Abstract
Potassium permanganate-modified bamboo biochar (MBB) was used to adsorb manganese from simulated groundwater and its performance was compared to that of unmodified bamboo biochar (BB), activated carbon, and manganese greensand. The adsorption kinetics, adsorption isotherms, and manganese fractions were investigated. The Langmuir model was the best fit for manganese adsorption by MBB and BB at the maximum adsorption capacities of 21.277 and 0.803 mg g−1, respectively. The heat of adsorption from the Temkin model indicated that manganese adsorption occurs via an ion exchange process for MBB and a physical adsorption process for BB. The sequential extraction results revealed that manganese was strongly bound to the iron/manganese oxide fraction, in accordance with the chemical adsorption established in pseudo-second order kinetic data records.
1. Introduction
Many water utilities strive to remove manganese (Mn) from sources of drinking water. Mn exhibits a range of valencies and colors depending on its oxidation state; Mn(II) is soluble in water and has a pale pink color, while Mn(IV) is insoluble and dark brown [1]. It is found naturally in the Earth’s crust; the geological characteristics of the Earth have led to the natural contamination of groundwater supplies. Mn removal from groundwater is chemically difficult; it accumulates in distribution systems and affects the taste and color of drinking water even at low concentrations [1]. Various studies have reported the negative impacts of Mn. Environmental Mn can affect verbal processing functions in adults [2], and another study revealed that school-aged children exposed to higher concentrations of Mn in drinking water had lower IQ levels [3]. In southern Quebec, Canada, groundwater sources for tap water have become contaminated with a high Mn content of 34 μg L−1 [3]. Further, high Mn consumption in southern China has significantly impacted people’s health, ultimately resulting in chronic illnesses and symptoms related to brain function [4]. In 2022, the World Health Organization (WHO) recommended that the Mn concentrations in safe drinking water be less than 0.08 mg L−1, while the maximum allowable concentration of Mn in groundwater for drinking purposes in Thailand was 0.3 mg L−1 [5]. Thus, groundwater contaminated with Mn to levels above those recommended must be treated to make drinking water safe for consumption.
Mn removal from groundwater can be carried out via physical, chemical, biological, and physicochemical processes [6]. The oxidation [7] and filtration [8] processes are currently popular due to their high efficiencies in Mn removal [9,10]. However, ozone oxidation is expensive, and chlorine oxidation often produces hazardous trihalomethane byproducts, which are carcinogenic agents. Thus, filtration is suitable for household Mn removal. Activated carbon (AC) is commonly used as filter media [11], however, it has no capacity with regard to oxidation. Thus, manganese greensand—sand coated with manganese oxide—has been employed to remove Mn. Recently, adsorbents coated with various oxidizing agents, such as zeolite tuff [12], kaolin [13], and manganese oxide-coated zeolite [14], have been developed for Mn removal. However, commercial adsorbents such as AC and greensand are expensive. Therefore, biochar and other natural materials have recently been studied more thoroughly for heavy metal removal. Biochar has high porosity and similar adsorption properties to AC, and serves as a low-cost alternative sorbent or adsorbent for the removal of metals, including Mn, from groundwater. Biochar from poultry and farmyard manure has served as an adsorbent for the removal of Mn from groundwater [15], and bamboo biochar (BB) has been used for the adsorption of lead(II) from aqueous solutions [16]. Biochar is a product of the thermal decomposition of biomass. Three different methods have been reported for thermal decomposition, namely, pyrolysis, hydrothermal carbonization, and microwave-assisted carbonization, in which pyrolysis temperature is between 400–700 °C. As biochar has low surface area and porosity, physical and chemical activation methods have been applied both pre- and post-carbonization [17]. For example, biochar coated with potassium permanganate has proven effective in the removal of cadmium [18,19] and lead [20] from aqueous solutions. Piispanen and Sallanko [21] reported that the addition of manganese oxide-coated media in filters can improve Mn removal to achieve Mn concentrations of less than 0.02 mg L−1. Furthermore, Chen et al. [22] reported that MnO2 can be produced at the surface of bamboo biochar (BB) for effective uranium(VI) adsorption. Among the biochars derived from biowastes and biomass, bamboo is a good choice, as it is abundantly available, has a high growth rate, and is easy to process. Moreover, the need for a cost-effective solution for Mn pollution control, particularly in developing countries and rural areas where advanced technologies such as ozone oxidation are not possible. The use of permanganate-modified bamboo biochar has previously been proposed as a novel technology. Although many adsorbents are used to remove heavy metals, they may be capable of both storing and releasing them. The chemical fractionation of Mn on the adsorbent can be used to determine the metal released from the adsorbent. The sequential extraction processes presented by Tessier [23] consist of numerous steps, resulting in the following fractions: water soluble, exchangeable, bound to carbonates, bound to iron and manganese oxide, bound to organic matter, and residual. The highly soluble and exchangeable fractions present the risk of heavy metals leaching into the environment. The mobility of Mn is often increased at low pH or redox potentials. Previous studies have examined the Mn fractions in water, soil, and sediments; for example, in soil amendments with sewage sludge, biochar reduced Mn mobility in contaminated sites, decreasing the acid-soluble fractions by 70.38% [24]. Another study found that dissolved and particulate Mn in the Seine River estuary were bound to calcium carbonate, leading to the stabilization of the Mn(II) form [25]. Furthermore, the intraparticle diffusion, pseudo-first order (PFO) and pseudo-second order (PSO) can be used to describe adsorption mechanisms and explain the uptake rate of adsorbate on biochar [26]. Therefore, it is important to refer to the results of such studies as a guide for effective heavy metal removal.
