Effect of Karst Microhabitats on the Structure and Function of the Rhizosphere Soil Microbial Community of Rhododendron pudingense
Abstract
:1. Introduction
2. Materials and Methods
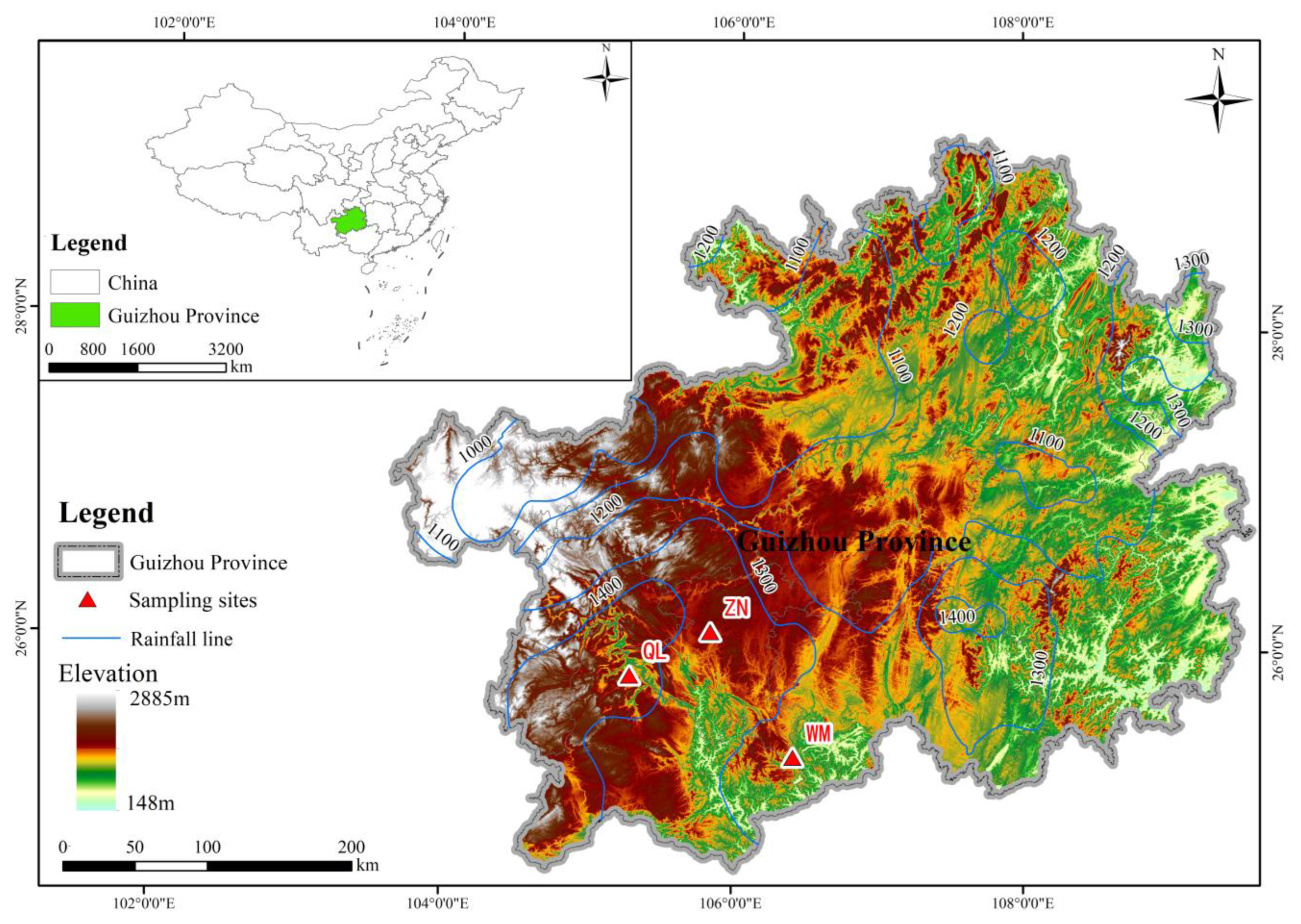
2.1. Study Area
2.2. Classification of Karst Microhabitats
2.3. Sample Collection
2.4. Determination of Physical and Chemical Properties of Soil
2.5. DNA Extraction and Sequencing
2.6. Data Analysis
3. Results
3.1. Soil Nutrient Content and Enzyme Activity
3.2. Dilution Curve
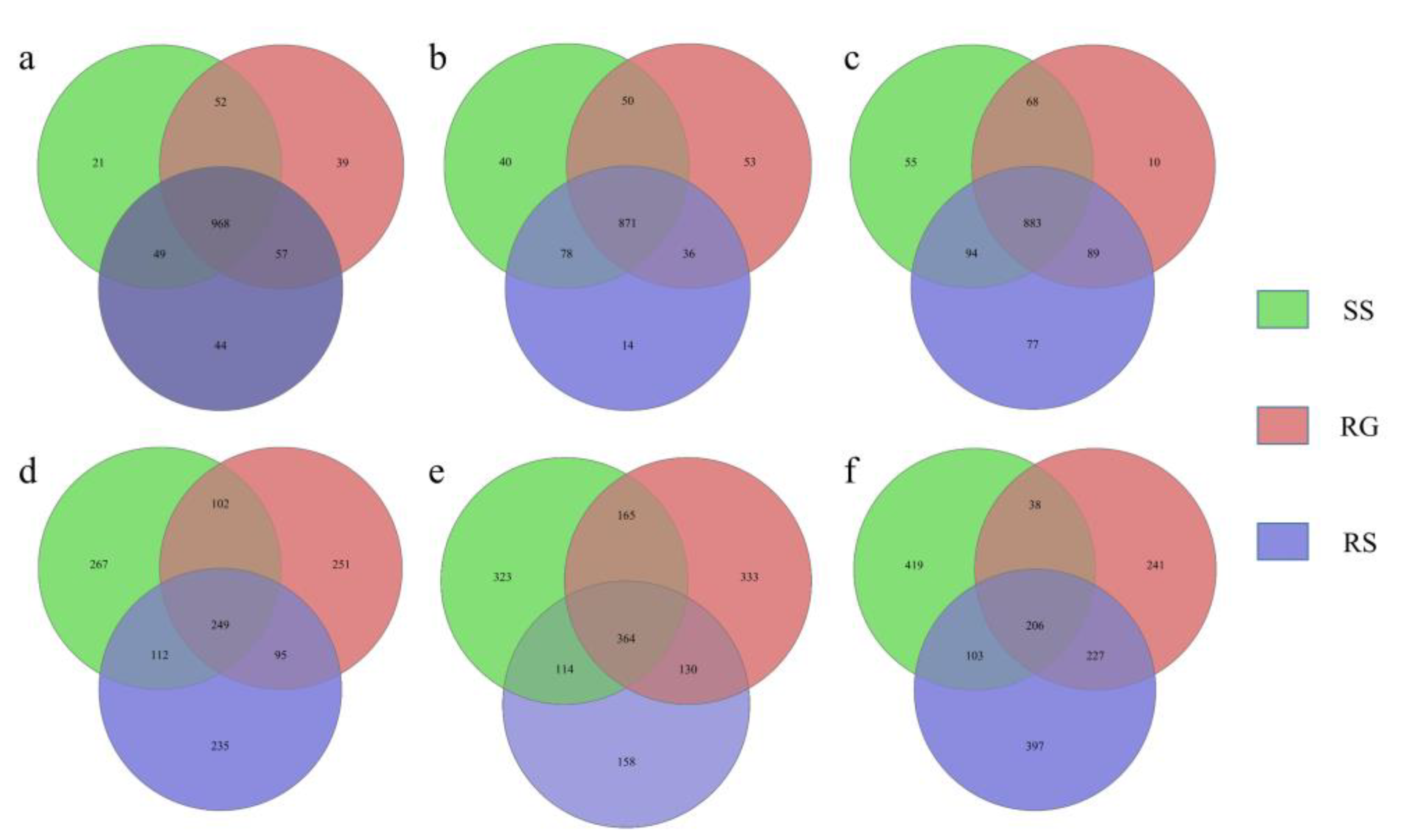
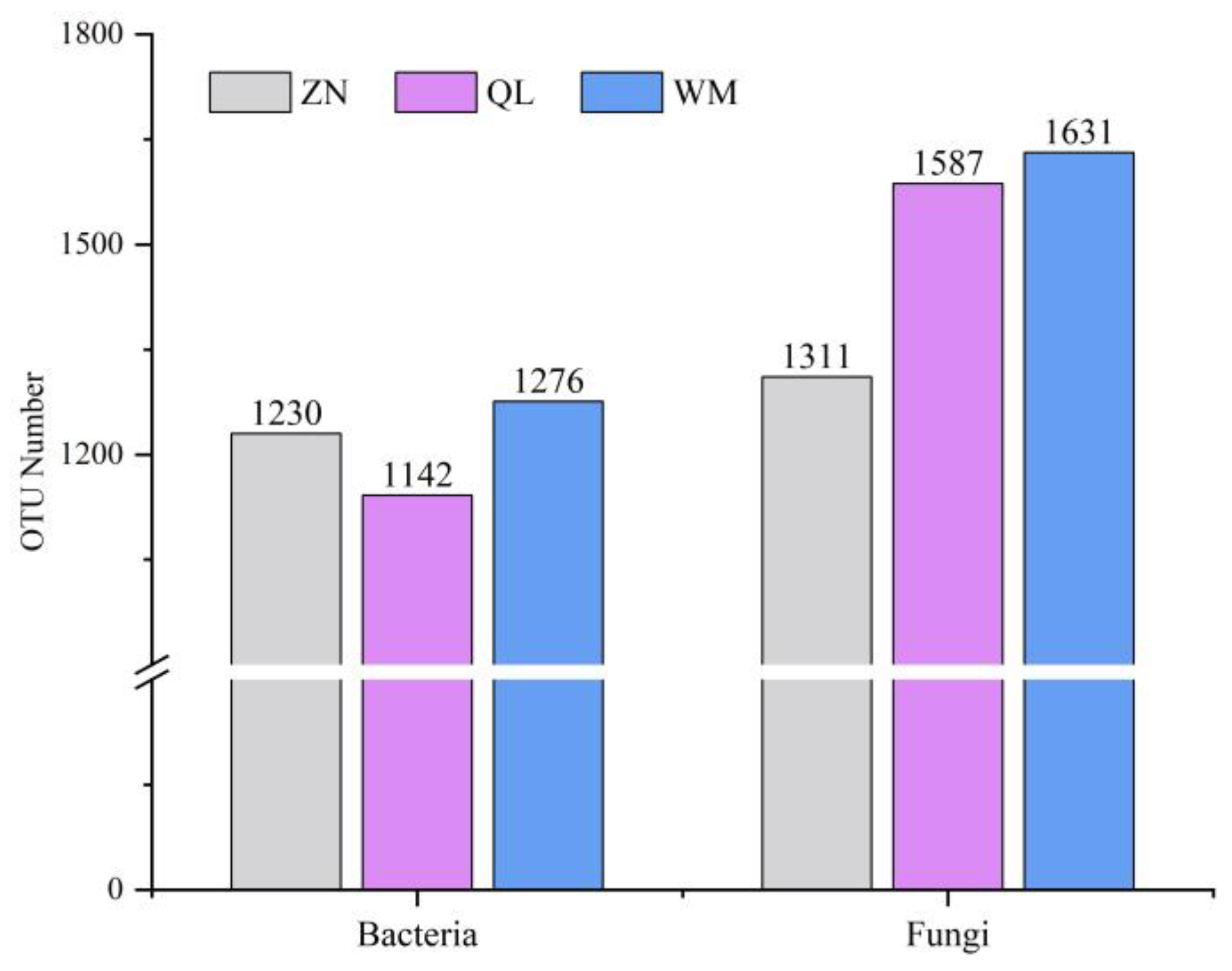
3.3. Alpha Diversity Index and OTU Analysis
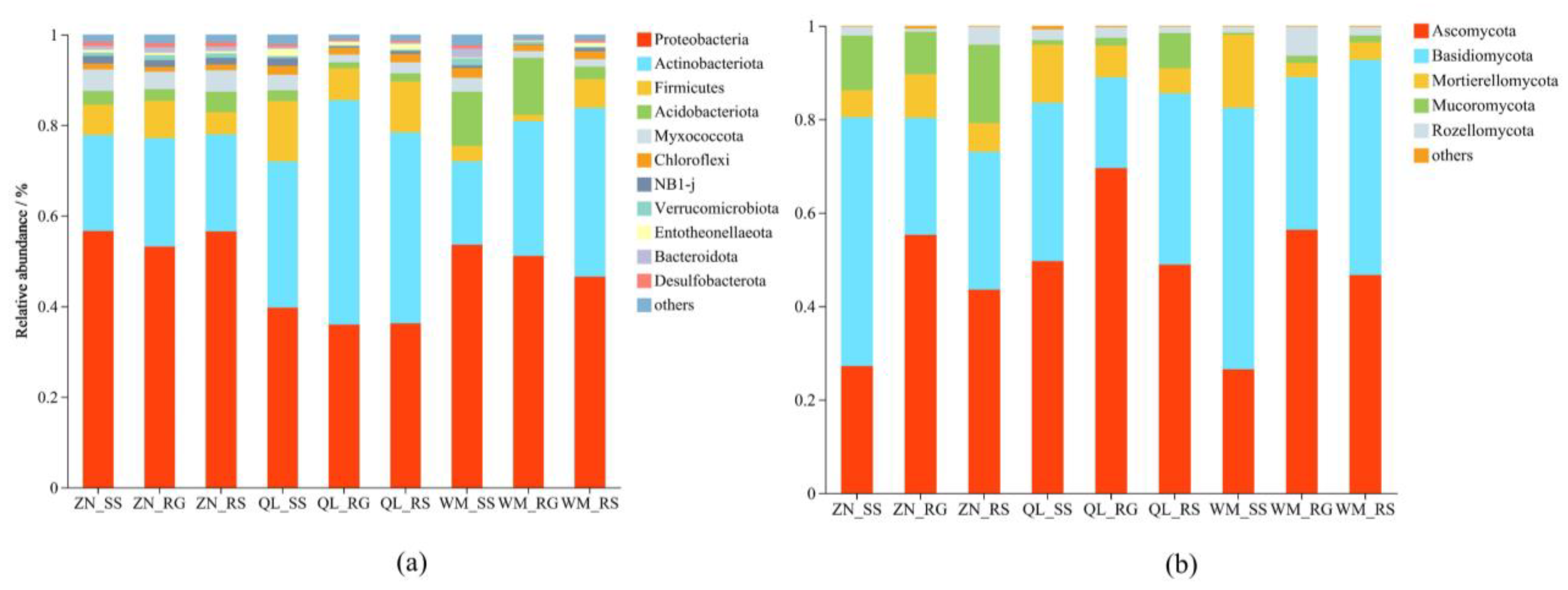
3.4. Analysis of Microbial Community Composition in Different Karst Microhabitats
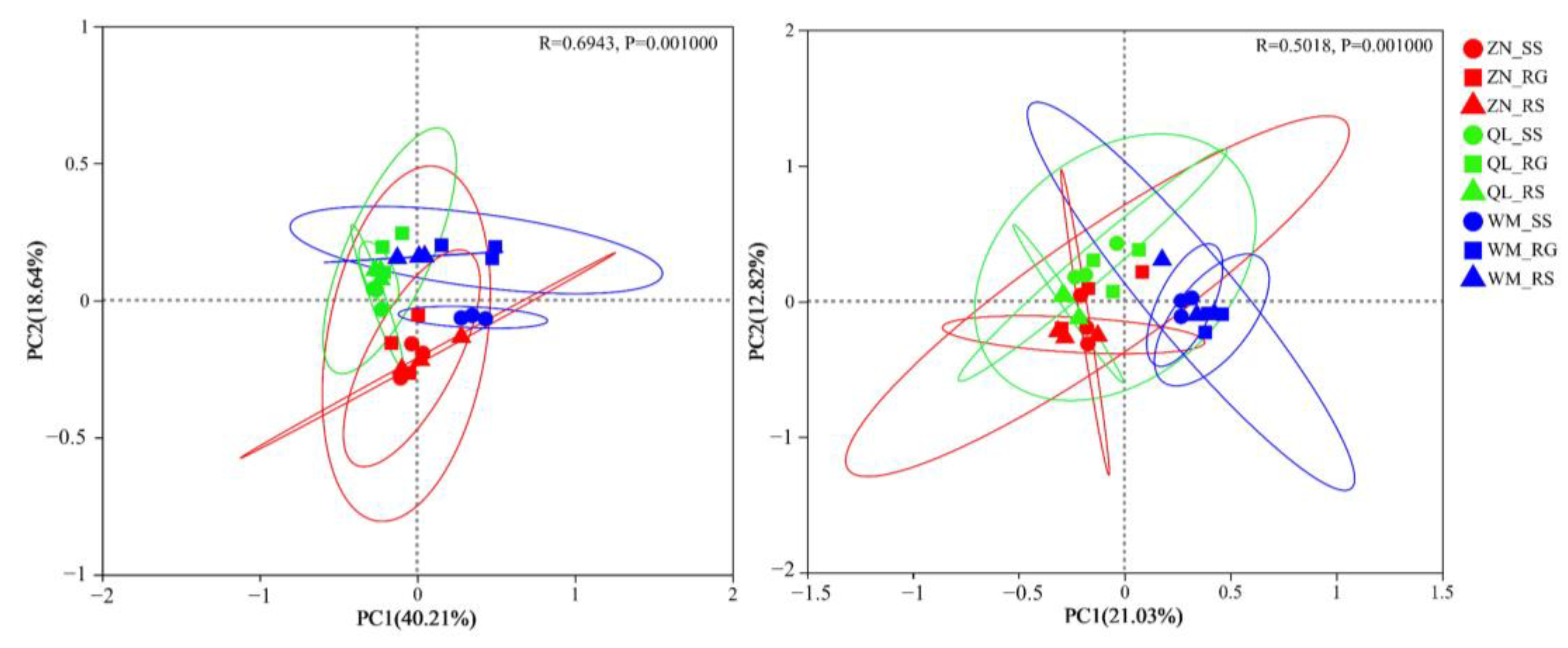
3.4.1. PCoA Analysis
3.4.2. Microbial Community Structure
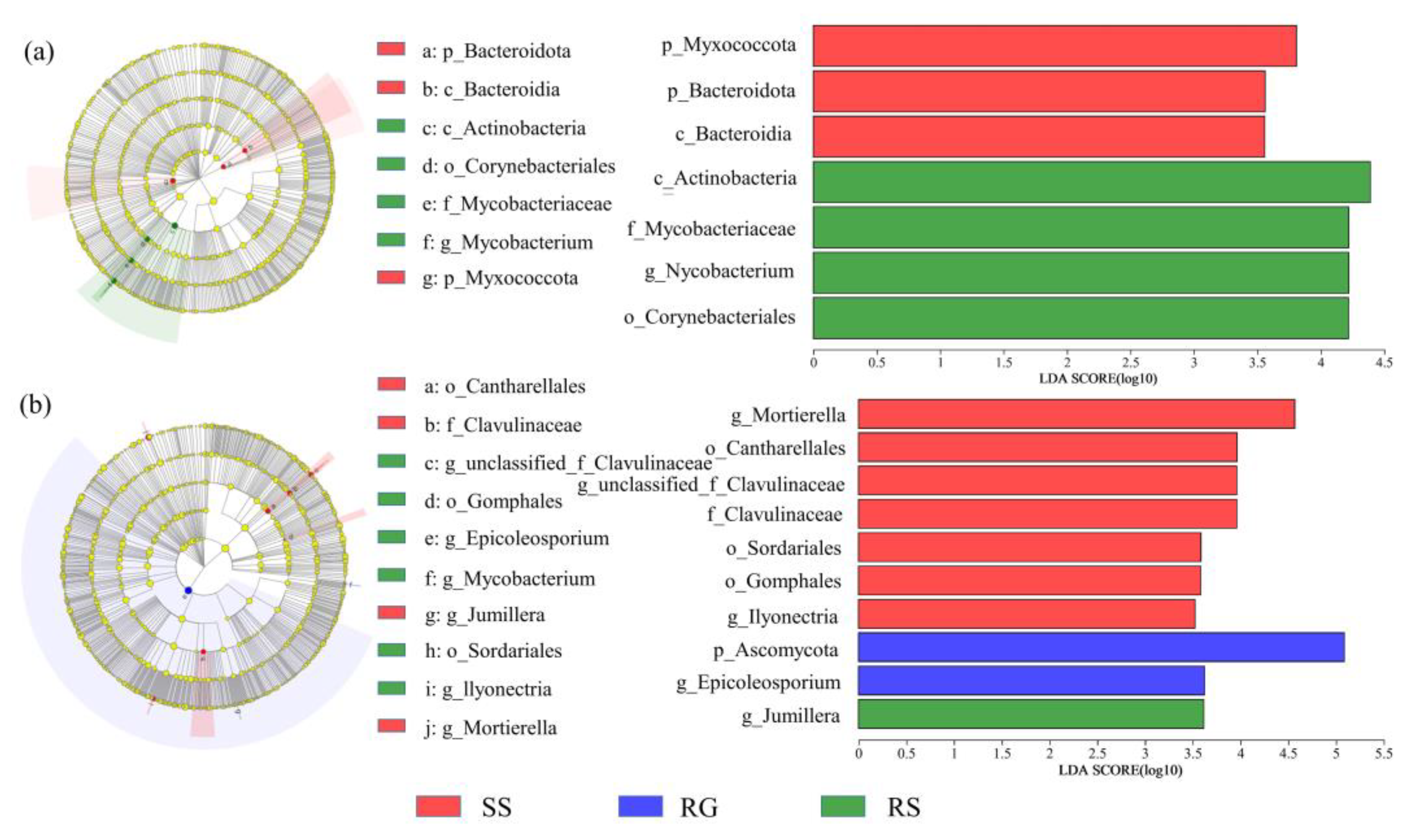
3.5. Differences in Soil Microbial Communities in Different Karst Microhabitat
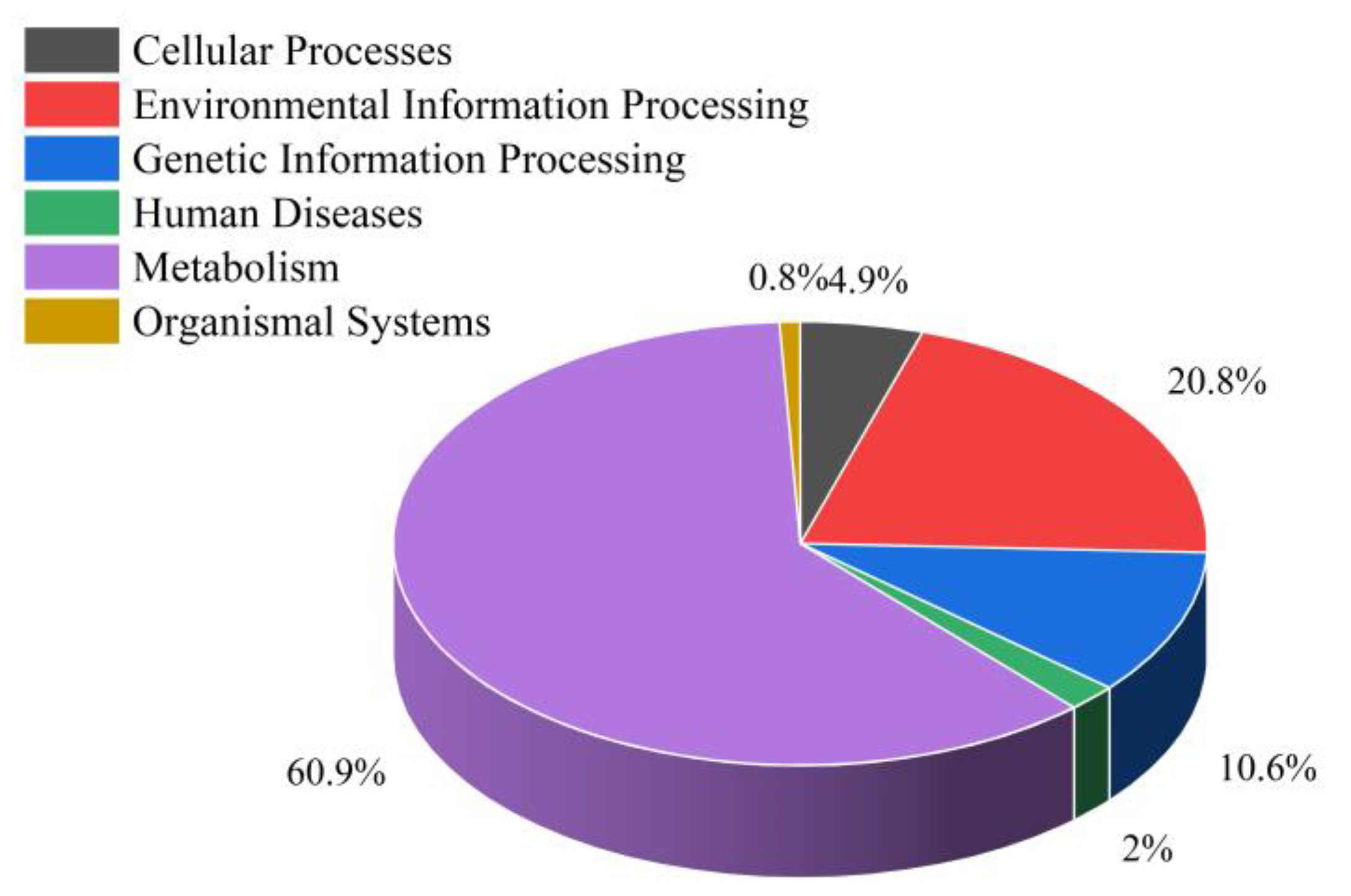
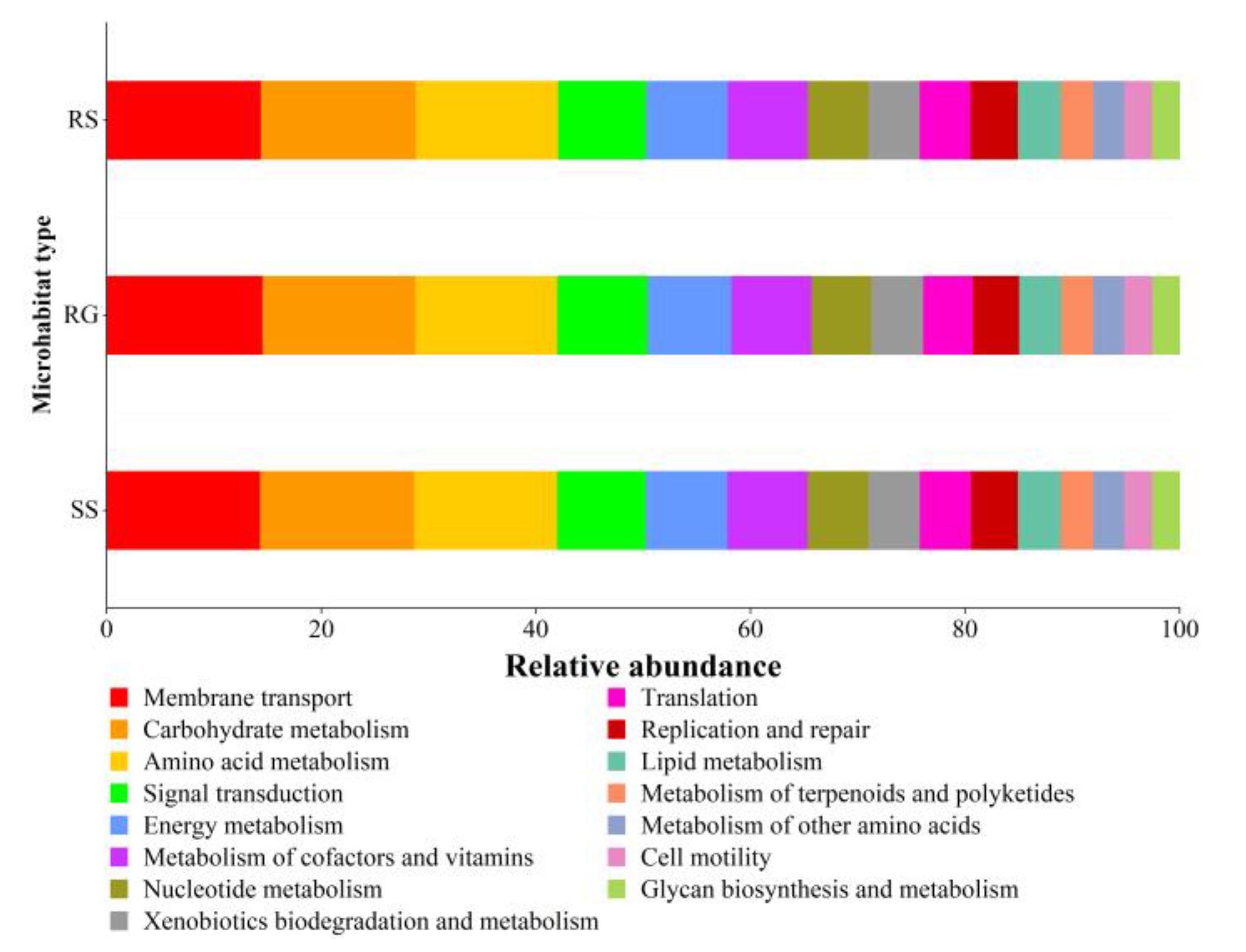
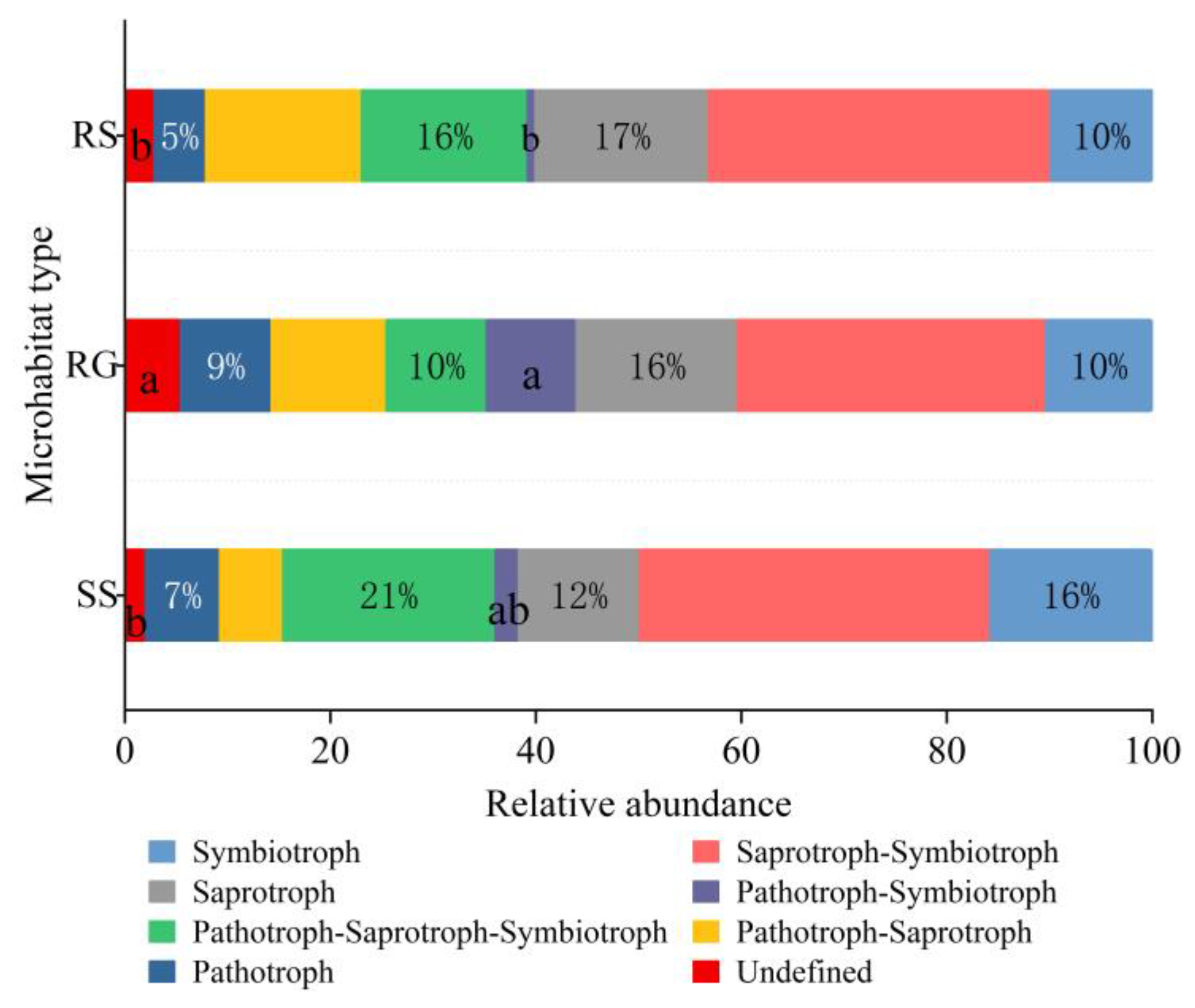
3.6. Notes on the Function of Soil Microorganisms
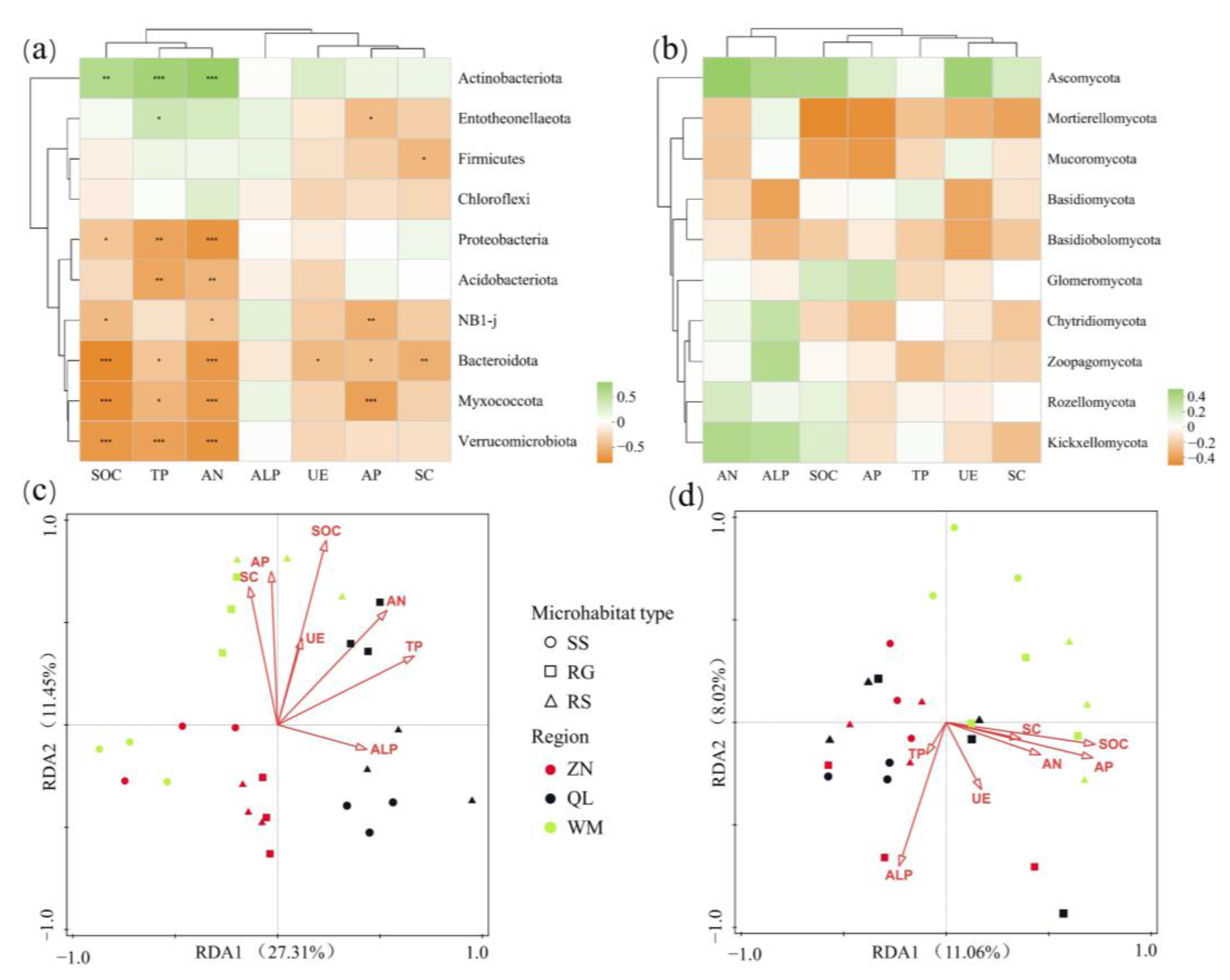
3.7. Relationship between Microbial Community Structure and Soil Factors
4. Discussion
4.1. Effects of Habitat Heterogeneity on Rhizosphere soil Microbial Diversity
4.2. Effects of Habitat Heterogeneity on Rhizosphere Soil Microbial Community Structure
4.3. Effects of Habitat Heterogeneity on the Functional Structure of Rhizosphere Soil Microorganisms
4.4. Limitation and Prospect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, R.F.; Zhao, X.Q. Role of soil microbes in the acquisition of nutrients by plants. Acta Ecol. Sin. 2015, 35, 6584–6591. [Google Scholar]
- Wu, C.H.; Liu, J.Z. Research progress on influencing factors of rhizosphere microorganisms and their interaction with plants. J. Hebei Norm. Univ. Nat. Sci. Ed. 2022, 46, 603–613. [Google Scholar]
- Zhao, H.; Zhou, Y.C. Characteristics of structure and abundance of the nitrogen-fixing bacterial community in Pinus massoniana soil developed from different parent rocks. Acta Ecol. Sin. 2020, 40, 6189–6201. [Google Scholar]
- Zhu, L.X.; Zhang, J.E.; Liu, W.G. Review of studies on interactions between root exudates and rhizopheric microorganisms. Ecol. Environ. 2003, 12, 102–105. [Google Scholar]
- Mahmoudi, T.R.; Yu, J.M.; Liu, S.; Pierson, L.R.; Pierson, E.A. Drought-stress tolerance in wheat seedlings conferred by phenazine-producing rhizobacteria. Front. Microbiol. 2019, 10, 1590. [Google Scholar] [CrossRef] [Green Version]
