.; Burton, A. Global Transcriptomic Profiling of Aspen Trees under Elevated CO2 to Identify Potential Molecular Mechanisms Responsible for Enhanced Radial Growth. J. Plant Res. 2013, 126, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Yanhong, Z.; Shibei, G.; Lijuan, J.; Kaiqian, Y.; Yu, W.; Xiaodan, W.; Jie, Z.; Xiaojian, X.; Kai, S.; Christine, H.F.; et al. A Novel CO2-responsive Systemic Signaling Pathway Controlling Plant Mycorrhizal Symbiosis. New Phytol. 2019, 224, 106–116. [Google Scholar] [CrossRef]
- Wu, F.; Sun, X.; Zou, B.; Zhu, P.; Ji, K. Transcriptional Analysis of Masson Pine (Pinus massoniana) under High CO2 Stress. Genes 2019, 10, 804. [Google Scholar] [CrossRef]
- Li, X.M.; He, X.Y.; Zhang, L.H.; Chen, W.; Chen, Q. Influence of Elevated CO2 and O3 on IAA, IAA Oxidase and Peroxidase in the Leaves of Ginkgo trees. Biol. Plant 2009, 53, 339–342. [Google Scholar] [CrossRef]
- Teng, N.J.; Wang, J.; Chen, T. Elevated CO2 Induces Physiological, Biochemical and Structural Changes in Leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Pan, C.; Huan, M.; Qiaomei, F.; Feijun, F.; Ruishuang, A.; Golam, J.; JingquanShi, K. Role of Ethylene Biosynthesis and Signaling in Elevated CO2-induced Heat Stress Response in Tomato. Planta 2019, 250, 563–572. [Google Scholar] [CrossRef]
- Li, X.; Ahammed Golam, J.; Li, Z.; Tang, M.; Yan, P.; Wenyan, H. Decreased Biosynthesis of Jasmonic Acid via Lipoxygenase Pathway Compromised Caffeine-Induced Resistance to Colletotrichum gloeosporioides under Elevated CO2 in Tea Seedlings. Biochem. Cell Biol. 2016, 106, 1270–1277. [Google Scholar] [CrossRef]
- Sytar, M. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Mohammad, S.; Kanchan, V.; Vinod, K.; Namira, A.; Susmita, D.; Riya, J.; Edappayil, J.; Puthur, J.T.; Sasan, A.; Devendra Kumar, C.; et al. Metal/Metalloid-Based Nanomaterials for Plant Abiotic Stress Tolerance: An Overview of the Mechanisms. Plants 2022, 11, 316. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, L.; Guo, W.; Yu, Y.; Tao, L.; Zhang, L.; Song, X.; Huang, W.; Cheng, L.; Chen, J.; et al. Exogenous Application of Phytohormones Promotes Growth and Regulates Expression of Wood Formation-Related Genes in Populus simonii × P. nigra. Int. J. Mol. Sci. 2019, 20, 792. [Google Scholar] [CrossRef]
- Fischer, U.; Kucukoglu, M.; Helariutta, Y.; Bhalerao, R.P. The Dynamics of Cambial Stem Cell Activity. Annu. Rev. Plant Biol. 2019, 70, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Ohashi-Ito, K.; Iwamoto, K.; Nagashima, Y.; Kojima, M.; Sakakibara, H.; Fukuda, H. A Positive Feedback Loop Comprising LHW-TMO5 and Local Auxin Biosynthesis Regulates Initial Vascular Development in Arabidopsis Roots. Plant Cell Physiol. 2019, 60, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Smetana, O.; Makila, R.; Lyu, M.; Amiryousefi, A.; Sanchez Rodriguez, F.; Wu, M.F.; Sole-Gil, A.; Leal Gavarron, M.; Siligato, R.; Miyashima, S.; et al. High Levels of Auxin Signalling Define the Stem-cell Organizer of the Vascular Cambium. Nature 2019, 565, 485–489. [Google Scholar] [CrossRef]
- Johnsson, C.; Jin, X.; Xue, W.; Dubreuil, C.; Lezhneva, L.; Fischer, U. The Plant Hormone Auxin Directs Timing of Xylem Development by Iinhibition of Secondary Cell Wall Deposition through Repression of Secondary Wall NAC-domain Transcription Factors. Physiol. Plant 2019, 165, 673–689. [Google Scholar] [CrossRef]
- Xu, C.; Shen, Y.; He, F.; Fu, X.; Yu, H.; Lu, W.; Li, Y.; Li, C.; Fan, D.; Wang, H.C.; et al. Auxin-mediated Aux/IAA-ARF-HB Signaling Cascade Regulates Secondary Xylem Development in Populus. New Phytol. 2019, 222, 752–767. [Google Scholar] [CrossRef]
