Figure 1.
This article distinguishes between two distinct areas of sustainable design research, namely, sustainability theory and design theory. Sustainability theory encompasses concepts such as LCA, C2C, and PEF, while design theory focuses on the principles and methods of design. In this article, we aim to integrate C2C theory from sustainability theory with the conceptual design method from design theory. This integration can enhance our understanding of sustainable design and provide practical solutions for creating environmentally friendly products.
Figure 1.
This article distinguishes between two distinct areas of sustainable design research, namely, sustainability theory and design theory. Sustainability theory encompasses concepts such as LCA, C2C, and PEF, while design theory focuses on the principles and methods of design. In this article, we aim to integrate C2C theory from sustainability theory with the conceptual design method from design theory. This integration can enhance our understanding of sustainable design and provide practical solutions for creating environmentally friendly products.
Figure 2.
This paper employs a combined qualitative and quantitative experimental approach to validate the feasibility of integrating C2C theory with conceptual product design. The qualitative and quantitative experiments provide valuable data support for the theory, while the theory provides a logical framework for the experiments. What sets this study apart is the approach taken, which begins with the key principles of C2C theory and combines them with conceptual product design methods to discuss the feasibility of the theory using a qualitative and quantitative experimental validation method.
Figure 2.
This paper employs a combined qualitative and quantitative experimental approach to validate the feasibility of integrating C2C theory with conceptual product design. The qualitative and quantitative experiments provide valuable data support for the theory, while the theory provides a logical framework for the experiments. What sets this study apart is the approach taken, which begins with the key principles of C2C theory and combines them with conceptual product design methods to discuss the feasibility of the theory using a qualitative and quantitative experimental validation method.
Figure 3.
The product undergoes ingestion and enters the human digestive system. During the first stage, the product and food pass through the stomach, where the vigorous movements break food down into smaller particles. Subsequently, the product gradually absorbs some of the food. In the second stage, the product traverses the small intestine, where the food undergoes further degradation due to the coordinated movements of the small intestine. The external cellulose of the product serves to prevent the villi of the small intestine from coming into contact with other digestive fluids and food that may be absorbed by the product, while allowing the product to absorb more food. Finally, the product enters the large intestine during the third stage, where peristalsis expels it from the body along with the absorbed food. Therefore, the workflow of the product obviates the need for users to restrict their diets to achieve a reduction in food absorption.
Figure 3.
The product undergoes ingestion and enters the human digestive system. During the first stage, the product and food pass through the stomach, where the vigorous movements break food down into smaller particles. Subsequently, the product gradually absorbs some of the food. In the second stage, the product traverses the small intestine, where the food undergoes further degradation due to the coordinated movements of the small intestine. The external cellulose of the product serves to prevent the villi of the small intestine from coming into contact with other digestive fluids and food that may be absorbed by the product, while allowing the product to absorb more food. Finally, the product enters the large intestine during the third stage, where peristalsis expels it from the body along with the absorbed food. Therefore, the workflow of the product obviates the need for users to restrict their diets to achieve a reduction in food absorption.
Figure 4.
The above figure illustrates the structural blueprint of a conceptual product. The upper part of the product features an angled aperture, which facilitates food intake, while the lower end has multiple small orifices to discharge digestive enzymes. Upon entry of food into the product, the downward sloping aperture creates a unidirectional pathway, making it difficult for the food to escape. Consequently, a state of ease in food ingestion but difficulty in excretion ensues. The bottle product, in turn, is a consequence of the packaging design.
Figure 4.
The above figure illustrates the structural blueprint of a conceptual product. The upper part of the product features an angled aperture, which facilitates food intake, while the lower end has multiple small orifices to discharge digestive enzymes. Upon entry of food into the product, the downward sloping aperture creates a unidirectional pathway, making it difficult for the food to escape. Consequently, a state of ease in food ingestion but difficulty in excretion ensues. The bottle product, in turn, is a consequence of the packaging design.
