Different Cropping Patterns to Restore Saline-Alkali Soils in Northeast China Affect the Abundance of Functional Genes in the Soil Nitrogen Cycle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sampling
2.4. Soil Chemical Analysis
2.5. Soil DNA Extraction and Quantitative PCR (qPCR)
2.6. Statistical Analysis
3. Results
3.1. Soil Chemical Properties and Soil Enzymes
3.2. N-Cycling Gene Abundance
3.3. N-Cycling Gene Abundance Ratio
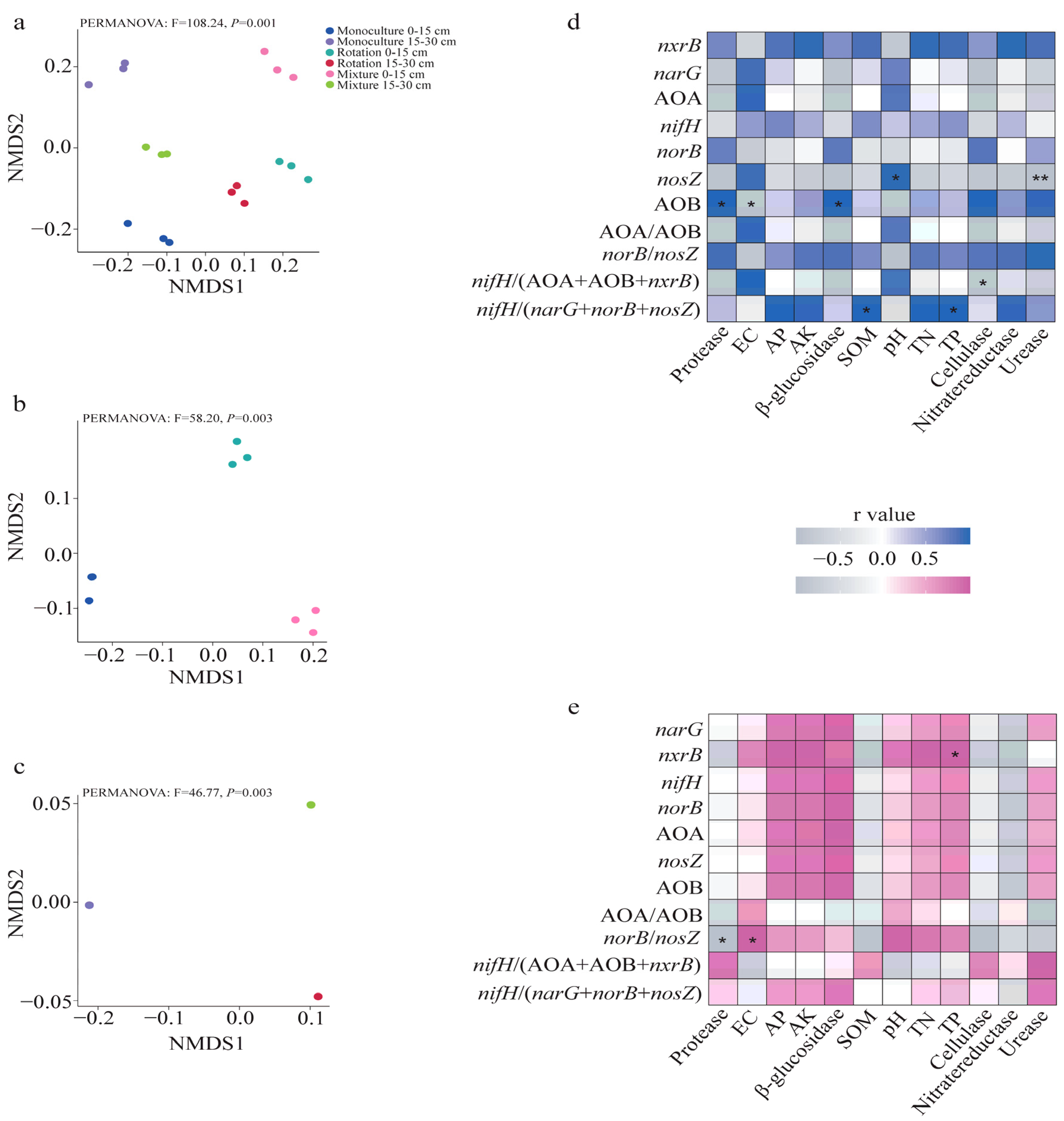
3.4. Association of Soil Properties and Enzyme Activity with Gene Abundance
3.5. Random Forest Analysis
4. Discussion
4.1. Complex Cropping Patterns Alter nifH Gene Expression
4.2. Cropping Patterns Ecological Niches Affect Nitrifying Bacteria Abundance
4.3. Mixture and Rotation Reduce N Losses by Reducing narG and nosZ Abundance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fagodiya, R.K.; Malyan, S.K.; Singh, D.; Kumar, A.; Yadav, R.K.; Sharma, P.C.; Pathak, H. Greenhouse gas emissions from salt-affected soils: Mechanistic understanding of interplay factors and reclamation approaches. Sustainability 2022, 14, 11876. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.-L.; Wang, S. Brief analysis on the trend of improve saline alkali soil in China. Hubei Agric. Sci. 2021, 59, 302–306. [Google Scholar]
- Ma, Y.; Tashpolat, N. Current Status and Development Trend of Soil Salinity Monitoring Research in China. Sustainability 2023, 15, 5874. [Google Scholar] [CrossRef]
- Shao, Q.; Han, N.; Ding, T.; Zhou, F.; Wang, B. SsHKT1; 1 is a potassium transporter of the C3 halophyte Suaeda salsa that is involved in salt tolerance. Funct. Plant Biol. 2014, 41, 790–802. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Lyu, M.J.A.; Leng, B.Y.; Zhu, X.G.; Wang, B.S. The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant Mol. Biol. 2016, 91, 241–256. [Google Scholar] [CrossRef]
- Yuan, F.; Liang, X.; Li, Y.; Yin, S.; Wang, B. Methyl jasmonate improves tolerance to high salt stress in the recretohalophyte Limonium bicolor. Funct. Plant Biol. 2019, 46, 82–92. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Yang, Z.; Wei, X.; Yuan, F.; Yin, S.; Wang, B. Evaluation of salt-tolerant germplasm and screening of the salt-tolerance traits of sweet sorghum in the germination stage. Funct. Plant Biol. 2018, 45, 1073–1081. [Google Scholar] [CrossRef]
- Wang, F.X.; Yin, C.H.; Song, Y.P.; Li, Q.; Tian, C.Y.; Song, J. Reproductive allocation and fruit-set pattern in the euhalophyte Suaeda salsa in controlled and field conditions. Plant Biosyst. 2018, 152, 749–758. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Yuan, F.; Chen, M. Exogenous salicylic acid improves the germination of Limonium bicolor seeds under salt stress. Plant Signal. Behav. 2019, 14, e1644595. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Wei, X.; Zhao, X.; Wang, B.; Sui, N. Transcription profiles of genes related to hormonal regulations under salt stress in sweet sorghum. Plant Mol. Biol. Rep. 2017, 35, 586–599. [Google Scholar] [CrossRef]
- Song, Y.; Ma, L.; Zhang, H.; Fu, R.; Liang, X.; Li, J.; Li, J.; Li, M.; Shan, Y.; Cheng, J.; et al. The diversity and structure of diazotrophic communities in the rhizosphere of coastal saline plants is mainly affected by soil physicochemical factors but not host plant species. Front. Microbiol. 2022, 9, 2634. [Google Scholar] [CrossRef]
- Boyrahmadi, M.; Raiesi, F. Plant roots and species moderate the salinity effect on microbial respiration, biomass, and enzyme activities in a sandy clay soil. Biol. Fert. Soils. 2018, 54, 509–521. [Google Scholar] [CrossRef]