Few studies have been conducted on the removal of Mn from water using bamboo biochar and modified bamboo biochar by permanganate impregnation. Thus, modified bamboo biochar may be a novel adsorbent for Mn adsorption. In addition, previous research has hardly investigated the comparison of adsorption performances of modified adsorbents with commercial greensand and activated carbon for Mn removal in real groundwater. Therefore, this study aims to investigate the removal efficiencies of manganese for bamboo biochar (BB) and potassium permanganate-modified bamboo biochar (MBB) as compared with those of AC and manganese greensand (MnG) in order to study the adsorption kinetics and isotherms and investigate the Mn fractionation in the adsorbents after the adsorption process using real groundwater with a certain Mn concentration.
2. Materials and Methods
2.1. Preparation of Simulated Groundwater
Groundwater was withdrawn from the NonNhon subdistrict, Warinchamrap district, Ubon Ratchathani province in Thailand. Simulated groundwater was prepared by adding MnSO4.H2O (analytical grade, 98%, KemAus™, Sydney, Australia) to the natural groundwater at specific concentrations of 1, 3, 5, 7, and 9 mg L−1 for the batch experiments. The chemical characteristics of the groundwater are presented in Table 1.

Table 1.
The chemical characteristics of the groundwater.
2.2. Adsorbents
The adsorbents in this study included BB, MBB, AC, and MnG. BB was prepared via the carbonization of bamboo (Bambusa beecheyana) from Ubon Rachathani province, Thailand, in a mound kiln under oxygen-limited conditions and within the temperature range of 400–600 °C. The obtained BB was crushed to a uniform size in the range of 2.0–3.0 mm. The preparation of MBB was adapted from the method of Taffarel and Rubio [27] and Xuwen et al. [28]. Here, 10 g of the crushed BB was placed in a 500-mL beaker containing 400 mL of 5% (w v−1) KMnO4 solution (KMnO4, analytical grade, 99%, KemAus™, Sydney, Australia) and then boiled and stirred at 90 °C for 1 h. The suspension was allowed to cool and then filtered and rinsed with tap water until the water washings became colorless. The biochar samples were then dried in an oven at 60 °C for 6 h. Commercial-grade AC and MnG were purchased from a domestic supplier (J.L. Intertrade Co., Ltd., Bangkok, Thailand) and were sieved to obtain particles 2.0–3.0 mm in size before use. All adsorbents were kept in closed containers and used for all the experiments.
2.3. Characterization of Adsorbents
The external surface structural morphology of all adsorbents was determined using scanning electron microscopy and energy dispersive X-ray spectrometry (SEM-EDS, JEOL Model JSM-7610F Plus, Tokyo, Japan) before and after Mn adsorption. The surface of the adsorbents was investigated using a surface area and pore size analyzer (BET, Quantachrome Model Quadrasorb evo, Anton Paar GmbH, Graz, Austria). The chemical bonds or functional groups in the adsorbent molecules were analyzed before and after adsorption by Fourier transform infrared spectroscopy (FT-IR, Thermo Scientific Nicolet 6700, Waltham, MA, USA). Elemental analysis of the adsorbent materials was conducted using a wavelength dispersive X-Ray fluorescence spectrometer (WDXRF, Model Rigaku ZSX Primus, Tokyo, Japan). The adsorption capacities of the adsorbents were tested by the adsorption of iodine following the ASTM D4607 standard.
The measurement of the point of zero charge (pHpzc) was adapted from the method of Yang et al. [8]. A 0.005 M CaCl2 solution (CaCl2.2H2O, analytical grade, 78%, Q RëC™, Auckland, New Zealand) was boiled for 15 min to remove CO2 and then allowed to cool to room temperature. The initial pH (pHI) of the solution was adjusted to 3, 5, 7, and 9 via the dropwise addition of 0.5 M HCl (HCl, analytical grade, 37%, Q RëC™, Auckland, New Zealand) or 0.5 M NaOH (NaOH, analytical grade, 99%, Q RëC™, Auckland, New Zealand). Subsequently, 1 g of the adsorbents was added to 30 mL of each pH solution in 50-mL screw cap test tubes, the mixtures were shaken at 150 rpm for 24 h at 28 ± 0.5 °C, and the pH value after adsorption (pHF) was measured. The pHF values were plotted against the pHI ones to determine pHpzc from the intersection point of the curves with the pHI = pHF line.
2.4. Adsorption Experiments
2.4.1. Study of Contact Time
Batch adsorption experiments were carried out to determine the equilibrium contact time of Mn adsorption. The simulated groundwater with an Mn concentration of 5 mg L−1 was adjusted to pH 6.7 ± 0.1 with 1 N HNO3 (HNO3, analytical grade, 65%, Q RëC™, Auckland, New Zealand) and 1 N NaOH. Dosages of 50 g L−1 of BB and 0.25 g L−1of MBB, AC, and MnG were used. The mixtures were shaken for various times—24, 48, 72, and 96 h—at 100 rpm and 28 ± 0.5 °C. The suspensions were then filtered through Grade 42 Whatman filter paper and the Mn concentration of the solutions was analyzed via inductively coupled plasma-optical emission spectroscopy (ICP-OES, Perkin ElmerOptima 8000, Rodgau, Germany).
Then, the amount of Mn adsorbed on each adsorbent and the percentage removal efficiencies were calculated following Equations (1) and (2), respectively:
where is the volume of the solution (L), is the initial Mn concentration (mg L−1), is the final Mn concentration at time (mg L−1), is the Mn concentration of the blank control without the addition of an adsorbent, and m is the sorbent mass (g).
2.4.2. Adsorption Kinetics
The kinetics of Mn adsorption from simulated groundwater at an Mn concentration of 5 mg L−1 were determined using BB, AC, MBB, and MnG in amounts of 50, 50, 0.25, and 0.25 g L−1, respectively. The mixtures were shaken in an incubator shaker at 30 °C and 100 rpm at an initial pH of 6.7 ± 0.1. In addition, in order to investigate the influence of temperature at 25 and 35 °C on thermodynamic adsorption, the solutions were separated from the mixtures via filtration through Grade 42 Whatman filter paper before analysis of the Mn concentration. All experiments were conducted in triplicate. The kinetics of Mn adsorption were evaluated by applying two different models: the pseudo-first order and pseudo-second-order kinetic models [29]. The pseudo-first order kinetic model is expressed as in Equation (3):
where and are the amounts of Mn (mg g−1) adsorbed at equilibrium and at time , respectively, and is the Lagergren pseudo-first order adsorption rate constant (min−1). The linear form of the pseudo-second order model can be represented by Equation (4):
where (g mg−1 h−1) is the pseudo-second order rate constant.
