Temperature Induced Flowering Phenology of Olea ferruginea Royle: A Climate Change Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Herbarium Records
2.3. Real–Time Field Observations
2.4. Climatic Data
2.5. Statistical Analysis
3. Results
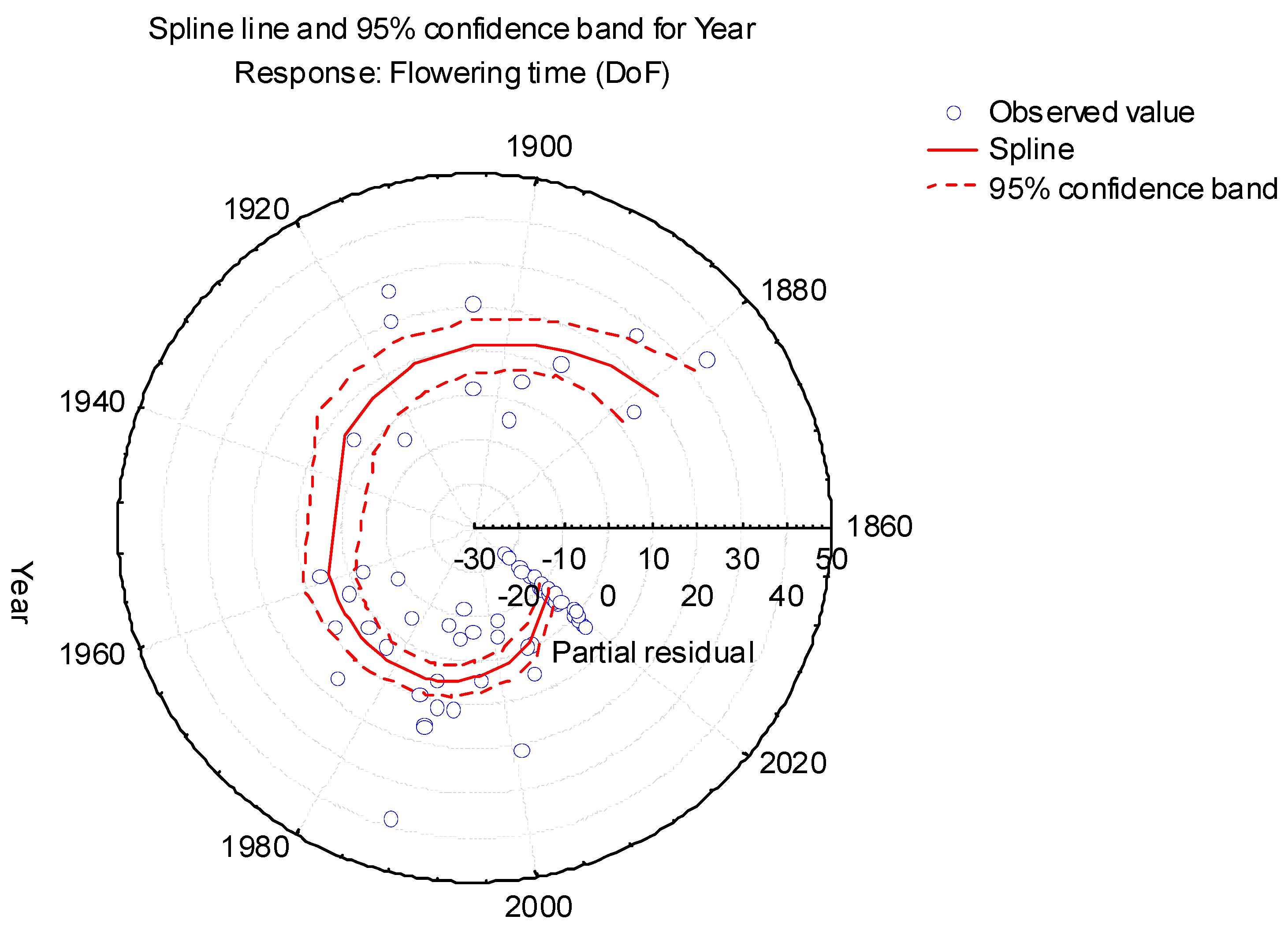
3.1. Flowering Phenological Shift
3.2. Seasonal Temperature Trends
3.3. Impacts of Warming Seasons on Flowering Phenology
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Yohe, G.A. Globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.G.; Ellwood, E.R.; Primack, R.B.; Davis, C.C.; Pearson, K.D.; Gallinat, A.S.; Yost, J.M.; Nelson, G.; Mazer, S.J.; Rossington, N.L.; et al. Old Plants New Tricks: Phenological Research Using Herbarium Specimens. Trends Ecol. Evol. 2017, 32, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Gaira, K.S.; Dhar, U. Phenological change modelling for selected Himalayan medicinal herbs using herbarium records: A case study. Ecol. Inform. 2020, 60, 101177. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.L.; Klein, A.M.G.; Rusticucci, M.; Alexander, L.V.; Brönnimann, S.; Charabi, Y.; Dentener, F.J.; Dlugokencky, E.J.; Easterling, D.R.; Kaplan, A.; et al. Observations: Atmosphere and Surface. In Climate Change 2013 the Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Mohandass, D.; Zhao, J.; Xia, Y.; Campbell, M.J.; Li, Q.J. Increasing temperature causes flowering onset time changes of alpine ginger Roscoea in the Central Himalayas. J. Asia Pac. Biodivers. 2015, 8, 191–198. [Google Scholar] [CrossRef]
- Guo, F.; Lenoir, J.; Bonebrake, T.C. Land–use change interacts with climate to determine elevational species redistribution. Nat. Commun. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Numata, S.; Yamaguchi, K.; Shimizu, M.; Sakurai, G.; Morimoto, A.; Alias, N.; Azman, N.Z.N.; Hosaka, T.; Satake, A. Impacts of climate change on reproductive phenology in tropical rainforests of Southeast Asia. Commun. Biol. 2022, 5, 311. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2022, Impacts Adaptation and Vulnerability Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Sparks, T.H.; Jeffree, E.P.; Jeffree, C.E. An examination of the relationship between flowering times and temperature at the national scale using long–time phenological records from the UK. Int. J. Biometeorol. 2000, 44, 82–87. [Google Scholar] [CrossRef]
- Fitter, A.H.; Fitter, R.S. Rapid changes in flowering time in British plants. Science 2002, 296, 1689–1691. [Google Scholar] [CrossRef]
- Miller–Rushing, A.J.; Primack, R.B. Global warming and flowering times in Thoreau’s concord: A community perspective. Ecology 2008, 80, 332–341. [Google Scholar] [CrossRef]
- Helama, S.; Tolvanen, A.; Karhu, J.; Poikolainen, J.; Kubin, E. Finnish National Phenological Network 1997–2017: From observations to trend detection. Int. J. Biometeorol. 2020, 64, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Peter, B.; Ol’ga, B.; Briede, A. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Suonan, J.; Classen, A.T.; Sanders, N.J.; He, J.S. Plant phenological sensitivity to climate change on the Tibetan Plateau and relative to other areas of the world. Ecosphere 2019, 10, e02543. [Google Scholar] [CrossRef]
