Migration and Conversion of Phosphorus in Hydrothermal Carbonization of Municipal Sludge with Hydrochloric Acid
Abstract
:1. Introduction
2. Methods and Materials
2.1. Municipal Sludge
2.2. Experimental Methods
2.2.1. HTC Experimental Procedure
2.2.2. Sample Characterization
3. Results
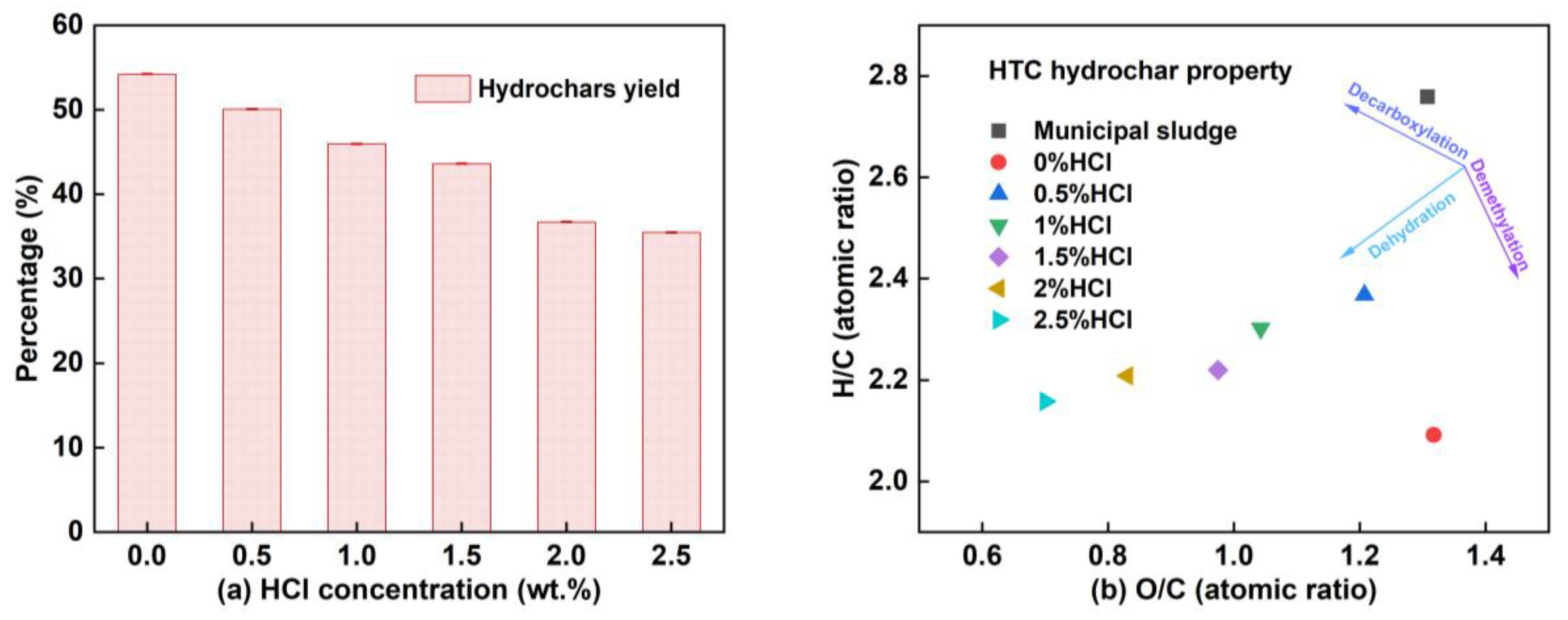
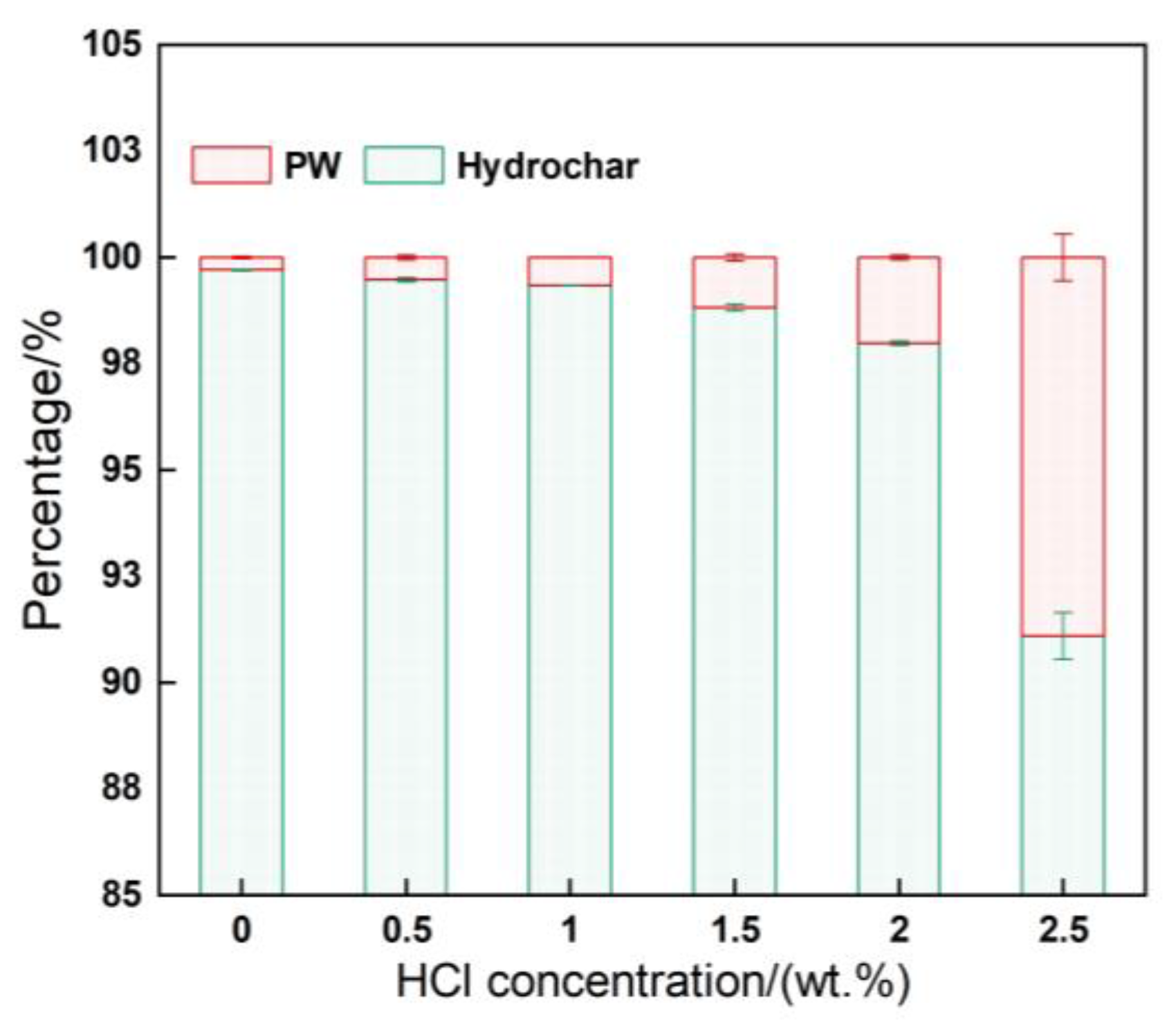
3.1. Variation of Hydrochar Yield
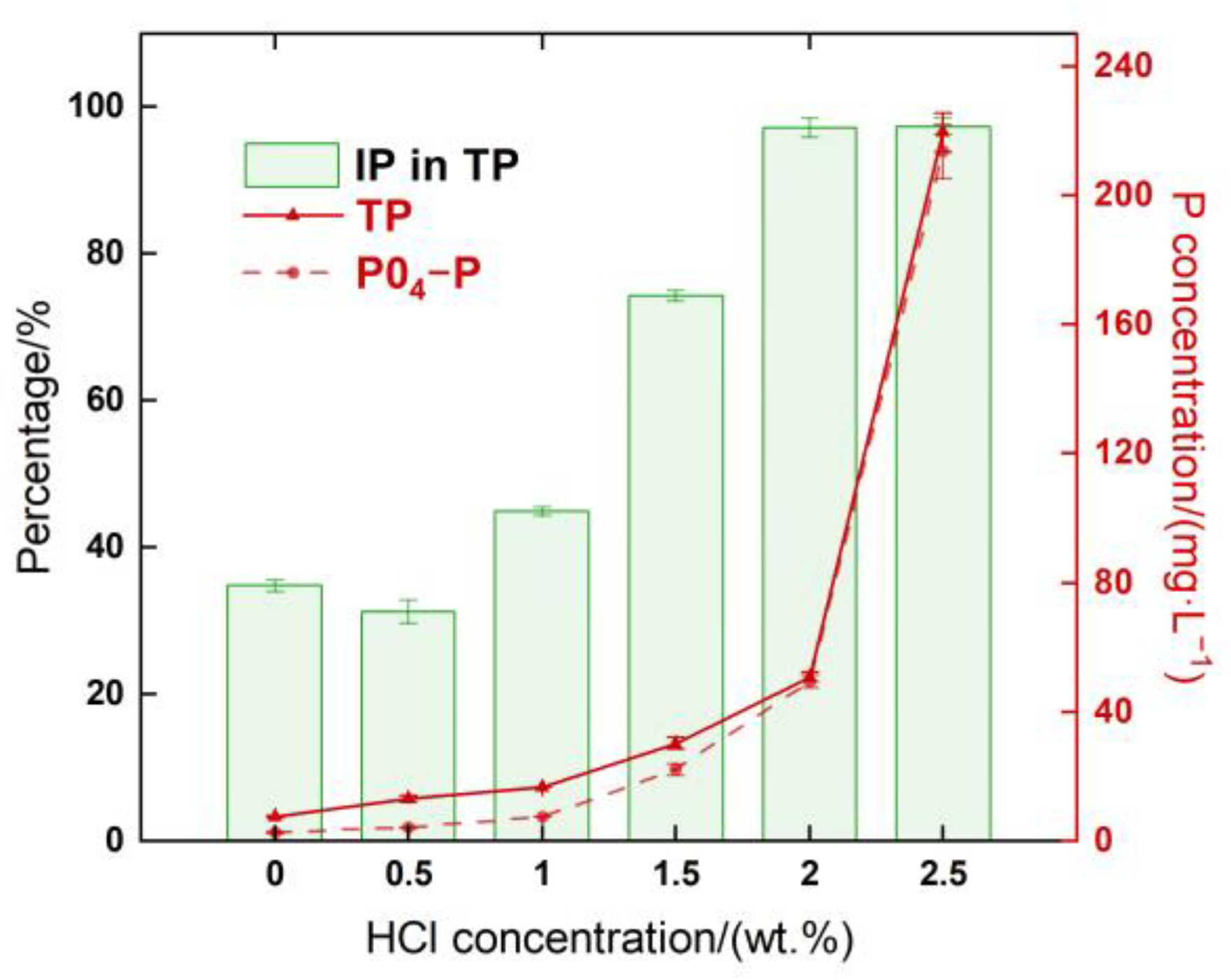
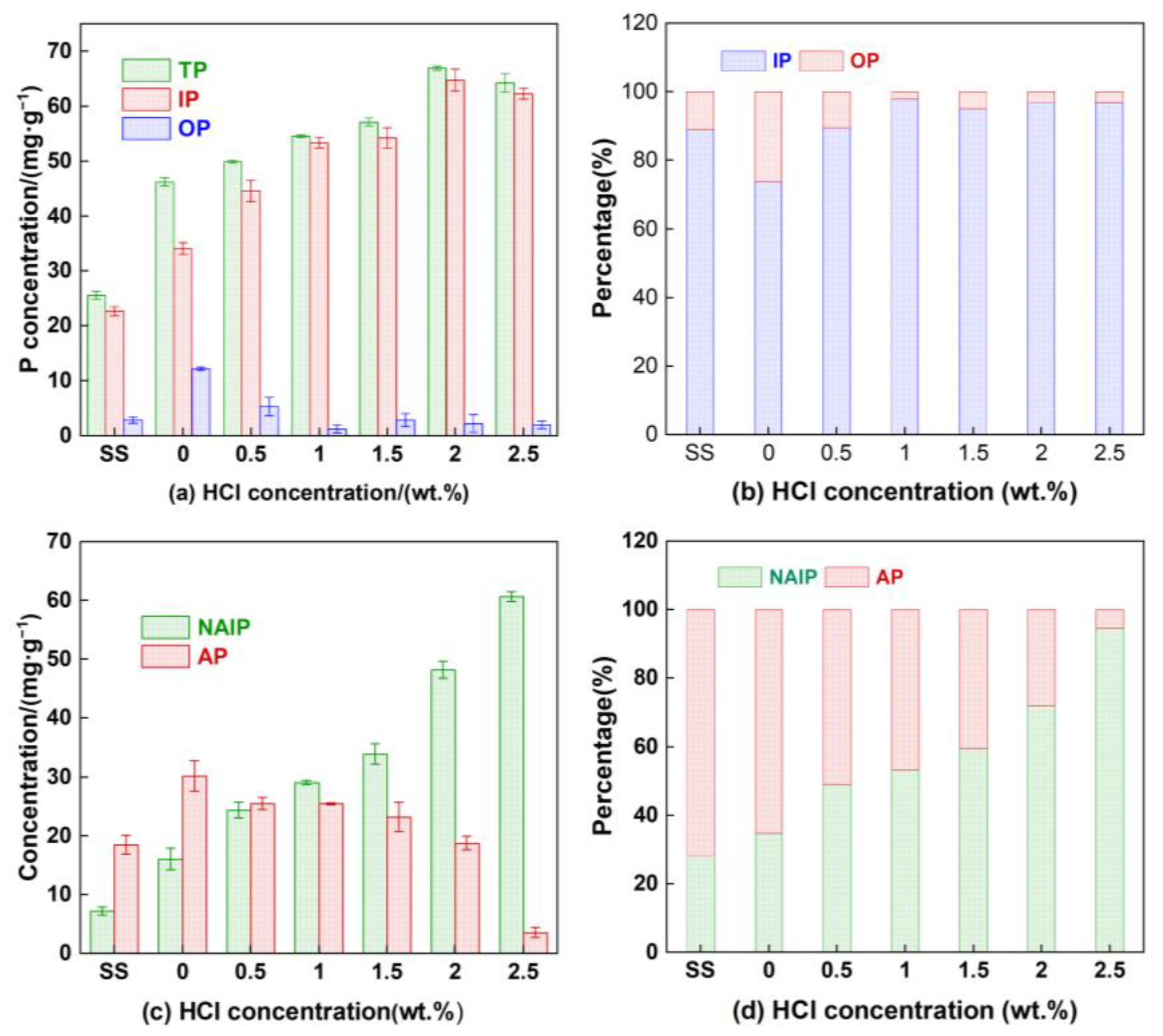
3.2. Distribution of Phosphorus
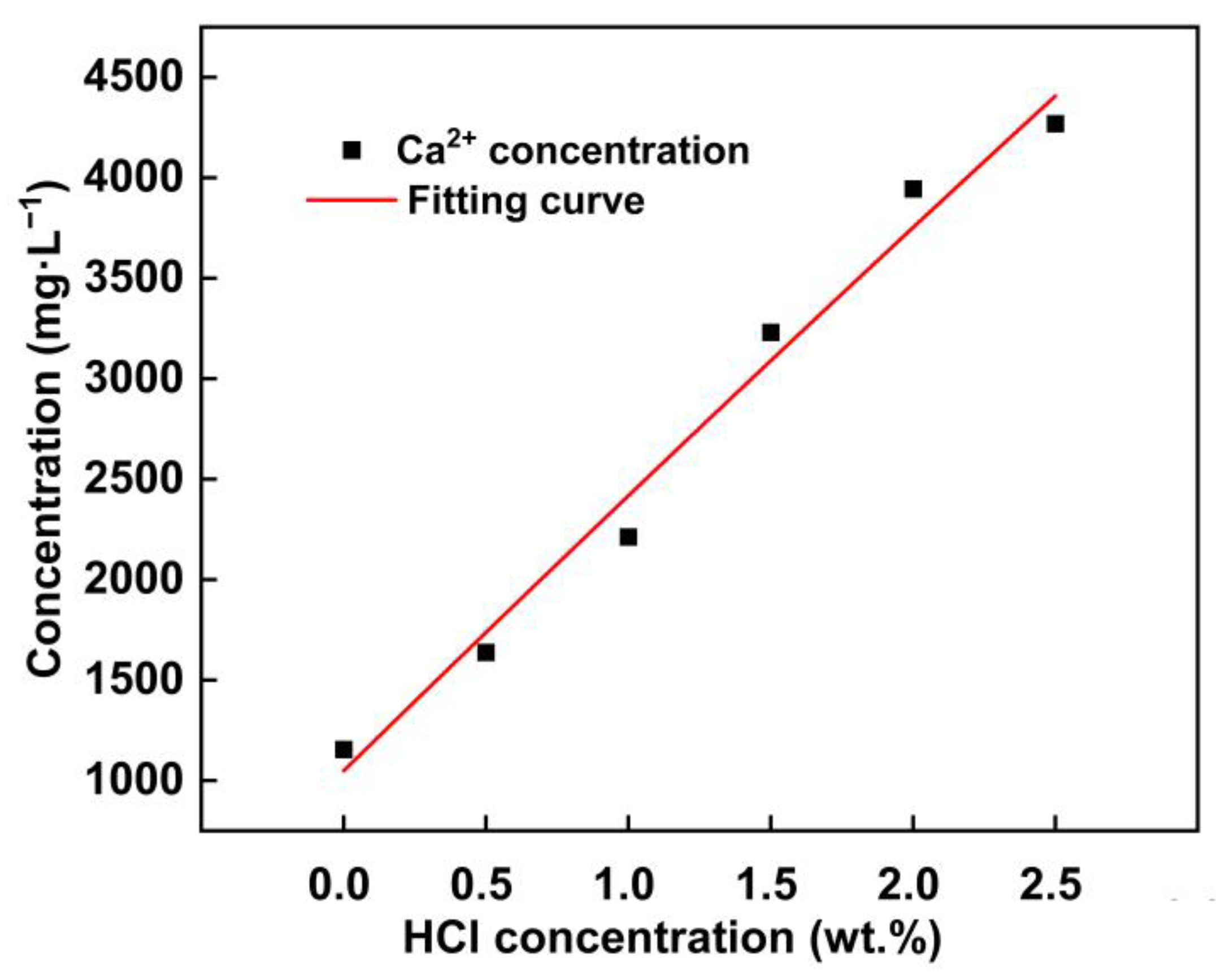
3.3. Morphological Transformation of Phosphorus in Process Water
3.4. Changes in Process Water pH
3.5. Transformation of Phosphorus in Hydrochar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Q.; Xu, J.; Gao, H.; Yan, J. A review of phosphorus recovery from different thermal treatment products of sewage sludge. Waste Dispos. Sustain. Energy 2019, 1, 99–115. [Google Scholar] [CrossRef]
- van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total Environ. 2016, 542, 1078–1093. [Google Scholar] [CrossRef]
- Zheng, X.; Ye, Y.; Jiang, Z.; Ying, Z.; Ji, S.; Chen, W.; Wang, B.; Dou, B. Enhanced transformation of phosphorus (P) in sewage sludge to hydroxyapatite via hydrothermal carbonization and calcium-based additive. Sci. Total Environ. 