- Ruan, L.; Ma, Z.H.; Liu, Z.Y.; Qin, F.; Wang, H.G. Isolation, identification and toxicity determination of pathogens causing alfalfa root rot. J. Chin. Agric. Univ. 2016, 21, 56–67. [Google Scholar]
- Zhang, L.; Xu, H.M.; Zhu, B.L. Association of rhizosphere soil microbiome with the occurrence and development of replant disease-A review. Acta Microbiol. Sin. 2016, 56, 1234–1241. [Google Scholar]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Qiu, Q.; Li, J.Y.; Wang, J.H.; Wang, N.; Sun, K.; He, Q.; Su, Y.; Pan, X. Microbes, enzyme activities and nutrient characteristics of rhizosphere and non-rhizosphere soils under four shrubs in Xining Nanshan, Prefecture, China. Acta Ecol. Sin. 2014, 34, 7411–7420. [Google Scholar]
- Allison, S.D.; Lu, Y.; Weihe, C.; Goulden, M.L.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef] [Green Version]
- Song, T.Q. Plants and the Environment in Karst Areas of Southwest China; Sci Press: Beijing, China, 2015; pp. 1–5. [Google Scholar]
- Xi, X.Q.; Zhao, Y.J.; Liu, Y.G.; Wang, X.; Gao, X.M. Variation and correlation of plant functional traits in karst area of central Guizhou Province, China. Chin. J. Plant Ecol. 2011, 35, 1000–1008. [Google Scholar] [CrossRef]
- Yu, L.F.; Zhu, S.Q.; Ye, J.Z.; Wei, L.M.; Chen, Z.R. Dynamics of adegraded karst forest in the process of natural restoration. Sci. Silvea Sin. 2002, 38, 1–7. [Google Scholar]
- Zhang, J.Y.; Dai, M.H.; Wang, L.C.; Su, W.C.; Cao, L.G. Plant selection and their ecological adaptation for rocky desertification control in karst region in the southwest of China. Earth Environ. 2015, 43, 269–278. [Google Scholar]
- Liu, F.; Wang, S.J.; Luo, H.B.; Liu, Y.S.; Liu, H.Y. Microhabitats in karst forest ecosystem and variability of soils. Acta Pedol. Sin. 2008, 45, 1055–1062. [Google Scholar]
- Zhu, S.Q. Ecological Research on Karst Forest(Ⅰ); Guizhou Science and Techlonogy Press: Guiyang, China, 1993; p. 168. [Google Scholar]
- Yan, L.B.; Zhang, J.L.; Zhu, S.Y.; Gao, F.; Yang, Y.Z.; Huang, Z.S.; Yu, L.F. Base on model experiment to study the effects of vertical space on the temperature of soil microhabitats in the process of karst rock desertification. Appl. Ecol. Environ. Res. 2019, 17, 15605–15614. [Google Scholar] [CrossRef]
- Li, A.D.; Jia, S.; Yu, L.F. Microclimates of different microhabitats in Huajiang karst area. J. Zhejiang For. Coll. 2010, 27, 374–378. [Google Scholar]