- Reed, P.B.; Pfeifer-Meister, L.E.; Roy, B.A.; Johnson, B.R.; Bailes, G.T.; Nelson, A.A.; Boulay, M.C.; Hamman, S.T.; Bridgham, S.D. Prairie plant phenology driven more by temperature than moisture in climate manipulations across a latitudinal gradient in the Pacific Northwest USA. Ecol. Evol. 2019, 9, 3637–3650. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Wolf, A.A.; Zavaleta, E.S.; Selmants, P.C. Flowering phenology shifts in response to biodiversity loss. Proc. Natl. Acad. Sci. USA 2017, 114, 3463–3468. [Google Scholar] [CrossRef]
- Howell, A.; Winkler, D.E.; Phillips, M.L.; McNellis, B.; Reed, S.C. Experimental Warming Changes Phenology and Shortens Growing Season of the Dominant Invasive Plant Bromus tectorum (Cheatgrass). Front. Plant Sci. 2020, 11, 570001. [Google Scholar] [CrossRef]
- Vogel, J. Drivers of phenological changes in southern Europe. Int. J. Biometeorol. 2022, 66, 1903–1914. [Google Scholar] [CrossRef]
- Ahmad, M.; Uniyal, S.K.; Batish, D.R.; Rathee, S.; Sharma, P.; Singh, H.P. Flower phenological events and duration pattern is influenced by temperature and elevation in Dhauladhar mountain range of Lesser Himalaya. Ecol. Indic. 2021, 129, 107902. [Google Scholar] [CrossRef]
- Negi, G.C.S.; Joshi, S.; Singh, P.; Joshi, R. Phenological response patterns of forest communities to annual weather variability at long–term ecological monitoring sites in Western Himalaya. Trees For. People 2022, 8, 100237. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T. Temperature and plant development: Phenology and seasonality. In Plant Growth and Climate Change; Morison, J.I., Morecroft, M.D., Eds.; Blackwell: Oxford, UK, 2006; pp. 70–94. [Google Scholar]
- Nord, E.A.; Lynch, J.P. Plant phenology: A critical controller of soil resource acquisition. J. Exp. Bot. 2009, 60, 1927–1937. [Google Scholar] [CrossRef]
- Hunt, K.M.R.; Zaz, S.N. Linking the North Atlantic Oscillation to winter precipitation over the Western Himalaya through disturbances of the subtropical jet. Clim. Dyn. 2022, 60, 2389–2403. [Google Scholar] [CrossRef]
- Mahdi, S.S.; Dhekale, B.S.; Jan, R.; Bhat, M.A.; Hussain, A.; Jehangir, I.A.; Sofi, N.R.; Ahmed, L.; Quereshi, A.M.I.; Aezum, A.M.; et al. Analysis and farmers’ perception of climate change in the Kashmir Valley, India. Theor. Appl. Climatol. 2022, 149, 727–741. [Google Scholar] [CrossRef]
- Lone, B.A.; Qayoom, S.; Nazir, A.; Ahanger, S.A.; Basu, U.; Bhat, T.A.; Dar, Z.A.; Mushtaq, M.; Sabagh, A.E.; Soufan, W.; et al. Climatic Trends of Variable Temperate Environment: A Complete Time Series Analysis during 1980–2020. Atmosphere 2022, 13, 749. [Google Scholar] [CrossRef]