Intraparticle diffusion is the mechanism of heavy metal adsorption onto adsorbent particles. The most widely applied intraparticle diffusion equation for adsorption systems is provided by Cheung et al. [30] as Equation (5):
where is the intraparticle diffusion rate constant (mgg−1 min1/2), is the amount of solute on the surface of the sorbent at time (mgg−1), and is the intraparticle diffusion rate constant (mgg−1 min1/2) and the intercept of the plot of versus t1/2. When film diffusion is present, the intercept is , which provides insight into the boundary layer’s thickness.
2.4.3. Adsorption Isotherms
The adsorption isotherms were determined using simulated groundwater at Mn concentrations of 1, 3, 5, 7, and 9 mg L−1 at an adjusted pH of 6.7 ± 0.1. Sorbent doses of 50 g L−1 of BB and 0.25 g L−1 of MBB, AC, and MnG were used. The mixtures were shaken in an incubator shaker at 100 rpm and 30 °C until the equilibration time had been reached (48 h for BB and AC; 72 h for MBB and MnG). Thereafter, the suspensions were filtered through Grade 42 Whatman filter paper and the Mn concentration of the solutions was analyzed by ICP-OES. All experiments were performed in triplicate. The amount of Mn adsorbed onto the adsorbent was calculated using Equation (1):
The isotherm data were then subjected to the most widely used empirical models: the Langmuir, Freundlich [31], and Temkin [32] isotherms and the Dubinin–Raduskevich (D-R) isotherm [33]. The linearized form of the Langmuir model is presented in Equation (6):
where is the Langmuir maximum adsorption capacity (mg g−1), is Mn concentration at the equilibrium time (mg L−1), and is the Langmuir affinity constant (L mg−1). is an important tool in the calculation of the equilibrium parameter () that explains the favorability of the adsorption process; is calculated using Equation (7):
The linearized form of the Freundlich model is presented in Equation (8):
where is the Freundlich constant (mg g−1) (mg L−1)1/n and is a dimensionless constant related to the adsorption intensity. The Temkin model is presented in Equation (9):
where is the Temkin isotherm equilibrium binding constant (L g−1), is the universal gas constant (8.314 J mol−1 K−1), is the temperature (K), and is a constant related to the heat of sorption (J mol−1).
The Dubinin–Radushkevich isotherm model (D-R), which proposes that pore filling mechanisms explain adsorption, is widely employed to differentiate between chemical and physical adsorption of metal ions. The linear form of the Dubinin–Radushkevich isotherm is defined by Equations (10)–(12):
where is the Dubinin–Radushkevich constant (mol2 kJ−2), qs is the theorical maximum adsorption capacity (mg g1), and ε describes the Polanyi potential, with R being the universal ideal gas constant (8.314 j mol−1 K−1) and T the absolute temperature (K). From this model, we can determine the mean adsorption energy E (kJ mol−1); when the value of E is <8 kJ mol−1, the process is physical adsorption, and when E is between 8 kJ mol−1 and 16 kJ mol−1, the process is chemical adsorption [34].
2.5. Thermodynamics Calculations
In determining the effect of temperature on Mn adsorption onto adsorbents, thermodynamic parameters provide further information on the inherent energy changes associated with the adsorption process. The most typical equations for thermodynamic parameters include the change in free energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°). ΔG° is calculated as in Equation (13) [35]:
where is the universal gas constant (8.314 Jmol−1 K−1), is the temperature (K), and is the equilibrium constant. If has units of L mg−1, then × Madsorbate × 1000 × 55.5 [35], where is the Langmuir affinity constant (L mg−1), Madsorbate is the molecular weight of Mn (54.94 g mol−1), and 55.5 is the molar concentration of water. Here, the values of ΔH° and ΔS° were respectively obtained from the slope and the intercept of the plot between lnK and 1/ by considering the following Equation (14):
ΔG° = −RTlnK
2.6. Chemical Fractionization
Here, 2 g of adsorbent for the pre- and post-adsorption stages were analyzed using sequential extraction techniques [22]. The chemical forms of Mn are classified into six fractions—water soluble (F1), exchangeable (F2), bound to carbonates (F3), bound to iron and manganese oxide (F4), bound to organic matter (F5), and residual (F6)—following the procedures shown in Table 2.

Table 2.
Sequential extraction procedures.
3. Results and Discussion
3.1. Characteristics of Biochar
The external surface structural morphology from the SEM-EDS images shows the porous structure of BB and AC (Figure 1a,b), with BB having longitudinal pores. According to the International Union of Pure and Applied Chemistry (IUPAC) pore size classification, it was found that BB and AC contained mostly macropores (>50 nm) and few mesopores (2–50 nm) and micropores (<2 nm), respectively. The high amount of macropores is suitable for removal of pollutants in liquid phase while providing liquid mass transfer, whereas mesopores provide solute diffusion to the active sites [36]. The pores of BB and AC range from micro- to macropore sizes (10–100 μm) [29]. The vascular bundles of the raw biomass are the origin of the large pores. As shown in Figure 1c, the Mn nanoparticles were successfully doped onto the surface of MBB, which has the same microparticle characteristics as the MnG surface in Figure 1d. The Mn composition on the MBB adsorbent surface increases to 50.07%, which is higher than the value for MnG (14.27%).

Figure 1.
