Sustainable Biodiesel Production from a New Oleaginous Fungus, Aspergillus carneus Strain OQ275240: Biomass and Lipid Production Optimization Using Box–Behnken Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. Morphological and Molecular Characterizations of the Tested Isolate
2.3. Box–Behnken Design (BBD) and Response Surface Analysis
2.4. Analytical Methods
2.4.1. Estimation of Dry Weight (Fungal Biomass)
2.4.2. Estimation of Lipids
2.4.3. Profiling of Fatty Acid Methyl Esters (FAMEs)
2.4.4. Estimation of Physicochemical Characteristics of Biodiesel
3. Results and Discussion
3.1. Molecular Identification of the Fungal Strain
3.2. Optimization of the Culture Conditions for the Dry Biomass, Lipid Content, and Lipid Yield from Aspergillus carneus
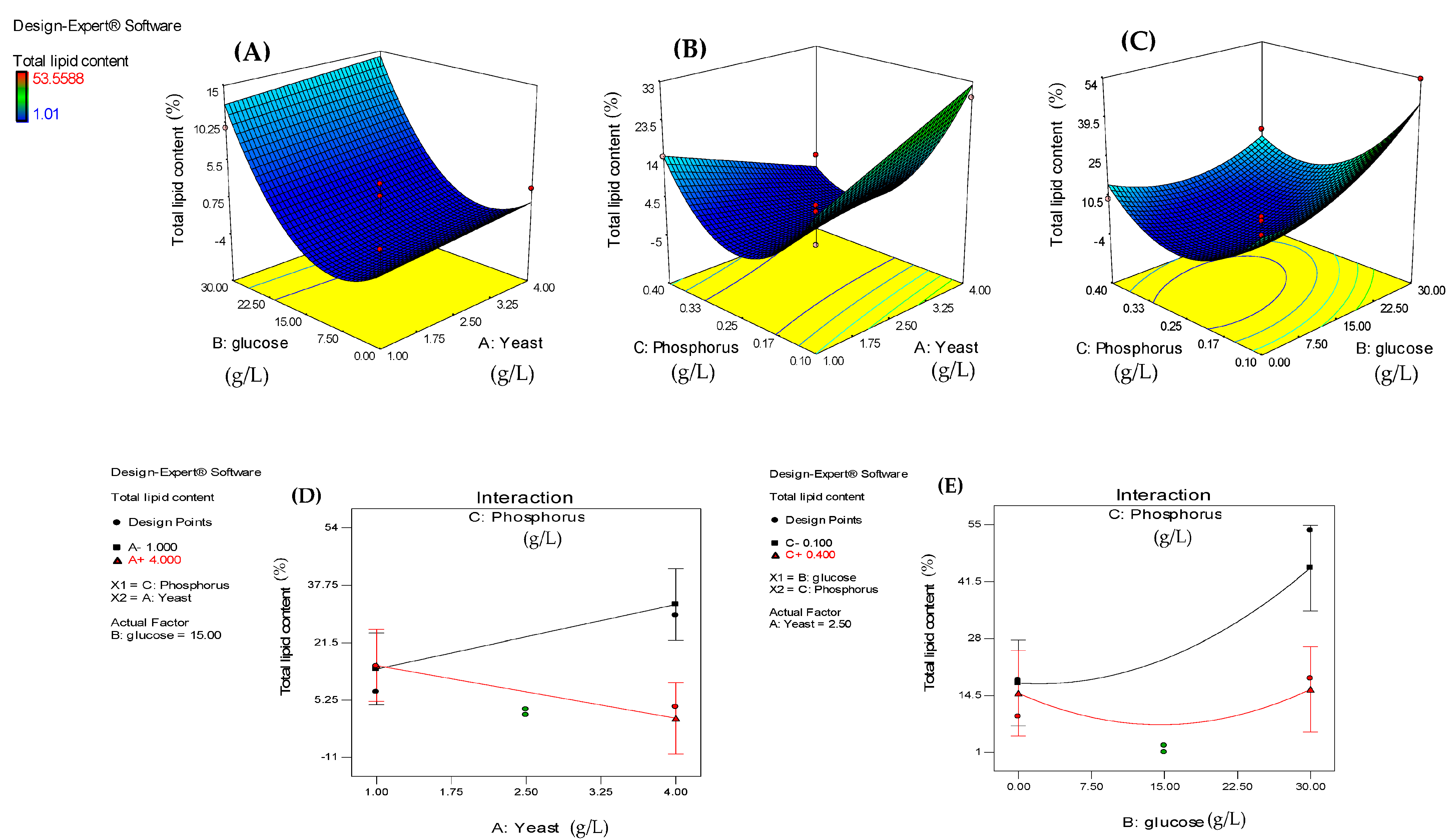
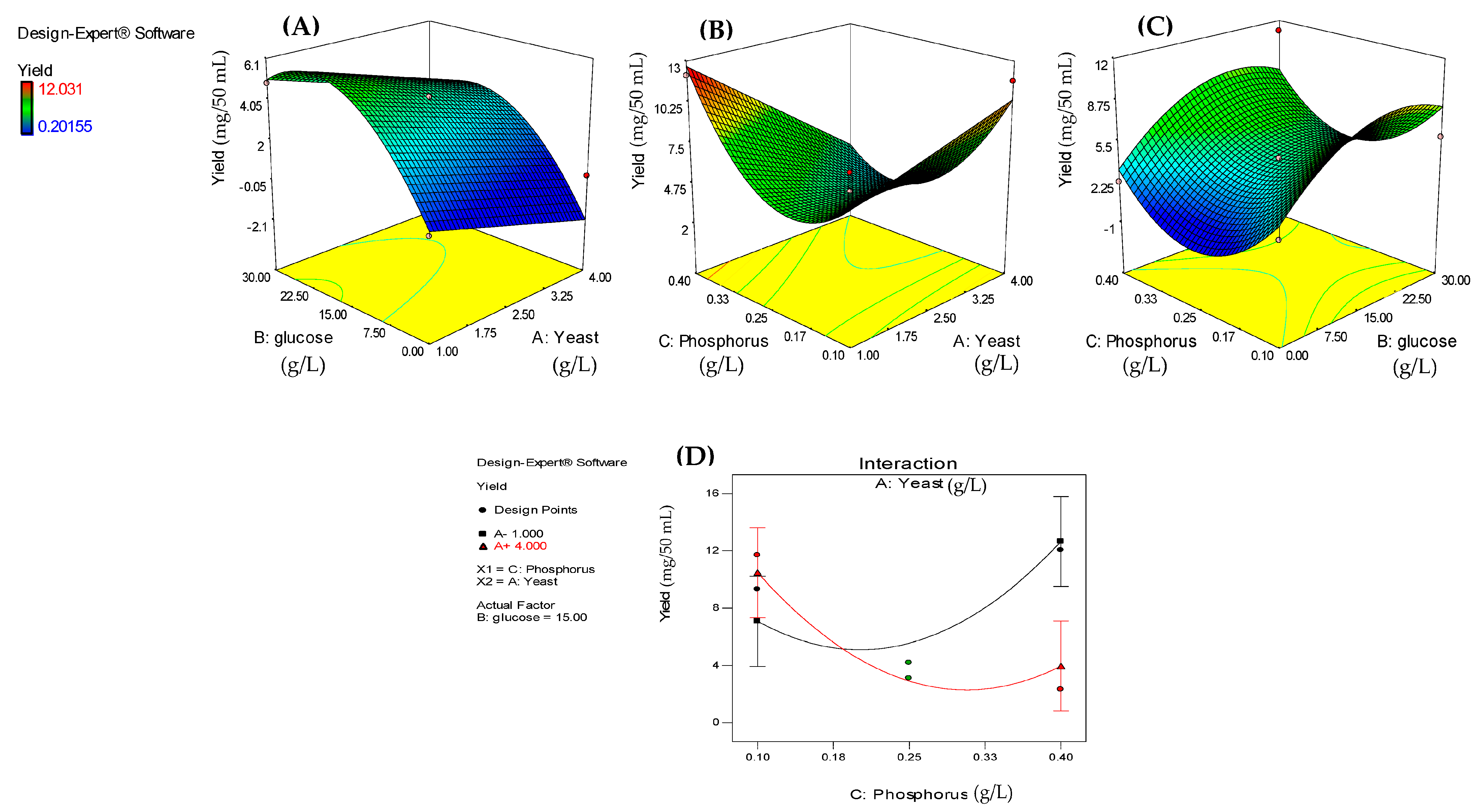
3.3. Impact of Process Variables on Dry Biomass, Lipid Content, and Lipid Yield of A. carneus
3.4. Validation of the Proposed Models
3.5. Fatty Acid Methyl Esters (FAMEs) Analysis
3.6. Properties of Fungal Biodiesel
| Biodiesel Properties | EN14214 | ASTM D6751-08 | WVO [49,50] | A. carneus |
|---|---|---|---|---|
| Saturated fatty acids (SFAs) (%) | - | - | - | 89.50 |
| Monounsaturated fatty acids (MUFAs) (%) | - | - | - | 9.50 |
| Polyunsaturated fatty acids (PUFAs) (%) | - | - | - | 1.00 |
| kinematic viscosity (ln(kυ)); mm2/s) | 3.5–5.0 | 1.9–6.0 | 4.54 | 4.48 |
| Density (ρ; g/cm3) | - | 0.86–0.9 | 0.88 | 0.87 |
| Iodine Number (IN; gI2100/g oil) | ≤120 | - | - | 10.29 |
| Oxidation Stability (OS; h) | ≥8 | ≥3 | 5.80 | 7.33 |
| Cetane Number (CN) | ≥51 | ≥47 | 58.30 | 71.81 |
| Saponification Value (SV; mg KOH/g) | - | - | - | 196.15 |
| High Heating Value (HHV; MJ/kg) | - | - | 40.11 | 39.57 |
| Pour Point (PP; °C) | - | - | −11.00 | −3.01 |
| Cloud Point (CP; °C) | - | - | −8.00 | 3.51 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Farooqi, Z.U.R.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Deora, P.S.; Verma, Y.; Muhal, R.A.; Goswami, C.; Singh, T. Biofuels: An alternative to conventional fuel and energy source. Mater. Today Proc. 2022, 48, 1178–1184. [Google Scholar]
