Carbon Fixation and Soil Aggregation Affected by Biochar Oxidized with Hydrogen Peroxide: Considering the Efficiency of Pyrolysis Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Treatments Preparation
2.3. Biochar Characterization
2.4. Soil Carbon Pool Analysis
2.5. Soil Aggregation
2.6. Soil pH, EC, BD
2.7. Data Analysis
3. Results
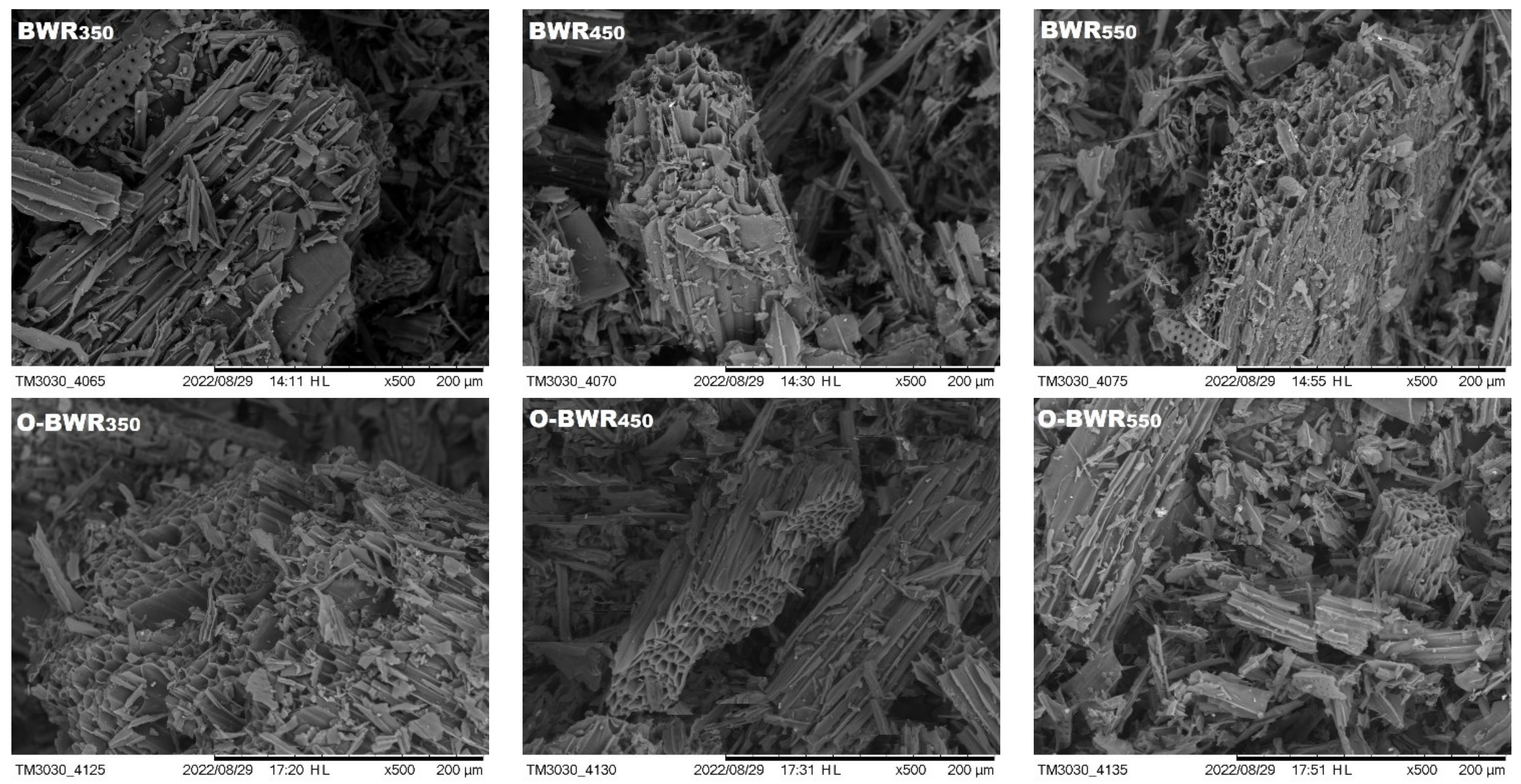
3.1. Biochar SEM Results
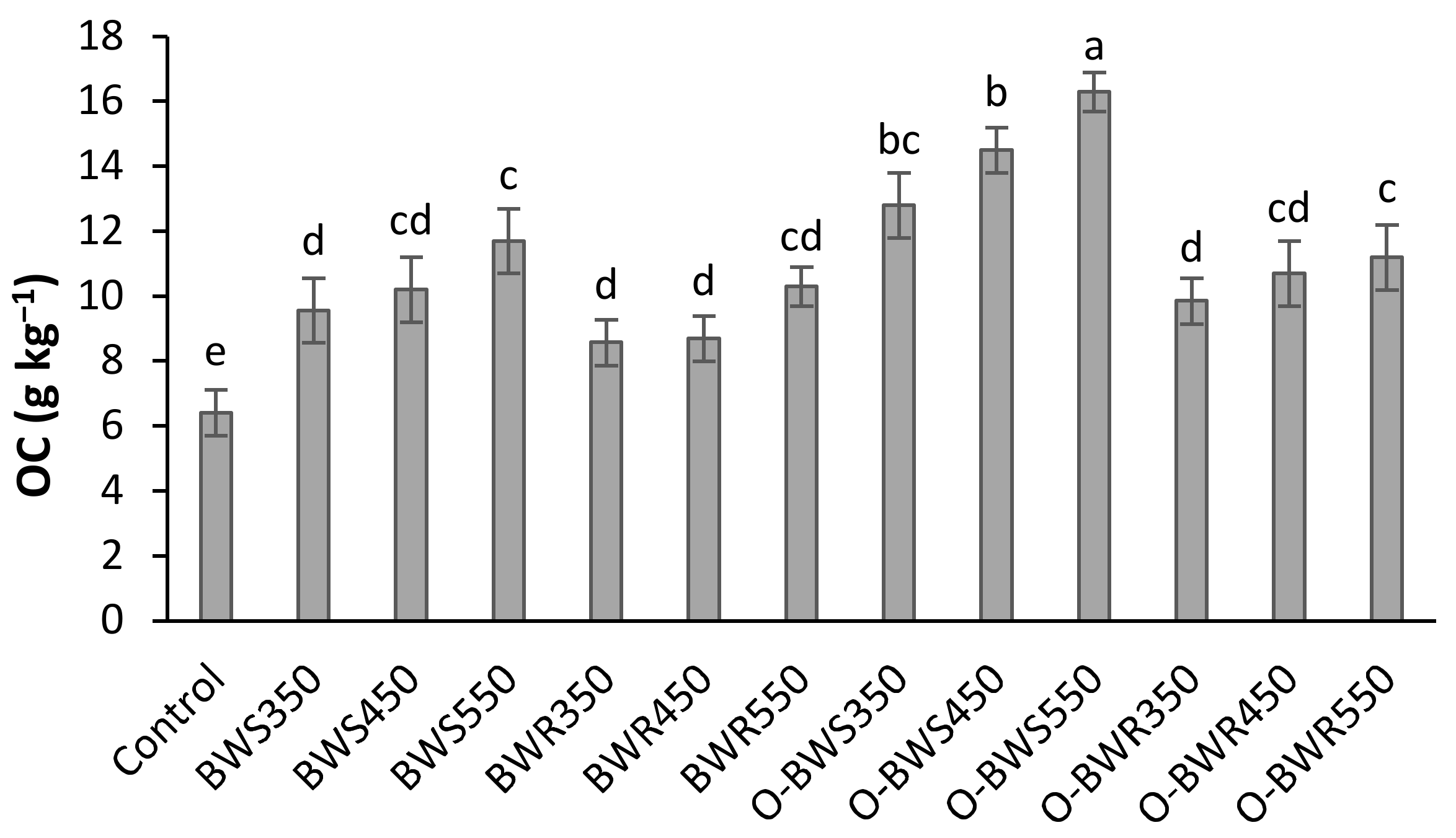
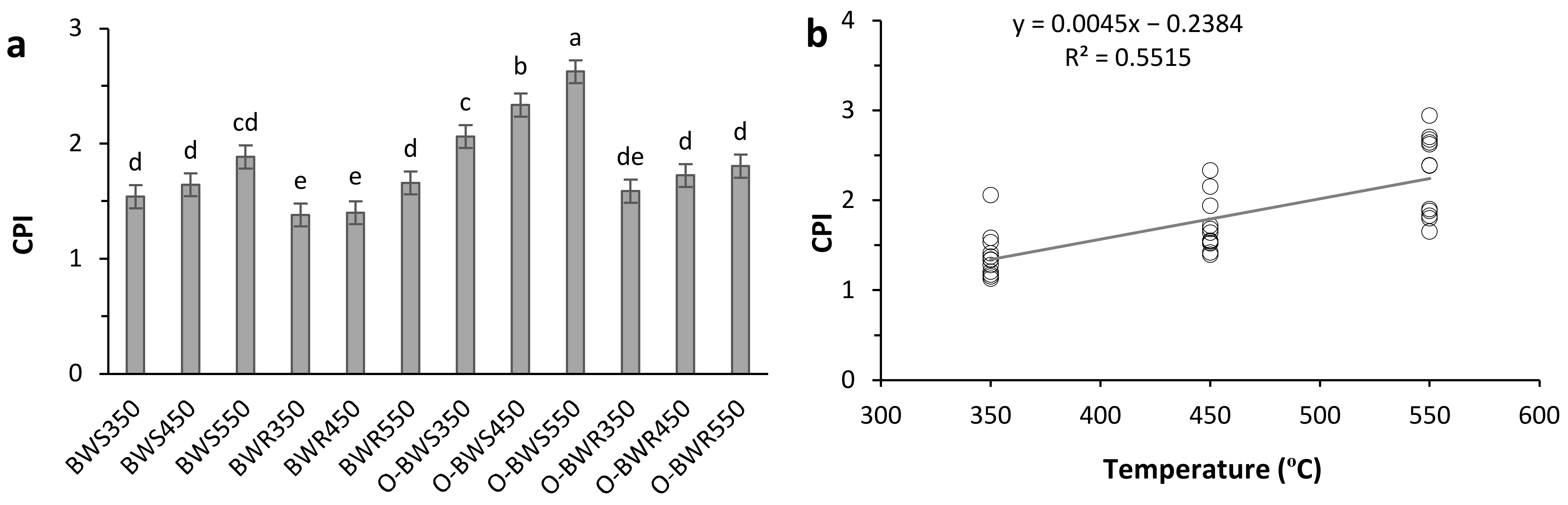
3.2. C Sequestration
3.3. Soil Aggregation
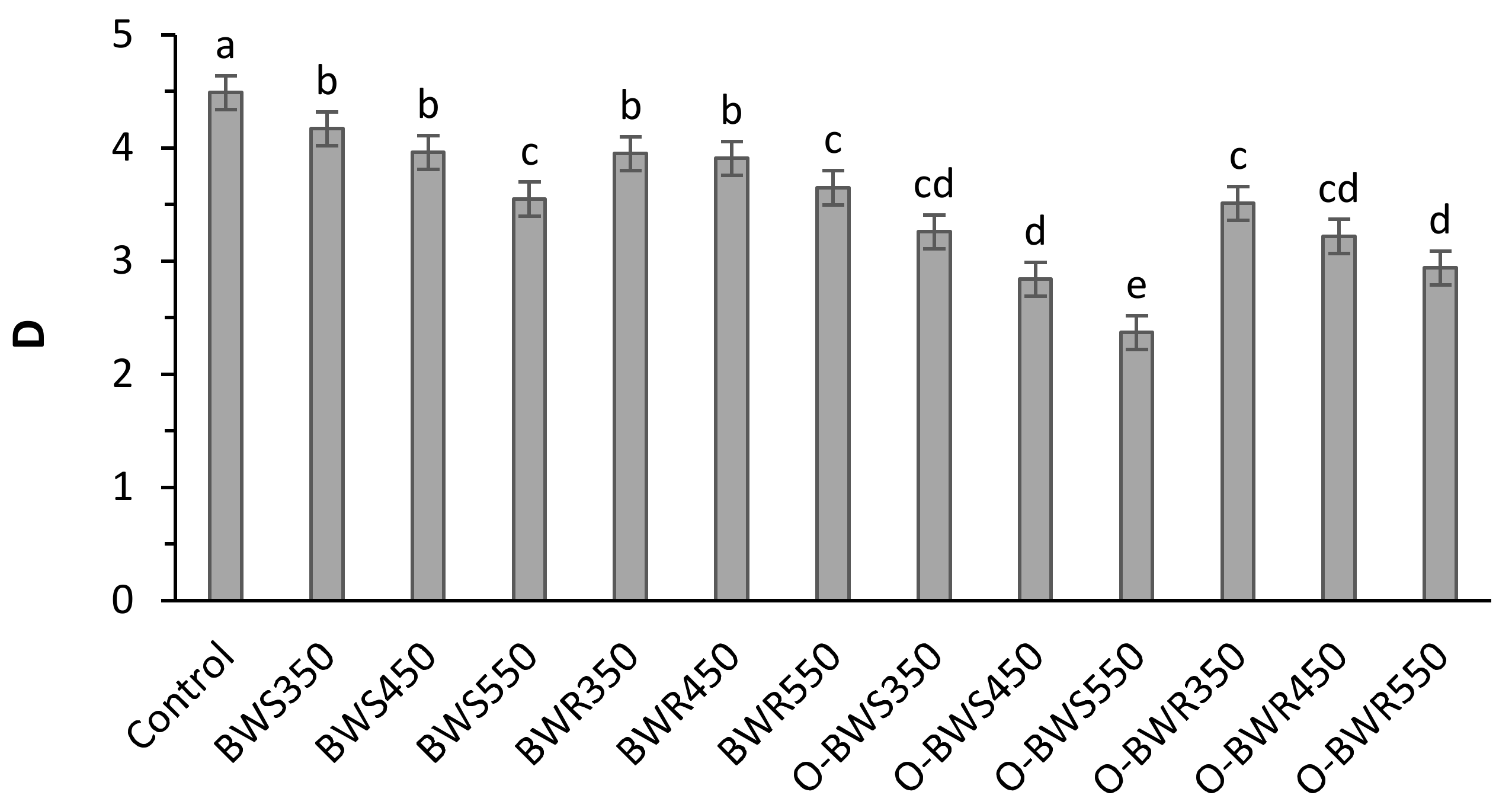
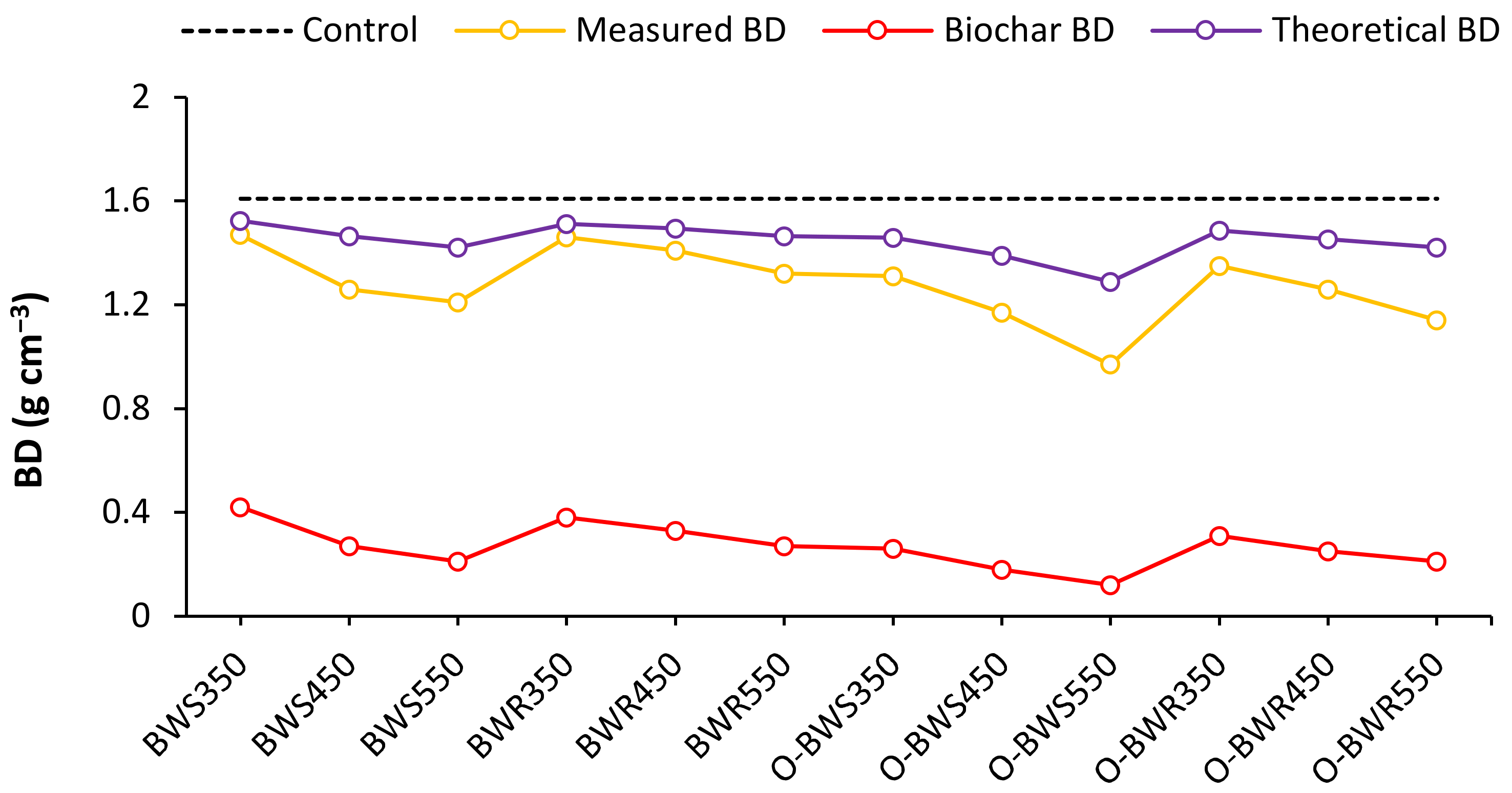
3.4. Soil Fractal Dimension (D) and Bulk Density (BD)
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Z.; Feng, W.; Luo, Y.; Baldock, J.; Wang, E. Soil Organic Carbon Dynamics Jointly Controlled by Climate, Carbon Inputs, Soil Properties and Soil Carbon Fractions. Glob. Chang. Biol. 2017, 23, 4430–4439. [Google Scholar] [CrossRef]
- Ge, X.; Cao, Y.; Zhou, B.; Wang, X.; Yang, Z.; Li, M.-H. Biochar Addition Increases Subsurface Soil Microbial Biomass but Has Limited Effects on Soil CO2 Emissions in Subtropical Moso Bamboo Plantations. Appl. Soil Ecol. 2019, 142, 155–165. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. Effect of Animal Waste Based Digestate Fertilization on Soil Microbial Activities, Greenhouse Gas Emissions and Spring Wheat Productivity in Loam and Sandy Loam Soil. Agronomy 2021, 11, 1281. [Google Scholar] [CrossRef]
- De Oliveira Silva, B.; Moitinho, M.R.; de Araujo Santos, G.A.; Teixeira, D.D.B.; Fernandes, C.; La Scala, N. Soil CO2 Emission and Short-Term Soil Pore Class Distribution after Tillage Operations. Soil Tillage Res. 2019, 186, 224–232. [Google Scholar] [CrossRef]
- Khan, N.; Jhariya, M.K.; Raj, A.; Banerjee, A.; Meena, R.S. Soil Carbon Stock and Sequestration: Implications for Climate Change Adaptation and Mitigation. In Ecological Intensification of Natural Resources for Sustainable Agriculture; Jhariya, M.K., Meena, R.S., Banerjee, A., Eds.; Springer: Singapore, 2021; pp. 461–489. ISBN 978-981-334-202-6. [Google Scholar]
- Ma, J.; Ullah, S.; Niu, A.; Liao, Z.; Qin, Q.; Xu, S.; Lin, C. Heavy Metal Pollution Increases CH4 and Decreases CO2 Emissions Due to Soil Microbial Changes in a Mangrove Wetland: Microcosm Experiment and Field Examination. Chemosphere 2021, 269, 128735. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Srinivasa Rao, C.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil Organic Carbon Dynamics: Impact of Land Use Changes and Management Practices: A Review. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 156, pp. 1–107. ISBN 978-0-12-817598-9. [Google Scholar]