SEM-EDS images (200×): (a) bamboo biochar (BB), (b) activated carbon (AC), (c) potassium permanganate-modified bamboo biochar (MBB), and (d) manganese green sand (MnG).
The BET surface area of the adsorbents is presented in Table 3. BB has a pore volume of 0.143 cc g−1, which correlates with the value of 0.14 cc g−1 reported by Sahoo et al. [37]. MBB has a BET surface area of 104.2 m2 g−1, smaller than the original BB (176.2 m2 g−1), which is due to Mn filling the pores, as shown in Figure 1c. The EDS spectral data reveal an Mn content of 50.07%. MnG is mainly composed of silicon dioxide, and has a BET surface area of 0.147 m2 g−1, smaller than the value reported by Xuwen et al. [28] (8.4 m2 g−1). In addition, Outram et al. [38] characterized MnO2-coated silica (quartz) as having a low surface area (<0.5 m2 g−1). Similarly, the iodine numbers for MBB (69.13 mg g−1) and BB (67.55 mg g−1) are higher than that of MnG (6.14 mg g−1), as shown in Table 3; all are lower than the value of 271.96 mg g−1 for AC.

Table 3.
Characteristics of adsorbents.
The pHpzc value is another useful measure; it indicates the pH at which the charge of the adsorbent surface is zero as compared to pHDI, where pHDI is the pH value of DI water with adsorbents added. The pHpzc of the adsorbents ranges from 7.43–8.10 for AC, BB, and MBB. The surface of an adsorbent is positive at pHDI < pHpzc, neutral at pHDI = pHpzc, and negative at pHDI > pHpzc; therefore, the adsorption of Mn(II) increases with an increase in pH due to the electrostatic attraction between the positively charged Mn(II) ions and the negatively charged surface of the adsorbent when pHDI > pHpzc. In the pHDI range of 6 to 9, dissolved Mn(II), a divalent cation, can be removed from solution via sorption onto a solid surface, most often manganese oxide [39]. Mn(II) adsorption onto MnOx surfaces is rapid [40] and is accompanied by the release of H+, as is the case with cation adsorption onto oxide surfaces, shown in Equation (15) [41]:
Mn2+(aq) + MnO(OH)2(s) → MnO2MnO(s) + 2H+(aq)
The FT-IR spectra of the adsorbents were recorded between 500 and 4000 cm−1 using a spectrometer. As depicted in Figure 2a, b, the FT-IR spectra of BB, AC, MBB, and MnG all display peaks at 3445.19, 3445.41, 3446.34, and 3448.29 cm−1, respectively, indicating the presence of O–H stretching vibrations [18]. BB shows peaks at 1625.26, 875.62, and 571.61 cm−1, attributed to C=C stretching, C–H bending, and C–H bending, respectively [38]. After adsorption, the respective peaks are shifted to wave numbers of 1602.42, 861.52, and 577.91 cm−1. The functional groups of BB and Mn2+ ions may have become coordinated to produce the newly observed peak at 489.39 cm−1 [42]. AC shows peaks at 1642.04, 1400.06, and 607.08 cm−1, assigned to C=C stretching into the carbonyl/carboxyl groups, C–H bending, and aromatic C–H bending, respectively; the functional groups and chemical binding after adsorption lead to peaks at 1641.88, 1401.96, and 584.35 cm−1, respectively.
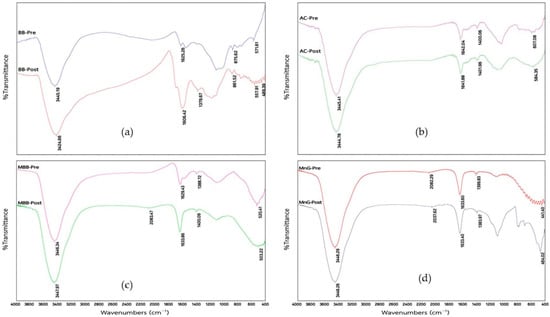
Figure 2.
FT-IR spectra of adsorbents: (a) bamboo biochar (BB), (b) activated carbon (AC), (c) potassium permanganate-modified bamboo biochar (MBB), and (d) manganese green sand (MnG).
The FT-IR spectra of MBB and MnG in Figure 2c, d show peaks at 1388.72 cm−1 (MBB) and 1399.83 cm−1 (MnG), arising from -OH stretching vibrations, while the peaks at 520.41 and 441.40 cm−1 indicate Mn–O stretching [43]. The spectral peaks are shifted to 3448.26, 1383.97, and 464.02 cm−1for MnG and 3447.97, 1400.09, and 503.22 cm−1 for MBB, indicating the binding of Mn to the O-H group.
Table 4 presents the elemental composition of the adsorbents. Silica (Si) was the main component of AC, while that of BB was potassium (K). BB contained an Mn content of 0.70%, while Mn was not detected in AC. However, higher levels of other cationic metals were present in AC compared to BB. BB had high contents of K (64.5%), Si (7.93%), and Ca (7.78%), with compositional values comparable to those reported by Liu et al. [44]. MBB had lower metal contents than BB, and contained no iron (Fe) due to the leaching of metals during preparation. MBB had an Mn content of 58.30%, approximately six-fold higher than that of MnG, which is because MnO2 is able to penetrate the micropores of MBB, whereas MnG does not possess a porous structure.

Table 4.
The elemental composition of adsorbents by WDXRF.