- Gujree, I.; Ahmad, I.; Zhang, F.; Arshad, A. Innovative Trend Analysis of High–Altitude Climatology of Kashmir Valley, North–West Himalayas. Atmosphere 2022, 13, 764. [Google Scholar] [CrossRef]
- Bhutiyani, M.R.; Kale, V.S.; Pawar, N.J. Climate change and the precipitation variations in the northwestern Himalaya: 1866–2006. Int. J. Clim. 2009, 30, 535–548. [Google Scholar] [CrossRef]
- Khan, S.; Verma, S. Ensemble modeling to predict the impact of future climate change on the global distribution of Olea europaea subsp. cuspidata. Front. For. Glob. Chang. 2022, 5, 977691. [Google Scholar] [CrossRef]
- Wani, I.A.; Khan, S.; Verma, S.; Al-Misned, F.A.; Shafik, H.M.; El-Serehy, H.A. Predicting habitat suitability and niche dynamics of Dactylorhiza hatagirea and Rheum webbianum in the Himalaya under projected climate change. Sci. Rep. 2022, 12, 13205. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ridwan, Q.; Khan, S.; Pant, S.; Siddiqui, S.; Moustafa, M.; Ahmad, A.E.; Yassin, H.M. Changing Climatic Scenarios Anticipate Dwindling of Suitable Habitats for Endemic Species of Himalaya—Predictions of Ensemble Modelling Using Aconitum heterophyllum as a Model Plant. Sustainability 2022, 14, 8491. [Google Scholar] [CrossRef]
- Kumari, P.; Wani, I.A.; Khan, S.; Verma, S.; Mushtaq, S.; Gulnaz, A.; Paray, B.A. Modeling of Valeriana wallichii Habitat Suitability and Niche Dynamics in the Himalayan Region under Anticipated Climate Change. Biology 2022, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.D. Spring- and fall-flowering species show diverging phenological responses to climate in the Southeast USA. Int. J. Biometeorol. 2019, 63, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.; Hamid, M.; Wani, S.A.; Malik, A.H.; Waza, S.A.; Khuroo, A.A. ubstantial shifts in flowering phenology of Sternbergia vernalis in the Himalaya: Supplementing decadal field records with historical and experimental evidences. Sci. Total. Environ. 2021, 795, 148811. [Google Scholar] [CrossRef] [PubMed]
- Calinger, K.M.; Queenborough, S.; Curtis, P.S. Herbarium specimens reveal the footprint of climate change on flowering trends across north–central North America. Ecol. Lett. 2013, 16, 1037–1044. [Google Scholar] [CrossRef]
- Lima, D.F.; Mello, J.H.F.; Lopes, I.T.; Forzza, R.C.; Goldenberg, R.; Freitas, L. Phenological responses to climate change based on a hundred years of herbarium collections of tropical Melastomataceae. PLoS ONE 2021, 16, e0251360. [Google Scholar] [CrossRef]
- Gaira, K.S.; Rawal, R.S.; Rawat, B.; Bhat, I.D. Impact of climate change on the flowering of Rhododendron arboreum in central Himalaya India. Curr. Sci. 2014, 106, 12. [Google Scholar]
- Hart, R.; Salick, J.; Ranjitkar, S.; Xu, J. Herbarium specimens show contrasting phenological responses to Himalayan climate. Proc. Natl. Acad. Sci. USA 2014, 111, 10615–10619. [Google Scholar] [CrossRef]