- John, C.B.; Raja, S.A.; Deepanraj, B.; Ong, H.C. Palm stearin biodiesel: Preparation, characterization using spectrometric techniques and the assessment of fuel properties. Biomass Convers. Biorefin. 2022, 12, 1679–1693. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M. Pretreated fucoidan and alginate from a brown seaweed as a substantial carbon source for promoting biomass lipid biochemical constituents and biodiesel quality of Dunaliella salina. Renew. Energy 2020, 157, 246–255. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Carvalho, A.K.F.; Bento, H.B.; de Castro, H.F. Integration of microbial biodiesel and bioethanol industries through utilization of vinasse as substrate for oleaginous fungi. Bioresour. Technol. Rep. 2019, 6, 46–53. [Google Scholar] [CrossRef]
- Athenaki, M.; Gardeli, C.; Diamantopoulou, P.; Tchakouteu, S.S.; Sarris, D.; Philippoussis, A.; Papanikolaou, S. Lipids from yeasts and fungi: Physiology, production and analytical considerations. J. Appl. Microbiol. 2018, 124, 336–367. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Subhash, G.V.; Mohan, S.V. Lipid accumulation for biodiesel production by oleaginous fungus Aspergillus awamori: Influence of critical factors. Fuel 2014, 116, 509–515. [Google Scholar] [CrossRef]
- Subhash, G.V.; Mohan, S.V. Biodiesel production from isolated oleaginous fungi Aspergillus sp. using corncob waste liquor as a substrate. Bioresour. Technol. 2011, 102, 9286–9290. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncaglia, L.; Lucia, M.; Leonardi, A.; Rossi, M. Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Fact. 2010, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Luo, H.; Mu, T.; Shen, Y.; Yuan, M.; Liu, J. Enhancement of lipid accumulation by oleaginous yeast through phosphorus limitation under high content of ammonia. Bioresour. Technol. 2018, 262, 9–14. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Komaitis, M.; Aggelis, G. Single cell oil (SCO) production by Mortierella isabellina grown on high-sugar content media. Bioresour. Technol. 2004, 95, 287–291. [Google Scholar] [CrossRef]
- Wynn, J.P.; Hamid, A.A.; Li, Y.; Ratledge, C. Biochemical events leading to the diversion of carbon into storage lipids in the oleaginous fungi Mucor circinelloides and Mortierella alpina. Microbiology 2001, 147, 2857–2864. [Google Scholar] [CrossRef]
- Abdellah, E.M.; Ali, T.H.; Abdou, D.A.M.; Hassanein, N.M.; Fadel, M.; El-Din, A.K.; El-Ghonemy, D.H. Enhancement of lipid productivity from a promising oleaginous fungus Aspergillus sp. strain EM2018 for biodiesel production: Optimization of culture conditions and identification. Grasas Aceites 2020, 71, e371. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Marques, S.S.I.; Cabanelas, I.T.D.; Pereira, S.A.; Druzian, J.I.; De souza, C.O.; Vich, D.V.; De Carvalho, G.C.; Nacimento, M.A. Screening microalgae strains for biodiesel production: Lipid productivity and estimation of fuel quality based on fatty acid profiles as selective criteria. Bioenergy Res. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Awad, G.E.A.; Salam, W.A.; El-diwany, A.; Salama, B.A.; Abdelkader, A.F.; Esawy, M.A.D. Keratinase production by Bacillus pumilus GHD in solid-state fermentation using sugar cane bagasse: Optimization of culture conditions using a box-Behnken experimental design. Ann. Microbiol. 2011, 61, 663–672. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Singh, R.; Bishnoi, N.R.; Kirrolia, A. Evaluation of Pseudomonas aeruginosa an innovative bioremediation tool in multi metals ions from simulated system using multi response methodology. Bioresour. Technol. 2013, 138, 222–234. [Google Scholar] [CrossRef]
- Hussein, A.A.; El Sayed, O.; Asker, M.S.; Mohamed, S.S.; Abdelhamid, S. Biodiesel production from local isolate Penicillium commune NRC 2016. J. Sci. Res. Sci. 2017, 34, 179–193. [Google Scholar] [CrossRef]
- Inouye, L.S.; Lotufo, G.R. Comparison of macro-gravimetric and micro-colorimetric lipid determination methods. Talanta 2006, 70, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.A.; Alharthi, S. Use of Response Surface Methodology in optimization of biomass, lipid productivity and fatty acid profiles of marine microalga Dunaliella parva for biodiesel production. Environ. Technol. Innov. 2021, 22, 101485. [Google Scholar] [CrossRef]
- Ghasemi, A.; Moosavi-Nasab, M. Production of second-generation biodiesel using low-quality date fruits. Biotechnol. Rep. 2020, 27, e00480. [Google Scholar] [CrossRef]
- Gnanasekaran, R.; Dhandapani, B.; Iyyappan, J. Improved itaconic acid production by Aspergillus niveus using blended algal biomass hydrolysate and glycerol as substrates. Bioresour. Technol. 2019, 283, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Iyyappan, J.; Baskar, G.; Bharathiraja, B.; Saravanathamizhan, R. Malic acid production from biodiesel derived crude glycerol using morphologically controlled Aspergillus niger in batch fermentation. Bioresour. Technol. 2018, 269, 393–399. [Google Scholar] [CrossRef]
- Fawzy, M.A. Biosorption of copper ions from aqueous solution by Codium vermilara: Optimization, kinetic, isotherm and thermodynamic studies. Adv. Powder Technol. 2020, 31, 3724–3735. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Al-Zaban, M.I.; Albarakaty, F.M.; Abdelwahab, S.F.; Hassan, S.H.; Fawzy, M.A. Kinetic, isotherm and thermodynamic aspects of Zn2+ biosorption by Spirulina platensis: Optimization of process variables by response surface methodology. Life 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.A.; Darwish, H.; Alharthi, S.; Al-Zaban, M.I.; Noureldeen, A.; Hassan, S.H. Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box–Behnken experimental design. Sci. Rep. 2022, 12, 3256. [Google Scholar] [CrossRef]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Ekeberg, D.; Shapaval, V. The influence of phosphorus source and the nature of nitrogen substrate on the biomass production and lipid accumulation in oleaginous Mucoromycota fungi. Appl. Microbiol. Biotechnol. 2020, 104, 8065–8076. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, W.; Shen, H.; Zhao, Z.K.; Yang, Z.; Yan, J.; Zhao, M. Co-utilization of corn stover hydrolysates and biodiesel-derived glycerol by Cryptococcus curvatus for lipid production. Bioresour. Technol. 2016, 219, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Thangavelu, K.; Sekar, A.; Sanjeev, B.; Uthandi, S. Aspergillus caespitosus ASEF14, an oleaginous fungus as a potential candidate for biodiesel production using sago processing wastewater (SWW). Microb. Cell Fact. 2021, 20, 179. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production strategies and applications of microbial single cell oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef] [PubMed]
- Jiru, T.M.; Abate, D. Oleaginous microorganisms, diversity, lipid biosynthesis pathway and strain improvement. Webpub. J. Sci. Res. 2014, 2, 55–65. [Google Scholar]
- Shoaib, A.; Bhran, A.; Rasmey, A.H.; Mikky, Y. Optimization of cultural conditions for lipid accumulation by Aspergillus wentii Ras101 and its transesterification to biodiesel: Application of response surface methodology. 3 Biotech 2018, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Al-Hawash, A.B.; Li, S.; Zhang, X.; Zhang, X.; Ma, F. Productivity of γ-Linoleic acid by oleaginous fungus Cunninghamella echinulata using a pulsed high magnetic field. Food Biosci. 2018, 21, 1–7. [Google Scholar] [CrossRef]
- Roopnarain, A.; Gray, V.; Sym, S. Phosphorus limitation and starvation effects on cell growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour. Technol. 2014, 156, 408–411. [Google Scholar] [CrossRef]
- Li, Y.; Han, F.; Xu, H.; Mu, J.; Chen, D.; Feng, B.; Zeng, H. Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour. Technol. 2014, 174, 24–32. [Google Scholar] [CrossRef]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical steps in carbon metabolism affecting lipid accumulation and their regulation in oleaginous microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef]