2020, 738, 139786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jiang, Z.; Ying, Z.; Ye, Y.; Chen, W.; Wang, B.; Dou, B. Migration and Transformation of Phosphorus during Hydrothermal Carbonization of Sewage Sludge: Focusing on the Role of pH and Calcium Additive and the Transformation Mechanism. ACS Sustain. Chem. Eng. 2020, 8, 7806–7814. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Zaccariello, L.; Battaglia, D.; Morrone, B.; Mastellone, M.L. Hydrothermal Carbonization: A Pilot-Scale Reactor Design for Bio-waste and Sludge Pre-treatment. Molecules 2022, 28, 781. [Google Scholar] [CrossRef]
- Chen, W.T.; Haque, M.A.; Lu, T.; Aierzhati, A.; Reimonn, G. A perspective on hydrothermal processing of sewage sludge. Curr. Opin. Environ. Sci. Health 2020, 14, 63–73. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Numviyimana, C.; Warchoł, J.; Khalaf, N.; Leahy, J.J.; Chojnacka, K. Phosphorus recovery as struvite from hydrothermal carbonization liquor of chemically produced dairy sludge by extraction and precipitation. J. Environ. Chem. Eng. 2022, 10, 106947. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Guo, D.; Munir, M.T.; Song, L.; Wu, X.; Huang, Y. Feasibility of using different hydrothermal processes for sewage sludge management in China. Sci. Total Environ. 