- Choudhary, M.; Chandra, P.; Dixit, B.; Nehra, V.; Choudhary, U.; Choudhary, S. Plant growth-promoting microbes: Role and prospective in amelioration of silk stress. Commun. Soil Sci. Plan. 2022, 53, 1692–1711. [Google Scholar] [CrossRef]
- Luo, S.S.; Tian, L.; Chang, C.L.; Wang, S.J.; Zhang, J.F.; Zhou, X.; Li, X.J.; Tran, L.S.P.; Tian, C.J. Grass and maize vegetation systems restore saline-sodic soils in the Songnen Plain of northeast China. Land Degrad. Dev. 2018, 29, 1107–1119. [Google Scholar] [CrossRef]
- Guo, Z.G.; Liu, H.X.; Wang, S.M.; Tian, F.P.; Cheng, G.D. Biomass, persistence and drought resistance of nine lucerne varieties in the dry environment of west China. Aust. J. Exp. Agr. 2005, 45, 59–64. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, W.; Xi, L. Alfalfa cover crops influence the soil fungal community and function in apple orchards in arid desert oases in northwest China. Sustainability 2022, 14, 11816. [Google Scholar] [CrossRef]
- Li, B.; Liu, X.; Zhu, D.; Su, H.; Guo, K.; Sun, G.; Li, X.; Sun, L. Crop diversity promotes the recovery of fungal communities in saline-alkali areas of the Western Songnen Plain. Front. Microbiol. 2023, 14, 52. [Google Scholar] [CrossRef]
- Singh, A.; Quinn, N.W.T.; Benes, S.E.; Cassel, F. Policy-driven sustainable saline drainage disposal and forage production in the western San Joaquin Valley of California. Sustainability 2020, 12, 6362. [Google Scholar] [CrossRef]
- Jensen, K.B.; Pearse, G.; Larson, S.R.; Robins, J.G. ‘AlkarXL’, a new tall wheatgrass cultivar for use on saline semiarid lands. J. Plant Regist. 2020, 14, 298–305. [Google Scholar] [CrossRef]
- Suyama, H.; Benes, S.E.; Robinson, P.H.; Getachew, G.; Grattan, S.R.; Grieve, C.M. Biomass yield and nutritional quality of forage species under long-term irrigation with saline-sodic drainage water: Field evaluation. Anim. Feed Sci. Technol. 2007, 135, 329–345. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi, A.; Andrade, D.S.; Dick, R.P. Microbial biomass in soils under different tillage and crop rotation systems. Biol. Fert. Soils 2003, 38, 15–20. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, F.; Zhou, H.; Lyu, S.; Liu, W.; Cui, G.; Fan, Z.; Liu, L.; Zhang, K. Preliminary Screening of Vegetable Varieties Suitable for Rotation to Prevent and Control Banana Fusarium Wilt. Chin. J. Trop. Crops 2021, 42, 1678–1684. [Google Scholar]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- Bainard, L.D.; Evans, B.; Malis, E.; Yang, T.; Bainard, J.D. Influence of Annual Plant Diversity on Forage Productivity and Nutrition, Soil Chemistry, and Soil Microbial Communities. Front. Microbiol. 2020, 4, 560479. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.; Jones, H.G.; Karley, A.J.J.N.P. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Gaba, S.; Lescourret, F.; Boudsocq, S.; Enjalbert, J.; Hinsinger, P.; Journet, E.P.; Navas, M.L.; Wery, J.; Louarn, G.; Malézieux, E.; et al. Multiple cropping systems as drivers for providing multiple ecosystem services: From concepts to design. Agron. Sustain. Dev. 2015, 35, 607–623. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.M.; Kraemer, F.B.; Soria, M.A.; Guerrero, L.D.; Morrás, H.J.; Figuerola, E.L.; Erijman, L.J.A.S.E. The influence of soil properties on denitrifying bacterial communities and denitrification potential in no-till production farms under contrasting management in the Argentinean Pampas. Appl. Soil Ecol. 2014, 75, 172–180. [Google Scholar] [CrossRef]