- Liao, H.K.; Li, J.; Long, J.; Zhang, W.J.; Liu, L.F. Soil characteristics of different microhabitats of chinese prickly ash in karst mountain areas of Guizhou province. J. Agro-Env. Sci. 2013, 32, 2429–2435. [Google Scholar]
- Yu, G.S.; Wang, S.J.; Rong, L. Microclimate characteristics of different microhabitats in successional stages of Maolan karst forest. Ear Environ. 2011, 39, 469–477. [Google Scholar]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [Green Version]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Lu, Z.C.; Wen, Y.G.; Zhou, X.G.; Wang, L.; Xun, D.J.; Zhu, H.G.; Li, J.W. Correlation analysis of plant and soil microbial diversity during forest natural restoration in karst region, southwest China. Guangxi Sci. 2022, 29, 108–119. [Google Scholar]
- Guo, W.; Gao, L.W.; Peng, Z.W.; Wei, M.Q.; Wang, Y.Z.; Hu, Y.L.; Liu, X. Characteristics of microbial community in rhizosphere and non-rhizosphere soil of Cunninghamia lanceolata plantation with different stand ages. Res. Soil Water Conserv. 2022, 29, 260–267. [Google Scholar]
- Ding, S.; Wei, S.Z.; Chen, Z.L.; Shao, J.; Duan, F.R.; Yan, Y.; Duan, X.W. Variation characteristics of microorganism at different soil depths of typical forests in southwest China. Chin. J. Appl. Ecol. 2022, 33, 1–11. [Google Scholar]
- Chen, X.L.; Shen, Y.; Huang, Y.; Luo, C.L.; Yan, W.D.; Liu, H.N.; He, G.X.; He, H.J. Responses of soil enzyme activities and microbial communities to dry and wet seasons in Cinnamomum camphora and Pinus massoniana plantations. J. Cent. South Uni. For. Technol. 2022, 42, 114–126. [Google Scholar]
- Wu, Q.S.; Long, J.; Li, J.; Liao, H.K.; Liu, L.F.; Wu, J.N.; Xiao, X. Effects of different microhabitat types on soil microbial community composition in the Maolan karst forest in Southwest China. Acta Ecol. Sin. 2019, 39, 1009–1018. [Google Scholar]
- Lucas-Borja, M.E.; Candel, D.; Jindo, K.; Moreno, J.L.; Andrés, M.; Bastida, F. Soil microbial community structure and activity in monospecific and mixed forest stands, under mediterranean humid conditions. Plant Soil 2012, 354, 359–370. [Google Scholar] [CrossRef]
- Patel, J.S.; Singh, A.; Singh, H.B.; Sarma, B.K. Plant genotype, microbial recruitment and nutritional security. Front. Plant Sci. 2015, 6, 608. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Yang, C.; Yang, B.; Chen, P.; Ma, Y. A new species of Rhododendron (Ericaceae) from Guizhou, China. Phytokeys 2020, 146, 53–59. [Google Scholar] [CrossRef]