2022, 838, 156154. [Google Scholar] [CrossRef] [PubMed]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Sarrion, A.; Diaz, E.; de la Rubia, M.A.; Mohedano, A.F. Fate of nutrients during hydrothermal treatment of food waste. Bioresour. Technol. 2021, 342, 125954. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Liu, S.; Zhou, X.; Gao, L.; Lu, Y.; Zhang, R. Phosphorus complexation of sewage sludge during thermal hydrolysis with different reaction temperature and reaction time by P K-edge XANES and (31)P NMR. Sci. Total Environ. 2019, 688, 1–9. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Arauzo, P.J.; Becker Gcapital Es, C.; Kruse, A. Experimental and thermodynamic studies of phosphate behavior during the hydrothermal carbonization of sewage sludge. Sci. Total Environ. 2019, 692, 147–156. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef]
- Dai, L.; Yang, B.; Li, H.; Tan, F.; Zhu, N.; Zhu, Q.; He, M.; Ran, Y.; Hu, G. A synergistic combination of nutrient reclamation from manure and resultant hydrochar upgradation by acid-supported hydrothermal carbonization. Bioresour. Technol. 2017, 243, 860–866. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhai, Y.; Li, S.; Liu, X.; Wang, B.; Liu, X.; Fan, Y.; Shi, H.; Li, C.; Zhu, Y. Thermal treatment of sewage sludge: A comparative review of the conversion principle, recovery methods and bioavailability-predicting of phosphorus. Chemosphere 2022, 291, 133053. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; Kruse, A. Fertilizer and activated carbon production by hydrothermal carbonization of digestate. Biomass Convers. Biorefin. 2017, 8, 423–436. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Speciation of Nutrients in Hydrochar Produced from Hydrothermal Carbonization of Poultry Litter under Different Treatment Conditions. ACS Sustain. Chem. Eng. 2018, 6, 11265–11272. [Google Scholar] [CrossRef]
- Pardo, P.; Lopez-Sanchez, J.F.; Rauret, G. Relationships between phosphorus fractionation and major components in sediments using the SMT harmonised extraction procedure. Anal. Bioanal. Chem. 2003, 376, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sarrion, A.; de la Rubia, A.; Coronella, C.; Mohedano, A.F.; Diaz, E. Acid-mediated hydrothermal treatment of sewage sludge for nutrient recovery. Sci. Total Environ. 2022, 838, 156494. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of organic wastes to carbonaceous solid fuel—A review of mechanisms and process parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Wust, D.; Rodriguez Correa, C.; Suwelack, K.U.; Kohler, H.; Kruse, A. Hydrothermal carbonization of dry toilet residues as an added-value strategy—Investigation of process parameters. J. Environ. Manag. 2019, 234, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, X.; Deng, Y.; Tsang, D.C.W.; Jiang, R.; Becker, G.C.; Kruse, A. Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. Sci. Total Environ. 2020, 698, 134240. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Fletcher, L.A. Influence of pH on hydrothermal treatment of swine manure: Impact on extraction of nitrogen and phosphorus in process water. Bioresour. Technol. 2016, 214, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, S.M.; Molde, J.S.; Timler, J.G.; Wood, B.M.; Mikula, A.L.; Vozhdayev, G.V.; Colosky, E.C.; Spokas, K.A.; Valentas, K.J. Phosphorus reclamation through hydrothermal carbonization of animal manures. Environ. Sci. Technol. 2014, 48, 10323–10329. [Google Scholar] [CrossRef]
- Fernandez, S.; Srinivas, K.; Schmidt, A.J.; Swita, M.S.; Ahring, B.K. Anaerobic digestion of organic fraction from hydrothermal liquefied algae wastewater byproduct. Bioresour. Technol. 2018, 247, 250–258. [Google Scholar] [CrossRef]
- Huang, R.; Tang, Y. Evolution of phosphorus complexation and mineralogy during (hydro)thermal treatments of activated and anaerobically digested sludge: Insights from sequential extraction and P K-edge XANES. Water Res. 2016, 100, 439–447. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, M.; Ying, Z.; Feng, Y.; Wang, B.; Dou, B. Correlating phosphorus transformation with process water during hydrothermal carbonization of sewage sludge via experimental study and mathematical modelling. Sci. Total Environ. 2022, 807, 150750. [Google Scholar] [CrossRef]