- Khot, M.; Kamat, S.; Zinjarde, S.; Pant, A.; Chopade, B.; RaviKumar, A. Single cell oil of oleaginous fungi from the tropical mangrove wetlands as a potential feedstock for biodiesel. Microb. Cell Fact. 2012, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Vicente, G.; Bautista, L.F.; Gutiérrez, F.J.; Rodríguez, R.; Martínez, V.; Rodríguez-Frómeta, R.A.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Direct transformation of fungal biomass from submerged cultures into biodiesel. Energy Fuel 2010, 24, 3173–3178. [Google Scholar] [CrossRef]
- Kraisintu, P.; Yongmanitchai, W.; Limtong, S. Selection and optimization for lipid production of a newly isolated oleaginous yeast, Rhodosporidium toruloides DMKU3-TK16. Agric. Nat. Resour. 2010, 44, 436–445. [Google Scholar]
- Hashem, A.H.; Suleiman, W.B.; Abu-elreesh, G.; Shehabeldine, A.M.; Khalil, A.M.A. Sustainable lipid production from oleaginous fungus Syncephalastrum racemosum using synthetic and watermelon peel waste media. Bioresour. Technol. Rep. 2020, 12, 100569. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.A. Fatty acid characterization and biodiesel production by the marine microalga Asteromonas gracilis: Statistical optimization of medium for biomass and lipid enhancement. Mar. Biotechnol. 2017, 19, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Chuppa-Tostain, G.; Hoarau, J.; Watson, M.; Adelard, L.; Shum Cheong Sing, A.; Caro, Y.; Grondin, I.; Bourven, I.; Francois, J.M.; Girbal-Neuhauser, E.; et al. Production of Aspergillus niger biomass on sugarcane distillery wastewater: Physiological aspects and potential for biodiesel production. Fungal Biol. Biotechnol. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Banerjee, R. Optimization of lipid enriched biomass production from oleaginous fungus using response surface methodology. Indian J. Exp. Biol. 2013, 51, 979–983. [Google Scholar]
- Fawzy, M.A.; El-Otify, A.M.; Adam, M.S.; Moustafa, S.S. The impact of abiotic factors on the growth and lipid accumulation of some green microalgae for sustainable biodiesel production. Environ. Sci. Pollut. Res. 2021, 28, 42547–42561. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Mohapatra, S.B.; Mohanty, R.C.; Das, P. Transesterified Olax scandens oil as a bio-additive: Production and engine performance studies. Tribol. Int. 2021, 153, 106653. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Zhao, M.; Kuang, L.; Nie, J.; Riley, W.W. Improving the cold flow properties of biodiesel from waste cooking oil by surfactants and detergent fractionation. Fuel 2011, 90, 1036–1040. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z. Biodiesel production from waste cooking oil by acidic ionic liquid as a catalyst. Renew. Energy 2015, 77, 521–526. [Google Scholar] [CrossRef]