- An, M.T. Studies on Maintenance Mechanism of Plant Species Diversityand Soil Moisture and Nutrient Pattern in Karst Forest Summary. Ph.D. Thesis, Guizhou University, Guiyang, China, 2019. [Google Scholar]
- Riley, D.; Barber, S.A. Bicarbonate accumulation and pH changes at the soybean (Glycine max (L.) Merr.) root-soil interface. Soil Sci. Soc. Am. J. 1969, 33, 905–908. [Google Scholar] [CrossRef]
- Duran-Pinedo, A.E.; Solbiati, J.; Frias-Lopez, J. The effect of the stress hormone cortisol on the metatranscriptome of the oral microbiome. Npj Biofilms Microbiomes 2018, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.L.; Xu, M.; Zhou, X.; Chen, J.; Zhang, J.; Zhang, J. Effects of different vegetation types on the characteristics of soil bacterial communities in the Hilly area of central Guizhou. J. Ecol. Rural Environ. 2021, 37, 518–525. [Google Scholar]
- Peng, J.G.; Gong, J.Y.; Fan, Y.H.; Zhang, H.; Zhang, Y.F.; Bai, Y.Q.; Wang, Y.M.; Xie, L.J. Diversity of soil microbial communities in rhizosphere and non-rhizosphere of Rhododendron moulmainense. Sci. Silvea Sin. 2022, 58, 89–99. [Google Scholar]
- Peng, L.M.; Jiang, X.F. Analysis of fungal community structure and diversity in the roots of Rhododendron rubiginosum. Southwest Chin. J. Agric. Sci. 2022, 35, 957–963. [Google Scholar]
- Zhou, J.; Huang, Y.; Mo, M. Phylogenetic analysis on the soil bacteria distributed in karst forest. Braz. J. Microbiol. 2009, 40, 827–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardle, D.A. Controls of temporal variability of the soil microbial biomass: A global-scale synthesis. Soil Biol. Biochem. 1998, 30, 1627–1637. [Google Scholar] [CrossRef]
- Zuo, P.; Ou, Z.J.; Jiang, Q.W.; Liu, M. Function diversity of soil microbial communities in originalcoastal wetlands, Yancheng, Jiangsu province. J. Nanjing Uni. Nat. Sci. 2014, 50, 715–722. [Google Scholar]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Liu, R.Y.; Chen, X. Study on microstructure and infections of Rhododendron annae mycorrhiza. J. Cent South Uni. For. Technol. 2012, 32, 28–33. [Google Scholar]
- Zhou, J.; Lei, T. Review and prospects on methodology and affecting factors of soil mi-crobial diversity. Biodivers. Sci. 2007, 15, 306. [Google Scholar]
- Wang, D.L. Study on Karst Rocky Desertification Forming Process and Its Control Technology. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2003. [Google Scholar]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran, C.F.; Taylor, M.; Lear, G. Bacteria as emerging indicators of soil condition. Appl. Environ. Microbiol. 2016, 83, e02826-16. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Wang, C.; Zhou, Z. Impacts of forest thinning on soil microbial community structure and extracellular enzyme activities: A global meta-analysis. Soil Bio. Biochem. 2020, 149, 107915. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, S.; Liu, S.; Guo, H.; Wang, T.; Zhou, J.; Peng, X. Changes in plant rhizosphere microbial communities under different vegetation restoration patterns in karst and non-karst ecosystems. Sci. Rep. 2019, 9, 8761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Ou, J.; Li, L.P.; Yang, S.T.; He, Y.J.; Li, Z.C. Community composition and ecological function analysis of endophytic fungi in the roots of Rhododendron simsii in Pinus massoniana forest on central Guizhou. Acta Ecol. Sin. 2020, 40, 1228–1239. [Google Scholar]
- Li, A.D.; Lu, Y.F.; Wei, X.L.; Yu, L.F. Studies on the regime of soil moisture under different microhabitats in Huajiang karst valley. Carsolog Sin. 2008, 27, 56–62. [Google Scholar]
- Harris, R.F. Effect of water potential on microbial growth and activity. Water Potential Relat. Soil Microbiol. 1981, 9, 23–95. [Google Scholar]
- Gong, X.; Liu, C.; Li, J.; Luo, Y.; Yang, Q.; Zhang, W.; Yang, P.; Feng, B. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Till. Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential growth responses of soil bacterial taxa to carbon substrates of varying chemical recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef] [Green Version]
- Nan, J.; Chao, L.; Ma, X.; Xu, D.; Mo, L.; Zhang, X.; Zhao, X.; Bao, Y. Microbial diversity in the rhizosphere soils of three Stipa species from the eastern Inner Mongolian grasslands. Glob. Ecol. Conserv. 2020, 22, e992. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Niu, J.; Dang, K.; Zhang, S.; Wang, S.; Wang, Z. Changes in physicochemical properties, enzymatic activities, and the microbial community of soil significantly influence the continuous cropping of Panax quinquefolius L.(American ginseng). Plant Soil. 2021, 463, 427–446. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, S.; Li, S.Z.; Deng, Y. Advances in molecular ecology on microbial functional genes of carbon cycle. Microbiol. Chin. 2017, 44, 1676–1689. [Google Scholar]
- Chaer, G.; Fernandes, M.; Myrold, D.; Bottomley, P. Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microb. Ecol. 2009, 58, 414–424. [Google Scholar] [CrossRef]