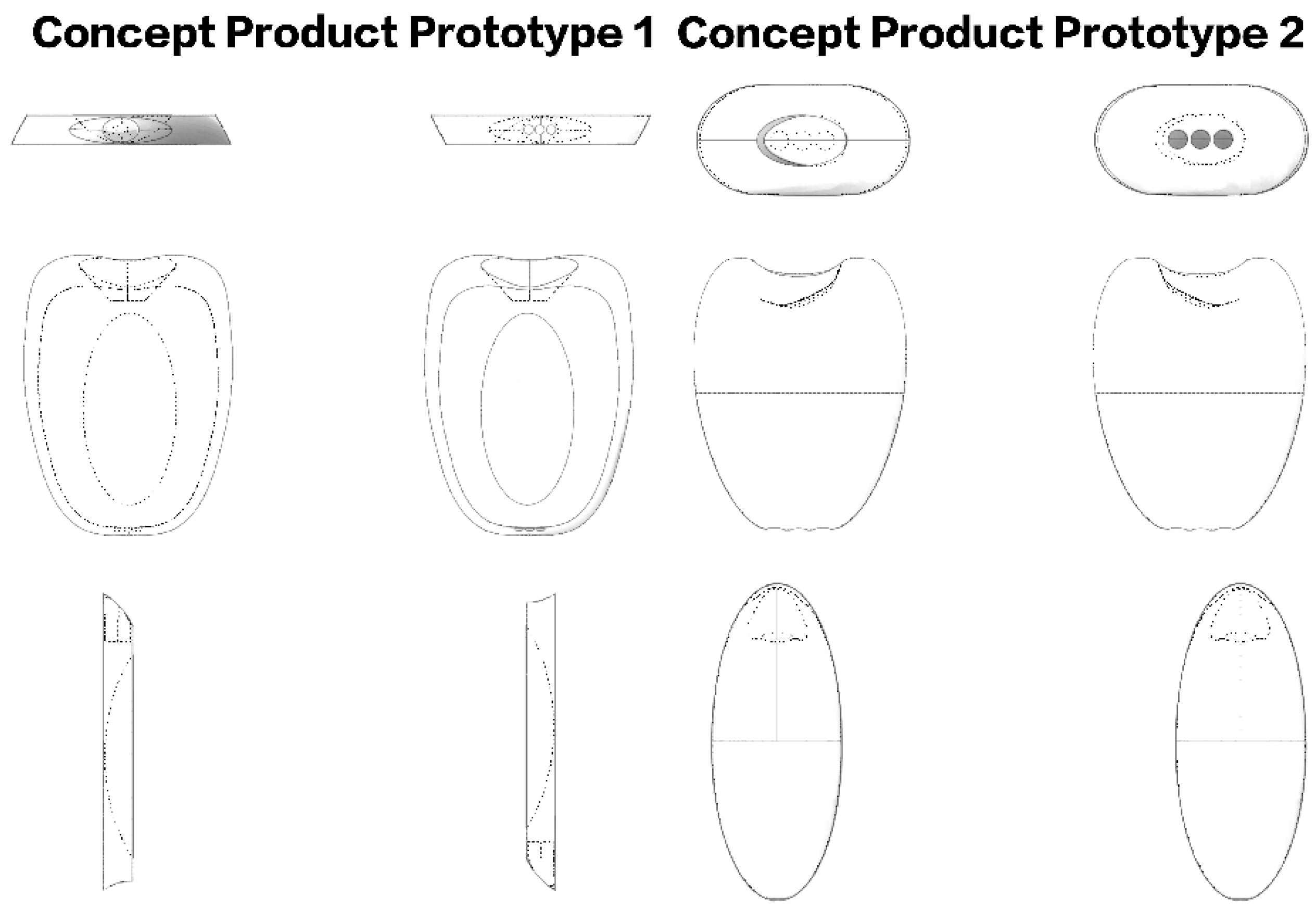
Figure 5.
