Polystyrene Nanoparticle Uptake and Deposition in Silkworm and Influence on Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of NaLuF4: 20% Yb, 2% Er@NaLuF4 UCNPs
2.3. In Situ Polymerization of Polystyrene (PS) on the Surface of UCNPs
2.4. Cultivation Experiment of Silkworms
2.5. Characterization of UCNPs-Labelled NPs Fluorescent Probes
2.6. Time Gated Imaging of Biological Samples
2.7. Distribution Analysis of NPs
2.8. In Situ Observation of NPs in Silk
3. Results and Discussion
3.1. Morphology and Optical Properties of UCNPs-Labelled NP Fluorescent Probe
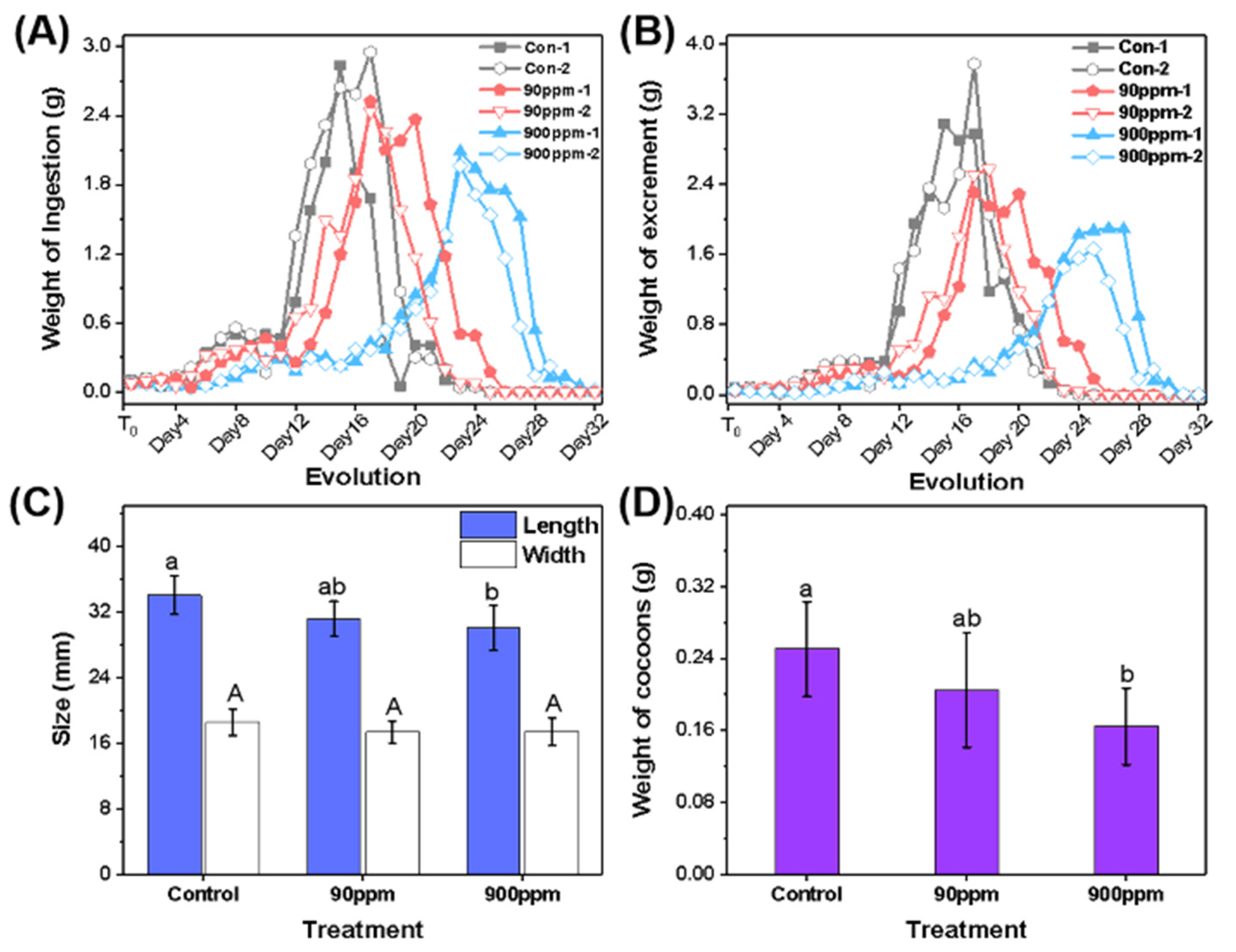
3.2. Comparison of Growth Evolution of Silkworms under Different Culture Conditions
3.3. Discussion of the Distribution of NPs
3.4. Investigations of Silk and Silkworm Eggs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Besseling, E.; Quik, J.T.K.; Sun, M.; Koelmans, A.A. Fate of nano- and microplastic in freshwater systems: A modeling study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wang, B.; Lurling, M.; Koelmans, A.A. Nanoplastic Affects Growth of S. obliquus and Reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.K.; Kim, K.S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.S.; et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Li, S.; Zhang, X.; Feng, H.; Dai, Y.; Zhao, J.; Yue, T. Molecular modeling of nanoplastic transformations in alveolar fluid and impacts on the lung surfactant film. J. Hazard. Mater. 2022, 427, 127872. [Google Scholar] [CrossRef]
- Al-Sid-Cheikh, M.; Rowland, S.J.; Stevenson, K.; Rouleau, C.; Henry, T.B.; Thompson, R.C. Uptake, Whole-Body Distribution, and Depuration of Nanoplastics by the Scallop Pecten maximus at Environmentally Realistic Concentrations. Environ. Sci. Technol. 2018, 52, 14480–14486. [Google Scholar] [CrossRef]
- Wang, S.; Zhong, Z.; Li, Z.; Wang, X.; Gu, H.; Huang, W.; Fang, J.K.-H.; Shi, H.; Hu, M.; Wang, Y. Physiological effects of plastic particles on mussels are mediated by food presence. J. Hazard. Mater. 2021, 404, 124136. [Google Scholar] [CrossRef]
- Guo, X.; Cai, Y.; Ma, C.; Han, L.; Yang, Z. Combined toxicity of micro/nano scale polystyrene plastics and ciprofloxacin to Corbicula fluminea in freshwater sediments. Sci. Total Environ. 2021, 789, 147887. [Google Scholar] [CrossRef]
- Manzo, S.; Schiavo, S. Physical and chemical threats posed by micro (nano)plastic to sea urchins. Sci. Total Environ. 2022, 808, 152105. [Google Scholar] [CrossRef] [PubMed]