- Martins, S.R.; Casalinho, H.; Silva, J.J.C.A.S. Technology, Qualidade do solo como indicador de sustentabilidade de agroecossistemas. Curr. Agr. Sci. Technol. 2007, 13, 195–203. [Google Scholar]
- Zhang, W.; Shen, Z.; Shao, Y.; Shi, L.; Liu, S.; Shi, N.; Fu, S. Soil biota and sustainable agriculture: A review. Acta Ecol. Sin. 2020, 40, 3183–3206. [Google Scholar]
- Lee, T.K.; Han, I.; Kim, M.S.; Seong, H.J.; Kim, J.S.; Sul, W.J. Characterization of a nifH-Harboring Bacterial Community in the Soil-Limited Gotjawal Forest. Front. Microbiol. 2019, 10, 1858. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Liu, Q.; Zhao, J.; Zhang, J. Ecological niche differentiation of ammonia-oxidising archaea and bacteria in acidic soils due to land use change. Soil Res. 2017, 56, 71–79. [Google Scholar] [CrossRef]
- Gross, C.; Hossen, S.; Hartmann, H.; Noll, M.; Borken, W. Biological nitrogen fixation and nifH gene abundance in dead wood of 13 different tree species. Biogeochemistry 2022, 161, 353–371. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ. Microbiol. 2008, 10, 2931–2941. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Dell, E.A.; Bowman, D.; Rufty, T.; Shi, W. The community composition of soil-denitrifying bacteria from a turfgrass environment. Res. Microbiol. 2010, 161, 315–325. [Google Scholar] [CrossRef]
- Rosa Portilho, I.I.; Savin, M.C.; Borges, C.D.; Tsai, S.M.; Mercante, F.M.; Roscoe, R.; de Carvalho, L.A. Maintenance of N cycling gene communities with crop-livestock integration management in tropical agriculture systems. Agr. Ecosyst. Environ. 2018, 267, 52–62. [Google Scholar] [CrossRef]
- Dai, Z.M.; Li, Y.; Zhang, X.J.; Wu, J.J.; Luo, Y.; Kuzyakov, Y.; Brookes, P.C.; Xu, J.M. Easily mineralizable carbon in manure-based biochar added to a soil influences N2O emissions and microbial-N cycling genes. Land Degrad. Dev. 2019, 30, 406–416. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Wang, Q.; Wang, S.; Liu, D.; Liu, G. Responses of soil ammonia oxidation and ammonia-oxidizing communities to land-use conversion and fertilization in an acidic red soil of southern China. Eur. J. Soil Biol. 2017, 80, 110–120. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Ye, S.; Liu, S.; Stirling, E.; Gilbert, J.A.; Faust, K.; Knight, R.; Jansson, J.K.; Cardona, C.; et al. Earth microbial co-occurrence network reveals interconnection pattern across microbiomes. Microbiome 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.; United States Department of Agriculture (USDA) & Natural Resources Conservation Service (NRCS): Washington, DC, USA, 2010. [Google Scholar]
- Chen, K.; Peng, J.; Li, J.; Yang, Q.; Zhan, X.M.; Liu, N.; Han, X.R. Stabilization of soil aggregate and organic matter under the application of three organic resources and biochar-based compound fertilizer. J. Soil Sediment. 2020, 20, 3633–3643. [Google Scholar] [CrossRef]
- Hu, X.J.; Liu, J.J.; Liang, A.Z.; Li, L.J.; Yao, Q.; Yu, Z.H.; Li, Y.S.; Jin, J.; Liu, X.B.; Wang, G.H. Conventional and conservation tillage practices affect soil microbial co-occurrence patterns and are associated with crop yields. Agric. Ecosyst. Environ. 2021, 319, 107534. [Google Scholar] [CrossRef]
- Guan, S.Y. Soil Enzymes and Their Research Method; Higher Education Press: Beijing, China, 1986; pp. 275–312. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package, Version 1.0.12.; R Project: Tartu, Estonia, 2019.