- Katre, G.; Ajmera, N.; Zinjarde, S.; RaviKumar, A. Mutants of Yarrowia lipolytica NCIM 3589 grown on waste cooking oil as a biofactory for biodiesel production. Microb. Cell Fact. 2017, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Rivaldi, J.D.; Carvalho, A.K.F.; da Conceição, L.R.V.; de Castro, H.F. Assessing the potential of fatty acids produced by filamentous fungi as feedstock for biodiesel production. Prep. Biochem. Biotechnol. 2017, 47, 970–976. [Google Scholar] [CrossRef]
- Siwina, S.; Leesing, R. Bioconversion of durian (Durio zibethinus Murr.) peel hydrolysate into biodiesel by newly isolated oleaginous yeast Rhodotorula mucilaginosa KKUSY14. Renew. Energy 2021, 163, 237–245. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energy Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Arguelles, E.D.; Martinez-Goss, M.R. Lipid accumulation and profiling in microalgae Chlorolobion sp. (BIOTECH 4031) and Chlorella sp. (BIOTECH 4026) during nitrogen starvation for biodiesel production. J. Appl. Phycol. 2020, 33, 1–11. [Google Scholar] [CrossRef]
- Sinha, S.K.; Gupta, A.; Bharalee, R. Production of biodiesel from freshwater microalgae and evaluation of fuel properties based on fatty acid methyl ester profile. Biofuels 2016, 7, 69–78. [Google Scholar] [CrossRef]
- Arguelles, E.D.; Laurena, A.C.; Monsalud, R.G.; Martinez-Goss, M.R. Fatty acid profile and fuel-derived physico-chemical properties of biodiesel obtained from an indigenous green microalga, Desmodesmus sp. (I-AU1), as potential source of renewable lipid and high quality biodiesel. J. Appl. Phycol. 2018, 30, 411–419. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Farrag, A.A.; Elsayed, M.A.; El-Khawaga, A.M. Biodiesel production by Aspergillus niger lipase immobilized on barium ferrite magnetic nanoparticles. Bioengineering 2016, 3, 14. [Google Scholar] [CrossRef]
- Canesin, E.A.; de Oliveira, C.C.; Matsushita, M.; Dias, L.F.; Pedrao, M.R.; de Souza, N.E. Characterization of residual oils for biodiesel production. Electron. J. Biotechnol. 2014, 17, 39–45. [Google Scholar] [CrossRef]