- Buttò, V.; Deslauriers, A.; Rossi, S.; Rozenberg, P.; Shishov, V.; Morin, H. The Role of Plant Hormones in Tree-ring Formation. Trees 2020, 34, 315–335. [Google Scholar] [CrossRef]
- Funada, R.; Yamagishi, Y.; Begum, S.; Kudo, K.; Nakaba, S. Xylogenesis in Trees: From Cambial Cell Division to Cell Death. In Secondary Xylem Biology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 25–43. [Google Scholar] [CrossRef]
- Fajstavr, M.; Paschova, Z.; Giagli, K.; Vavrcik, H.; Gryc, V.; Urban, J. Auxin (IAA) and Soluble Carbohydrate Seasonal Dynamics monitored During Xylogenesis and Phloemogenesis in Scots pine. Ifor.-Bioge For. 2018, 11, 553–562. [Google Scholar] [CrossRef]
- Johnson, D.; Eckart, P.; Alsamadisi, N.; Noble, H.; Martin, C.; Spicer, R. Polar Auxin Transport is Implicated in Vessel Differentiation and Spatial Patterning during Secondary Growth in Populus. Am. J. Bot. 2018, 105, 186–196. [Google Scholar] [CrossRef]
- Garcia-Rojas, M.; Meneses, M.; Oviedo, K.; Carrasco, C.; Defilippi, B.; Gonzalez-Aguero, M.; Leon, G.; Hinrichsen, P. Exogenous Gibberellic acid Application Induces the Overexpression of Key Genes for Pedicel Lignification and an Increase in Berry Drop in Table Grape. Plant Physiol. Biochem. 2018, 126, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Felipo-Benavent, A.; Cristina, R.; Blanco-Tourián, N.; Serrano-Mislata, A.; Nicolas, B.; Achard, P.; Javier, A.B.M.; Alabadí, D. Regulation of Xylem Fiber Differentiation by Gibberellins through DELLA-KNAT1 Interaction. Development 2018, 145, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Jeon, W.; Kim, H.; Vo, K.; Kim, J.; Park, J.; Choi, I.; Lee, H.; Han, H.; Ko, H. Wood Forming Tissue-specific Bicistronic Expression of PdGA20ox1 and PtrMYB221 Improves Both the Quality and Quantity of Woody Biomass Production in a Hybrid Poplar. Plant Biotechnol. J. 2019, 17, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Gerttula, S.; Zinkgraf, M.; Muday, G.K. Transcriptional and Hormonal Regulation of Gravitropism of Woody Stems in Populus. Plant Cell 2015, 27, 2800–2813. [Google Scholar] [CrossRef]
- Ragni, L.; Nieminen, K.; Pacheco-Villalobos, D.; Sibout, R.; Schwechheimer, C.; Hardtke, C.S. Mobile Gibberellin Directly Stimulates Arabidopsis Hypocotyl Xylem Expansion. Plant Cell 2011, 23, 1322–1336. [Google Scholar] [CrossRef]
- Israelsson, M.; Moritz, T. Tissue-specific Localization of Gibberellins and Expression of Gibberellin-biosynthetic and Signaling Genes in Wood-forming Tissues in Aspen. Plant J. 2005, 44, 494–504. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Guo, G.S.; Qiu, Z.F.; Li, X.D.; Zeng, B.S.; Fan, C.J. Exogenous GA3 Application Altered Morphology, Anatomic and Transcriptional Regulatory Networks of Hormones in Eucalyptus grandis. Protoplasma 2018, 255, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Moriwaki, T.; de Oliveira, D.M.; Andreotti, G.C.; de Souza, L.A.; Dos Santos, W.D.; Bonato, C.M.; Antunes, W.C. Increased Gibberellins and Light Levels Promotes Cell Wall Thickness and Enhance Lignin Deposition in Xylem Fibers. Front. Plant Sci. 2018, 9, 1391. [Google Scholar] [CrossRef]
- Digby, J.; Wareing, P.F. The Effect of Applied Growth Hormones on Cambial Division and the Differentiation of the Cambial Derivatives. Ann. Bot. 1966, 30, 539–548. [Google Scholar] [CrossRef]