3.2. Batch Experiments
The contact time significantly influences the removal of metal ions from aqueous solutions. The Mn adsorption capacities (mg g−1) and percentage removal efficiencies at different times for each adsorbent are shown in Figure 3a, b, respectively. Adsorption of Mn by all adsorbents reaches the equilibration time at 72 h. However, MnG shows the maximum adsorption at 24 h and then plateaus, indicating that Mn was rapidly adsorbed onto active sites. This phenomenon corresponds to the study of Köse et al. 2020 [45], and is caused by the collision of adsorbent, implying that chemical adsorption occurs at the active sites. Reaching sorption equilibrium within a short contact time is an indication of the efficiency of a material, and short contact times are preferred for efficient metal removal [46]. We found that when using an adsorbent dosage of 5% w v−1 for BB and AC we could achieve rates of 87.18% and 96.85%, respectively, whereas for MBB and MnG the rates were 51.07% and 31.48%, respectively, with the same adsorbent dosage of 0.03% w v−1. Thus, BB and MBB could be used as low-cost adsorbents for Mn removal to replace AC and MnG, respectively. Furthermore, these results suggest that chemical adsorption may be stronger than physical adsorption thanks to its shorter reaction time [47]. MBB initially exhibits good removal efficiency because of its empty surfaces, which adsorb manganese(IV) oxide (MnO2). According to Equation (16), Mn2+ in groundwater is adsorbed onto the adsorbent’s surface as follows [48]:
Mn2+(aq) + MnO2(s) → MnO2(s) − Mn2+
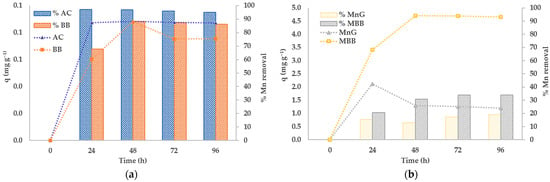
Figure 3.
Effect of contact time and Mn removal efficiency of adsorbents: (a) BB and AC; (b) MBB and MnG. Initial Mn 4.562 mg L−1,; dosage 5%w v−1 for BB and AC, 0.03%w v−1 for MBB and MnG.
Our investigation of contact time revealed that pHDI is significantly lower than pHpzc, which is due to positive discharge of Mn2+, resulting in lower Mn adsorption.
3.3. Adsorption Kinetics
The PFO and PSO kinetic models were applied to interpret the mechanisms of Mn adsorption onto the adsorbents. The kinetic parameters are shown in Table 5. In the case of the pseudo-second order model, the theoretical values for all adsorbents are close to the experimental values. The pseudo-second order model was the best fit for the kinetic data of Mn adsorption based on the values of the correlation coefficient (R2) and . This implies that the rate-limiting step comprised chemical adsorption involving valence forces by the sharing or exchanging of electrons between the adsorbent and adsorbate [49], revealing a chemisorption relationship generated by both the adsorbent and the solution [50].

Table 5.
Adsorption kinetics parameters.
Table 5 displays the values, which were derived from the slopes of the linear portions of the curves. A linear line can be seen on the intra-particle diffusion plot, which indicates that intra-particle diffusion has an impact. This shows that diffusion occurs within the adsorbent’s pores and controls adsorption [51]. The highest diffusion rate is represented by the value of 0.0114 mg g−1 min−1/2 for MnG, followed by MBB at 0.0059 mg g−1 min−1/2. AC and BB are similar, and have lower diffusion rates. The higher diffusion rates of MnG and MBB may be due to several processes on adsorbents, such as adsorption, oxidation, and precipitation.
3.4. Adsorption Performance and Isotherm Fitting
The linear patterns of the Freundlich and Langmuir models were used to represent the equilibrium adsorption isotherm data. Table 6 shows the constant parameters and correlation coefficients for each isotherm model. For all adsorbents, the Langmuir model leads to better correlation values (R2 > 0.959) than the others. The value of indicates the separation factor, showing that the behavior of Mn2+ adsorption is favorable (0 < < 1). The value of BB is slightly higher than that of AC, and the value of MBB is higher than that of MnG. The Temkin plot shows that the adsorption energies of Mn for BB and AC are less than 8 kJ mol−1; thus, adsorption is a physical process in this case. On the other hand, adsorption by MBB and MnG involves energies between 8 and 16 kJ mol−1, indicating that the adsorption process proceeds via ion exchange [48].

Table 6.
Parameters and constants for selected isotherms.
Adsorption is assessed via the D-R isotherm model in terms of energy [52]. If is between 8–16 kJ mol−1, the process is chemical adsorption, while if it is between 1–8 kJ mol−1 it is physical adsorption. In this study, we discovered that a value of in the range of 1.67–3.773 kJ mol−1 indicates a physical process for all adsorbents. D-R isotherm descriptions of vapor and gas adsorption on microporous sorbents such as activated carbon and zeolites typically use this model [53]. As a result, the D-R model might not be appropriate for describing the lower R2 of Mn adsorption on MBB and MnG [54]. In general, it is less appropriate for metal adsorption.
Table 7 compares the values from the Langmuir adsorption model of BB and MBB with those from other studies. MBB has a higher than other adsorbents when using an initial Mn concentration lower than or equal to 5 mg L−1.

Table 7.
Adsorption capacity of Mn compared to other adsorbents.
The commercial costs of AC, BB, and MnG in Thailand are within the respective ranges of 2.79–3.52, 0.88, and 5.29–7.34 USD kg−1. The cost of MBB can be estimated from the cost of the chemicals use for preparation, notably the cost of permanganate at 2.35–4.14 USD kg−1. Thus, MBB costs around 2.63 USDkg−1. However, in the present study it was found that MBB can be used in lower amounts than BB, leading to a lower-cost adsorbent.
3.5. Thermodynamic Studies
Thermodynamic parameters are calculated as shown in Table 8. Adsorption in the case of all adsorbents was a spontaneous process according, as shown by the negative values of ΔG° [57,58]. The positive values of ΔS° demonstrate that the adsorption process increased the randomness at the interface of the solid/solution system [58].

Table 8.
Calculation of thermodynamic parameters.