Six views of two conceptual product prototypes based on maize seeds are displayed. These designs aim to retain the natural product’s appearance, potentially enhancing user acceptance psychologically.
Figure 5.
Six views of two conceptual product prototypes based on maize seeds are displayed. These designs aim to retain the natural product’s appearance, potentially enhancing user acceptance psychologically.
Figure 6.
In accordance with the C2C principle of valuing diversity, the design of the derivative ball departs from the original concept product. As a product with conceptual diversity, the purpose of the derivative ball is to maintain the functionality of the concept product while enhancing its performance. To that end, four derivative ball were designed, with each following the principles of C2C theory.
Figure 6.
In accordance with the C2C principle of valuing diversity, the design of the derivative ball departs from the original concept product. As a product with conceptual diversity, the purpose of the derivative ball is to maintain the functionality of the concept product while enhancing its performance. To that end, four derivative ball were designed, with each following the principles of C2C theory.
Figure 7.
After careful evaluation of four derivative ball designs, we chose the final experimental prototype. Our evaluation was based on the complexity of the 3D printing process and the internal space of each prototype. Ultimately, we determined that Prototype 4 was the optimal choice.
Figure 7.
After careful evaluation of four derivative ball designs, we chose the final experimental prototype. Our evaluation was based on the complexity of the 3D printing process and the internal space of each prototype. Ultimately, we determined that Prototype 4 was the optimal choice.
Figure 8.
The design of qualitative experiments for the concept product involved four different solutions: black sesame pastes, maize paste, lotus root powder, and brown rice flour. Furthermore, the design of quantitative experiments for the concept product involved three different concentrations of starch solutions.
Figure 8.
The design of qualitative experiments for the concept product involved four different solutions: black sesame pastes, maize paste, lotus root powder, and brown rice flour. Furthermore, the design of quantitative experiments for the concept product involved three different concentrations of starch solutions.
Figure 9.
The experimental procedures were carried out utilizing a maize paste solution with high viscosity and a lotus root powder solution.
Figure 9.
The experimental procedures were carried out utilizing a maize paste solution with high viscosity and a lotus root powder solution.
Figure 10.
The experimental procedures were carried out utilizing a maize paste solution with high viscosity and a lotus root powder solution.
Figure 10.
The experimental procedures were carried out utilizing a maize paste solution with high viscosity and a lotus root powder solution.
Figure 11.
The weight changes of eight hollow dry maize seeds absorbing food in 100 mL/80 g starch solution at different times of the quantitative experiment.
Figure 11.
The weight changes of eight hollow dry maize seeds absorbing food in 100 mL/80 g starch solution at different times of the quantitative experiment.
Figure 12.
The weight changes of eight hollow dry maize seeds absorbing food in 100 mL/120 g starch solution at different times of the quantitative experiment.
Figure 12.
The weight changes of eight hollow dry maize seeds absorbing food in 100 mL/120 g starch solution at different times of the quantitative experiment.
Figure 13.
The weight changes of eight hollow dry maize seeds absorbing food in 100 mL/160 g starch solution at different times of the quantitative experiment.
Figure 13.
The weight changes of eight hollow dry maize seeds absorbing food in 100 mL/160 g starch solution at different times of the quantitative experiment.
Figure 14.
The absorption data of each maize seed in the starch solution in Experiment 1.
Figure 14.
The absorption data of each maize seed in the starch solution in Experiment 1.
Figure 15.
The absorption data of each maize seed in the starch solution in Experiment 2.
Figure 15.
The absorption data of each maize seed in the starch solution in Experiment 2.
Figure 16.
The absorption data of each maize seed in the starch solution in Experiment 3.
Figure 16.
The absorption data of each maize seed in the starch solution in Experiment 3.
Figure 17.
In the qualitative experiment, the absorption capacity of hollow maize seed was tested by four different solutions, aiming to investigate the absorption ability of the conceptual product in various solutions.
Figure 17.