- Dedman, C.J.; Christie-Oleza, J.A.; Fernandez-Juarez, V.; Echeveste, P. Cell size matters: Nano- and micro-plastics preferentially drive declines of large marine phytoplankton due to co-aggregation. J. Hazard. Mater. 2022, 424, 127488. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Jiang, R.; Xiong, Y.; Wu, J.; Xu, J.; Zheng, J.; Zhu, F.; Ouyang, G. Quantification of the combined toxic effect of polychlorinated biphenyls and nano-sized polystyrene on Daphnia magna. J. Hazard. Mater. 2019, 364, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Perez, E.; Jiang, Q.; Chen, Q.; Jiao, Y.; Huang, Y.; Yang, Y.; Zhao, Y. Polystyrene nanoplastic induces oxidative stress, immune defense, and glycometabolism change in Daphnia pulex: Application of transcriptome profiling in risk assessment of nanoplastics. J. Hazard. Mater. 2021, 402, 123778. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, L.; Huang, Q.; Dong, S.; Wang, X.; Yan, C. Combined effects of micro-/nano-plastics and oxytetracycline on the intestinal histopathology and microbiome in zebrafish (Danio rerio). Sci. Total Environ. 2022, 843, 156917. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Sun, X.; Chen, B.; Li, Q.; Liu, N.; Xia, B.; Zhu, L.; Qu, K. Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphile. Sci. Total Environ. 2018, 642, 1378–1385. [Google Scholar] [CrossRef]
- Chu, Q.; Zhang, S.; Yu, X.; Wang, Y.; Zhang, M.; Zheng, X. Fecal microbiota transplantation attenuates nano-plastics induced toxicity in Caenorhabditis elegans. Sci. Total Environ. 2021, 779, 146454. [Google Scholar] [CrossRef]
- Nyeste, K.; Dobrocsi, P.; Czegledi, I.; Czedli, H.; Harangi, S.; Baranyai, E.; Simon, E.; Nagy, S.A.; Antal, L. Age and diet-specific trace element accumulation patterns in different tissues of chub (Squalius cephalus): Juveniles are useful bioindicators of recent pollution. Ecol. Indic. 2019, 101, 1–10. [Google Scholar] [CrossRef]
- Yancheva, V.; Georgieva, E.; Velcheva, I.; Iliev, I.; Stoyanova, S.; Vasileva, T.; Bivolarski, V.; Todorova-Bambaldokova, D.; Zulkipli, N.; Antal, L.; et al. Assessment of the exposure of two pesticides on common carp (Cyprinus carpio Linnaeus, 1758): Are the prolonged biomarker responses adaptive or destructive? Comp. Biochem. Phys. C. 2022, 261, 109446. [Google Scholar]