3.6. Mn Fractionization of Adsorbents
The highest content of Mn in the pre-adsorption AC and BB was present in the fractions bound to organic matter (F5, 31.37%) and in those bound to iron and manganese oxide (F4, 25.17%), as shown in Figure 4. After adsorption, the main fraction of Mn was F4 in both AC (32.15%) and BB (26.34%). This finding corresponds to the findings of previous research by Inam et al. [59], who reported that the iron/manganese oxide bound fraction is a possible main source of Mn binding. The binding of Mn with iron and manganese oxide in this study was strong, in agreement with pseudo-second order kinetics data, leading to chemical adsorption. The behavior of Mn in binding to iron/manganese oxide in water is controlled by environmental conditions such as pH changes [60]. After adsorption by AC, the percentage of exchangeable Mn(F2) was above 10%, indicating that there were remaining portions that could be released. Metals exhibit thermodynamic instability in the F2 fraction, as they are sensitive to redox and pH changes [61].
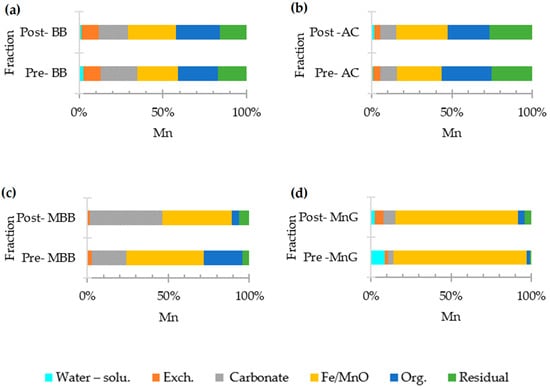
Figure 4.
Mn fractionization of adsorbents: (a) bamboo biochar (BB), (b) activated carbon (AC), (c) potassium permanganate-modified bamboo biochar (MBB), and (d) manganese greensand (MnG).
The highest Mn fraction of MnG both pre- and post-adsorption was the F4 fraction at 76.70%. These was due to the main component of sand being oxides of silica and alumina; on the other hand, the main component of MBB is Mn oxide. When MBB contacted Mn ions in the solution, oxidation occurred and Mn was precipitated into its solid form [41]. Thus, the Mn fraction bound to carbonates (F3) increased from 21.25% to 44.6%.
According to the present study, the binding of Mn in the carbonate phase was poor [22], and dependent on the pHDI of MBB and MnG. The properties and structure of biochar are critical to the immobilization process of the heavy metals on it. The pyrolysis conditions and feedstock type have a significant impact on the properties of biochar [62]. Physical properties such as the surface area, pore volume, and pore size favor the preliminary diffusion of elemental metals through the pores of the biochar surfaces [59]. The functional groups that appear on the surface of the sorbent are shown in Figure 2. Of these, the O-H group is important for binding of Mn to iron and manganese oxide(F4). Research has suggested that ion exchange processes involve manganese ions (Mn2+) that attach themselves to adjacent hydroxyl groups [62], resulting in the formation of complexes with OH in groundwater, such as Mn(OH)+, Mn(OH)2, Mn2(OH)3+, Mn2OH3+, and Mn(OH)42− [63].
4. Conclusions
In this study, MBB displayed the maximum Mn adsorption capacity, which was due to adsorption in the biochar pores and Mn oxidation and precipitation on the surfaces. MBB is a low-cost adsorbent material, and can be used in smaller quantities than BB; moreover, it can be used as a substitute for MnG because it is cheaper. However, the use of MBB for the removal of low Mn concentrations in groundwater should be investigated further, as excess Mn from MBB may leach out. Thus, it is recommended to use a small amount of MBB and an up-flow adsorption filter. This could provide the reaction times and entrap the precipitated in the filter, thereby achieving both high removal efficiency and safety. Further research on Mn adsorption with biochar is needed in order to optimize the process and understand the mechanisms involved. Moreover, additional research on use of MBB as an adsorbent should be conducted in groundwater with high manganese concentrations and using column filtration.
Author Contributions
Conceptualization, A.W., S.V. and A.P.; methodology, A.W.; software, A.W.; validation, S.V. and A.P.; formal analysis, S.V. and A.P.; investigation, A.W.; resources, S.V. and A.P.; data curation, S.V. and A.P.; writing—original draft preparation, A.W.; writing—review and editing, S.V., A.P. and A.W.; supervision, S.V. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by National Research Council of Thailand, grant number NRCT.MHESI(A)(PS)/122/2563; the APC was funded by the Research, Innovation, and Partnerships Office, King Mongkut’s University of Technology Thonburi.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the School of Energy, Environment, and Materials, King Mongkut’s University of Technology Thonburi, for partially supporting the research fund for chemicals and facilities, as well as to the Department of Chemistry, Faculty of Science at King Mongkut’s University of Technology Thonburi and to the Faculty of Science at Ubon Ratchathani for assisting the laboratory in conducting research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World-Health-Organization. Drinking Water Safety Management. 2022. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 25 November 2022).
- Riojas-Rodríguez, H.; Solís-Vivanco, R.; Schilmann, A.; Montes, S.; Rodríguez, S.; Ríos, C.; Rodríguez-Agudelo, Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ. Health Perspect. 2010, 118, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.F.; Sauvé, S.; Barbeau, B.; Legrand, M.; Brodeur, M.È.; Bouffard, T.; Limoges, E.; Bellinger, D.C.; Mergler, D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ. Health Perspect. 2011, 119, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Crossgrove, J.; Zheng, W. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Groundwater Analysis Division, Notification of the Ministry of Natural Resources and Environment, B.E.2551 (2008). Water Quality Standards Groundwater Used for Consumption. Available online: http://www.dgr.go.th/dga/th/about/350 (accessed on 11 November 2022).