- Wan, W.; Tan, J.; Wang, Y.; Qin, Y.; He, H.; Wu, H.; Zuo, W.; He, D. Responses of the rhizosphere bacterial community in acidic crop soil to pH: Changes in diversity, composition, interaction, and function. Sci. Total Environ. 2020, 700. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Ji, L.; Wang, Z.; Li, K.; Xu, X.; Guo, D. Functional diversity and CO2 emission characteristics of soil bacteria during the succession of halophyte vegetation in the Yellow River Delta. Int. J. Environ. Res. Public Health 2022, 19, 12919. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Xia, W.Y.; Zhao, H.; Zeng, M. Effects of deep vertical rotary tillage on soil enzyme activity, microbialcommunity structure and functional diversity of cultivated land. Acta Ecol. Sin. 2022, 42, 5009–5021. [Google Scholar]
- Balami, S.; Vašutová, M.; Košnar, J.; Karki, R.; Khadka, C.; Tripathi, G.; Cudlin, P. Soil fungal communities in abandoned agricultural land has not yet moved towards the seminatural forest. For. Ecol. Manag. 2021, 491, 119181. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhou, G.N.; Zhu, X.Y.; Gao, B.J.; Xu, H.D. Effects of litter on soil organic carbon and microbial functional diversity. Acta Ecol. Sin. 2021, 41, 2709–2718. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Viles, H.A.; Gorbushina, A.A. Soiling and microbial colonisation on urban roadside limestone: A three year study in Oxford, England. Build. Environ. 2003, 38, 1217–1224. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 2014, 17, 155–164. [Google Scholar] [CrossRef]
- Yu, G.S.; Wang, S.J.; Rong, L.; Ran, J.C. Litter dynamics of major successional communities in Maolan karst forest of China. Chin. J. Plant Ecol. 2011, 35, 1019–1028. [Google Scholar]
- Zhang, Z.H.; Hu, G.; Zhu, J.D.; Ni, J. Spatial heterogeneity of soil nutrients and its impact on tree species distribution in a karst forest of Southwest China. Chin. J. Plant Ecol. 2011, 35, 1038–1049. [Google Scholar]


| Microhabitats | Grown Form | Soil Water Characteristics |
|---|---|---|
| Soil surface (SS) | Contiguous soil covering area is greater than 1 m2; or, although the contiguous soil covers an area less than 1 m2, the bare rate of bedrock is less than 50%, and the soil is the main body, and no rock groove or groove depth more than 30 cm has been formed. | The soil layer is thicker, the ventilation condition is better, and the water is lost slowly. |