In the qualitative experiment, the absorption capacity of hollow maize seed was tested by four different solutions, aiming to investigate the absorption ability of the conceptual product in various solutions.
Figure 18.
In the qualitative experiment, a comparison was made of the four different qualitative experimental data, and a summary was made of the material weight used in the experimental solutions, the average absorption of the conceptual product in different solutions, and the absorption rate in different solutions.
Figure 18.
In the qualitative experiment, a comparison was made of the four different qualitative experimental data, and a summary was made of the material weight used in the experimental solutions, the average absorption of the conceptual product in different solutions, and the absorption rate in different solutions.
Figure 19.
A comparison was made between the absorption rates of the derivative small balls of the conceptual product and the hollow dry maize kernels of the conceptual product in viscous lotus root powder solution and viscous maize starch solution.
Figure 19.
A comparison was made between the absorption rates of the derivative small balls of the conceptual product and the hollow dry maize kernels of the conceptual product in viscous lotus root powder solution and viscous maize starch solution.
Table 1.
Experimental Solution Proportioning in Quantitative Analysis.
Table 1.
Experimental Solution Proportioning in Quantitative Analysis.
| Material Weight/Material Classification | Starch 1 | Starch 2 | Starch 3 |
|---|
| Self-weight (g) | 80.00 | 120.00 | 160.00 |
| Boiling water (mL) | 100 | 100 | 100 |
Table 2.
Experimental Solution Proportioning in Qualitative Analysis.
Table 2.
Experimental Solution Proportioning in Qualitative Analysis.
| Material Weight/Material Classification | Black Sesame Aleurone | Maize Flour | Lotus Root Starch | Brown Rice Flour |
|---|
| Self-weight (g) | 65.02 | 60.23 | 60.55 | 100.58 |
| Boiling water (mL) | 250 | 250 | 250 | 250 |
Table 3.
Experimental Solution Proportioning in Qualitative Analysis.
Table 3.
Experimental Solution Proportioning in Qualitative Analysis.
| Material Weight/Material Classification | Maize Paste Solution | Lotus Root Powder Solution |
|---|
| Weight (g) | 50.03 | 50.05 |
| Boiling water (mL) | 250 | 250 |
| Cool the solution for 1 h before the experiment. |
Table 4.
Experimental Solution Proportioning in Qualitative Analysis.
Table 4.
Experimental Solution Proportioning in Qualitative Analysis.
| Material Weight/Material Classification | Maize Flour | Lotus Root Starch |
|---|
| Weight (g) | 50.00 | 49.95 |
| Boiling water (mL) | 250 | 250 |
| Cool the solution for 1 h before the experiment. |
Table 5.
Starch Solution Experiment 1 data results.
Table 5.
Starch Solution Experiment 1 data results.
| Starch Solution | | | After the Experiment (g) |
|---|
| 100 mL/80 g | Before Experiment (g) | Mean Value (g) | 5 min | 10 min | 15 min | 20 min | 25 min |
|---|
| Maize Seed 1 | 0.30 | 0.34 | 0.52 | 0.46 | 0.63 | 0.66 | 0.65 |
| Maize Seed 2 | 0.34 | 0.43 | 0.52 | 0.56 | 0.60 | 0.55 |
| Maize Seed 3 | 0.35 | 0.43 | 0.43 | 0.57 | 0.64 | 0.58 |
| Maize Seed 4 | 0.37 | 0.56 | 0.47 | 0.47 | 0.64 | 0.61 |
| Maize Seed 5 | 0.33 | 0.48 | 0.48 | 0.54 | 0.54 | 0.56 |
| Maize Seed 6 | 0.34 | 0.49 | 0.55 | 0.58 | 0.58 | 0.63 |
| Maize Seed 7 | 0.35 | 0.42 | 0.56 | 0.50 | 0.54 | 0.66 |
| Maize Seed 8 | 0.35 | 0.56 | 0.46 | 0.47 | 0.67 | 0.65 |
Table 6.