- Primack, D.; Imbres, C.; Primack, R.B.; Miller-Rushing, A.J.; Tredici, P.D. Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am. J. Bot. 2004, 91, 1260–1264. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Hughes, L.; Leishman, M.R. Phenological trends among Australian alpine species using herbarium records to identify climate–change indicators. Aust. J. Bot. 2004, 57, 1–9. [Google Scholar] [CrossRef]
- Rawal, D.S.; Kasel, S.; Keatley, M.R.; Nitschke, C.R. Herbarium records identify sensitivity of flowering phenology of eucalypts to climate: Implications for species response to climate change. Austral Ecol. 2015, 40, 117–125. [Google Scholar] [CrossRef]
- Yao, T.; Xue, Y.; Chen, D.; Chen, F.; Thompson, L.; Cui, P.; Koike, T.; Lau, W.K.M.; Lettenmaier, D.; Mosbrugger, V.; et al. Recent Third Pole’s rapid warming accompanies cryospheric melt and water cycle intensification and interactions between monsoon and environment: Multi–disciplinary approach with observation modeling and analysis. Bull. Am. Meteorol. Soc. 2018, 100, 423–444. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change 2007, Impacts Adaptation and Vulnerability Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 7–22. [Google Scholar]
- Kattel, G.R. Climate warming in the Himalayas threatens biodiversity ecosystem functioning and ecosystem services in the 21st century: Is there a better solution? Biodivers. Conserv. 2022, 31, 2017–2044. [Google Scholar] [CrossRef]
- Tewari, V.P.; Verma, R.K.; von Gadow, K. Climate change effects in the Western Himalayan ecosystems of India: Evidence and strategies. For. Ecosyst. 2017, 4, 13. [Google Scholar] [CrossRef]
- Rashid, I.; Romshoo, S.A.; Chaturvedi, R.K.; Ravindranath, N.H.; Sukumar, R.; Jayaraman, M.; Lakshni, T.V.; Sharma, J. Projected climate change impacts on vegetation distribution over Kashmir Himalayas. Clim. Chang. 2015, 132, 601–613. [Google Scholar] [CrossRef]
- Shafiq, M.U.; Rasool, R.; Ahmed, P.; Dimri, A.P. Temperature and precipitation trends in Kashmir Valley north western Himalayas. Theor. Appl. Clim. 2019, 135, 293–304. [Google Scholar] [CrossRef]
- Romshoo, S.A.; Bashir, J.; Rashid, I. Twenty–first century–end climate scenario of Jammu and Kashmir Himalaya India using ensemble climate models. Clim. Chang. 2020, 162, 1473–1491. [Google Scholar] [CrossRef]
- Dad, J.M.; Muslim, M.; Rashid, I.; Rashid, I.; Reshi, Z.A. Time series analysis of climate variability and trends in Kashmir Himalaya. Ecol. Indic. 2021, 126, 107690. [Google Scholar] [CrossRef]
- Sabin, T.P.; Krishnan, R.; Vellore, R.; Priya, P.; Borgaonkar, H.P.; Singh, B.B.; Sagar, A. Climate Change Over the Himalayas. In Assessment of Climate Change over the Indian Region; Springer: Singapore, 2020; pp. 207–222. [Google Scholar]
- Gaira, K.S.; Dhar, U.; Belwal, O.K. Potential of herbarium records to sequence phenological pattern: A case study of Aconitum heterophyllum in the Himalaya. Biodivers. Conserv. 2011, 20, 2201–2210. [Google Scholar] [CrossRef]
