Zinc Nanoparticles (ZnNPs): High-Fidelity Amelioration in Turnip (Brassica rapa L.) Production under Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition of ZnNPs and Experimental Design
2.2. Seed Selection and Germination
2.3. Measurement of Plant Height, Shoot Length, Diameter, and Weight
2.4. Analysis of Chlorophyll and Carotenoid Contents
2.5. Analysis of Activity of Antioxidant Enzymes
2.6. Analysis of Superoxide Dismutase (SOD) Activity
2.7. Analysis of Catalase (CAT) Activity
2.8. Analysis of Peroxidase (POD) Activity
2.9. Analysis of Malondialdehyde (MDA) Contents
2.10. Measurement of Contents of Ascorbic Acid (AA)
2.11. Measurement of Total Phenolic Contents
2.12. Analysis of Total Soluble Proteins (TSPs)
2.13. Analysis of Total Soluble Sugar (TSS) Contents
2.14. Analysis of Total Free Amino Acid (TFA) Contents
2.15. Analysis of Flavonoid Contents
2.16. Statistical Analysis
3. Results
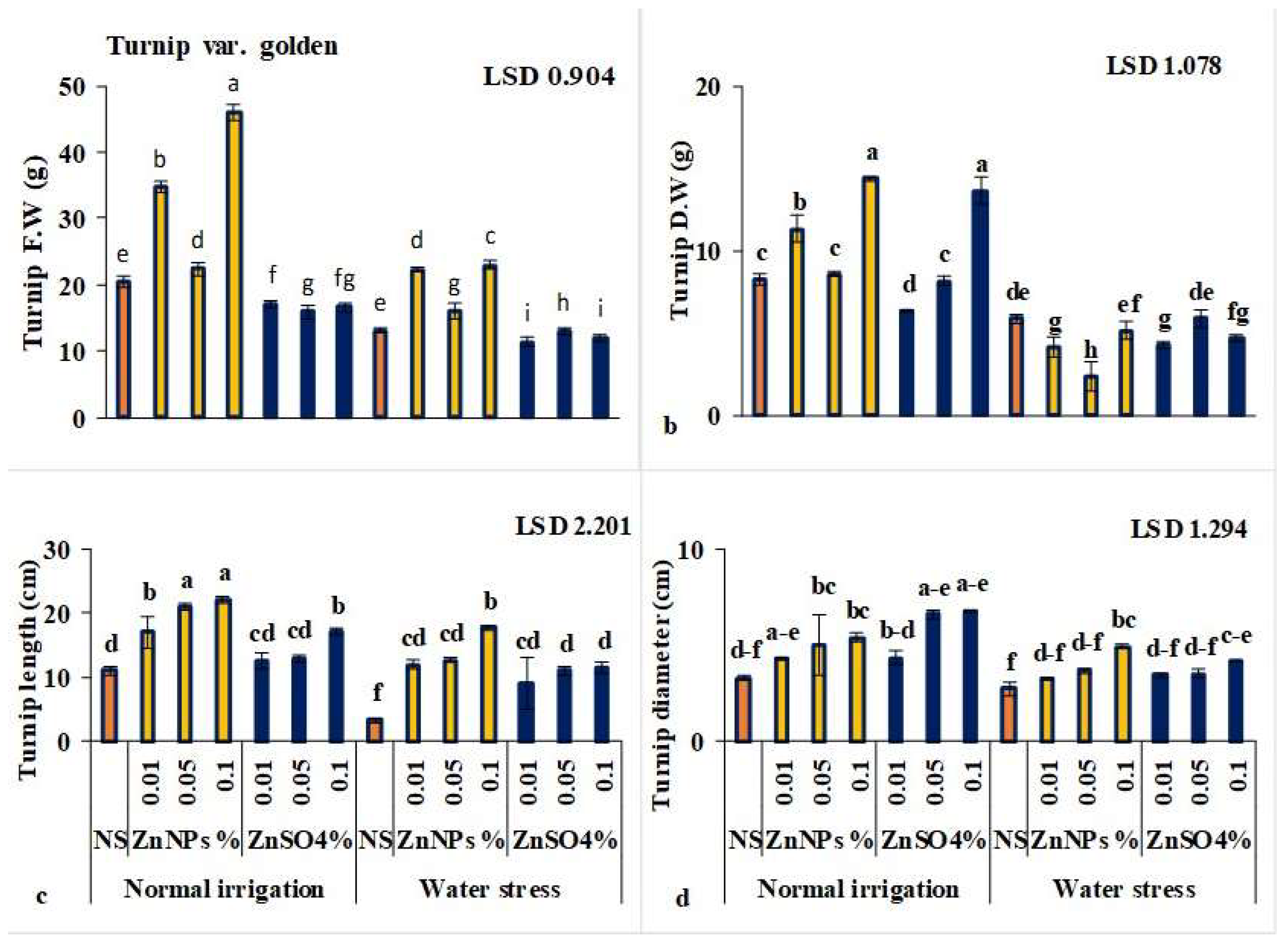
3.1. ZnNPs Enhanced Leaf Growth Attributes
3.1.1. ZnNPs Enhanced Shoot Length of Turnip Plants
3.1.2. ZnNPs Ameliorate Chlorophyll and Carotenoid Contents in Leaves
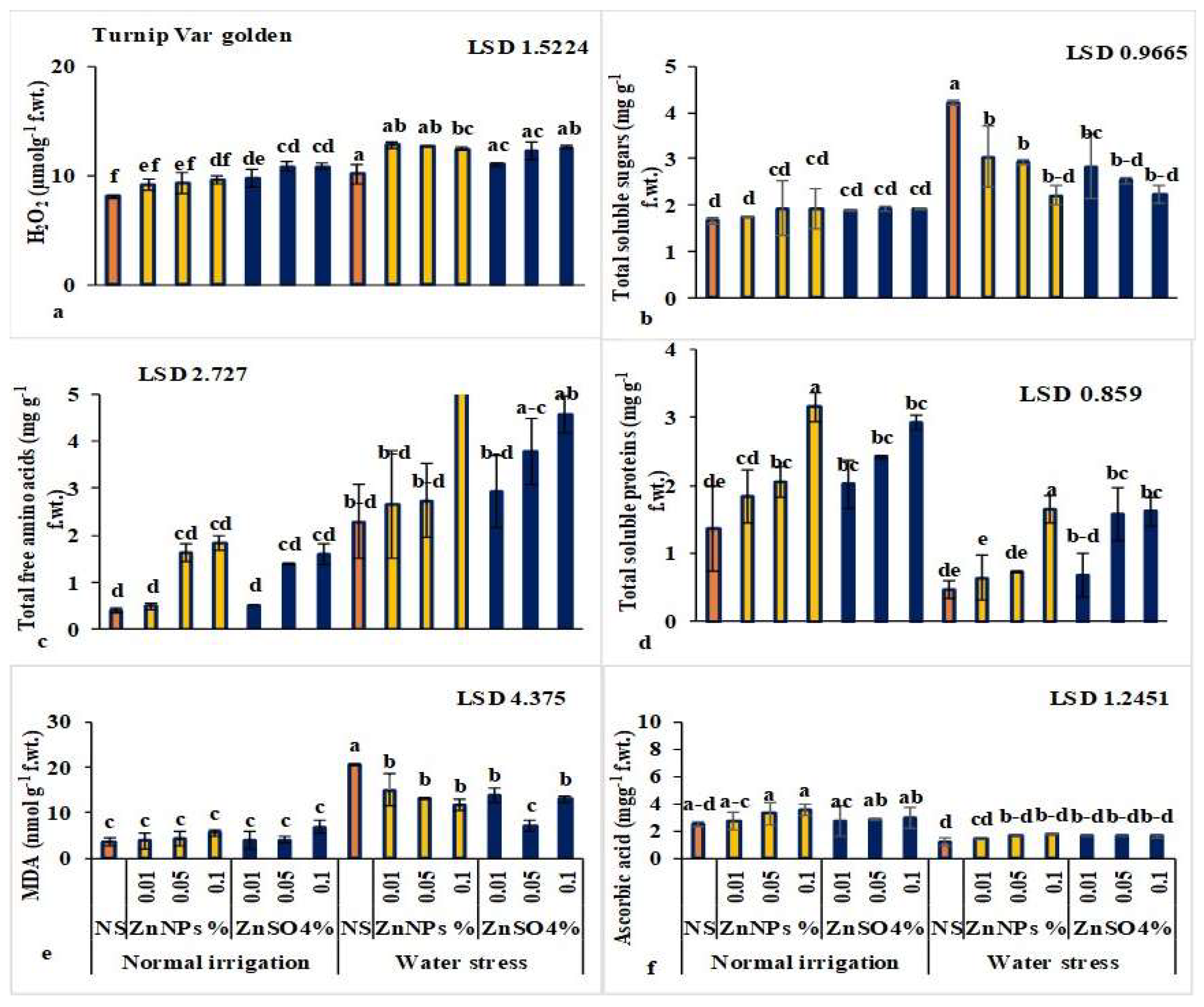
3.1.3. ZnNPs Enhanced Antioxidant Enzyme Activity in Leaves
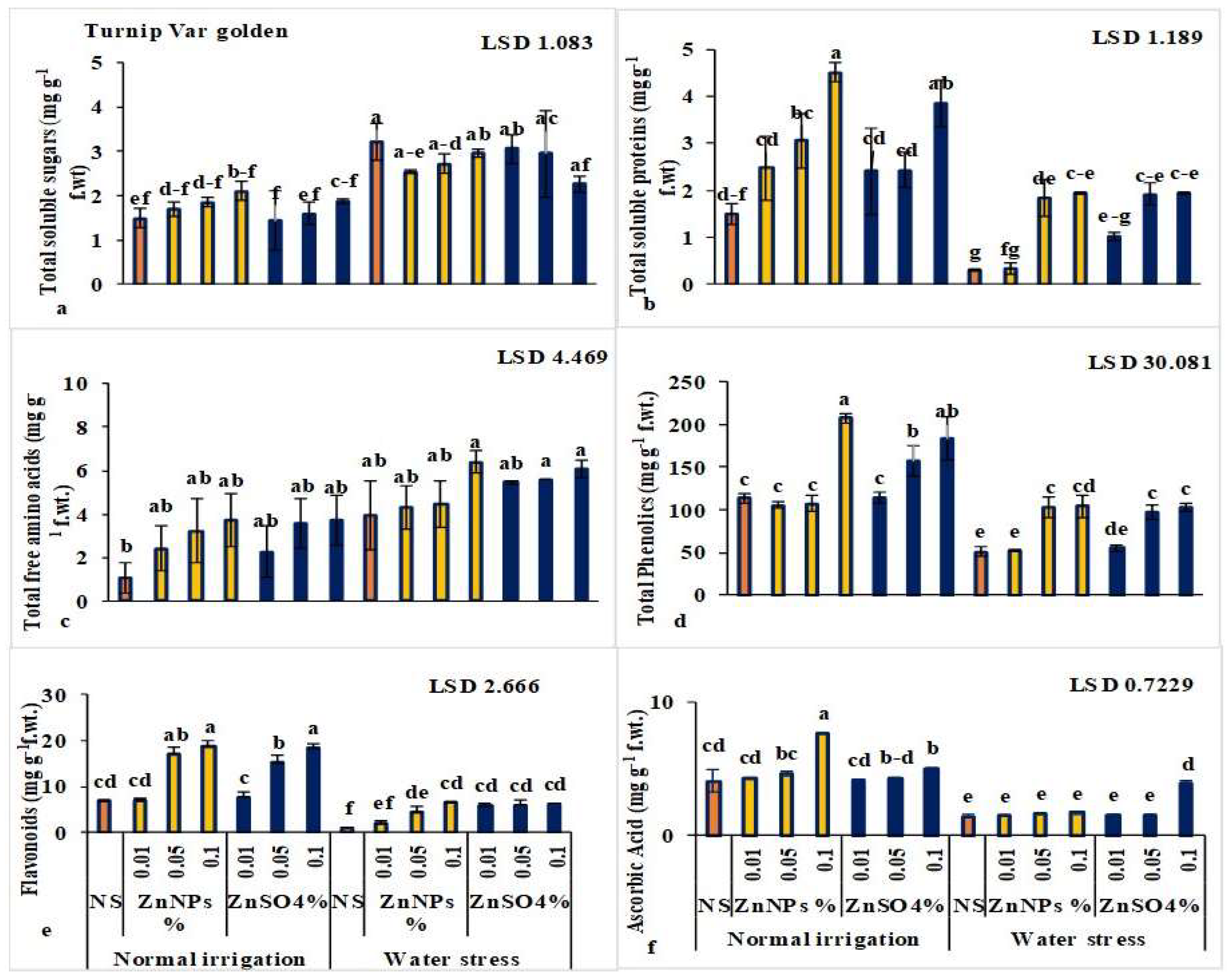
3.1.4. ZnNPs Enhanced Sugars, Proteins, and Secondary Metabolites in Leaves
3.2. ZnNPs Enhanced Growth Attributes of Root/Turnip
3.2.1. ZnNPs Enhanced Root/Turnip Length
3.2.2. ZnNPs Enhanced Sugars, Proteins, and Secondary Metabolites in Root/Turnip
3.2.3. ZnNPs Enhanced Antioxidant Enzyme Activity in Root/Turnip
3.2.4. Correlation among Growth Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafar, S.; Perveen, S.; Kamran Khan, M.; Shaheen, M.R.; Hussain, R.; Sarwar, N.; Rashid, S.; Nafees, M.; Farid, G.; Alamri, S.J.P.o. Effect of zinc nanoparticles seed priming and foliar application on the growth and physio-biochemical indices of spinach (Spinacia oleracea L.) under salt stress. PLoS ONE 2022, 17, e0263194. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Parvaiz, M.; Shahbaz, M.; Saeed, M.; Zafar, S. Triacontanol positively influences growth, yield, biochemical attributes and antioxidant enzymes of two linseed (Linum usitatissimum L.) accessions differing in drought tolerance. Pak. J. Bot. 2022, 54, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Toubali, S.; Ait-El-Mokhtar, M.; Boutasknit, A.; Anli, M.; Ait-Rahou, Y.; Benaffari, W.; Ben-Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. Root reinforcement improved performance, productivity, and grain bioactive quality of field-droughted quinoa (Chenopodium quinoa). Front. Plant Sci. 2022, 13, 860484. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Abida, K.; Madiha, I.; Basra, S.M.A.; Sara, Z. Effect of different seed priming treatments on growth and nutrients uptake in wheat (Triticum aestivum) under salt stress. Int. J. Agric. Biol. 2019, 21, 964–970. [Google Scholar] [CrossRef]
- Naveen, N.; Kumari, N.; Avtar, R.; Jattan, M.; Ahlawat, S.; Rani, B.; Malik, K.; Sharma, A.; Singh, M. Evaluation of effect of brassinolide in Brassica juncea leaves under drought stress in field conditions. Horticulturae 2021, 7, 514. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Taha, T.F.; Najjar, A.A.; Zabermawi, N.M.; Nader, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Salama, A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021, 28, 6782–6794. [Google Scholar] [CrossRef]
- Zafar, S.; Akhtar, M.; Perveen, S.; Hasnain, Z.; Khalil, A. Attenuating the adverse aspects of water stress on wheat genotypes by foliar spray of melatonin and indole-3-acetic acid. Physiol. Mol. Biol. Plants 2020, 26, 1751–1762. [Google Scholar] [CrossRef]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Muhammad Sabir Tariq, R.; Ayub, M.A.; Hossain, A.; Hassan, M.M.; Brestic, M.; Sohidul Islam, M. Selenium alleviates the adverse effect of drought in oilseed crops camelina (Camelina sativa L.) and canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Amjad, S.F.; Mansoora, N.; Din, I.U.; Khalid Iqbal, R.; Jatoi, G.H.; Murtaza, G.; Yaseen, S.; Naz, M.; Danish, S.; Fahad, S.; et al. Application of zinc fertilizer and mycorrhizal inoculation on physio-biochemical parameters of wheat grown under water-stressed environment. Sustainability 2021, 13, 11007. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, F.; Farooq, M. Application of micronutrients in rice-wheat cropping system of South Asia. Rice Sci. 2019, 26, 356–371. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Jia, K.; Yan, C.; Yan, H.; Gao, J. Physiological responses of turnip (Brassica rapa L. subsp. rapa) seedlings to salt stress. HortScience 2020, 55, 1567–1574. [Google Scholar] [CrossRef]
- Wahocho, N.; Wahocho, S.; Memon, N.; Leghari, M.; Baloch, Q. Growth and yield response of turnip to various nitrogen application rates. Pak. J. Agric. Agric. Eng. Vet. Sci. 2016, 32, 143–149. [Google Scholar]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Davies, B. Carotenoids in higher plants. In Lipids and Lipid Polymers in Higher Plants; Springer: Berlin/Heidelberg, Germany, 1977; pp. 199–217. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Maxie, E.; Rae, H.L.; Eaks, I.; Sommer, N. Studies on radiation-induced ethylene production by lemon fruits. Radiat. Bot. 1966, 6, 445–455. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, T.; Phang, I.Y.; Shen, L.; Chow, S.Y.; Zhang, W.-D. Morphology and mechanical properties of multiwalled carbon nanotubes reinforced nylon-6 composites. Macromolecules 2004, 37, 7214–7222. [Google Scholar] [CrossRef]
- Crampton, C.F.; Stein, W.H.; Moore, S. Comparative studies on chromatographically purified histones. J. Biol. Chem. 1957, 225, 363–386. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Raatz, B.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24× SEA 5 of common bean. Front. Plant Sci. 2017, 8, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.-H. Root response to drought stress in rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 1513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ding, J.; Wang, H.; Su, L.; Zhao, C. Biochar addition alleviate the negative effects of drought and salinity stress on soybean productivity and water use efficiency. BMC Plant Biol. 2020, 20, 288. [Google Scholar] [CrossRef]