- Oksanen, J.J. Vegan: Community Ecology Package; R Project: Helsinki, Finland, 2010. [Google Scholar]
- Archer, E. rfPermute: Estimate Permutation p-Values for Random Forest Importance Metrics; R Project: Indianapolis, IN, USA, 2016. [Google Scholar]
- Team, R.J.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009; Volume 14, pp. 12–21. [Google Scholar]
- Guo, Y.D.; Cheng, M.; Zhao, X.F.; Hao, B.P.; Zhang, Y.F.; Cao, W.D.; Zheng, P.S. Effects of green manure rotation on soil properties and yield and quality of silage maize in saline-alkali soils. Chin. J. Eco-Agric. 2018, 26, 856–864. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. Biomed. Res. Int. 2014, 2014, 589341. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Shao, T.; Zhou, Y.; Zhang, X.; Gao, X.; Long, X.; Rengel, Z.J.L.D. Periphyton improves soil conditions and offers a suitable environment for rice growth in coastal saline alkali soil. Land Degrad. Dev. 2021, 32, 2775–2788. [Google Scholar] [CrossRef]
- Alaylar, B.; Gulluce, M.; Karadayi, M. Detection of the nifH gene in nitrogen fixing bacteria from agricultural areas in erzurum. Fresen Environ. Bull. 2020, 29, 809–814. [Google Scholar]
- Zhou, L.T.; Li, J.J.; Pokhrel, G.R.; Chen, J.; Zhao, Y.L.; Bai, Y.; Zhang, C.; Lin, W.X.; Wu, Z.Y.; Wu, C.Z. nifH Gene sequencing reveals the effects of successive monoculture on the soil diazotrophic microbial community in Casuarina equisetifolia plantations. Front. Plant Sci. 2021, 11, 578812. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Li, Q.Q.; Tang, Y.N.; Zhang, H.; Han, C.; Wang, X.; Zhao, X.H.; Shi, G.Y. Selenium enhanced nitrogen accumulation in legumes in soil with rhizobia bacteria. J. Clean. Prod. 2022, 380, 134960. [Google Scholar] [CrossRef]
- Dai, X.L.; Song, D.L.; Guo, Q.K.; Zhou, W.; Liu, G.R.; Ma, R.P.; Liang, G.Q.; He, P.; Sun, G.; Yuan, F.S.; et al. Predicting the influence of fertilization regimes on potential N fixation through their effect on free-living diazotrophic community structure in double rice cropping systems. Soil Boil. Biochem. 2021, 156, 108220. [Google Scholar] [CrossRef]
- Wang, L.N.; Yu, Z.; Yang, J.; Zhou, J. Diazotrophic bacterial community variability in a subtropical deep reservoir is correlated with seasonal changes in nitrogen. Environ. Sci. Pollut. Res. 2015, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.Z.; Hong, H.S.; Zhang, Y.Z.; Chen, N.W.; Zeng, Y.; Wang, W.P. Health, Anthropogenic nitrogen sources and exports in a village-scale catchment in Southeast China. Environ. Geochem. Health 2006, 28, 45. [Google Scholar] [CrossRef]
- Hao, J.Q.; Feng, Y.Z.; Wang, X.; Yu, Q.; Zhang, F.; Yang, G.H.; Ren, G.X.; Han, X.H.; Wang, X.J.; Ren, C.J. Soil microbial nitrogen-cycling gene abundances in response to crop diversification: A meta-analysis. Sci. Total Environ. 2022, 838, 156621. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Wang, J.; Wang, J.D.; Zhang, Y.C. Long-term fertilization with high nitrogen rates decreased diversity and stability of diazotroph communities in soils of sweet potato. Appl. Soil Ecol. 2022, 170, 104266. [Google Scholar] [CrossRef]
- Xu, H.; Wu, X.; Zhao, L.; Zhao, Y.; Wu, T.; Hu, G.; Li, W.; Ding, Y. Changes in soil enzyme activities under different vegetation types of the northern fringe of the permafrost regions in the Qinghai-Tibetan Plateau. Fresen. Environ. Bull. 2015, 24, 4720–4728. [Google Scholar]
- Graham, E.B.; Yang, F.; Bell, S.; Hofmockel, K.S. High Genetic Potential for Proteolytic Decomposition in Northern Peatland Ecosystems. Appl. Environ. Microb. 2019, 85, e02851-18. [Google Scholar] [CrossRef] [Green Version]
- Geisseler, D.; Horwath, W.R. Regulation of extracellular protease activity in soil in response to different sources and concentrations of nitrogen and carbon. Soil Boil. Biochem. 2008, 40, 3040–3048. [Google Scholar] [CrossRef]
- Ladd, J.; Butler, J. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.M.; Song, W.F.; Wen, S.L.; Wang, B.R.; Zhu, C.Q.; Shen, R.F. Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 2017, 113, 240–249. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, D.; Zhang, L.; Li, J.; Raza, W.; Huang, Q.W.; Shen, Q.R. Temporal variation of diazotrophic community abundance and structure in surface and subsoil under four fertilization regimes during a wheat growing season. Agric. Ecosyst. Environ. 2016, 216, 116–124. [Google Scholar] [CrossRef]