- Ghafoor, G.Z.; Sharif, F.; Khan, H.A.U.; Shahid, M.G.; Siddiq, Z.; Shahzad, L. Effect of climate warming on seedling growth and biomass accumulation of Acacia modesta and Olea ferruginea in a subtropical scrub forest of Pakistan. Écoscience 2022, 29, 133–146. [Google Scholar] [CrossRef]
- Haq, F.; Ahmad, H.; Alam, M. Traditional uses of medicinal plants of NandiarKhuwarr catchment (District Battagram) Pakistan. J. Med. Plants Res. 2011, 5, 39–48. [Google Scholar]
- Murad, W.; Ahmad, A.; Gilani, S.A.; Khan, M.A. Indigenous knowledge and folk use of medicinal plants by the tribal communities of Hazar Nao Forest Malakand District North Pakistan. J. Med. Plants Res. 2011, 5, 1072–1086. [Google Scholar]
- Yousaf, Z.; Shinwari, Z.K.; Ali, S.M. Medicinally important flora of Dhibbia Karsal Village (Mianwali district Punjab). Asian J. Plant Sci. 2004, 3, 757–762. [Google Scholar] [CrossRef]
- Haq, I.; Hussain, Z. Medicinal plants of Palandri district Poonch (AJK). Pak. J. Plant Sci. 1995, 1, 115–126. [Google Scholar]
- Abbasi, A.M.; Khan, M.; Ahmad, M.; Zafar, M.; Jahan, S.; Sultana, S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North–West Frontier Province Pakistan. J. Ethnopharmacol. 2010, 128, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Mann, H. Nonparametric tests against trend. Econometrica 1945, 13, 124–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods; Charles Graffin: London, UK, 1975. [Google Scholar]
- Mahmood, R.; Jia, S.; Zhu, W. Analysis of climate variability trends and prediction in the most active parts of the Lake Chad basin Africa. Sci. Rep. 2019, 9, 6317. [Google Scholar] [CrossRef]
- Ren, H.; Han, G.; Li, M.H.; Gao, C.; Jiang, L. Ethylene–regulated leaf lifespan explains divergent responses of plant productivity to warming among three hydrologically different growing seasons. Glob. Chang. Biol. 2021, 27, 4169–4180. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.U.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Pudas, E.; Leppälä, M.; Tolvanen, A.; Poikolainen, J.; Venäläinen, A.; Kubin, E. Trends in phenology of Betula pubescens across the boreal zone in Finland. Int. J. Biometeorol. 2008, 52, 251–259. [Google Scholar] [CrossRef]
- Menzel, A.; Yuan, Y.; Matiu, M.; Sparks, T.; Scheifinger, H.; Gehrig, R.; Estrella, N. Climate change fingerprints in recent European plant phenology. Glob. Chang. Biol. 2020, 26, 2599–2612. [Google Scholar] [CrossRef]
- Cerlini, P.B.; Saraceni, M.; Orlandi, F.; Selvestri, L.; Fornaciari, M. Phenological response to temperature variability and orography in Central Italy. Int. J. Biometeorol. 2022, 66, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Bhardwaj, D.R.; Sharma, P.; Handa, A.K.; Kumar, D. Impact of climatic patterns on phenophase and growth of multi–purpose trees of north–western mid Himalayan ecosystem. Trees For. People 2021, 6, 100143. [Google Scholar] [CrossRef]
- Pérez–Ramos, I.M.; Cambrollé, J.; Hidalgo–Galvez, M.D.; Matías, L.; Montero-Ramírez, A.; Santolaya, S.; Godoy, Ó. Phenological responses to climate change in communities of plants species with contrasting functional strategies. Environ. Exp. Bot. 2019, 170, 103852. [Google Scholar] [CrossRef]