- Zhuang, X.; Huang, Y.; Song, Y.; Zhan, H.; Yin, X.; Wu, C. The transformation pathways of nitrogen in sewage sludge during hydrothermal treatment. Bioresour. Technol. 2017, 245, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fang, C.; Zhang, B.; Tang, Y. Transformations of Phosphorus Speciation during (Hydro)thermal Treatments of Animal Manures. Environ. Sci. Technol. 2018, 52, 3016–3026. [Google Scholar] [CrossRef]
- Qaramaleki, S.V.; Villamil, J.A.; Mohedano, A.F.; Coronella, C.J. Factors Affecting Solubilization of Phosphorus and Nitrogen through Hydrothermal Carbonization of Animal Manure. ACS Sustain. Chem. Eng. 2020, 8, 12462–12470. [Google Scholar] [CrossRef]
- Mekmene, O.; Quillard, S.; Rouillon, T.; Bouler, J.-M.; Piot, M.; Gaucheron, F. Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci. Technol. 2009, 89, 301–316. [Google Scholar] [CrossRef]
- Huang, R.; Fang, C.; Lu, X.; Jiang, R.; Tang, Y. Transformation of Phosphorus during (Hydro)thermal Treatments of Solid Biowastes: Reaction Mechanisms and Implications for P Reclamation and Recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. [Google Scholar] [CrossRef] [PubMed]



| Proximately Analysis (wt.%) | Ultimately Analysis (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Samples | Ash | Volatile | Fixed Carbon | C | H | N | S | O a |
