Vertical Transportation Diversity of Petroleum Pollutants under Groundwater Fluctuations and the Instructions for Remediation Strategy
Abstract
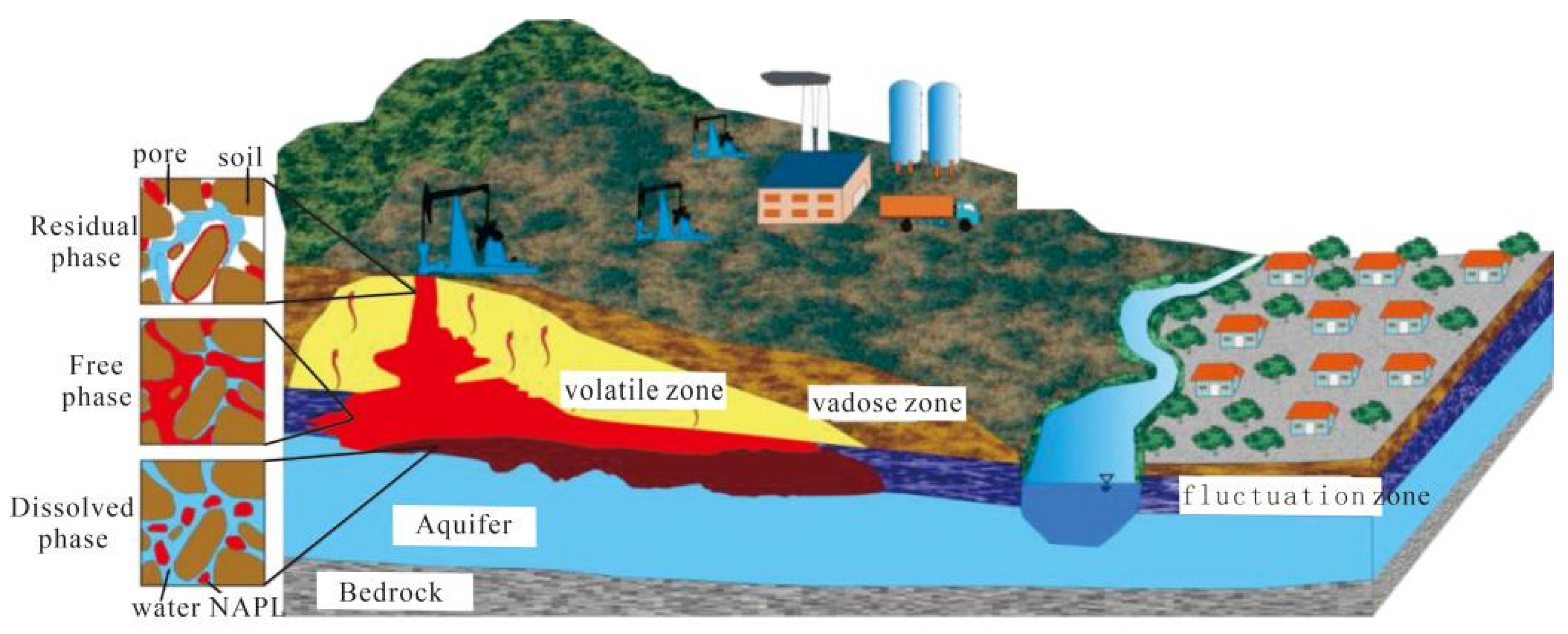
:1. Introduction
2. Materials and Methods
2.1. Experimental Pollutants and Soil Media
2.1.1. Experimental Pollutants
2.1.2. Experimental Soil Media and Device
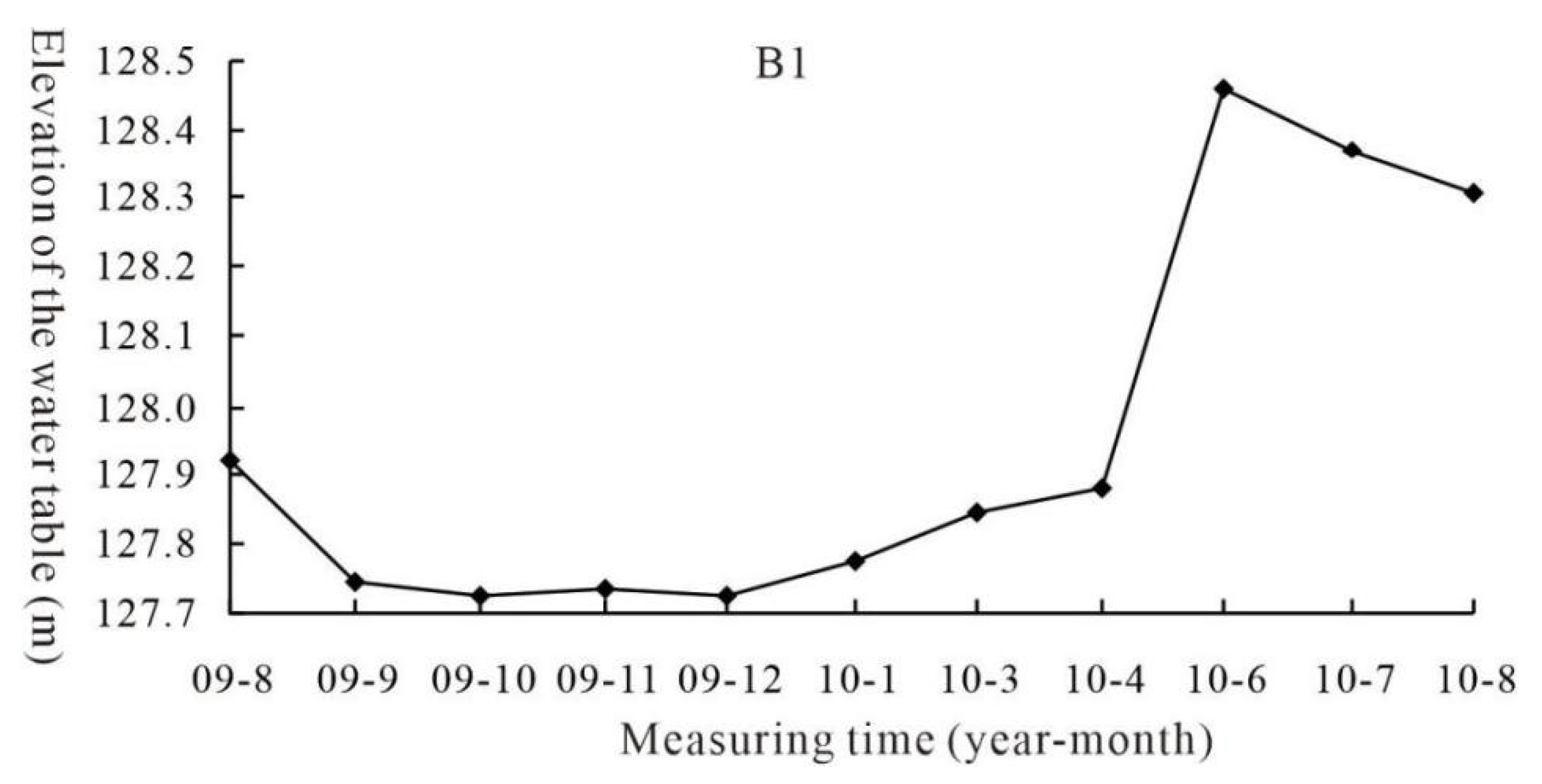
2.2. Simulation Design of the Water Table Fluctuation Zone
2.3. Adding Mode of Pollutants
2.4. Sample Collection and Test Analysis
- (1)
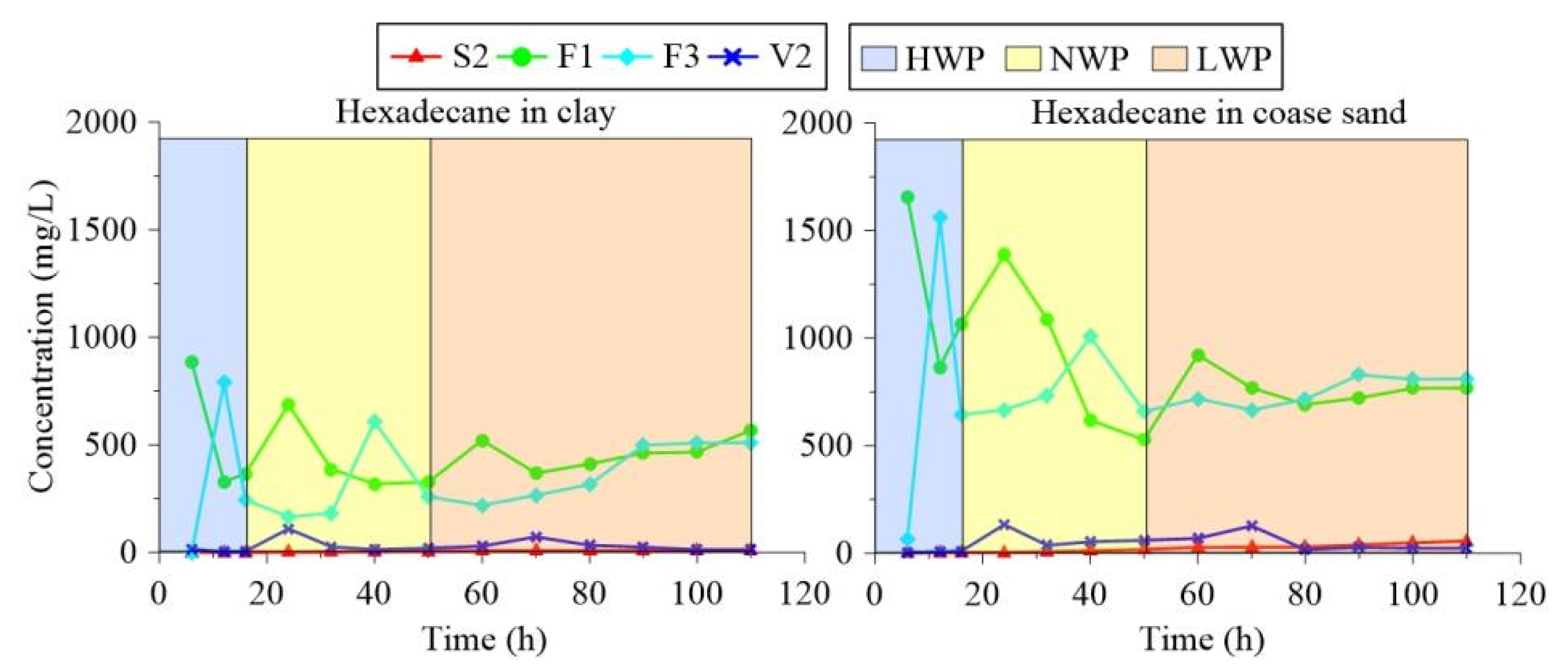
- Soil sampling: During the experiment, soil in the column was taken out, and the infiltration of groundwater was changed, which caused inconsistencies in the simulation. Therefore, parallel experiments were designed. More specifically, experiments on four columns filled with coarse sand were conducted simultaneously under the same conditions. The columns were numbered column 1-1, column 1-2, column 1-3, and column 1-4. The experiment in column 1-1 was terminated after six hours, while the experiments in columns 2, 3, and 4 continued. Then, column 1-1 was unpacked, and soil samples were collected from various layers for measurement. Figure 5 presents S1, S2, and S3 as samples from the aquifer; V1 and V2 as samples from the vadose zone; and F1, F2, and F3 as samples from the fluctuation zone. Similarly, the experiment in column 1-2 was terminated after 32 h, while columns 1-3 and 1-4 continued. Using the sampling method described above, the columns filled with clay (column 2-1, column 2-2, column 2-3, and column 2-4) were collected in the same way as the coarse sand columns. These settings ensured that the soil media were not disturbed and that the experimental conditions did not change with soil sampling. The sampling times for the four soil columns were 6 h, 32 h, 82 h, and 110 h, respectively.
- (2)
- Water sampling: After 3 h, a water sample was collected from the lateral-face sampling hole. The sampling volume was 10 mL, and all sampling holes were accessible during sample collection. Whereas the samples from hole S2 represented the aquifer, samples F1 and F3 represented the water table fluctuation zone. According to the primary test and references [42], the sampling times were 6 h, 12 h, 16 h, 24 h, 32 h, 40 h, 50 h, 60 h, 70 h, 80 h, 90 h, 100 h, and 110 h.