- Robbirt, K.M.; Davy, A.J.; Hutchings, M.J.; Roberts, D.L. Validation of biological collections as a source of phenological data for use in climate change studies: A case study with the orchid Ophrys sphegodes. J. Ecol. 2011, 99, 235–241. [Google Scholar] [CrossRef]
- Lavoie, C.; Lachance, D. A new herbarium–based method for reconstructing the phenology of plant species across large areas. Am. J. Bot. 2006, 93, 512–516. [Google Scholar] [CrossRef]
- Bowers, J.E. Has climatic warming altered spring flowering date of Sonoran desert shrubs? Southwest. Nat. 2007, 52, 347–355. [Google Scholar] [CrossRef]
- Fox, N.; Jönsson, A.M. Climate effects on the onset of flowering in the United Kingdom. Environ. Sci. Eur. 2019, 31, 89. [Google Scholar] [CrossRef]
- Du, Y.; Pan, Y.; Ma, K. Moderate chilling requirement controls budburst for subtropical species in China. Agric. For. Meteorol. 2019, 278, 107693. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Dai, J.; Ge, Q.; Lin, S. Stronger Spring Phenological Advance in Future Warming Scenarios for Temperate Species with a Lower Chilling Sensitivity. Front. Plant Sci. 2022, 13, 830573. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Luedeling, E.; Shrestha, K.K.; Guan, K.; Xu, J. Flowering phenology of tree Rhododendron along an elevation gradient in two sites in the Eastern Himalayas. Int. J. Biometeorol. 2013, 57, 225–240. [Google Scholar] [CrossRef]
- Abu–Asab, M.S.; Peterson, P.M.; Shetler, S.G. Earlier plant flowering in spring as a response to global warming in the Washington DC area. Biodivers. Conserv. 2001, 10, 597–612. [Google Scholar] [CrossRef]
- Archer, D.R.; Fowler, H.J. Spatial and temporal variations in precipitation in the Upper Indus Basin, global teleconnections and hydrological implications. Hydrol. Earth Sys. Sci. 2004, 8, 47–61. [Google Scholar] [CrossRef]
- Sheikh, M.M.; Manzoor, N.; Adnan, M.; Ashraf, J.; Khan, A.M. Climate Profile and pastclimate changes in Pakistan. In Global Change Impact Studies Center Islamabad, Pakistan (GCISC)-RR-01; National Centre for Physics: Islamabad, Pakistan, 2009. [Google Scholar]
- Roe, G.H.; Montgomery, D.R.; Hallet, B. Orographic climate feedbacks and the relief of mountain ranges. J. Geophys. Res. 2003, 108, 2315. [Google Scholar] [CrossRef]
- Yunling, H.; Yiping, Z. Climate change from 1960–2000 in the Lancang River Valley, China. Mount. Res. Dev. 2005, 25, 341–348. [Google Scholar] [CrossRef]
- Liu, X.B.; Chen, B. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Jaswal, A.K.; Rao, G.S.P. Recent trends in meteorological parameters over Jammu and Kashmir. Mausam 2010, 61, 369–382. [Google Scholar] [CrossRef]
- Joshi, P.K.; Rawat, A.; Narula, S.; Sinha, V. Assessing impact of climate change on forest cover type shifts in Western Himalayan Eco-region. J. For. Res. 2012, 23, 75–80. [Google Scholar] [CrossRef]