- Patil, D.S.; Chavan, S.M.; Oubagaranadin, J.U.K. A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 2006, 4, 468–487. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiong, W.; Huang, T. Catalytic oxidation removal of manganese from groundwater by iron–manganese co-oxide filter films under anaerobic conditions. Sci. Total Environ. 2020, 737, 139525. [Google Scholar] [CrossRef]
- Yang, H.; Yan, Z.; Du, X.; Bai, L.; Yu, H.; Ding, A.; Li, G.; Liang, H.; Aminabhavi, T.M. Removal of manganese from groundwater in the ripened sand filtration: Biological oxidation versus chemical auto-catalytic oxidation. Chem. Eng. J. 2020, 382, 123033. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, T.; Wen, G.; Cao, X. The simultaneous removal of ammonium and manganese from groundwater by iron-manganese co-oxide filter film: The role of chemical catalytic oxidation for ammonium removal. Chem. Eng. J. 2017, 308, 322–329. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, T.; Liu, C.; Zhang, S. Effects of dissolved oxygen on the start-up of manganese oxides filter for catalytic oxidative removal of manganese from groundwater. Chem. Eng. J. 2019, 371, 88–95. [Google Scholar] [CrossRef]
- Rahpeima, S.; Javanbakht, V.; Esmaili, J. Synthesis and characterization of activated carbon/maghemite/starch magnetic bionanocomposite and its application for permanganate removal from aqueous solution. J. Inorg. Organomet. Polym. Mater. 2018, 28, 195–211. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Miner. Eng. 2010, 23, 1131–1138. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Altunkaynak, Y.; Güzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Bastida, C.; Martínez-Miranda, V.; Solache-Ríos, M.; Linares-Hernández, I.; Teutli-Sequeira, A.; Vázquez-Mejía, G. Drinking water characterization and removal of manganese. Removal of manganese from water. J. Environ. Chem. Eng. 2018, 6, 2119–2125. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Ullah, H.; Hussain, Q.; Al-Wabel, M.I.; Ahmad, M.; Hussain, A.; Riaz, M.; Sik, O.Y.; Kong, J. Adsorption and thermodynamic mechanisms of manganese removal from aqueous media by biowaste-derived biochars. J. Liq. 2018, 266, 373–380. [Google Scholar] [CrossRef]
- Lalhruaitluanga, H.; Jayaram, K.; Prasad, M.N.V.; Kumar, K.K. Lead (II) adsorption from aqueous solutions by raw and activated charcoals of Melocanna baccifera Roxburgh (bamboo)—A comparative study. J. Hazard. Mater. 2010, 175, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Li, M.; Niu, D.; Sang, W.; Verpoort, F. Investigating the sorption behavior of cadmium from aqueous solution by potassium permanganate-modified biochar: Quantify mechanism and evaluate the modification method. Environ. Sci. Pollut. Res. 2018, 25, 8330–8339. [Google Scholar] [CrossRef]
- Mo, Z.; Shi, Q.; Zeng, H.; Lu, Z.; Bi, J.; Zhang, H.; Rinklebe, J.; Lima, E.C.; Rashid, A.; Shahab, A. Efficient removal of Cd (II) from aqueous environment by potassium permanganate-modified eucalyptus biochar. Biomass Convers. Biorefinery 2021, 1–13. [Google Scholar] [CrossRef]
- Mo, Z.L.; Zeng, H.H.; Lin, H.; Asfandyar, S.; Shi, Q.L.; Zhang, H. Adsorption Characteristics of Pb (Ⅱ) on Eucalyptus Biochar Modified by Potassium Permanganate. Huan Jing Ke Xue Huanjing Kexue 2021, 42, 5440–5449. [Google Scholar]
- Piispanen, J.K.; Sallanko, J.T. Mn (II) removal from groundwater with manganese oxide-coated filter media. J. Environ. Sci. Health Part A 2010, 45, 1732–1740. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Lv, J.; Feng, Z.; Liu, Y.; Xia, H.; Li, Y.; Wang, C.; Zeng, K.; Liu, Y.; et al. Simple one-pot synthesis of manganese dioxide modified bamboo-derived biochar composites for uranium (vi) removal. New J. Chem. 2022, 46, 14427–14438. [Google Scholar] [CrossRef]
- Tessier, A.P.G.C.; Campbell, P.G.; Bisson, M.J.A.C. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Gao, F.; Li, M.; Luo, X. Effects of biochar-derived sewage sludge on heavy metal adsorption and immobilization in soils. Int. J. Environ. Res. Public Health 2017, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Ouddane, B.; Martin, E.; Boughriet, A.; Fischer, J.C.; Wartel, M. Speciation of dissolved and particulate manganese in the Seine River estuary. Mar. Chem. 1997, 58, 189–201. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. On the removal of Mn2+ ions by adsorption onto natural and activated Chilean zeolites. Miner. Eng. 2009, 22, 336–343. [Google Scholar] [CrossRef]
- Xuwen, H.E.; Huimin, Y.A.N.G.; Yong, H.E. Treatment of mine water high in Fe and Mn by modified manganese sand. Min. Sci. Technol. 2010, 20, 571–575. [Google Scholar]
- Hou, Q.; Zhang, Q.; Huang, G.; Liu, C.; Zhang, Y. Elevated manganese concentrations in shallow groundwater of various aquifers in a rapidly urbanized delta, south China. Sci. Total Environ. 2020, 701, 134777. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Tempkin, M.I.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327. [Google Scholar]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel (II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. 2006, 133, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chem. Eng. J. 2006, 125, 111–117. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Are the thermodynamic parameters correctly estimated in liquid-phase adsorption phenomena. J. Mol. Liq. 2016, 218, 174–185. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.; Luque, R.; Clark, J.H.; Macquarrie, D.J. Tuneable porous carbonaceous materials from renewable resources. Chem. Soc. Rev. 2009, 38, 3401–3418. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Outram, J.G.; Couperthwaite, S.J.; Millar, G.J. Comparitve analysis of the physical, chemical and structural characteristics and performance of manganese greensands. J. Water Process Eng. 2016, 13, 16–26. [Google Scholar] [CrossRef]