- (3)
- Gas sampling: The majority of the gas samples were taken from the V2 hole in the aeration zone. The gas-sampling system was installed in the sampling hole. The injector was subsequently adopted. Sampling times were 6 h, 12 h, 16 h, 24 h, 32 h, 40 h, 50 h, 60 h, 70 h, 80 h, 90 h, 100 h, and 110 h.
3. Results and Discussion
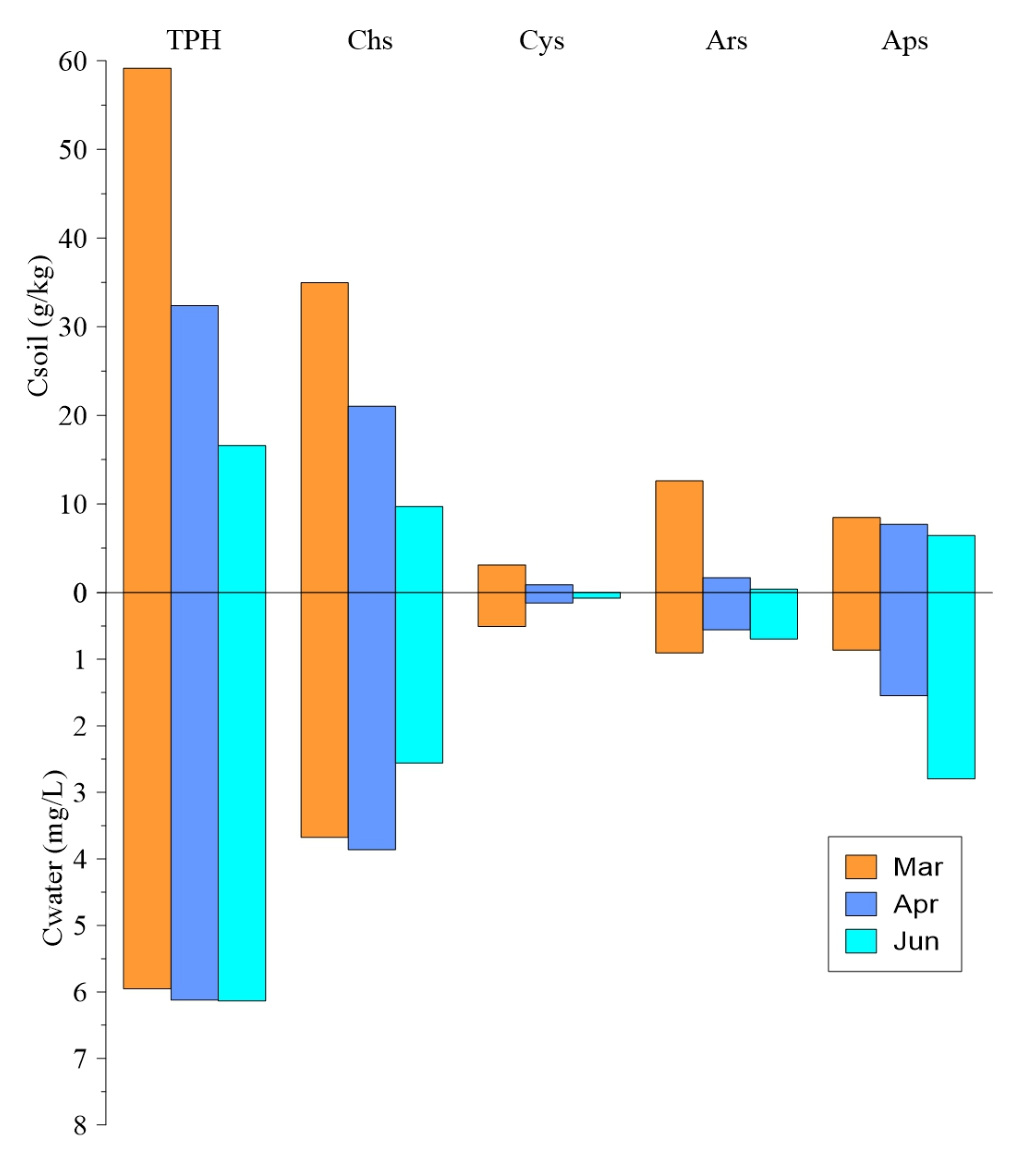
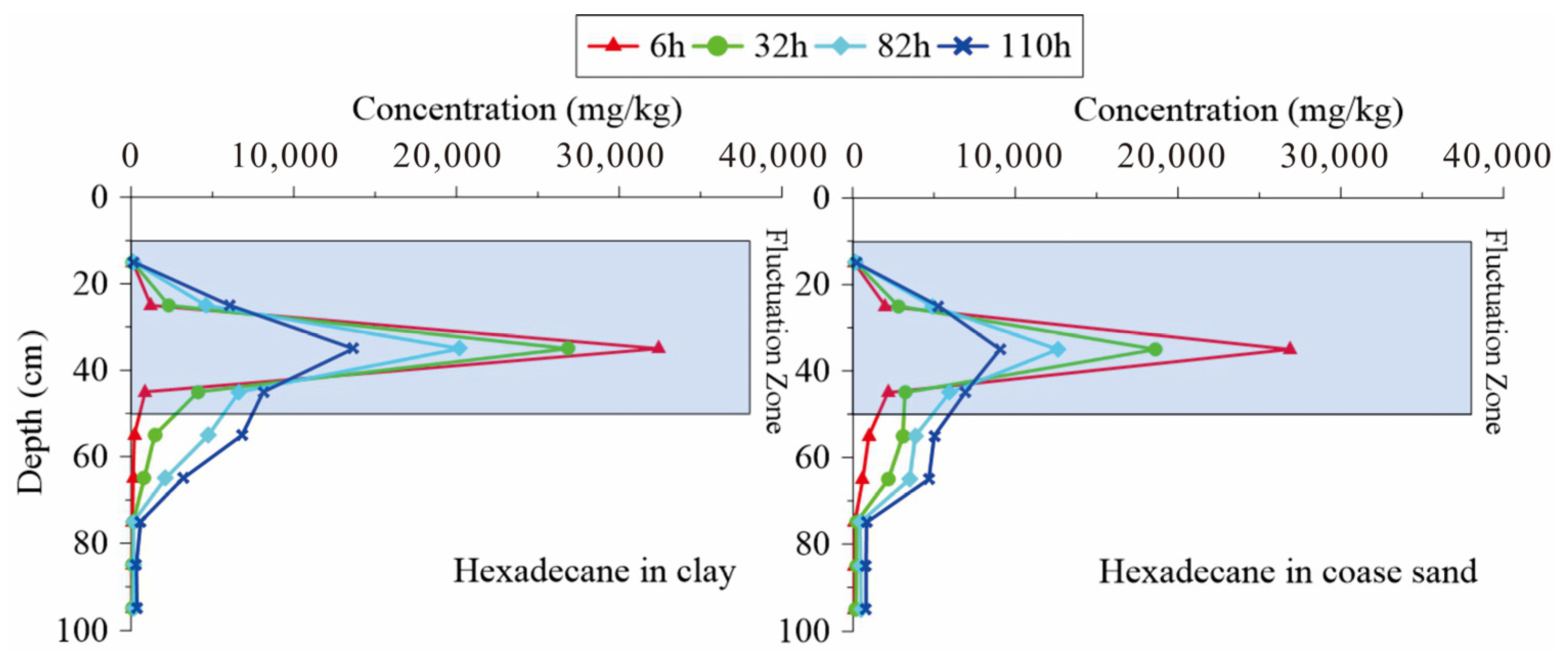
3.1. Change Rules of Chain Alkanes in the Water Fluctuation Process
3.2. Change Rules of Cycloalkane in the Water Fluctuation Process
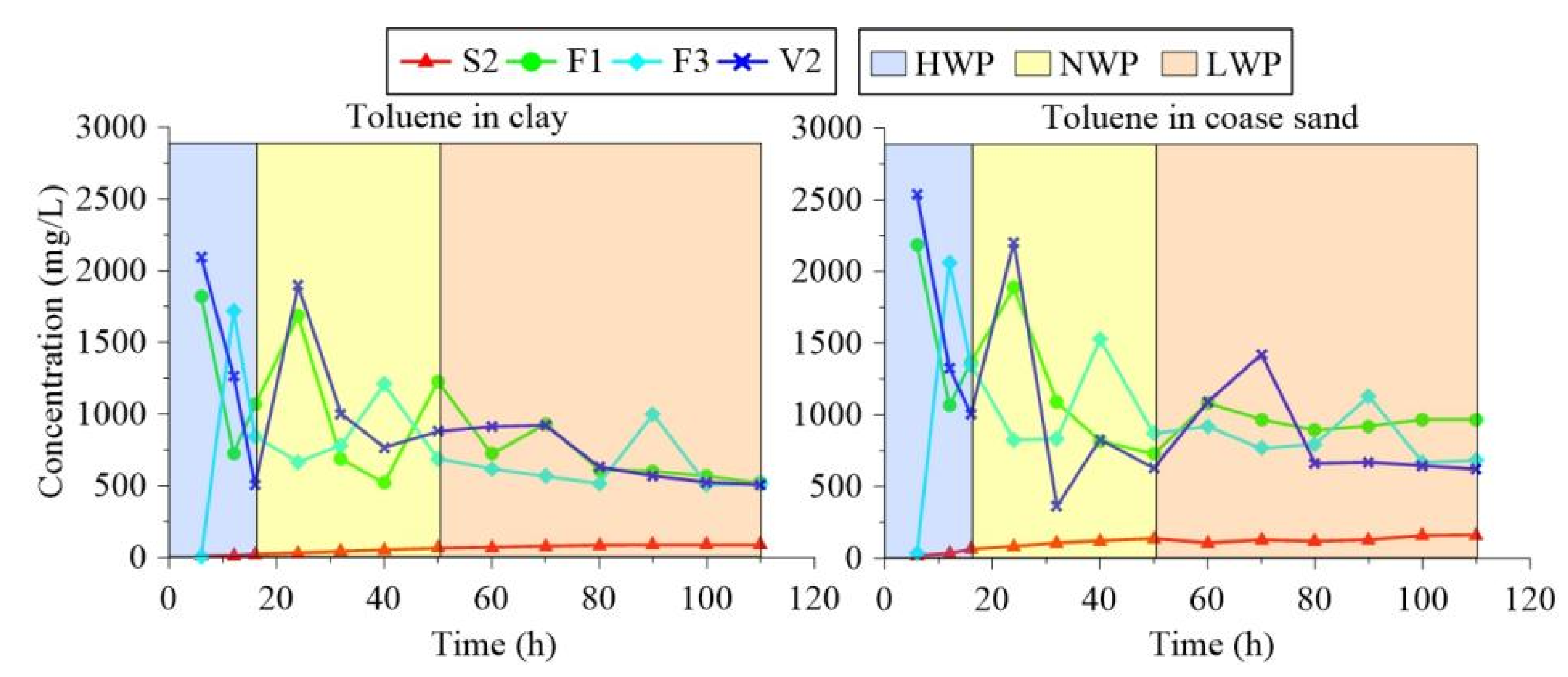
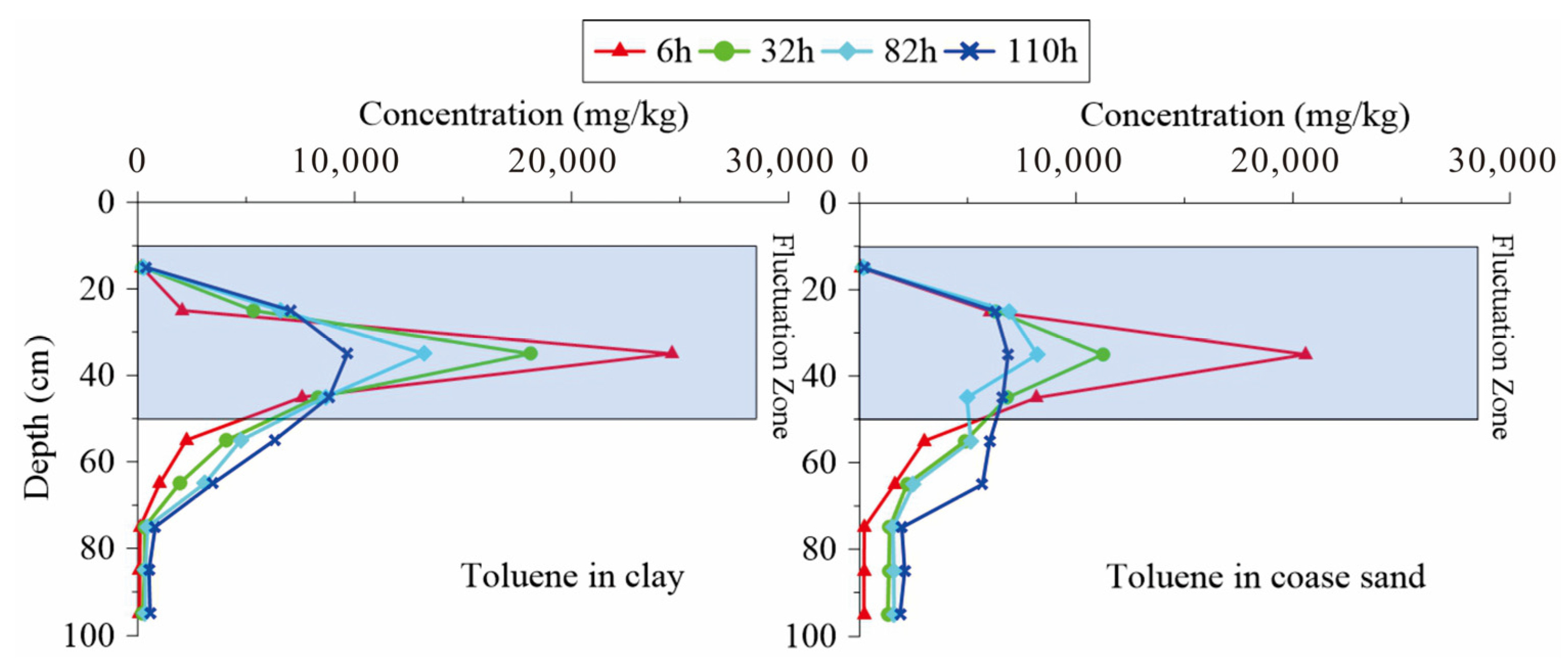
3.3. Change Rules of Aromatic Hydrocarbon in the Water Fluctuation Process
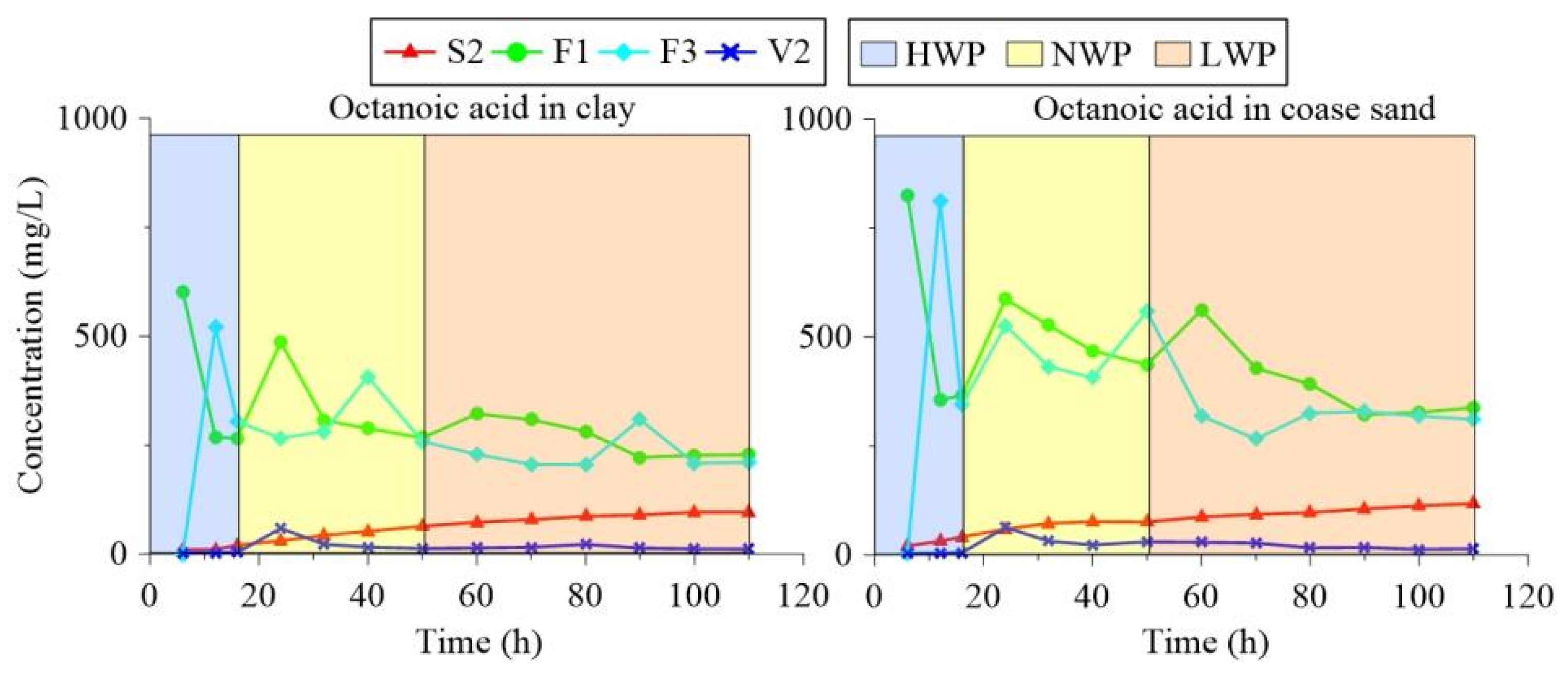
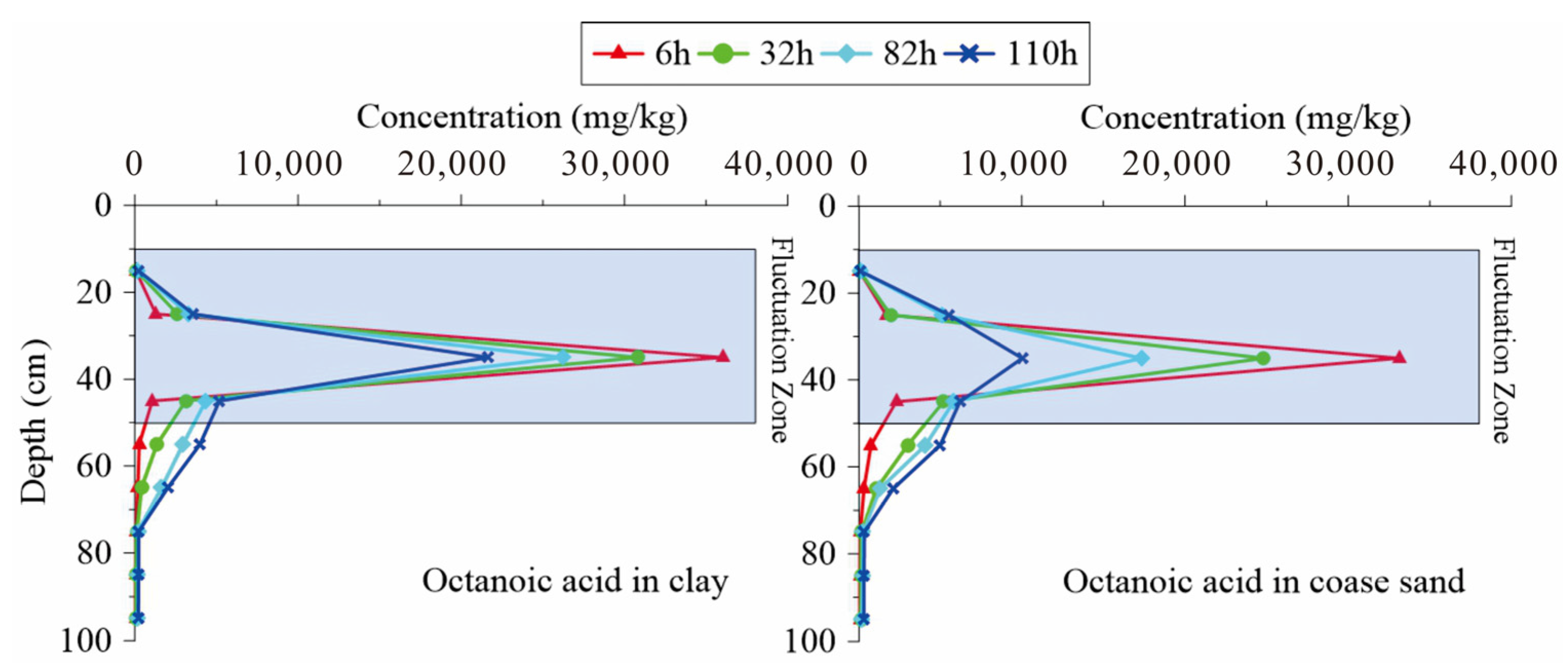
3.4. Change Rules of NOS Organic Substances in the Water Table Fluctuation Process
3.5. Instructions for Remediation Strategy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bajagain, R.; Jeong, S.W. Degradation of petroleum hydrocarbons in soil via advanced oxidation process using peroxymonosulfate activated by nanoscale zero-valent iron. Chemosphere 2021, 270, 128627. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Verpoort, F.; Dong, C.D.; Chen, C.W.; Chen, S.; Kao, C.M. Remediation of petroleum-hydrocarbon contaminated groundwater using optimized in situ chemical oxidation system: Batch and column studies. Process. Saf. Environ. 2020, 138, 18–26. [Google Scholar] [CrossRef]
- Atlas, R.M.; Hazen, T.C. Oil biodegradation and bioremediation: A tale of the two worst spills in US history. Environ. Sci. Technol. 2011, 45, 6709–6715. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Mao, F.; Xiao, Y.; Luo, L. Modeling capillary fringe effect on petroleum vapor intrusion from groundwater contamination. Water Res. 2019, 150, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, G.; Sugiura, N.; Utsumi, M.; Zhang, Z.; Yang, Y. Distribution of petroleum hydrocarbons in soils and the underlying unsaturated subsurface at an abandoned petrochemical site, North China. Hydrol. Process. 2014, 28, 2185–2191. [Google Scholar] [CrossRef]
- Chandra, S.; Sharma, R.; Singh, K.; Sharma, A. Application of bioremediation technology in the environment contaminated with petroleum hydrocarbon. Ann. Microbiol. 2013, 63, 417–431. [Google Scholar] [CrossRef]
