Aspergillus niger Enhances the Efficiency of Sewage Sludge Biochar as a Sustainable Phosphorus Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Cultivation Conditions
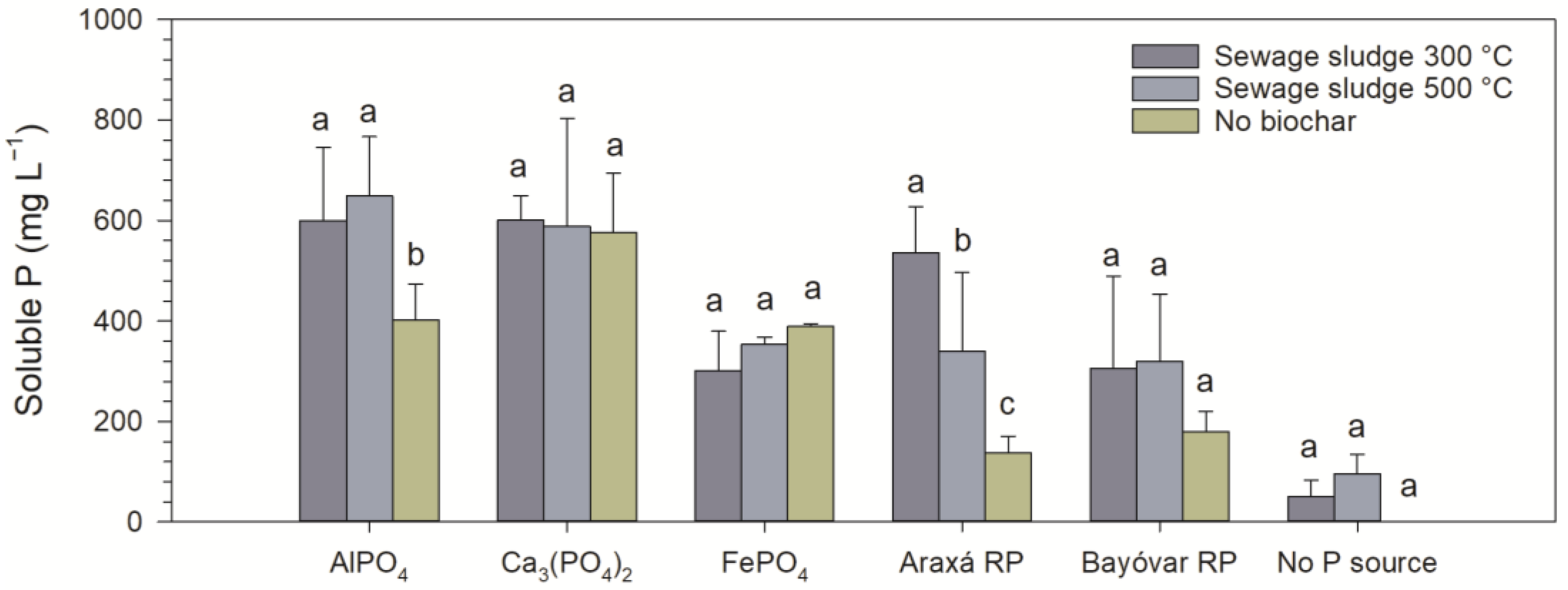
2.2. Experiment I: Effect of Biochars on the Solubilization of P Sources
2.3. Experiment II: P Release from Sewage Sludge Biochar by A. niger into Soil Solution
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012. [Google Scholar]
- FAO. FAOSTAT: Fertilizers by Nutrient. Available online: http://www.fao.org/faostat/en/#data/RFN (accessed on 12 March 2023).