- Zou, J.X.; Yao, Q.; Liu, J.J.; Li, Y.S.; Song, F.Q.; Liu, X.B.; Wang, G.H. Changes of diazotrophic communities in response to cropping systems in a Mollisol of Northeast China. PeerJ 2020, 8, e9550. [Google Scholar] [CrossRef] [PubMed]
- Collavino, M.M.; Tripp, H.J.; Frank, I.E.; Vidoz, M.L.; Calderoli, P.A.; Donato, M.; Zehr, J.P.; Aguilar, O.M. nifH pyrosequencing reveals the potential for location-specific soil chemistry to influence N2-fixing community dynamics. Environ. Microbiol. 2014, 16, 3211–3223. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Yu, Z.; Li, Y.; Wang, G.; Tang, C.; Mathesius, U.; Liu, X.; Liu, J.; Liu, J.; Herbert, S.J.; et al. Linking rhizospheric diazotrophs to the stimulation of soybean N2 fixation in a Mollisol amended with maize straw. Plant Soil 2021, 463, 279–289. [Google Scholar] [CrossRef]
- Colloff, M.J.; Wakelin, S.A.; Gomez, D.; Rogers, S.L. Detection of nitrogen cycle genes in soils for measuring the effects of changes in land use and management. Soil Biol. Biochem. 2008, 40, 1637–1645. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2014, 8, 660–674. [Google Scholar] [CrossRef]
- Ventouses, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B.; et al. Towards an Ecological Understanding of Biological Nitrogen Fixation. In The Nitrogen Cycle at Regional to Global Scales; Springer: Dordrecht, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Liu, H.Y.; Qin, S.Y.; Liu, H.E. Comammox Nitrospira and AOB communities are more sensitive than AOA community to different fertilization strategies in a fluvo-aquic soil. Agr. Ecosyst. Environ. 2023, 342. [Google Scholar] [CrossRef]
- Sias, C.; Wolters, B.R.; Reiter, M.S.; Flessner, M.L. Cover crops as a weed seed bank management tool: A soil down review. Ital. J. Agron. 2021, 16, 1852. [Google Scholar] [CrossRef]
- Ile, O.J.; Aguilos, M.; Morkoc, S.; Heitman, J.; King, J.S. Root biomass distribution and soil physical properties of short-rotation coppice American sycamore (Platanus occidentalis L.) grown at different planting densities. Forests 2021, 12, 1806. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Chen, L.; Wu, Z. Response of soil saccharidase activities to free-air carbon dioxide enrichment (FACE) under rice-wheat rotation. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2004, 15, 1019–1024. [Google Scholar]
- Munroe, J.W.; McCormick, I.; Deen, W.; Dunfield, K.E. Effects of 30 years of crop rotation and tillage on bacterial and archaeal ammonia oxidizers. J. Environ. Qual. 2016, 45, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Bai, N.L.; Sun, H.F.; Zhou, S.; Zheng, X.Q.; Li, S.X.; Zhang, J.Q.; Zhang, H.Y.; Lv, W.G. Spatial and temporal responses of ammonia-oxidizing bacteria and archaea to organic amendments in rice-wheat rotation system. Appl. Soil Ecol. 2019, 139, 94–99. [Google Scholar] [CrossRef]
- Zhang, H.L.; Sun, H.F.; Zhou, S.; Bai, N.L.; Zheng, X.Q.; Li, S.X.; Zhang, J.Q.; Lv, W.G. Effect of straw and straw biochar on the community structure and diversity of ammonia-oxidizing bacteria and archaea in rice-wheat rotation ecosystems. Sci. Rep. 2019, 9, 9367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, H.J.; Tang, C.Y.; Cao, Y.J.; Li, X.; Huang, P.Y. Contribution of ammonia-oxidizing archaea and bacteria to nitrification under different biogeochemical factors in acidic soils. Environ. Sci. Pollut. R. 2022, 29, 17209–17222. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Dong, Z.X.; Wang, Z.; Xiao, L.W.; Zhu, B. The contributions of ammonia oxidizing bacteria and archaea to nitrification-dependent N2O emission in alkaline and neutral purple soils. Sci. Rep. 2022, 12, 19928. [Google Scholar] [CrossRef]
- Ying, J.Y.; Li, X.X.; Wang, N.N.; Lan, Z.C.; He, J.Z.; Bai, Y.F. Contrasting effects of nitrogen forms and soil pH on ammonia oxidizing microorganisms and their responses to long-term nitrogen fertilization in a typical steppe ecosystem. Soil Biol. Biochem. 2017, 107, 10–18. [Google Scholar] [CrossRef]
- Robinson, A.; Di, H.J.; Cameron, K.C.; Podolyan, A.; He, J. The effect of soil pH and dicyandiamide (DCD) on N2O emissions and ammonia oxidiser abundance in a stimulated grazed pasture soil. J. Soil. Sediment. 2014, 14, 1434–1444. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, G.; Myrold, D.D.; Zhou, W. Variable responses of ammonia oxidizers across soil particle-size fractions affect nitrification in a long-term fertilizer experiment. Soil Biol. Biochem. 2017, 105, 25–36. [Google Scholar] [CrossRef]