- Wang, Q.; Little, C.H.A.; Odén, P.C. Control of Longitudinal and Cambial Growth by Gibberellins and Indole-3-acetic acid in Current-year Shoots of Pinus sylvestris. Tree Physiol. 1997, 17, 715–721. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Chhin, S.; Zhang, J. A Bimodal Pattern and Age-Related Growth of Intra-Annual Wood Cell Development of Chinese Fir in Subtropical China. Front. Plant Sci. 2021, 12, 757438. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Li, Y.; Yang, L.; Zhang, Y.; Cai, Y. Effect of Exogenous Gibberellin, Paclobutrazol, Abscisic Acid, and Ethrel Application on Bulblet Development in Lycoris radiata. Front. Plant Sci. 2020, 11, 615547. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.M.; Ji, J.; Yue, J.Y.; Shi, S.Q.; Chang, E.M. Exogenous Abscisic Acid Modulates Reactive Oxygen Metabolism and Related Gene Expression in Platycladus orientalis under H2O2-Induced Stress. Russ. J. Plant Physiol. 2020, 67, 85–93. [Google Scholar] [CrossRef]
- Ewers, F.W.; Aloni, R. Effects of Applied Auxin and Gibberellin on Phloem and Xylem Production in Needle Leaves of Pinus. Bot. Gaz. 1985, 146, 466–471. Available online: http://www.jstor.org/stable/2474621 (accessed on 29 October 2015). [CrossRef]
- Mehdi, B.T.; Dagmar, R.; Martin, B.; Laura, R. Auxin and Gibberellin Signaling Cross-talk Promotes Hypocotyl Xylem Expansion and Cambium Homeostasis. J. Exp. Bot. 2021, 72, 3647–3660. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kondo, Y.; Kojima, M.; Takebayashi, Y.; Sakakibara, H.; Fukuda, H. Suppression of DELLA Signaling Induces Procambial Cell Formation in culture. Plant J. 2018, 94, 48–59. [Google Scholar] [CrossRef]
- Bjorklund, S.; Antti, H.; Uddestrand, I.; Moritz, T.; Sundberg, B. Cross-talk Between Gibberellin and Auxin in Development of Populus wood: Gibberellin Stimulates Polar Auxin Transport and Has a Common Transcriptome with Auxin. Plant J. 2007, 52, 499–511. [Google Scholar] [CrossRef]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous Gibberellin Enhances Secondary Xylem Development and Lignification in Carrot Taproot. Protoplasma 2016, 254, 839–848. [Google Scholar] [CrossRef]
- Siposova, K.; Kollarova, K.; Liskova, D.; Vivodova, Z. The Effects of IBA on the Composition of Maize Root Cell Walls. J. Plant Physiol. 2019, 239, 10–17. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, J.; Wang, K.; Li, D.; Zhang, W. MiR396GRF Module Associates with Switchgrass Biomass Yield and Feedstock Quality. Plant Biotechnol. J. 2021, 19, 1523–1536. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Lu, L.; Yi, X.; Yao, N.; Lai, Z.; Zhang, J. Comparative Transcriptome Study of the Elongating Internode in Elephant Grass (Cenchrus purpureus) seedlings in response to exogenous gibberellin applications. Ind. Crops Prod. 2022, 178, 114653. [Google Scholar] [CrossRef]
- Yang, X.; Hill, K.A.; Austin, R.S.; Tian, L. Differential Gene Expression of Brachypodium distachyon Roots Colonized by Gluconacetobacter diazotrophicus and the Role of BdCESA8 in the Colonization. Mol. Plant-Microbe Interact. 2021, 34, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Hassan, M.J.; Li, Z.; Peng, Y. Indole-3-acetic acid Improves Drought Tolerance of White Clover via Activating Auxin, Abscisic acid and Jasmonic acid Related Genes and Inhibiting Senescence Genes. BMC Plant Biol. 2020, 20, 150. [Google Scholar] [CrossRef] [PubMed]
- Shun-Ying, C.S.-R.K.; Ching-Te, C. Roles of Gibberellins and Abscisic acid in Dormancy and Germination of Red Bayberry (Myrica rubra) Seeds. Tree Physiol. 2008, 28, 1431–1439. [Google Scholar] [CrossRef]
- Qibing, L.; Fuqing, W.; Peike, S.; Zhe, Z.; Xin, Z.; Xiuping, G.; Jiulin, W.; Zhijun, C.; Jie, W.; Haiyang, W.; et al. The SnRK2-APC/CTE Regulatory Module Mediates the Antagonistic Action of Gibberellic acid and Abscisic acid Pathways. Nat. Commun. 2015, 6, 7981. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).