- Roy, E.D.; Richards, P.D.; Martinelli, L.A.; Coletta, L.D.; Lins, S.R.M.; Vazquez, F.F.; Willig, E.; Spera, S.A.; VanWey, L.K.; Porder, S. The Phosphorus Cost of Agricultural Intensification in the Tropics. Nat. Plants 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- Vassilev, N.; Martos, E.; Mendes, G.; Martos, V.; Vassileva, M. Biochar of Animal Origin: A Sustainable Solution to the Global Problem of High-Grade Rock Phosphate Scarcity? J. Sci. Food Agric. 2013, 93, 1799–1804. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A Safe Operating Space for Humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Elser, J.J.; Hilton, J.; Ohtake, H.; Schipper, W.J.; Van Dijk, K.C. Greening the Global Phosphorus Cycle: How Green Chemistry Can Help Achieve Planetary P Sustainability. Green Chem. 2015, 17, 2087–2099. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A Comprehensive Review of Phosphorus Recovery from Wastewater by Crystallization Processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus Recovery from Municipal Wastewater Treatment: Critical Review of Challenges and Opportunities for Developing Countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Recent Advances in Removing Phosphorus from Wastewater and Its Future Use as Fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Zhu, F.; Cakmak, E.K.; Cetecioglu, Z. Phosphorus Recovery for Circular Economy: Application Potential of Feasible Resources and Engineering Processes in Europe. Chem. Eng. J. 2023, 454, 140153. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Pinheiro, T.D.; de Oliveira, L.E.Z.; de Araujo, A.S.; Coser, T.R.; Paz-Ferreiro, J. Direct and Residual Effect of Biochar Derived from Biosolids on Soil Phosphorus Pools: A Four-Year Field Assessment. Sci. Total Environ. 2020, 739, 140013. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.C.; Chagas, J.K.M.; da Silva, J.; Paz-Ferreiro, J. Short-Term Effects of a Sewage Sludge Biochar Amendment on Total and Available Heavy Metal Content of a Tropical Soil. Geoderma 2019, 344, 31–39. [Google Scholar] [CrossRef]
- Liang, S.; Chen, H.; Zeng, X.; Li, Z.; Yu, W.; Xiao, K.; Hu, J.; Hou, H.; Liu, B.; Tao, S.; et al. A Comparison between Sulfuric Acid and Oxalic Acid Leaching with Subsequent Purification and Precipitation for Phosphorus Recovery from Sewage Sludge Incineration Ash. Water Res. 2019, 159, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Bridle, T.R.; Pritchard, D. Energy and Nutrient Recovery from Sewage Sludge via Pyrolysis. Water Sci. Technol. 2004, 50, 169–175. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.A.T.C.; Figueiredo, C.C. Sewage Sludge Biochar: Effects on Soil Fertility and Growth of Radish. Biol. Agric. Hortic. 2016, 32, 127–138. [Google Scholar] [CrossRef]
- Li, F.; Liang, X.; Niyungeko, C.; Sun, T.; Liu, F.; Arai, Y. Effects of Biochar Amendments on Soil Phosphorus Transformation in Agricultural Soils. Adv. Agron. 2019, 158, 131–172. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Farias, W.M.; Coser, T.R.; Monteiro de Paula, A.; Sartori da Silva, M.R.; Paz-Ferreiro, J. Sewage Sludge Biochar Alters Root Colonization of Mycorrhizal Fungi in a Soil Cultivated with Corn. Eur. J. Soil Biol. 2019, 93, 103092. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, D.; Li, Z.; Guo, S.; Chen, Z.; Wu, L.; Zhao, Y. Greenhouse Gas Emissions from Soils Amended with Cornstalk Biochar at Different Addition Ratios. Int. J. Environ. Res. Public Health 2023, 20, 927. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ma, J.; Chen, F.; Yu, R.; Hu, G.; Zhang, S. Remediation of Soil Polluted with Cd in a Postmining Area Using Thiourea-Modified Biochar. Int. J. Environ. Res. Public Health 2020, 17, 7654. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Wang, X.; Naveed, M.; Mustafa, A.; Ashraf, S.; Samreen, T.; Nadeem, S.M.; Jamil, M. Biochar Mediated-Alleviation of Chromium Stress and Growth Improvement of Different Maize Cultivars in Tannery Polluted Soils. Int. J. Environ. Res. Public Health 2021, 18, 4461. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Haderlein, S.B. Potential Effects of Biochar on the Availability of Phosphorus—Mechanistic Insights. Geoderma 2016, 277, 83–90. [Google Scholar] [CrossRef]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of Pyrolysis Parameters on Phosphorus Fractions of Biosolids Derived Biochar. Sci. Total Environ. 2019, 695, 133846. [Google Scholar] [CrossRef] [PubMed]
- Steckenmesser, D.; Vogel, C.; Adam, C.; Steffens, D. Effect of Various Types of Thermochemical Processing of Sewage Sludges on Phosphorus Speciation, Solubility, and Fertilization Performance. Waste Manag. 2017, 62, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.O.; Freitas, A.L.M.; Pereira, O.L.; Silva, I.R.; Vassilev, N.B.; Costa, M.D. Mechanisms of Phosphate Solubilization by Fungal Isolates When Exposed to Different P Sources. Ann. Microbiol. 2014, 64, 239–249. [Google Scholar] [CrossRef]
- Mendes, G.O.; Bahri-Esfahani, J.; Csetenyi, L.; Hillier, S.; George, T.S.; Gadd, G.M. Chemical and Physical Mechanisms of Fungal Bioweathering of Rock Phosphate. Geomicrobiol. J. 2021, 38, 384–394. [Google Scholar] [CrossRef]
- Mendes, G.O.; Zafra, D.L.; Vassilev, N.B.; da Silva, I.R.; Ribeiro, J.I.; Costa, M.D. Biochar Enhances Aspergillus Niger Rock Phosphate Solubilization by Increasing Organic Acid Production and Alleviating Fluoride Toxicity. Appl. Environ. Microbiol. 2014, 80, 3081–3085. [Google Scholar] [CrossRef]
- Mendes, G.O.; da Silva, N.M.R.M.; Anastácio, T.C.; Vassilev, N.B.; Ribeiro, J.I.; da Silva, I.R.; Costa, M.D. Optimization of Aspergillus Niger Rock Phosphate Solubilization in Solid-State Fermentation and Use of the Resulting Product as a P Fertilizer. Microb. Biotechnol. 2015, 8, 930–939. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef] [PubMed]
- USEPA Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-3050b-acid-digestion-sediments-sludges-and-soils (accessed on 9 April 2023).
- Braga, J.M.; Defelipo, B. V Determinação Espectrofotométrica de Fósforo Em Extratos de Solo e Material Vegetal. Rev. Ceres 1974, 21, 73–85. [Google Scholar]
- Manual de Métodos de Análise de Solo, 3rd ed.; Teixeira, P.C., Donagemma, G.K., Fontana, A., Teixeira, W.G., Eds.; Embrapa: Brasília, Brazil, 2017; ISBN 9788570357717. [Google Scholar]
- Oliveira, C.M.B.; Gatiboni, L.C.; Miquelluti, D.J.; Smyth, T.J.; Almeida, J.A. Maximum Phosphorus Adsorption Capacity and Binding Energy Constant of an Oxisol Fitting Different Langmuir Models. Rev. Bras. Cienc. Solo 2014, 38, 1805–1815. [Google Scholar] [CrossRef]