The average absorption rate and absorption rate of maize seeds in the starch solution at each time period were recorded in Experiment 1.
Table 6.
The average absorption rate and absorption rate of maize seeds in the starch solution at each time period were recorded in Experiment 1.
| Mean/Absorption Rate | | | After the Experiment (g) |
|---|
| 100 mL/80 g | Before Experiment (g) | Mean Value (g) | 5 min | 10 min | 15 min | 20 min | 25 min |
|---|
| Maize Seed 1 | 0.30 | 0.34 | Mean value (g) |
| Maize Seed 2 | 0.34 | 0.49 | 0.49 | 0.54 | 0.61 | 0.61 |
| Maize Seed 3 | 0.35 |
| Maize Seed 4 | 0.37 |
| Maize Seed 5 | 0.33 | Absorption rate |
| Maize Seed 6 | 0.34 | 44% | 44% | 59% | 79% | 79% |
| Maize Seed 7 | 0.35 |
| Maize Seed 8 | 0.35 |
Table 7.
Starch Solution Experiment 2 data results.
Table 7.
Starch Solution Experiment 2 data results.
| Starch Solution | | | After the Experiment (g) |
|---|
| 100 mL/120 g | Before Experiment (g) | Mean Value (g) | 5 min | 10 min | 15 min | 20 min | 25 min |
|---|
| Maize Seed 1 | 0.35 | 0.35 | 0.46 | 0.56 | 0.57 | 0.50 | 0.64 |
| Maize Seed 2 | 0.34 | 0.45 | 0.47 | 0.62 | 0.58 | 0.66 |
| Maize Seed 3 | 0.31 | 0.38 | 0.50 | 0.58 | 0.51 | 0.71 |
| Maize Seed 4 | 0.37 | 0.46 | 0.43 | 0.58 | 0.65 | 0.64 |
| Maize Seed 5 | 0.37 | 0.47 | 0.54 | 0.60 | 0.69 | 0.65 |
| Maize Seed 6 | 0.34 | 0.46 | 0.48 | 0.52 | 0.53 | 0.55 |
| Maize Seed 7 | 0.35 | 0.41 | 0.41 | 0.47 | 0.52 | 0.52 |
| Maize Seed 8 | 0.35 | 0.51 | 0.47 | 0.58 | 0.55 | 0.52 |
Table 8.
The average absorption rate and absorption rate of maize seeds in the starch solution at each time period were recorded in Experiment 2.
Table 8.
The average absorption rate and absorption rate of maize seeds in the starch solution at each time period were recorded in Experiment 2.
| Mean/Absorption Rate | | | After the Experiment (g) |
|---|
| 100 mL/120 g | Before Experiment (g) | Mean Value (g) | 5 min | 10 min | 15 min | 20 min | 25 min |
|---|
| Maize Seed 1 | 0.35 | 0.35 | Mean value (g) |
| Maize Seed 2 | 0.34 | 0.45 | 0.48 | 0.57 | 0.57 | 0.61 |
| Maize Seed 3 | 0.31 |
| Maize Seed 4 | 0.37 |
| Maize Seed 5 | 0.37 | Absorption rate |
| Maize Seed 6 | 0.34 | 29% | 37% | 63% | 63% | 74% |
| Maize Seed 7 | 0.35 |
| Maize Seed 8 | 0.35 |
Table 9.
Starch Solution Experiment 3 data results.
Table 9.