- Guo, J.X.; Zhou, Y.X.; Guo, H.J.; Min, W. Saline and alkaline stresses alter soil properties and composition and structure of gene-based nitrifier and denitrifier communities in a calcareous desert soil. BMC Microbiol. 2021, 21, 246. [Google Scholar] [CrossRef] [PubMed]
- Balume, I.; Agumas, B.; Musyoki, M.; Marhan, S.; Cadisch, G.; Rasche, F. Potential proteolytic enzyme activities modulate archaeal and bacterial nitrifier abundance in soils differing in acidity and organic residue treatment. Appl. Soil Ecol. 2022, 169, 104188. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of beta-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant Nut. 2017, 17, 794–807. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Hu, W.; Ma, D.; Yang, Y.; Lan, H.; Gao, Y. Diversity and abundance of ammonia-oxidizing microorganisms in relation to soil environment in rhizosphere soil of Halocnemum strobilaceum in Ebinur Lake wetland. Acta Sci. Circumstantiae 2017, 37, 1967–1975. [Google Scholar]
- Luo, P.; Fan, Y.; Yang, J.; Ge, Y.; Cai, F.; Han, X. Influence of long-term fertilization on abundance of ammonia oxidizing bacteria and archaea in brown soil. J. Plant Nutr. Fert. 2017, 23, 678–685. [Google Scholar]
- Li, S.; Jiang, X.; Wang, X.; Wright, A.L. Tillage effects on soil nitrification and the dynamic changes in nitrifying microorganisms in a subtropical rice-based ecosystem: A long-term field study. Soil. Till. Res. 2015, 150, 132–138. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Tiemann, L.K.; Grandy, A.S. Does agricultural crop diversity enhance soil microbial biomass and organic matter dynamics? A meta-analysis. Ecol. Appl. 2014, 24, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Zeng, S.; Hou, D.; Zhou, R.; Xing, C.; Deng, X.; Yu, L.; Wang, H.; Deng, Z.; Weng, S.; et al. Community diversity and abundance of ammonia-oxidizing archaea and bacteria in shrimp pond sediment at different culture stages. J. Appl. Microbiol. 2021, 130, 1442–1455. [Google Scholar] [CrossRef]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Luecker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.L.; Mei, L.L.; Wei, Q.H.; Li, B.; Zhang, P.; Sun, S.X.; Cui, G.W. Leymus chinensis resists degraded soil stress by modulating root exudate components to attract beneficial microorganisms. Front. Microbiol. 2022, 13, 951838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Shi, Y.C.; Dong, Y.X.; Lapen, D.R.; Liu, J.H.; Chen, W. Subsoiling and conversion to conservation tillage enriched nitrogen cycling bacterial communities in sandy soils under long-term maize monoculture. Soil Till. Res. 2022, 215, 105197. [Google Scholar] [CrossRef]
- Koch, H.; Lucker, S.; Albertsen, M.; Kitzinger, K.; Herbold, C.; Spieck, E.; Nielsen, P.H.; Wagner, M.; Daims, H. Expanded metabolic versatility of ubiquitous nitriteoxidiz-ing bacteria from the genus Nitrospira. Proc. Natl. Acad. Sci. USA 2015, 112, 11371–11376. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.F.; Wang, H.Y.; Chen, L.M.; Wang, J.W.; Zheng, M.S.; Liu, S.T.; Chen, Q.; Ni, J.R. Comammox Nitrospira within the Yangtze River continuum: Community, biogeography, and ecological drivers. ISME J. 2020, 14, 2488–2504. [Google Scholar] [CrossRef]
- Yu, J.-L.; Xia, J.-J.; Li, C.-H.; Zhang, S.-H.; Li, X.; Lu, Y.; Xininigen. Niche differentiation of Nitrospira and associated environmental driving forces in Xilin river basin. Microbiol. China 2020, 47, 1418–1429. [Google Scholar]
- Hu, H.W.; Zhang, L.M.; Dai, Y.; Di, H.J.; He, J.Z. pH-dependent distribution of soil ammonia oxidizers across a large geographical scale as revealed by high-throughput pyrosequencing. J. Soil Sediment. 2013, 13, 1439–1449. [Google Scholar] [CrossRef]
- Behnke, G.D.; Zabaloy, M.C.; Riggins, C.W.; Rodriguez-Zas, S.; Huang, L.; Villamil, M.B. Acidification in corn monocultures favor fungi, ammonia oxidizing bacteria, and nirK-denitrifier groups. Sci. Total Environ. 2020, 720, 137514. [Google Scholar] [CrossRef]