- Mendes, G.O.; Murta, H.M.; Valadares, R.V.; Silveira, W.B.d.; Silva, I.R.d.; Costa, M.D. Oxalic Acid Is More Efficient than Sulfuric Acid for Rock Phosphate Solubilization. Miner. Eng. 2020, 155, 106458. [Google Scholar] [CrossRef]
- Robinson, J.S.; Syers, J.K.; Bolan, N.S. Importance of Proton Supply and Calcium-Sink Size in the Dissolution of Phosphate Rock Materials of Different Reactivity in Soil. J. Soil Sci. 1992, 43, 447–459. [Google Scholar] [CrossRef]
- Mendes, G.d.O.; Dyer, T.; Csetenyi, L.; Gadd, G.M. Rock Phosphate Solubilization by Abiotic and Fungal-produced Oxalic Acid: Reaction Parameters and Bioleaching Potential. Microb. Biotechnol. 2022, 15, 1189–1202. [Google Scholar] [CrossRef]
- Mendes, G.O.; Vassilev, N.B.; Bonduki, V.H.A.; Silva, I.R.; Ribeiro, J.I.; Costa, M.D. Inhibition of Aspergillus Niger Phosphate Solubilization by Fluoride Released from Rock Phosphate. Appl. Environ. Microbiol. 2013, 79, 4906–4913. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb Immobilization via the Combination of Biochar and Phosphate Solubilizing Bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Hu, D. Effective Alleviation of Aluminum Phytotoxicity by Manure-Derived Biochar. Environ. Sci. Technol. 2013, 47, 2737–2745. [Google Scholar] [CrossRef]
- Silva, U.d.C.C.; Mendes, G.d.O.; Silva, N.M.R.M.; Duarte, J.L.; Silva, I.R.; Tótola, M.R.; Costa, M.D.M.D.; Totola, M.R.; Costa, M.D.M.D. Fluoride-Tolerant Mutants of Aspergillus Niger Show Enhanced Phosphate Solubilization Capacity. PLoS ONE 2014, 9, e110246. [Google Scholar] [CrossRef]
- Smith, E.A.; Mayfield, C.I.; Wong, P.T.S. Physical and Chemical Characterization of Selected Natural Apatites in Synthetic and Natural Aqueous Solutions. Water Air Soil Pollut. 1977, 8, 401–415. [Google Scholar] [CrossRef]
- Schneider, K.D.; van Straaten, P.; de Orduna, R.M.; Glasauer, S.; Trevors, J.; Fallow, D.; Smith, P.S. Comparing Phosphorus Mobilization Strategies Using Aspergillus Niger for the Mineral Dissolution of Three Phosphate Rocks. J. Appl. Microbiol. 2010, 108, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Raymond, N.S.; Jensen, L.S.; Müller Stöver, D. Enhancing the Phosphorus Bioavailability of Thermally Converted Sewage Sludge by Phosphate-Solubilising Fungi. Ecol. Eng. 2018, 120, 44–53. [Google Scholar] [CrossRef]
- Figueiredo, C.C.d.; Reis, A.d.S.P.J.; Araujo, A.S.d.; Blum, L.E.B.; Shah, K.; Paz-Ferreiro, J. Assessing the Potential of Sewage Sludge-Derived Biochar as a Novel Phosphorus Fertilizer: Influence of Extractant Solutions and Pyrolysis Temperatures. Waste Manag. 2021, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ker, J.C.; Fontes, M.P.F.; Souza, A.R.; Resende, M. Adsorção de Fósforo Em Alguns Solos Latossólicos: Relação Entre Mineralogia e Efeito de Calagem. Rev. Ceres 1996, 43, 216–226. [Google Scholar]
- Novais, R.F.; Smith, T.J. Fósforo Em Solo e Planta Em Condições Tropicais; Editora UFV: Viçosa, Brazil, 1999. [Google Scholar]
- Araújo, V.C.; Rossati, K.F.; Xavier, L.V.; Oliveira, V.A.d.; Carmo, G.J.d.S.; Assis, G.A.d.; Mendes, G.d.O. Enhanced Growth in Nursery of Coffee Seedlings Inoculated with the Rhizosphere Fungus Aspergillus Niger for Field Transplantation. Rhizosphere 2020, 15, 100236. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L.; Guo, D.; Wang, G.; Song, X.; Ma, Y. The Continuous Application of Biochar in Field: Effects on P Fraction, P Sorption and Release. Chemosphere 2020, 263, 128084. [Google Scholar] [CrossRef]