Starch Solution Experiment 3 data results.
| Starch Solution | | | After the Experiment (g) |
|---|
| 100 mL/160 g | Before Experiment (g) | Mean Value (g) | 5 min | 10 min | 15 min | 20 min | 25 min |
|---|
| Maize Seed 1 | 0.32 | 0.36 | 0.48 | 0.65 | 0.53 | 0.61 | 0.62 |
| Maize Seed 2 | 0.37 | 0.52 | 0.49 | 0.66 | 0.62 | 0.64 |
| Maize Seed 3 | 0.35 | 0.41 | 0.51 | 0.64 | 0.67 | 0.61 |
| Maize Seed 4 | 0.37 | 0.50 | 0.56 | 0.53 | 0.56 | 0.75 |
| Maize Seed 5 | 0.37 | 0.43 | 0.55 | 0.52 | 0.55 | 0.67 |
| Maize Seed 6 | 0.35 | 0.55 | 0.42 | 0.61 | 0.69 | 0.67 |
| Maize Seed 7 | 0.36 | 0.59 | 0.51 | 0.53 | 0.67 | 0.59 |
| Maize Seed 8 | 0.39 | 0.44 | 0.48 | 0.64 | 0.69 | 0.66 |
Table 10.
The average absorption rate and absorption rate of maize seeds in the starch solution at each time period were recorded in Experiment 3.
Table 10.
The average absorption rate and absorption rate of maize seeds in the starch solution at each time period were recorded in Experiment 3.
| Mean/Absorption Rate | | | After the Experiment (g) |
|---|
| 100 mL/160 g | Before Experiment (g) | Mean Value (g) | 5 min | 10 min | 15 min | 20 min | 25 min |
|---|
| Maize Seed 1 | 0.32 | 0.36 | Mean value (g) |
| Maize Seed 2 | 0.37 | 0.49 | 0.48 | 0.49 | 0.57 | 0.49 |
| Maize Seed 3 | 0.35 |
| Maize Seed 4 | 0.37 |
| Maize Seed 5 | 0.37 | Absorption rate |
| Maize Seed 6 | 0.35 | 36% | 33% | 36% | 58% | 36% |
| Maize Seed 7 | 0.36 |
| Maize Seed 8 | 0.39 |
Table 11.
Experimental Data of Black Sesame Paste Solution.
Table 11.
Experimental Data of Black Sesame Paste Solution.
| Black Sesame Aleurone | Before Experiment (g) | Mean Value (g) | 10 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Maize 1 | 0.27 | 0.34 | - | 0.50 | 0.57 | 68% |
| Maize 2 | 0.34 | 0.58 |
| Maize 3 | 0.32 | 0.50 |
| Maize 4 | 0.35 | 0.53 |
| Maize 5 | 0.37 | 0.68 |
| Maize 6 | 0.36 | 0.65 |
Table 12.
Experimental Data of Maize Paste Solution.
Table 12.
Experimental Data of Maize Paste Solution.
| Maize Paste | Before Experiment (g) | Mean Value (g) | 10 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Maize Seed 1 | 0.26 | 0.34 | - | 0.56 | 0.64 | 88% |
| Maize Seed 2 | 0.36 | 0.66 |
| Maize Seed 3 | 0.32 | 0.62 |
| Maize Seed 4 | 0.34 | 0.71 |
| Maize Seed 5 | 0.37 | 0.61 |
| Maize Seed 6 | 0.36 | 0.65 |
Table 13.
Experimental Data of Lotus Root Starch Solution.
Table 13.
Experimental Data of Lotus Root Starch Solution.
| Lotus Root Powder | Before Experiment (g) | Mean Value (g) | 10 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Maize Seed 1 | 0.27 | 0.33 | - | 0.59 | 0.63 | 91% |
| Maize Seed 2 | 0.35 | 0.62 |
| Maize Seed 3 | 0.32 | 0.59 |
| Maize Seed 4 | 0.34 | 0.65 |
| Maize Seed 5 | 0.36 | 0.65 |
| Maize Seed 6 | 0.36 | 0.65 |
Table 14.
Experimental Data of Brown Rice Flour Solution.
Table 14.
Experimental Data of Brown Rice Flour Solution.
| Maize Flour | Before Experiment (g) | Mean Value (g) | 10 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Maize Seed 1 | 0.28 | 0.34 | - | 0.48 | 0.52 | 53% |
| Maize Seed 2 | 0.36 | 0.47 |
| Maize Seed 3 | 0.31 | 0.45 |
| Maize Seed 4 | 0.35 | 0.71 |
| Maize Seed 5 | 0.37 | 0.51 |
| Maize Seed 6 | 0.36 | 0.50 |
Table 15.