- Zhang, T.R.; Wang, C.X.; Dong, F.Q.; Gao, Z.Y.; Zhang, C.J.; Zhang, X.J.; Fu, L.M.; Wang, Y.; Zhang, J.P. Uptake and Translocation of Styrene Maleic Anhydride Nanoparticles in Murraya exotica Plants as Revealed by Noninvasive, Real-Time Optical Bioimaging. Environ. Sci. Technol. 2019, 53, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef]
- Du, J.; Qv, W.; Niu, Y.; Qv, M.; Jin, K.; Xie, J.; Li, Z. Nanoplastic pollution inhibits stream leaf decomposition through modulating microbial metabolic activity and fungal community structure. J. Hazard. Mater. 2022, 424, 127392. [Google Scholar] [CrossRef] [PubMed]
- Tansil, N.C.; Li, Y.; Koh, L.D.; Peng, T.C.; Win, K.Y.; Liu, X.Y.; Han, M.Y. The use of molecular fluorescent markers to monitor absorption and distribution of xenobiotics in a silkworm model. Biomaterials 2011, 32, 9576–9583. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, M.; Zhang, H.; Fan, S.; Shao, H.; Hu, X.; Zheng, Y. Intrinsically Fluorescent Silks from Silkworms Fed with Rare-Earth Upconverting Phosphors. ACS Biomater. Sci. Eng. 2018, 4, 4021–4027. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Jiang, S. Multicolor Core/Shell-Structured Upconversion Fluorescent Nanoparticles. Adv. Mater. 2008, 20, 4765. [Google Scholar] [CrossRef]
- Kamimura, M.; Yano, Y.; Kuraoka, S.; Suyari, S.; Ube, T.; Wortmann, L.; Soga, K. Near-Infrared to Visible Upconversion Emission Induced Photopolymerization: Polystyrene Shell Coated NaYF4 Nanoparticles for Fluorescence Bioimaging and Nanothermometry. J. Photopolym. Sci. Technol. 2017, 30, 265–270. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.; Wang, J.; Wang, Q.; Han, Y.; Zhu, H.; Chen, X.; Liu, X. Tuning upconversion through energy migration in core-shell nanoparticles. Nat. Mater. 2011, 10, 968–973. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, C.; Gu, Z.; Xu, Y.; Li, S.; Hao, X.; Liu, X. Time-gated stimulated emission depletion nanoscopy. Opt. Eng. 2013, 52, 093107. [Google Scholar] [CrossRef]
- Clark, N.J.; Khan, F.R.; Mitrano, D.M.; Boyle, D.; Thompson, R.C. Demonstrating the translocation of nanoplastics across the fish intestine using palladium-doped polystyrene in a salmon gut-sac. Environ. Int. 2022, 159, 106994. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Chen, Q.; Jiang, W.; Wang, L.; Xie, H.; Kalogerakis, N.; Ma, Y.; Ji, R. A carbon-14 radiotracer-based study on the phototransformation of polystyrene nanoplastics in water versus in air. Environ. Sci.-Nano 2019, 6, 2907–2917. [Google Scholar] [CrossRef]