- Sijapati, S.; Bhatti, M.T.; Pradhan, N.S. Climate change impact on water availability, and farmers’ adaptation strategies: Case studies from Pakistan and Nepal. In Research Insights on Climate and Water in the Hindu Kush Himalayas; Vaidya, R.R., Sharma, E., Eds.; International Centre for Integrated Mountain Development (ICIMOD): Kathmandu, Nepal, 2004; pp. 109–128. [Google Scholar]
- Ockendon, N.; Baker, D.J.; Carr, J.A.; White, E.C.; Almond, R.E.A.; Amano, T.; Bertram, E.; Bradbury, R.B.; Bradley, C.; Butchart, S.H.; et al. Mechanisms underpinning climatic impacts on natural populations: Altered species interactions are more important than direct effects. Glob. Chang. Biol. 2014, 20, 2221–2229. [Google Scholar] [CrossRef]
- Khan, S.; Kumari, P.; Verma, S. Ambophily in Olea ferruginea: A transitional state in the pollination syndrome. Plant Biosyst. 2022, 157, 221–232. [Google Scholar] [CrossRef]



| Season | TMax (°C) | TMin (°C) | |
|---|---|---|---|
| Spring | Z-score | 2.0105 | 4.0213 |
| p-value | 0.04 | 0.00005 | |
| Trend | Positive | Positive | |
| Summer | Z-score | −0.9886 | 4.9988 |
| p-value | 0.32 | 5.768 × 10−7 | |
| Trend | No trend | Positive | |
| Autumn | Z-score | −0.191 | 4.8756 |
| p-value | 0.85 | 0.000001 | |
| Trend | No trend | Positive | |
| Winter | Z-score | 1.7192 | 3.6394 |
| p-value | 0.08 | 0.0002 | |
| Trend | No trend | Positive |
| Season | Sen’s Slope Estimation | |
|---|---|---|
| TMax (°C a−1) | TMin (°C a−1) | |
| Spring | 0.052 * (0.003 to 0.093) | 0.067 *** (0.036 to 0.100) |
| Summer | −0.012 ns (−0.033 to 0.011) | 0.052 *** (0.035 to 0.070) |
| Autumn | −0.003 ns (−0.027 to 0.018) | 0.060 *** (0.042 to 0.079) |
| Winter | 0.027 ns (−0.002 to 0.071) | 0.057 *** (0.027 to 0.089) |
| Season | n | Flowering Change (DoF) | |||
|---|---|---|---|---|---|
| GAM Coefficient (β) | SE | R2 | p Value | ||
| Winter | 50 | 5.4 | 2.32 | 14.9 | 0.623 |
| Spring | 50 | −1.05 | 2.02 | 25.78 | 0.004 |
| Summer | 50 | −7.5997 | 1.724 | 41.347 | 0.0153 |
| Autumn | 50 | −5.887 | 2.142 | 33.39 | 0.0047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Gaira, K.S.; Asgher, M.; Verma, S.; Pant, S.; Agrawala, D.K.; Alamri, S.; Siddiqui, M.H.; Kesawat, M.S. Temperature Induced Flowering Phenology of Olea ferruginea Royle: A Climate Change Effect. Sustainability 2023, 15, 6936. https://doi.org/10.3390/su15086936
Khan S, Gaira KS, Asgher M, Verma S, Pant S, Agrawala DK, Alamri S, Siddiqui MH, Kesawat MS. Temperature Induced Flowering Phenology of Olea ferruginea Royle: A Climate Change Effect. Sustainability. 2023; 15(8):6936. https://doi.org/10.3390/su15086936
Chicago/Turabian StyleKhan, Sajid, Kailash S. Gaira, Mohd Asgher, Susheel Verma, Shreekar Pant, Dinesh K. Agrawala, Saud Alamri, Manzer H. Siddiqui, and Mahipal Singh Kesawat. 2023. "Temperature Induced Flowering Phenology of Olea ferruginea Royle: A Climate Change Effect" Sustainability 15, no. 8: 6936. https://doi.org/10.3390/su15086936