- Hossain, F.M.; Kosinkova, J.; Brown, R.J.; Ristovski, Z.; Hankamer, B.; Stephens, E.; Rainey, T.J. Experimental investigations of physical and chemical properties for microalgae HTL bio-crude using a large batch reactor. Energies 2017, 10, 467. [Google Scholar] [CrossRef]
- Shrestha, D.S.; Van Gerpen, J.; Thompson, J. Effectiveness of cold flow additives on various biodiesels, diesel, and their blends. Trans. ASABE 2008, 51, 1365–1370. [Google Scholar] [CrossRef]
- Dunn, R.O. Cold flow properties of biodiesel: A guide to getting an accurate analysis. Biofuels 2015, 6, 115–128. [Google Scholar] [CrossRef]
- Ibiari, N.N.; El-Enin, S.A.A.; Attia, N.K.; El-Diwani, G. Ultrasonic comparative assessment for biodiesel production from rapeseed. J. Am. Sci. 2010, 6, 937–943. [Google Scholar]



| Range and Levels | |||
|---|---|---|---|
| Factors (mg/L) | Low (−1) | Medium (0) | High (+1) |
| Yeast | 1.0 | 2.5 | 4.0 |
| Glucose | 0.0 | 15.0 | 30.0 |
| Phosphorus | 0.1 | 0.25 | 0.4 |
| Run | Yeast (g/L) | Glucose (g/L) | Phosphorus (g/L) | Experimental Responses | Predicted Responses | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dry Biomass | Lipid Content | Lipid Yield | Dry Biomass | Lipid Content | Lipid Yield | ||||
| 1 | 1 | 0 | 0.25 | 0.020 ± 0.002 | 1.630 ± 0.278 | 0.330 ± 0.061 | 0.033 | 1.410 | 0.550 |
| 2 | 4 | 0 | 0.25 | 0.010 ± 0.002 | 2.020 ± 0.095 | 0.200 ± 0.040 | 0.002 | 1.220 | 0.190 |
| 3 | 1 | 30 | 0.25 | 0.050 ± 0.004 | 9.740 ± 0.238 | 4.870 ± 0.725 | 0.053 | 12.660 | 5.070 |
| 4 | 4 | 30 | 0.25 | 0.030 ± 0.003 | 5.790 ± 0.564 | 1.740 ± 0.325 | 0.022 | 14.290 | 2.420 |
| 5 | 1 | 15 | 0.1 | 0.123 ± 0.020 | 7.560 ± 0.867 | 9.300 ± 0.851 | 0.106 | 14.060 | 7.080 |
| 6 | 4 | 15 | 0.1 | 0.040 ± 0.004 | 29.190 ± 0.884 | 11.670 ± 0.785 | 0.039 | 32.270 | 10.470 |
| 7 | 1 | 15 | 0.4 | 0.081 ± 0.005 | 14.850 ± 1.340 | 12.030 ± 0.446 | 0.082 | 15.070 | 12.650 |
| 8 | 4 | 15 | 0.4 | 0.070 ± 0.007 | 3.320 ± 0.412 | 2.330 ± 0.229 | 0.087 | 2.120 | 3.960 |
| 9 | 2.5 | 0 | 0.1 | 0.015 ± 0.005 | 18.190 ± 0.923 | 2.730 ± 0.420 | 0.012 | 17.470 | 3.770 |
| 10 | 2.5 | 30 | 0.1 | 0.011 ± 0.003 | 53.550 ± 1.480 | 5.890 ± 0.628 | 0.032 | 44.700 | 8.280 |
| 11 | 2.5 | 0 | 0.4 | 0.024 ± 0.003 | 9.500 ± 1.100 | 2.280 ± 0.950 | 0.023 | 15.060 | 3.300 |
| 12 | 2.5 | 30 | 0.4 | 0.060 ± 0.005 | 18.550 ± 1.560 | 11.100 ± 0.592 | 0.044 | 15.980 | 7.820 |
| 13–17 a | 2.5 | 15 | 0.25 | 0.160 ± 0.035 | 2.620 ± 0.541 | 4.190 ± 0.685 | 0.155 | 1.480 | 4.230 |