- Szukics, U.; Grigulis, K.; Legay, N.; Kastl, E.M.; Baxendale, C.; Bardgett, R.D.; Clement, J.C.; Lavorel, S.; Schloter, M.; Bahn, M. Management versus site effects on the abundance of nitrifiers and denitrifiers in European mountain grasslands. Sci. Total Environ. 2019, 648, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Banning, N.C.; Maccarone, L.D.; Fisk, L.M.; Murphy, D.V. Ammonia-oxidising bacteria not archaea dominate nitrification activity in semi-arid agricultural soil. Sci. Rep. 2015, 5, e11146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Cui, R.Y.; Fu, B.; Yang, Y.X.; Wang, P.L.; Mao, Y.T.; Chen, A.Q.; Lei, B.K. Shallow groundwater table fluctuations affect bacterial communities and nitrogen functional genes along the soil profile in a vegetable field. Appl. Soil Ecol. 2020, 146, 103368. [Google Scholar] [CrossRef]
- Luan, L.; Zhang, G.; Sun, L.; Geng, R.; Wang, H. Spatial variation in water-holding properties of typical plant litters in the loess hilly region. J. Soil Water Conserv. 2015, 29, 225–230. [Google Scholar]
- Lobet, G.; Couvreur, V.; Meunier, F.; Javaux, M.; Draye, X. Plant water uptake in drying soils. Plant Physiol. 2014, 164, 1619–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.Z.; Guo, B.L.; Liu, H.S.; Wu, Y.Q.; Yu, J.Q.; Ding, H.; Jiang, X.H.; Luo, Q.D.; Zhang, Y.S. Low pH inhibits soil nosZ without affecting N2O uptake. J. Soil. Sediment. 2023, 23, 422–430. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Dong, L.; Wang, P.; Lu, X.; Zhang, X.; Su, Z.; Guo, Q.; Ma, P. Effects of different crop rotations on the incidence of cotton Verticillium wilt and structure and function of the rhizospheric microbial community. Plant Soil 2023. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Martens-Habbena, W.; Strauss, S.L. Cover crop composition drives changes in the abundance and diversity of nitrifiers and denitrifiers in citrus orchards with critical effects on N2O emissions. Geoderma 2022, 422, 115952. [Google Scholar] [CrossRef]
- Pereira, E.I.P.; Chung, H.; Scow, K.; Six, J. Microbial communities and soil structure are affected by reduced precipitation, but not by elevated carbon dioxide. Soil Sci. Soc. Am. J. 2013, 77, 482–488. [Google Scholar] [CrossRef]
- Jones, C.M.; Spor, A.; Brennan, F.P.; Breuil, M.-C.; Bru, D.; Lemanceau, P.; Griffiths, B.; Hallin, S.; Philippot, L. Recently identified microbial guild mediates soil N2O sink capacity. Nat. Clim. Chang. 2014, 4, 801–805. [Google Scholar] [CrossRef]
- Graf, D.R.; Saghaï, A.; Zhao, M.; Carlsson, G.; Jones, C.M.; Hallin, S.J.S.B. Lucerne (Medicago sativa) alters N2O-reducing communities associated with cocksfoot (Dactylis glomerata) roots and promotes N2O production in intercropping in a greenhouse experiment. Soil Biol. Biochem. 2019, 137, 107547. [Google Scholar] [CrossRef]
- Cantarel, A.A.; Pommier, T.; Desclos-Theveniau, M.; Diquélou, S.; Dumont, M.; Grassein, F.; Kastl, E.-M.; Grigulis, K.; Laîné, P.; Lavorel, S.J.E. Using plant traits to explain plant–microbe relationships involved in nitrogen acquisition. Ecology 2015, 96, 788–799. [Google Scholar] [CrossRef]
- Moreau, D.; Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Faivre, C.; Matejicek, A.; Strbik, F.; Philippot, L.; Mougel, C.J.E. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology 2015, 96, 2300–2310. [Google Scholar] [CrossRef] [Green Version]
- Adviento-Borbe, M.A.A.; Doran, J.W.; Drijber, R.A.; Dobermann, A. Soil electrical conductivity and water content affect nitrous oxide and carbon dioxide emissions in intensively managed soils. J. Environ. Qual. 2006, 35, 1999–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.S.; Qu, T.H.; Li, Y.F.; Van Zwieten, L.; Wang, H.L.; Chen, J.H.; Song, X.Z.; Lin, Z.; Zhang, X.P.; Luo, Y.; et al. Biochar-based fertilizer decreased while chemical fertilizer increased soil N2O emissions in a subtropical Moso bamboo plantation. Catena 2021, 202, 105257. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.; Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, K.A.; Bertagnolli, A.; Pannu, M.W.; Strand, S.E.; Brown, S.L.; Stahl, D.A. Evaluation of revised polymerase chain reaction primers for more inclusive quantification of ammonia-oxidizing archaea and bacteria. Environ. Microbiol. Rep. 2015, 7, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Lu, Q.; Raza, W.; Huang, Q.; Shen, Q. Ammonia oxidizer abundance in paddy soil profile with different fertilizer regimes. Appl. Soil Ecol. 2014, 84, 38–44. [Google Scholar] [CrossRef]