| Rock gully (RG) | The bare rate of bedrock is greater than 50%, the covering area of contiguous soil is less than 1 m2, and the depth of rock groove or groove is more than 30 cm. Or, although the depth of the rock groove or groove is not more than 30 cm, the thickness of the soil layer is more than 30 cm; or, although the bare rate of bedrock is less than 50%, it is mainly soil, and the rock groove or groove depth is more than 30 cm. | The soil layer is thick, and water and fertilizer preservation is better. |
| Rock surface (RS) | The bare rate of bedrock is greater than 50%, the contiguous soil covers an area less than 1 m2, the depth of rock groove or groove formed is less than 30 cm, and the thickness of soil layer is less than 30 cm. | There are good ventilation conditions, there is fast water loss, the water and fertilizer retention ability is weak, and it is easy to form frequent temporary drought in a short time. |
| Region | Microhabitat Type | Ground Diameter (cm) | Plant Height (m) | Altitude (m) |
|---|---|---|---|---|
| ZN | SS | 1.74 ± 0.13 | 1.90 ± 0.62 | 1389.66 ± 7.77 |
| RG | 2.46 ± 0.62 | 2.08 ± 1.14 | 1394.90 ± 18.72 | |
| RS | 1.24 ± 0.27 | 1.70 ± 0.28 | 1393.04 ± 9.25 | |
| QL | SS | 2.00 ± 0.13 | 2.27 ± 0.21 | 1426.30 ± 11.27 |
| RG | 2.33 ± 0.31 | 1.73 ± 0.81 | 1431.43 ± 16.71 | |
| RS | 2.00 ± 0.61 | 2.00 ± 0.20 | 1437.99 ± 13.84 | |
| WM | SS | 2.39 ± 0.50 | 2.20 ± 0.52 | 1291.42 ± 2.66 |
| RG | 1.75 ± 0.12 | 2.07 ± 0.81 | 1248.51 ± 52.50 | |
| RS | 2.05 ± 0.60 | 1.70 ± 1.01 | 1277.21 ± 49.24 |
| Region | Microhabitat | SOC (g/kg) | TN (g/kg) | TP (g/kg) | SAN (mg/kg) | SAP (mg/kg) | SC (μmol/L·min) | UE (μmol/L·min) | ALP (μmol/L·min) |
|---|---|---|---|---|---|---|---|---|---|
| ZN | SS | 97.23 ± 41.91 Aa | 8.54 ± 3.45 ABa | 0.77 ± 0.20 Aa | 448.52 ± 142.86 Aa | 30.20 ± 15.38 Aa | 1104.06 ± 16.34 Ba | 935.82 ± 5.67 Ba | 68.43 ± 0.23 Cc |
| RG | 74.43 ± 41.07 Ba | 8.01 ± 1.65 Ba | 0.61 ± 0.23 Ba | 385.70 ± 222.52 Ba | 27.89 ± 9.44 Aa | 1091.11 ± 13.9 Bb | 834.21 ± 23.54 Aa | 75.14 ± 1.20 Ba | |
| RS | 94.77 ± 28.45 Ba | 8.12 ± 1.84 Ca | 0.61 ± 0.12 Ba | 580.41 ± 29.11 Aa | 24.08 ± 10.79 Ba | 998.51 ± 26.74 Ab | 808.93 ± 19.33 ABb | 72.24 ± 0.96 Ab | |
| QL | SS | 137.90 ± 11.33 Aa | 13.76 ± 2.74 Aa | 0.72 ± 0.10 Ab | 750.99 ± 64.70 Aa | 22.02 ± 1.43 Aa | 1204.85 ± 26.74 Aa | 989.06 ± 13.02 Aa | 70.26 ± 0.48 Bb |
| RG | 208.93 ± 29.87 Aa | 19.44 ± 3.30 Ab | 1.36 ± 0.14 Aa | 951.63 ± 324.54 Aa | 60.09 ± 44.76 Aa | 984.65 ± 25.33 Cb | 775.20 ± 3.00 Bb | 80.24 ± 0.69 Aa | |
| RS | 134.88 ± 33.88 Ba | 14.12 ± 1.74 Bab | 1.16 ± 0.26 Aa | 761.58 ± 67.23 Aa | 25.45 ± 6.65 Ba | 1031.98 ± 19.45 Bb | 770.55 ± 23.05 Ac | 64.94 ± 1.18 Bc | |