- Knocke, W.R.; Hamon, J.R.; Thompson, C.P. Soluble manganese removal on oxide-coated filter media. J. Am. Water Work. Assoc. 1988, 80, 65–70. [Google Scholar] [CrossRef]
- Islam, A.A.; Goodwill, J.E.; Bouchard, R.; Tobiason, J.E.; Knocke, W.R. Characterization of filter media MnOx (s) surfaces and Mn removal capability. J. Am. Water Work. Assoc. 2010, 102, 71–83. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.; Zhong, S.; Zhang, F.; Xu, Z.; Gong, X.; Lu, C. Manganese oxide coated river sand for Mn (II) removal from groundwater. J. Chem. Technol. Biotechnol. 2015, 90, 1727–1734. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Zhang, S.; Wang, Q.; Chen, M.; Hu, T.; and Meng, C. Synthesis and characterization of Mn-Silicalite-1 by the hydrothermal conversion of Mn-magadiite under the neutral condition and its catalytic performance on selective oxidation of styrene. Microporous Mesoporous Mater. 2018, 268, 16–24. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, T.; Zhang, J.; Xiang, H.; Yang, X.; Hu, W.; Liang, F.; Mi, B. Ash fusion characteristics of bamboo, wood and coal. Energy 2018, 161, 517–522. [Google Scholar] [CrossRef]
- Köse, K.; Mavlan, M.; Uzun, L.; Youngblood, J.P. Cholesterol removal via cyclodextrin-decoration on cellulose nanocrystal (CNC)-grafted poly (HEMA-GMA) nanocomposite adsorbent. Cellulose 2021, 28, 471–487. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Removal of heavy metals from wastewater using date palm as a biosorbent: A comparative review. Sains Malays. 2018, 47, 35–49. [Google Scholar]
- Li, J.; Hu, J.; Sheng, G.; Zhao, G.; Huang, Q. Effect of pH, ionic strength, foreign ions and temperature on the adsorption of Cu (II) from aqueous solution to GMZ bentonite. Colloids Surf. A Physicochem. Eng. Asp. 2009, 349, 195–201. [Google Scholar] [CrossRef]
- Letterman, R.D.; Amirtharajah, A.; O’Melia, C.R. Coagulation and Flocculation. In Water Quality and Treatment, a Handbook of Community Water Supplies, American Water Works Association, 5th ed.; Letterman, R.D., Ed.; McGraw-Hill, Inc.: New York, NY, USA, 1999; Available online: http://www.aeb-water.com/book/water_quality_and_treatment.pdf (accessed on 25 November 2022).
- Liu, J.; Liu, H.; Yang, X.; Jia, X.; Cai, M.; Bao, Y. Preparation of Si–Mn/biochar composite and discussions about characterizations, advances in application and adsorption mechanisms. Chemosphere 2021, 281, 130946. [Google Scholar] [CrossRef]
- Baltrėnaitė-Gedienė, E.; Leonavičienė, T.; Baltrėnas, P. Comparison of Cu (II), Mn (II) and Zn (II) adsorption on biochar using diagnostic and simulation models. Chemosphere 2020, 245, 125562. [Google Scholar] [CrossRef]
- Lunge, S.; Singh, S.; Sinha, A. Magnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removal. J. Magn. Magn. Mater. 2014, 356, 21–31. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Şimşek, S. Insights into effective adsorption of lead ions from aqueous solutions by using chitosan-bentonite composite beads. J. Polym. Environ. 2022, 30, 3677–3687. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da'ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Singh, M.; Ahsan, M.; Pandey, V.; Singh, A.; Mishra, D.; Tiwari, N.; Singh, P.; Karak, T.; Khare, P. Comparative assessment for removal of anionic dye from water by different waste-derived biochar vis a vis reusability of generated sludge. Biochar 2022, 4, 13. [Google Scholar] [CrossRef]
- Akl, M.A.; Yousef, A.M.; AbdElnasser, S. Removal of iron and manganese in water samples using activated carbon derived from local agro-residues. J. Chem. Eng. Process Technol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Emmanuel, K.A.; Rao, A.V. Adsorption of Mn (II) from aqueous solutions using pithacelobium dulce carbon. Rasayan J. Chem. 2008, 1, 840–852. [Google Scholar]
- Şenol, Z.M.; Keskin, Z.S.; Şimşek, S. Synthesis and characterization of a new hybrid polymer composite (pollene@ polyacrylamide) and its applicability in uranyl ions adsorption. J. Radioanal. Nucl. Chem. 2023, 1–10. [Google Scholar] [CrossRef]
- Tavlieva, M.P.; Geneiva, S.D.; Georgieva, V.G.; Vlaev, L.T. Thermodynamics and kinetics of the removal of manganese (II) ions from aqueous solutions by white rice husk ash. J. Molecular Liquids 2017, 211, 938–947. [Google Scholar] [CrossRef]
- Inam, E.; Etim, U.J.; Akpabio, E.G.; Umoren, S.A. Process optimization for the application of carbon from plantain peels in dye abstraction. J. Taibah Univ. Sci. 2017, 11, 173–185. [Google Scholar] [CrossRef]
- Bandara, T.; Herath, I.; Kumarathilaka, P.; Heu, Z.Y.; Ok, Y.S.; Vithanage, M. Efficacy of woody biomass and biochar for alleviating heavy metal bioavailability in serpentine soil. Environ. Geochem. Health 2017, 39, 391–401. [Google Scholar] [CrossRef]
- Fuentes, A.; Lloréns, M.; Sáez, J.; Aguilar, M.I.; Ortuño, J.F.; Meseguer, V.F. Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour. Technol. 2008, 99, 517–525. [Google Scholar] [CrossRef]
- Omri, A.; Benzina, M. Removal of manganese (II) ions from aqueous solutions by adsorption on activated carbon derived a new precursor: Ziziphus spina-christi seeds. Alex. Eng. J. 2012, 51, 343–350. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E. Critical Stability Constants. Inorganic Complexes; Plenum Press: New York, NY, USA, 1976; Volume 4, p. 5. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).