- Ali, A.; Salman, A.; Khan, G.D.; Khan, A.A.; Hassan, S.S.; Goheer, M.A.; Khan, M.A.; Ahmed, S. Growth and yield response of turnip to different deficit irrigation levels and sowing dates under the agro-ecological conditions of Khyber Pakhtuankhwa. Pak. J. Agric. Res. 2020, 33, 480. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, M.Y.; Saleem, M. Shift in physiological and biochemical processes in wheat supplied with zinc and potassium under saline condition. J. Plant Nutr. 2018, 41, 19–28. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Aslam, N.; Mumtaz, S.; Jaafar, H.Z.; Wahab, P.E.M.; Qayum, M.; Ormenisan, A.N. Impact of Zn nanoparticles synthesized via green and chemical approach on okra (Abelmoschus esculentus L.) growth under salt stress. Sustainability 2021, 13, 3694. [Google Scholar] [CrossRef]
- Mahajan, P.; Dhoke, S.; Khanna, A. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J. Nanotechnol. 2011, 2011, 696535. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Yan, K.; Li, J.; Zafar, S.; Hasnain, Z.; Aslam, N.; Iqbal, N.; Hussain, S.S.; Usman, M.; Abbas, M.; et al. Agri-nanotechnology and tree nanobionics: Augmentation in crop yield, biosafety, and biomass accumulation. Front. Bioeng. Biotechnol. 2022, 10, 853045. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [Green Version]
- Sattar, A.; Wang, X.; Abbas, T.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Irfan, M.; Butt, M.; Wahid, M.A.; Cheema, M. Combined application of zinc and silicon alleviates terminal drought stress in wheat by triggering morpho-physiological and antioxidants defense mechanisms. PLoS ONE 2021, 16, e0256984. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Gu, W.; Zhang, L.; Li, L.; Qu, D.; Li, C.; Meng, Y.; Li, J.; Wei, S.; Li, W. Modulating the antioxidant system by exogenous 2-(3, 4-dichlorophenoxy) triethylamine in maize seedlings exposed to polyethylene glycol-simulated drought stress. PLoS ONE 2018, 13, e0203626. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Yasmeen, A.; Yousaf, M. Antioxidant status and their enhancements strategies for water stress tolerance in chickpea. Braz. J. Biol. 2022, 82. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Ain, N.U.; Naveed, M.; Hussain, A.; Mumtaz, M.Z.; Rafique, M.; Bashir, M.A.; Alamri, S.; Siddiqui, M.H. Impact of coating of urea with bacillus-augmented zinc oxide on wheat grown under salinity stress. Plants 2020, 9, 1375. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Kasivelu, G.; Raguraman, V.; Malaichamy, K.; Sevathapandian, S.K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatal. Agric. Biotechnol. 2020, 29, 101778. [Google Scholar] [CrossRef]
- Živanović, B.; Milić Komić, S.; Tosti, T.; Vidović, M.; Prokić, L.; Veljović Jovanović, S. Leaf soluble sugars and free amino acids as important components of abscisic acid—Mediated drought response in tomato. Plants 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Sultan, M.; Akhter, M.S.; Shah, K.H.; Ummara, U.; Manzoor, H.; Ulfat, M.; Alyemeni, M.N.; Ahmad, P. Foliar fertigation of ascorbic acid and zinc improves growth, antioxidant enzyme activity and harvest index in barley (Hordeum vulgare L.) grown under salt stress. Plant Physiol. Biochem. 2021, 158, 244–254. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]





| Soil Texture | Loam | CO32− (meq/L) | Nil |
|---|---|---|---|
| ECe (dS/m) | 2.04 | HCO3− (meq/L) | 2.75 |
| pH | 8.3 | Zn (ppm) | 2 |
| Organic Matter (%) | 0.76 | Available P (ppm) | 3.1 |
| Saturation | 35 | Available K+ (ppm) | 80 |
| Treatment | Df | Plant Height | Shoot Length | Root Length | Root F.wt. | Root Dry.wt. |
|---|---|---|---|---|---|---|
| Treatment (T) | 6 | 56.769 *** | 10.706 *** | 102.039 *** | 278.404 *** | 17.229 *** |
| Drought (D) | 1 | 712.595 *** | 94.5 *** | 288.095 *** | 1069.086 *** | 313.786 *** |
| T × D | 6 | 18.595 *** | 3.388 ns | 7.373 ns | 169.156 *** | 15.288 *** |
| Error | 26 | 2.934 | 1.5 | 5.731 | 1.547 | 0.825 |
| Treatment | Df | Root Diameter | Chl a | Chl b | Total Chl | Carotenoids |
| Treatment (T) | 6 | 4.769 *** | 0.098 ns | 0.057 ns | 0.275 ns | 0.023 ns |
| Drought (D) | 1 | 21.715 *** | 0.922 *** | 0.417 *** | 2.577 *** | 3.020 *** |
| T × D | 6 | 1.562 * | 0.017 ns | 0.013 ns | 0.027 ns | 0.224 ns |
| Error | 26 | 0.594 | 0.050 | 0.027 | 0.112 | 0.102 |