| WM | SS | 75.30 ± 13.29 Ac | 5.65 ± 0.92 Bc | 0.59 ± 0.19 Aa | 378.07 ± 24.79 Ab | 36.24 ± 4.05 Ab | 1161.75 ± 29.55 Aa | 1016.24 ± 16.86 Aa | 75.32 ± 0.45 Aa |
| RG | 237.02 ± 38.44 Ab | 17.25 ± 3.69 Ab | 0.71 ± 0.08 Ba | 858.03 ± 202.08 ABab | 57.66 ± 25.11 Ab | 1003.03 ± 6.81 Ab | 869.37 ± 9.33 Bb | 63.74 ± 0.62 Cb | |
| RS | 338.30 ± 58.49 Aa | 23.07 ± 1.68 Aa | 0.92 ± 0.07 ABa | 950.52 ± 535.62 Aa | 143.46 ± 71.89 Aa | 965.75 ± 9.51 ABc | 775.55 ± 22.65 Bb | 48.18 ± 0.41 Cc |
| Factor | SOC | TN | TP | AN | AP | UE | SC | ALP |
|---|---|---|---|---|---|---|---|---|
| Microhabitat | 29.028 *** | 25.559 *** | 15.955 *** | 5.187 * | 7.508 ** | 5.085 * | 14.367 *** | 452.735 *** |
| Region | 14.700 *** | 15.382 *** | 4.226 * | 2.637 | 2.968 | 130.997 *** | 160.722 *** | 276.331 *** |
| Microhabitat × Region | 15.560 *** | 13.439 *** | 5.584 ** | 1.761 | 4.485 * | 29.208 *** | 100.604 *** | 520.353 *** |
| Microbial | Microhabitat | Shannon Index | Simpson Index | Ace Index | Chao Index | Coverage Index |
|---|---|---|---|---|---|---|
| Bacterial | SS | 5.31 ± 0.07 a | 0.0153 ± 0.0023 a | 1367.67 ± 109.27 a | 1360.15 ± 113.82 a | 0.9860 ± 0.0026 a |
| RG | 5.25 ± 0.21 a | 0.0146 ± 0.0028 a | 1253.81 ± 91.88 a | 1253.47 ± 101.49 a | 0.9817 ± 0.0048 b | |
| RS | 5.23 ± 0.18 a | 0.0160 ± 0.0052 a | 1288.92 ± 172.51 a | 1220.39 ± 133.62 a | 0.9823 ± 0.0033 b | |
| Fungi | SS | 3.16 ± 0.94 a | 0.173 ± 0.152 a | 442.23 ± 95.57 a | 439.09 ± 98.13 a | 0.9984 ± 0.0007 a |
| RG | 3.29 ± 0.47 a | 0.114 ± 0.078 a | 453.73 ± 140.50 a | 448.98 ± 137.86 a | 0.9977 ± 0.0014 a | |
| RS | 3.14 ± 0.32 a | 0.117 ± 0.038 a | 462.63 ± 75.03 a | 457.03 ± 75.56 a | 0.9976 ± 0.0010 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Wang, H.; Dai, X.; Chen, M.; Luo, J.; Yang, R.; Ding, F. Effect of Karst Microhabitats on the Structure and Function of the Rhizosphere Soil Microbial Community of Rhododendron pudingense. Sustainability 2023, 15, 7104. https://doi.org/10.3390/su15097104
Yuan C, Wang H, Dai X, Chen M, Luo J, Yang R, Ding F. Effect of Karst Microhabitats on the Structure and Function of the Rhizosphere Soil Microbial Community of Rhododendron pudingense. Sustainability. 2023; 15(9):7104. https://doi.org/10.3390/su15097104
Chicago/Turabian StyleYuan, Congjun, Haodong Wang, Xiaoyong Dai, Meng Chen, Jun Luo, Rui Yang, and Fangjun Ding. 2023. "Effect of Karst Microhabitats on the Structure and Function of the Rhizosphere Soil Microbial Community of Rhododendron pudingense" Sustainability 15, no. 9: 7104. https://doi.org/10.3390/su15097104