- Bolade, O.P.; Adeniyi, K.O.; Williams, A.B.; Benson, N.U. Remediation and optimization of petroleum hydrocarbons degradation in contaminated water using alkaline activated persulphate. J. Environ. Chem. Eng. 2021, 9, 105801. [Google Scholar] [CrossRef]
- Xia, C.; Liu, Q.; Zhao, L.; Wang, L.; Tang, J. Enhanced degradation of petroleum hydrocarbons in soil by FeS@BC activated persulfate and its mechanism. Sep. Purif. Technol. 2022, 282, 120060. [Google Scholar] [CrossRef]
- Liu, B.; Liu, F.; Chen, H. Sorption characteristics of the major components of crude oil to vadose zone soil in Xinjiang oilfield, China. Fresenius Environ. Bull. 2013, 22, 3–10. [Google Scholar]
- Liu, J.; Wei, K.; Xu, S.; Cui, J.; Ma, J.; Xiao, X.; Xi, B.; He, X. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef]
- Li, K.; Ding, W.; Wang, F.; Lu, Z.; Chen, Y. Countermeasure and suggestions for soil contamination prevention and control of oil exploration industry. Environ. Prot. Chem. Ind. 2019, 39, 603–607. (In Chinese) [Google Scholar]
- Wang, B.; Liu, Q.; Zhang, S.; You, H.; Ma, Y.; An, J. Adsorption of petroleum pollutants released from soil by biocha. Acta Pet. Sin. Pet. Process. Sect. 2019, 35, 603–612. (In Chinese) [Google Scholar]
- Li, J.; Cao, X.; Sui, H.; He, L.; Li, X. Research status and prospect of petroleum contaminated soil remediation technology. Acta Pet. Sin. Pet. Process. Sect. 2017, 33, 811–833. (In Chinese) [Google Scholar]
- API. Technical Data Book-Petroleum Refining, 5th ed.; American Petroleum Institute: Washington, DC, USA, 1992. [Google Scholar]
- Song, D.; Kitamura, M.; Katayama, A.A. Approach for estimating microbial growth and biodegradation of hydrocarbon contaminants in subsoil based on field measurements: 2. Application in a field lysimeter experiment. Environ. Sci. Technol. 2010, 44, 6795–6801. [Google Scholar] [CrossRef]
- Ding, A.; Liu, B.; Liang, X.; Li, S.; Zhang, L.; Yin, H. A review of the petroleum hydrocarbon contamination transformation performance in the zone of intermittent saturation. Sci. Technol. Eng. 2018, 18, 172–178. (In Chinese) [Google Scholar]