- Thompson, H.W.; Mera, R.; Prasad, C. A Description of the Appropriate Use of Student’s t-test. Nutr. Neurosci. 1998, 1, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Wang, R.-Y.; Wang, Z.-J.; Shao, Q.-Q.; Hu, Y.-W.; Jia, H.-B.; Liu, X.-J.; Dong, F.-Q.; Fu, L.-M.; Zhang, J.-P. Daphnia magna uptake and excretion of luminescence-labelled polystyrene nanoparticle as visualized by high sensitivity real-time optical imaging. Chemosphere 2023, 326, 138341. [Google Scholar] [CrossRef]
- Jimenez-Lamana, J.; Marigliano, L.; Allouche, J.; Grassl, B.; Szpunar, J.; Reynaud, S. A Novel Strategy for the Detection and Quantification of Nanoplastics by Single Particle Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Anal. Chem. 2020, 92, 11664–11672. [Google Scholar] [CrossRef]
- Demir, E.; Demir, F.T. Drosophila melanogaster as a dynamic in vivo model organism reveals the hidden effects of interactions between microplastic/nanoplastic and heavy metals. J. Appl. Toxicol. 2023, 43, 212–219. [Google Scholar] [CrossRef]






| Treatment | Organ | Dry Weight (g) | Concentration of NPs (mg/kg)/Detection Limit | Content of NPs (μg) |
|---|---|---|---|---|
| Control | Head | 0.0074 | 0.2/0.4 | / |
| Digestive tract | 0.0198 | 0.95/0.09 | / | |
| Silk gland | 0.2521 | 0.12/0.01 | / | |
| Fat body | 0.2079 | 0.15/0.01 | / | |
| Excrement | 2.8806 | 0.03/0.03 | / | |
| NPs-900 ppm | Head | 0.0045 | 34.0/0.3 | 0.142 |
| Digestive tract | 0.0136 | 25.58/0.07 | 0.340 | |
| Silk gland | 0.1747 | 0.72/0.02 | 0.126 | |
| Fat body | 0.1640 | 7.78/0.02 | 1.275 | |
| Excrement | 1.7358 | 1344.07/0.03 | 2.3 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-J.; Zhang, Y.-H.; Gao, R.-Y.; Jia, H.-B.; Liu, X.-J.; Hu, Y.-W.; Shao, Q.-Q.; Fu, L.-M.; Zhang, J.-P. Polystyrene Nanoparticle Uptake and Deposition in Silkworm and Influence on Growth. Sustainability 2023, 15, 7090. https://doi.org/10.3390/su15097090
Wang Z-J, Zhang Y-H, Gao R-Y, Jia H-B, Liu X-J, Hu Y-W, Shao Q-Q, Fu L-M, Zhang J-P. Polystyrene Nanoparticle Uptake and Deposition in Silkworm and Influence on Growth. Sustainability. 2023; 15(9):7090. https://doi.org/10.3390/su15097090
Chicago/Turabian StyleWang, Ze-Jun, Yu-Hang Zhang, Rong-Yao Gao, Hua-Bing Jia, Xiao-Jing Liu, Ya-Wen Hu, Qian-Qian Shao, Li-Min Fu, and Jian-Ping Zhang. 2023. "Polystyrene Nanoparticle Uptake and Deposition in Silkworm and Influence on Growth" Sustainability 15, no. 9: 7090. https://doi.org/10.3390/su15097090