- Cassman, N.A.; Soares, J.R.; Pijl, A.; Lourenço, K.S.; van Veen, J.A.; Cantarella, H.; Kuramae, E.E. Nitrification inhibitors effectively target N2O-producing Nitrosospira spp. in tropical soil. Environ. Microbiol. 2019, 21, 1241–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bru, D.; Sarr, A.; Philippot, L. Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl. Environ. Microbiol. 2007, 73, 5971–5974. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.P.; Meng, F.G.; Li, W. The short- and long-term effects of formic acid on rapid nitritation start-up. Environ. Int. 2020, 135. [Google Scholar] [CrossRef]




| 0–15 cm | 15–30 cm | |||||
|---|---|---|---|---|---|---|
| Monoculture | Rotation | Mixture | Monoculture | Rotation | Mixture | |
| pH | 8.19 ± 0.08 a | 7.98 ± 0.14 a | 7.72 ± 0.11 b | 8.26 ± 0.13 a | 8.15 ± 0.06 a | 7.73 ± 0.20 b |
| SOM (g·kg−1) | 49.02 ± 1.87 b | 42.05 ± 8.85 ab | 55.44 ± 1.06 a | 48.78 ± 2.44 a | 50.95 ± 14.84 a | 46.81 ± 2.14 a |
| TP (g·kg−1) | 0.54 ± 0.21 a | 0.37 ± 0.09 a | 0.73 ± 0.35 a | 0.39 ± 0.00 a | 0.43 ± 0.05 a | 0.31 ± 0.33 b |
| TN (g·kg−1) | 2.74 ± 0.99 a | 2.41 ± 0.24 a | 3.42 ± 0.20 a | 2.11 ± 0.40 a | 2.15 ± 0.17 a | 1.91 ± 0.15 a |
| AP (mg·kg−1) | 61.95 ± 11.53 a | 52.31 ± 14.57 a | 69.24 ± 29.00 a | 49.16 ± 2.07 b | 58.57 ± 0.93 a | 39.31 ± 4.35 c |
| AK (mg·kg−1) | 50.01 ± 4.23 b | 41.48 ± 6.05 b | 71.85 ± 8.80 a | 43.61 ± 0.04 b | 51.61 ± 3.20 a | 40.95 ± 0.92 b |
| EC (mS·cm−1) | 417.84 ± 1.95 a | 392.63 ± 3.03 b | 382.32 ± 2.49 c | 443.23 ± 12.80 a | 440.75 ± 20.31 a | 435.98 ± 6.47 a |
| Urease (mg·g−1·d−1) | 0.09 ± 0.00 c | 0.12 ± 0.01 b | 0.17 ± 0.01 a | 0.09 ± 0.00 c | 0.10 ± 0.00 ab | 0.10 ± 0.01 a |
| Nitrate reductase (mg·g−1·d−1) | 0.13 ± 0.01 b | 0.12 ± 0.00 b | 0.17 ± 0.01 a | 0.11 ± 0.02 b | 0.09 ± 0.01 c | 0.13 ± 0.01 a |
| Cellulase (μg·10 g−1·d−1) | 89.91 ± 0.05 c | 188.81 ± 0.02 b | 216.45 ± 0.02 a | 53.28 ± 0.01 c | 69.93 ± 0.01 b | 136.53 ± 0.02 a |
| β-glucosidase (μg·g−1·h−1) | 34.37 ± 2.82 b | 58.03 ± 6.32 a | 66.75 ± 5.26 a | 23.82 ± 0.72 b | 24.20 ± 1.61 b | 30.71 ± 3.09 a |
| Protease (μg·g−1·h−1) | 511.86 ± 98.68 b | 848.40 ± 87.25 ab | 1021.47 ± 278.07 a | 399.68 ± 251.54 a | 502.24 ± 64.02 a | 684.94 ± 187.52 a |
| CP | SD | CP × SD | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| pH | 23.345 | <0.001 | 1.813 | 0.203 | 0.656 | 0.537 |
| SOM | 0.614 | 0.557 | <0.001 | 0.988 | 2.204 | 0.153 |
| TP | 0.692 | 0.519 | 4.342 | 0.059 | 2.846 | 0.091 |
| TN | 1.072 | 0.373 | 13.346 | 0.003 | 2.903 | 0.094 |
| AP | 0.015 | 0.985 | 3.294 | 0.095 | 2.436 | 0.129 |
| AK | 7.935 | 0.006 | 15.482 | 0.002 | 26.804 | <0.001 |
| EC | 6.647 | 0.011 | 76.099 | <0.001 | 3.169 | 0.079 |
| Urease | 74.454 | <0.001 | 71.61 | <0.001 | 32.763 | <0.001 |
| Nitrate reductase | 64.508 | <0.001 | 56.702 | <0.001 | 2.419 | 0.131 |
| Cellulase | 204.903 | <0.001 | 348.367 | <0.001 | 32.880 | <0.001 |
| β-glucosidase | 39.943 | <0.001 | 219.684 | <0.001 | 20.329 | <0.001 |
| Protease | 7.235 | 0.009 | 9.612 | 0.009 | 0.8 | 0.472 |
| CP | SD | CP × SD | ||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| nifH | 2.34 | 0.014 | 47.58 | <0.001 | 8.46 | 0.01 |
| AOA | 18,587.73 | <0.001 | 23,284.08 | <0.001 | 19,630.12 | <0.001 |
| AOB | 31.88 | <0.001 | 247.85 | <0.001 | 24.57 | <0.001 |
| nxrB | 74.17 | <0.001 | 339.44 | <0.001 | 82.10 | <0.001 |
| narG | 8.39 | <0.001 | 68.31 | <0.001 | 17.38 | <0.001 |
| norB | 9.67 | 0.03 | 76.96 | <0.001 | 1.04 | 0.39 |
| nosZ | 29.90 | <0.001 | 200.08 | <0.001 | 29.29 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Li, B.; Sun, M.; Li, X. Different Cropping Patterns to Restore Saline-Alkali Soils in Northeast China Affect the Abundance of Functional Genes in the Soil Nitrogen Cycle. Sustainability 2023, 15, 6592. https://doi.org/10.3390/su15086592
Ding J, Li B, Sun M, Li X. Different Cropping Patterns to Restore Saline-Alkali Soils in Northeast China Affect the Abundance of Functional Genes in the Soil Nitrogen Cycle. Sustainability. 2023; 15(8):6592. https://doi.org/10.3390/su15086592
Chicago/Turabian StyleDing, Junnan, Bin Li, Minglong Sun, and Xin Li. 2023. "Different Cropping Patterns to Restore Saline-Alkali Soils in Northeast China Affect the Abundance of Functional Genes in the Soil Nitrogen Cycle" Sustainability 15, no. 8: 6592. https://doi.org/10.3390/su15086592
APA StyleDing, J., Li, B., Sun, M., & Li, X. (2023). Different Cropping Patterns to Restore Saline-Alkali Soils in Northeast China Affect the Abundance of Functional Genes in the Soil Nitrogen Cycle. Sustainability, 15(8), 6592. https://doi.org/10.3390/su15086592