Summary of Qualitative Experiment Results.
Table 15.
Summary of Qualitative Experiment Results.
| Summary of four aspects of qualitative experimental research |
| Used material weight (g): |
| Brown rice flour (100.58) > Black sesame aleurone (65.02) > Lotus root flour (60.55) > Maize paste (60.23) |
| Solution viscosity: |
| Maize paste solution > Lotus root flour solution > Black sesame paste solution > Brown rice flour solution |
| Average absorption (g): |
| Maize paste solution (0.64) > Lotus root starch solution (0.63) > Black sesame paste solution (0.57) > Brown rice flour solution (0.52) |
| Absorption rate: |
| Maize paste solution (91%) > Lotus root starch solution (88%) > Black sesame paste solution (68%) > Brown rice flour solution (53%) |
Table 16.
Experimental Data of Viscous Maize Paste Solution.
Table 16.
Experimental Data of Viscous Maize Paste Solution.
| Viscous Maize Paste Solution | Before Experiment (g) | Mean Value (g) | 25 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Maize Seed 1 | 0.28 | 0.35 | - | 0.56 | 0.64 | 83% |
| Maize Seed 2 | 0.37 | 0.70 |
| Maize Seed 3 | 0.34 | 0.53 |
| Maize Seed 4 | 0.37 | 0.64 |
| Maize Seed 5 | 0.38 | 0.72 |
| Maize Seed 6 | 0.38 | 0.67 |
Table 17.
Experimental Data of Viscous Lotus Root Starch Solution.
Table 17.
Experimental Data of Viscous Lotus Root Starch Solution.
| Viscous Lotus Root Starch Solution | Before Experiment (g) | Mean Value (g) | 25 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Maize Seed 1 | 0.27 | 0.34 | - | 0.59 | 0.63 | 85% |
| Maize Seed 2 | 0.36 | 0.69 |
| Maize Seed 3 | 0.31 | 0.52 |
| Maize Seed 4 | 0.36 | 0.67 |
| Maize Seed 5 | 0.37 | 0.62 |
| Maize Seed 6 | 0.37 | 0.71 |
Table 18.
Experimental Data of Viscous Maize Paste Solution.
Table 18.
Experimental Data of Viscous Maize Paste Solution.
| Viscous Maize Paste Solution | Before Experiment (g) | Mean Value (g) | 25 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Ball 1 | 1.20 | 1.19 | - | 5.65 | 5.34 | 349% |
| Ball 2 | 1.18 | 5.22 |
| Ball 3 | 1.21 | 4.93 |
| Ball 4 | 1.15 | 5.54 |
Table 19.
Experimental Data of Viscous Lotus Root Starch Solution.
Table 19.
Experimental Data of Viscous Lotus Root Starch Solution.
| Viscous Lotus Root Starch Solution | Before Experiment (g) | Mean Value (g) | 25 min Later | After the Experiment (g) | Mean Value (g) | Absorption Rate |
|---|
| Experimental Subjects |
|---|
| Ball 1 | 1.21 | 1.19 | - | 2.84 | 3.15 | 165% |
| Ball 2 | 1.18 | 2.71 |
| Ball 3 | 1.21 | 3.72 |
| Ball 4 | 1.15 | 3.33 |
Table 20.
Comparison of Absorption Rate of Different Products in Viscous solution.
Table 20.
Comparison of Absorption Rate of Different Products in Viscous solution.
| Comparison of the Two Concept Products |
|---|
| Viscous Maize Paste Solution | Viscous Lotus Root Starch Solution |
|---|
| Experimental subjects/Absorption rate |
| Maize Seed | Ball | Maize Seed | Ball |
| 83% | 349% | 85% | 165% |