| Dry Biomass | Lipid Content | Lipid Yield | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model Term | CE | df | SE | F Value | p-Value Prob. > F | CE | df | SE | F Value | p-Value Prob. > F | CE | Df | SE | F Value | p-Value Prob. > F |
| Intercept | 0.155 | 1 | 0.012 | - | - | −1.480 | 1 | 3.480 | - | - | 4.230 | 1 | 1.230 | - | - |
| A-Yeast (g/L) | −0.015 | 1 | 0.006 | 7.030 | 0.0379 | 0.820 | 1 | 2.480 | 0.110 | 0.753 | −1.320 | 1 | 0.790 | 2.790 | 0.139 |
| B-Glucose (g/L) | 0.0103 | 1 | 0.006 | 3.080 | 0.1300 | 7.040 | 1 | 2.480 | 8.060 | 0.030 | 2.260 | 1 | 0.790 | 8.110 | 0.025 |
| C-Phos. (g/L) | 0.0058 | 1 | 0.006 | 0.970 | 0.3632 | −7.780 | 1 | 2.480 | 9.870 | 0.020 | −0.230 | 1 | 0.790 | 0.090 | 0.778 |
| AC | 0.0180 | 1 | 0.008 | 4.740 | 0.0401 | −8.290 | 1 | 3.500 | 5.590 | 0.056 | −3.020 | 1 | 1.120 | 7.250 | 0.031 |
| BC | - | - | - | - | - | −6.580 | 1 | 3.500 | 3.520 | 0.110 | - | - | - | - | - |
| A2 | −0.038 | 1 | 0.009 | 17.130 | 0.0061 | - | - | - | - | - | - | - | - | - | - |
| B2 | −0.089 | 1 | 0.009 | 93.260 | <0.0001 | 7.920 | 1 | 3.840 | 4.260 | 0.085 | −2.740 | - | 1.230 | 4.990 | 0.061 |
| C2 | −0.038 | 1 | 0.009 | 17.130 | 0.0061 | 16.860 | 1 | 3.840 | 19.290 | 0.005 | 4.310 | - | 1.230 | 12.290 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.G.; Baazeem, A.; Al-Zaban, M.I.; Fawzy, M.A.; Hassan, S.H.A.; Koutb, M. Sustainable Biodiesel Production from a New Oleaginous Fungus, Aspergillus carneus Strain OQ275240: Biomass and Lipid Production Optimization Using Box–Behnken Design. Sustainability 2023, 15, 6836. https://doi.org/10.3390/su15086836
Ibrahim AG, Baazeem A, Al-Zaban MI, Fawzy MA, Hassan SHA, Koutb M. Sustainable Biodiesel Production from a New Oleaginous Fungus, Aspergillus carneus Strain OQ275240: Biomass and Lipid Production Optimization Using Box–Behnken Design. Sustainability. 2023; 15(8):6836. https://doi.org/10.3390/su15086836
Chicago/Turabian StyleIbrahim, Amany G., Alaa Baazeem, Mayasar I. Al-Zaban, Mustafa A. Fawzy, Sedky H. A. Hassan, and Mostafa Koutb. 2023. "Sustainable Biodiesel Production from a New Oleaginous Fungus, Aspergillus carneus Strain OQ275240: Biomass and Lipid Production Optimization Using Box–Behnken Design" Sustainability 15, no. 8: 6836. https://doi.org/10.3390/su15086836
APA StyleIbrahim, A. G., Baazeem, A., Al-Zaban, M. I., Fawzy, M. A., Hassan, S. H. A., & Koutb, M. (2023). Sustainable Biodiesel Production from a New Oleaginous Fungus, Aspergillus carneus Strain OQ275240: Biomass and Lipid Production Optimization Using Box–Behnken Design. Sustainability, 15(8), 6836. https://doi.org/10.3390/su15086836