- Podgorski, D.C.; Zito, P.; Kellerman, A.M.; Bekins, B.A.; Spencer, R.G.M. Hydrocarbons to carboxyl-rich alicyclic molecules: A continuum model to describe biodegradation of petroleum-derived dissolved organic matter in contaminated groundwater plumes. Environ. Technol. Innov. 2018, 10, 175–193. [Google Scholar] [CrossRef]
- Abbas, O.; Rebufa, C.; Dupuy, N.; Permanyer, A.; Kister, J. Assessing petroleum oils biodegradation by chemometric analysis of spectroscopic data. Talanta 2008, 75, 857–871. [Google Scholar] [CrossRef]
- Rayner, J.L.; Snape, I.; Walworth, J.L.; Harvey, P.; Ferguson, S.H. Petroleum–hydrocarbon contamination and remediation by microbioventing at sub-Antarctic Macquarie Island. Cold Reg. Sci. Technol. 2015, 48, 139–153. [Google Scholar] [CrossRef]
- Weishaar, J.A.; Tsao, D.; Burken, J.G. Phytoremediation of BTEX hydrocarbons: Potential impacts of diurnal groundwater fluctuation on microbial degradation. Int. J. Phytoremediat. 2009, 11, 509–523. [Google Scholar] [CrossRef]
- Lenhard, R.J.; Oostrom, M.; Dane, J.H. A constitutive model for air-NAPL-water flow in the vadose zone accounting for immobile, non-occluded (residual) NAPL in strongly water-wet porous media. J. Contam. Hydrol. 2004, 71, 261–282. [Google Scholar] [CrossRef]
- Oostrom, M.; Hofstee, C.; Lenhard, R.J.; Wietsma, T.W. Flow behavior and residual saturation formation of liquid carbon tetrachloride in unsaturated heterogeneous porous media. Contam. Hydrol. 2003, 4, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Jarsjö, J.; Destouni, G.; Yaron, B. Retention and volatilisation of kerosene: Laboratory experiments on glacial and post-glacial soils. J. Contam. Hydrol. 1994, 17, 167–185. [Google Scholar] [CrossRef]
- Atekwana, E.A.; Atekwana, E.A. Geophysical signatures of microbial activity at hydrocarbon contaminated sites: A review. Surv. Geophys. 2010, 31, 247–283. [Google Scholar] [CrossRef]
- Bekins, B.A.; Cozzarelli, I.M.; Godsy, E.M.; Warren, E.; Essaid, H.I.; Tuccillo, M.E. Progression of natural attenuation processes at a crude oil spill site: II. Controls on spatial distribution of microbial populations. J. Contam. Hydrol. 2001, 53, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Rijal, M.L.; Appel, E.; Petrovský, E.; Blaha, U. Change of magnetic properties due to fluctuations of hydrocarbon contaminated groundwater in unconsolidated sediments. Environ. Pollut. 2010, 158, 1756–1762. [Google Scholar] [CrossRef]