| P Source | Total P (g kg−1) | Water-Soluble P (g kg−1) | pH (H2O) |
|---|---|---|---|
| AlPO4 | 254 | 0.59 | 6.86 |
| Ca3(PO4)2 | 200 | 0.13 | 6.36 |
| FePO4.2H2O | 166 | 0.39 | 6.74 |
| Araxá RP | 140 | 0.39 | 6.88 |
| Bayóvar RP | 146 | 0.02 | 6.78 |
| Property | Units | SS | Biochar 300 | Biochar 500 |
|---|---|---|---|---|
| pH (CaCl2) | - | 4.8 ± 0.4 | 5.8 ± 0.2 | 6.5 ± 0.3 |
| C | % | 21.0 ± 0.4 | 23.4 ± 0.4 | 19.0 ± 0.2 |
| H | % | 4.2 ± 0.1 | 3.6 ± 0.1 | 1.7 ± 0.1 |
| N | % | 3.0 ± 0.1 | 3.3 ± 0.1 | 2.3 ± 0.1 |
| NO3- | mg kg−1 | 23.3 ± 3.4 | 17.5 ± 1.2 | 5.84 ± 0.5 |
| NH4+ | mg kg−1 | 461.2 ± 36.0 | 431.9 ± 20.2 | 169.3 ± 10.5 |
| H/C | - | 2.4 ± 0.1 | 1.8 ± 0.1 | 1.1 ± 0.1 |
| C/N | - | 7.0 ± 0.1 | 7.0 ± 0.1 | 8.3 ± 0.1 |
| Total P | g kg−1 | 35.7 ± 2.8 | 41.1 ± 3.2 | 61.3 ± 5.6 |
| Water-soluble P | g kg−1 | nd | 0.02 ± 0.02 | 0.04 ± 0.02 |
| K | g kg−1 | 0.8 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 |
| Ca | g kg−1 | 6.6 ± 0.1 | 6.7 ± 0.2 | 8.2 ± 0.3 |
| Mg | g kg−1 | 0.8 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 |
| S | g kg−1 | 6.7 ± 0.2 | 15.1 ± 1.0 | 7.4 ± 0.4 |
| Total Cu | mg kg−1 | 115 ± 1.0 | 148 ± 1.0 | 145 ± 1.0 |
| Total Pb | mg kg−1 | 207 ± 1.0 | 256 ± 3.0 | 265 ± 1.0 |
| Total Zn | mg kg−1 | 306 ± 1.0 | 321 ± 1.0 | 411 ± 5.0 |
| Total Cr | mg kg−1 | 100 ± 1.0 | 106 ± 2.0 | 136 ± 1.0 |
| Total Co | mg kg−1 | 20 ± 1.0 | 22 ± 1.0 | 25 ± 1.0 |
| Total Mn | mg kg−1 | 56 ± 1.0 | 58 ± 1.0 | 80 ± 2.0 |
| Total Cu | mg kg−1 | 115 ± 1.0 | 148 ± 1.0 | 145 ± 1.0 |
| PV | mL g−1 | 0.022 ± 0.001 | 0.027 ± 0.001 | 0.053 ± 0.002 |
| SSA | m² g−1 | 18.2 ± 1.2 | 20.2 ± 1.8 | 52.5 ± 4.3 |
| Volatile matter | % (db) | 45 ± 4.0 | 36.8 ± 4.4 | 17.8 ± 0.6 |
| Ash | % (db) | 54 ± 3.0 | 56.6 ± 2.6 | 77.6 ± 0.6 |
| Fixed carbon | % (db) | - | 6.5 ± 1.8 | 4.7 ± 0.1 |
| P Source | SS Biochar 300 °C a | SS Biochar 500 °C a | No Biochar |
|---|---|---|---|
| AlPO4 | 72 | 73 | 53 |
| Ca3(PO4)2 | 92 | 82 | 96 |
| FePO4.2H2O | 50 | 52 | 78 |
| Araxá RP | 107 | 58 | 33 |
| Bayóvar RP | 58 | 51 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossati, K.F.; Figueiredo, C.C.d.; Mendes, G.d.O. Aspergillus niger Enhances the Efficiency of Sewage Sludge Biochar as a Sustainable Phosphorus Source. Sustainability 2023, 15, 6940. https://doi.org/10.3390/su15086940
Rossati KF, Figueiredo CCd, Mendes GdO. Aspergillus niger Enhances the Efficiency of Sewage Sludge Biochar as a Sustainable Phosphorus Source. Sustainability. 2023; 15(8):6940. https://doi.org/10.3390/su15086940
Chicago/Turabian StyleRossati, Kamila Fernanda, Cícero Célio de Figueiredo, and Gilberto de Oliveira Mendes. 2023. "Aspergillus niger Enhances the Efficiency of Sewage Sludge Biochar as a Sustainable Phosphorus Source" Sustainability 15, no. 8: 6940. https://doi.org/10.3390/su15086940
APA StyleRossati, K. F., Figueiredo, C. C. d., & Mendes, G. d. O. (2023). Aspergillus niger Enhances the Efficiency of Sewage Sludge Biochar as a Sustainable Phosphorus Source. Sustainability, 15(8), 6940. https://doi.org/10.3390/su15086940