| Dewatered sludge | 55.48 | 41.18 | 3.34 | 13.38 | 3.08 | 1.12 | 3.61 | 23.33 |
| HTC-0% HCl | 57.31 | 36.12 | 6.57 | 11.79 | 2.06 | 0.44 | 3.72 | 24.69 |
| HTC-0.5% HCl | 61.54 | 30.78 | 7.68 | 11.91 | 2.35 | 0.43 | 3.95 | 19.83 |
| HTC-1% HCl | 63.47 | 27.87 | 8.66 | 12.58 | 2.41 | 0.42 | 4.22 | 16.89 |
| HTC-1.5% HCl | 62.65 | 27.48 | 9.87 | 13.11 | 2.43 | 0.42 | 4.36 | 17.04 |
| HTC-2% HCl | 63.03 | 24.63 | 12.34 | 14.01 | 2.58 | 0.41 | 4.46 | 15.52 |
| HTC-2.5% HCl | 64.01 | 21.4 | 14.59 | 14.62 | 2.63 | 0.40 | 4.67 | 13.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Wang, Z.; Wu, Y.; Wu, R.; Zhao, F. Migration and Conversion of Phosphorus in Hydrothermal Carbonization of Municipal Sludge with Hydrochloric Acid. Sustainability 2023, 15, 6799. https://doi.org/10.3390/su15086799
Xue Y, Wang Z, Wu Y, Wu R, Zhao F. Migration and Conversion of Phosphorus in Hydrothermal Carbonization of Municipal Sludge with Hydrochloric Acid. Sustainability. 2023; 15(8):6799. https://doi.org/10.3390/su15086799
Chicago/Turabian StyleXue, Yang, Zhipu Wang, Yue Wu, Ruiqi Wu, and Fengtao Zhao. 2023. "Migration and Conversion of Phosphorus in Hydrothermal Carbonization of Municipal Sludge with Hydrochloric Acid" Sustainability 15, no. 8: 6799. https://doi.org/10.3390/su15086799