- McCray, J.E.; Tick, G.R.; Jawitz, J.W.; Gierke, J.; Brusseau, M.; Falta, R.; Knox, R.; Sabatini, D.; Annable, M.; Harwell, J.; et al. Remediation of NAPL source zones: Lessons learned from field studies at Hill and Dover AFB. Groundwater 2011, 49, 727–744. [Google Scholar] [CrossRef] [Green Version]
- Tzovolou, D.N.; Benoit, Y.; Haeseler, F.; Klintd, K.E.; Tsakiroglou, C.D. Spatial distribution of jet fuel in the vadoze zone of a heterogeneous and fractured soil. Sci. Total Environ. 2009, 407, 3044–3054. [Google Scholar] [CrossRef]
- Giampaolo, V.; Rizzo, E.; Titov, K.; Konosavsky, P.; Laletina, D.; Maineult, A.; Lapenna, V. Self-potential monitoring of a crude oil-contaminated site (Trecate, Italy). Environ. Sci. Pollut. Res. 2014, 21, 8932–8947. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.S.; Du, X.; Cao, Y.; Lei, Y. Fate and Transport of Petroleum Hydrocarbons in Vadose Zone: Compound-specific Natural Attenuation. Water Air Soil Pollut. 2013, 224, 1439. [Google Scholar] [CrossRef]
- Lopes de Castro, D.; Branco, R.M.G.C. 4-D ground penetrating radar monitoring of a hydrocarbon leakage site in Fortaleza (Brazil) during its remediation process: A case history. J. Appl. Geophy. 2003, 54, 127–144. [Google Scholar] [CrossRef]
- Thorstad, J.L. Influence of Borehole Construction on LNAPL Thickness Measurements; Oklahoma State University: Stillwater, OK, USA, 2007. [Google Scholar]
- Vermaak, K.H. Characterisation and Management of a LNAPL Pollution Site along the Coastal Regions; University of the Free State: Bloemfontein, South Africa, 2009. [Google Scholar]
- Luo, J.; Lu, W.; Yang, Q.; Ji, Y.; Xin, X. An adaptive dynamic surrogate model using a constrained trust region algorithm: Application to DNAPL-contaminated-groundwater-remediation design. Hydrogeol. J. 2020, 28, 1285–1298. [Google Scholar] [CrossRef]
- Villaume, J.F. Investigations at Sites Contaminated with Dense, Non-Aqueous Phase Liquids (NAPLs). Groundw. Monit. R. 2010, 5, 60–74. [Google Scholar] [CrossRef]
- Shi, J.; Deng, F.; Xiao, L.; Liu, H.; Ma, F.; Wang, M.; Zhao, R.; Chen, S.; Zhang, J.; Xiong, C. A proposed NMR solution for multi-phase flow fluid detection. Pet. Sci. 2019, 16, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Gudbjerg, J.; Sonnenborg, T.O.; Jensen, K.H. Remediation of NAPL below the water table by steam-induced heat conduction. J. Contam. Hydrol. 2004, 72, 207–225. [Google Scholar] [CrossRef]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 266, 100526. [Google Scholar] [CrossRef]