| Treatment | Df | TSS | Total Soluble Proteins | Total Free Amino Acids | Phenolics | Flavonoids |
| Treatment (T) | 6 | 0.138 ns | 4.095 *** | 4.916 ns | 5087.534 *** | 80.230 *** |
| Drought (D) | 1 | 12.565 *** | 25.582 *** | 56.619 * | 41979.97 *** | 728.29 *** |
| T × D | 6 | 0.359 ns | 0.699 ns | 0.695 ns | 1900.97 *** | 26.758 *** |
| Error | 26 | 0.417 | 0.347 | 7.336 | 297.354 | 2.251 |
| Treatment | Df | Ascorbic Acid | MDA | SOD | POD | CAT |
| Treatment (T) | 6 | 3.959 *** | 42.619 ns | 273.537 *** | 13.307 ** | 1.029 * |
| Drought (D) | 1 | 94.109 *** | 1004.729 *** | 4234.264 *** | 131.109 *** | 17.379 *** |
| T × D | 6 | 3.063 *** | 61.158 ns | 5.520 ns | 6.245 ns | 0.135 ns |
| Error | 26 | 0.188 | 32.567 | 48.853 | 2.686 | 0.388 |
| Treatment | Df | TSS | Total Soluble Proteins | Total Free Amino Acids | MDA | H2O2 |
| Treatment (T) | 6 | 0.526 ns | 1.949 *** | 5.329 ns | 22.424 * | 0.993 ns |
| Drought (D) | 1 | 10.327 *** | 15.113 *** | 63.385 *** | 827.807 *** | 85.347 *** |
| T × D | 6 | 0.906 * | 0.090 ns | 1.512 ns | 30.321 ** | 3.085 * |
| Error | 26 | 0.352 | 0.281 | 2.74 | 7.096 | 0.875 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zafar, S.; Javaid, A.; Perveen, S.; Hasnain, Z.; Ihtisham, M.; Abbas, A.; Usman, M.; El-Sappah, A.H.; Abbas, M. Zinc Nanoparticles (ZnNPs): High-Fidelity Amelioration in Turnip (Brassica rapa L.) Production under Drought Stress. Sustainability 2023, 15, 6512. https://doi.org/10.3390/su15086512
Li J, Zafar S, Javaid A, Perveen S, Hasnain Z, Ihtisham M, Abbas A, Usman M, El-Sappah AH, Abbas M. Zinc Nanoparticles (ZnNPs): High-Fidelity Amelioration in Turnip (Brassica rapa L.) Production under Drought Stress. Sustainability. 2023; 15(8):6512. https://doi.org/10.3390/su15086512
Chicago/Turabian StyleLi, Jia, Sara Zafar, Ayesha Javaid, Shagufta Perveen, Zuhair Hasnain, Muhammad Ihtisham, Adeel Abbas, Muhammad Usman, Ahmed H. El-Sappah, and Manzar Abbas. 2023. "Zinc Nanoparticles (ZnNPs): High-Fidelity Amelioration in Turnip (Brassica rapa L.) Production under Drought Stress" Sustainability 15, no. 8: 6512. https://doi.org/10.3390/su15086512