- Marcon, L.; Oliveras, J.; Puntes, V.F. In situ nanoremediation of soils and groundwaters from the nanoparticle’s standpoint: A review. Sci. Total. Environ. 2021, 791, 148324. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Monterroso, C.; Casares, J.J. Environmental Fate of Petroleum Hydrocarbons in Soil: Review of Multiphase Transport, Mass Transfer, and Natural Attenuation Processes. Pedosphere 2018, 28, 3–17. [Google Scholar] [CrossRef]
- Oostrom, M.; Hofstee, C.; Wietsma, T.W. LNAPLs do not Always Float: An Example Case of a Viscous LNAPL under Variable Water Table Conditions. J. Hydrol. Days 2006. [CrossRef]
- Miller, C.D.; Durnford, D.S.; Fowler, A.B. Equilibrium nonaqueous phase liquid pool geometry in coarse soils with discrete textural interfaces. J. Contam. Hydrol. 2004, 71, 239–260. [Google Scholar] [CrossRef]
- Roy, J.W.; Smith, J.E. Multiphase flow and transport caused by spontaneous gas phase growth in the presence of dense non-aqueous phase liquid. J. Contam. Hydrol. 2007, 89, 251–269. [Google Scholar] [CrossRef]
- Zhen, X.L.; Wang, B.C.; She, Z.L. Principle and Application of Oil Pollution in Soil—Groundwater System; Geological Publishing House: Beijing, China, 2004. (In Chinese) [Google Scholar]







| PS | P | SM & D (m) | WFA (m) | C (g/kg) | R |
|---|---|---|---|---|---|
| Perth (Australia) | Diesel oil | mixture of fine, medium and coarse sand (4) | 0.8 | 40.1 | [23] |
| Crystal Refinery (Mexico) | Crude oil | medium sand (3.9), coarse sand (0.4), sandy gravel (0.6) | 0.39 | 36.5 | [24] |
| Bemidji (USA) | Crude oil | medium sand (5.0) | 0.37 | 112.6 | [25] |
| Hradcany AFB (USA) | Gasoline | mixture of fine, medium and coarse sand (8) | 0.33 | 316.9 | [26] |
| Northern Refinery (China) | Crude oil | loam (1.7–3), clay (5), mixture of fine, medium and coarse sand (4) | 2.0 | 56.3 | [5] |
| AFB (USA) | Crude oil | mixture of fine, medium and coarse sand (9) | 2 | 89.6 | [27] |
| Kluczewo (Poland) | Gasoline | sandy clay (1.7), medium sand (3.4), clay (1.9) | 0.5–1.0 | 68.6 | [28] |
| Trecate (Italy) | Crude oil | silt (2.5), fine sand (7.5), clay (0.8), medium sand (5.0), sandy gravel (5.2) | 6.0 | 125.8 | [29] |
| Northeastern oilfield (China) | Crude oil | fine sand (1.5), caly (1.5), medium sand (3.0), coarse sand (4.0) | 0.5 | 50.8 | [30] |
| Fortakeza (Brazil) | Crude oil | clay (2.3) | 1.8 | 299.1 | [31] |
| Golden Oklahoma (USA) | Crude oil | silt clay (4.6), sandy gravel (0.31), coarse sand (0.15) | 1.3–2.8 | 121.2 | [32] |
| East London (England) | Crude oil | medium sand (4), sandy clay (7) | 0.3–3 | 39.6 | [33] |
| MW (g/mol) | FG | A (cm−1) | S (mg/L) | ρ (g/cm3) | BP (℃) | η (mPa·s) | H (cm3/cm3) | Log Kow | Koc (cm3/g) | Dair (cm2/s) | Dwater (cm2/s) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexadecane | 226.4 | -(CH2)n- | 2960 | 26 | 0.773 | 287 | 3.34 | 1.6 × 102 | 8.24 | 8.47 × 106 | 0.037 | 4.2 × 10−6 |
| Cyclohexane | 84.2 |  | 1450 | 55 | 0.779 | 80.7 | 0.98 | 7.8 × 100 | 3.44 | 9.63 × 102 | 0.084 | 9.1 × 10−6 |
| Toluene | 92.1 |  | 748 | 515 | 0.866 | 110.6 | 0.59 | 2.7 × 10−1 | 2.69 | 2.34 × 102 | 0.087 | 8.6 × 10−6 |
| Octanoic acid | 144.2 | -COOH | 1244 | 680 | 0.911 | 239.7 | 5.83 | 5.0× 102 | 3.05 | 1.10 × 104 | 0.023 | 3.6 × 10−6 |
| Characteristics | Column 1 | Column 2 |
|---|---|---|
| Media type | Coarse sand | Clay |
| Packing weight (kg) | 12.94 | 10.75 |
| Packing height (cm) | 90 | 90 |
| Packing density (g/cm3) | 1.83 | 1.52 |
| Porosity (%) | 30.9 | 42.7 |
| Specific surface area (m2/kg) | 58.8 | 449.6 |
| Organic matter content (%) | 1.5 | 3.8 |
| Mean diameter (μm) | 653.55 | 45.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Yang, M.; Tan, T.; Song, X. Vertical Transportation Diversity of Petroleum Pollutants under Groundwater Fluctuations and the Instructions for Remediation Strategy. Sustainability 2023, 15, 6514. https://doi.org/10.3390/su15086514
Cao Z, Yang M, Tan T, Song X. Vertical Transportation Diversity of Petroleum Pollutants under Groundwater Fluctuations and the Instructions for Remediation Strategy. Sustainability. 2023; 15(8):6514. https://doi.org/10.3390/su15086514
Chicago/Turabian StyleCao, Zhendong, Mingxing Yang, Tingjing Tan, and Xiaoqing Song. 2023. "Vertical Transportation Diversity of Petroleum Pollutants under Groundwater Fluctuations and the Instructions for Remediation Strategy" Sustainability 15, no. 8: 6514. https://doi.org/10.3390/su15086514