- Lal, R. Carbon Sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Wei, W.; He, Z.; Kuzyakov, Y.; Bol, R.; Hu, R. Soil Organic Matter Priming and Carbon Balance after Straw Addition Is Regulated by Long-Term Fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
- Cheng, L.; Leavitt, S.W.; Kimball, B.A.; Pinter, P.J.; Ottman, M.J.; Matthias, A.; Wall, G.W.; Brooks, T.; Williams, D.G.; Thompson, T.L. Dynamics of Labile and Recalcitrant Soil Carbon Pools in a Sorghum Free-Air CO2 Enrichment (FACE) Agroecosystem. Soil Biol. Biochem. 2007, 39, 2250–2263. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F.; Besharati, H.; Khalaj, M.A. The Effects of Biochar on Soil Nutrients Status, Microbial Activity and Carbon Sequestration Potential in Two Calcareous Soils. Biochar 2021, 3, 105–116. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo, Y.T. The Impact of Pyrolysis Temperature on Biochar Properties and Its Effects on Soil Hydrological Properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Gupta, D.K.; Gupta, C.K.; Dubey, R.; Fagodiya, R.K.; Sharma, G.; Keerthika, A.; Noor Mohamed, M.B.; Dev, R.; Shukla, A.K. Role of Biochar in Carbon Sequestration and Greenhouse Gas Mitigation. In Biochar Applications in Agriculture and Environment Management; Singh, J.S., Singh, C., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 141–165. ISBN 978-3-030-40996-8. [Google Scholar]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of Biochar on Availability and Plant Uptake of Heavy Metals—A Meta-Analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative Effects of Biochar and Compost Applications on Water Holding Capacity and Crop Yield of Rice under Evaporation Stress: A Two-Years Field Study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, C.; Li, M.; Zheng, Y.; Ge, C.; Gu, J.; Li, H.; Duan, M.; Wang, X.; Chen, R. Research Progress and Prospects for Using Biochar to Mitigate Greenhouse Gas Emissions during Composting: A Review. Sci. Total Environ. 2021, 798, 149294. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and Its Importance on Nutrient Dynamics in Soil and Plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.; Wang, Z.; Xing, B. Biochar-Induced Negative Carbon Mineralization Priming Effects in a Coastal Wetland Soil: Roles of Soil Aggregation and Microbial Modulation. Sci. Total Environ. 2018, 610–611, 951–960. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar Stability in Soil: Meta-analysis of Decomposition and Priming Effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Z.; Huang, Y.; Tsang, D.C.W.; Ok, Y.S.; Zhao, L.; Qiu, H.; Xu, X.; Cao, X. Stabilization of Dissolvable Biochar by Soil Minerals: Release Reduction and Organo-Mineral Complexes Formation. J. Hazard. Mater. 2021, 412, 125213. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The Mechanisms of Biochar Interactions with Microorganisms in Soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Niu, Q.; Zeng, G.; Lai, C.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. New Notion of Biochar: A Review on the Mechanism of Biochar Applications in Advannced Oxidation Processes. Chem. Eng. J. 2021, 416, 129027. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Praveen Akre, A.; Sreedharan, V.; Suresh Kumar, M. Potential Role of Biochar in Advanced Oxidation Processes: A Sustainable Approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of Chemical Oxidation on Surface Oxygen-Containing Functional Groups and Adsorption Behavior of Biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar Modification to Enhance Sorption of Inorganics from Water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Kopecký, M.; Kolář, L. A Meta-analysis on the Impacts of Different Oxidation Methods on the Surface Area Properties of Biochar. Land Degrad. Dev. 2023, 34, 299–312. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Cui, X.; Fang, S.; Yao, Y.; Li, T.; Ni, Q.; Yang, X.; He, Z. Potential Mechanisms of Cadmium Removal from Aqueous Solution by Canna Indica Derived Biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Wang, Z.; Yin, K.; Chen, T.; Liu, C. Enhanced Removal of As(III) by Heterogeneous Catalytic Oxidation of As(III) on Fe-Biochar Fibers with H2O2 and Hydroxylamine. Chem. Eng. J. 2022, 428, 131200. [Google Scholar] [CrossRef]
- Bian, S.; Xu, S.; Yin, Z.; Liu, S.; Li, J.; Xu, S.; Zhang, Y. An Efficient Strategy for Enhancing the Adsorption Capabilities of Biochar via Sequential KMnO4-Promoted Oxidative Pyrolysis and H2O2 Oxidation. Sustainability 2021, 13, 2641. [Google Scholar] [CrossRef]
- Tomczyk, A.; Szewczuk-Karpisz, K. Effect of Biochar Modification by Vitamin C, Hydrogen Peroxide or Silver Nanoparticles on Its Physicochemistry and Tetracycline Removal. Materials 2022, 15, 5379. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459. [Google Scholar] [CrossRef]
- Kemper, W.D.; Chepil, W.S. Size Distribution of Aggregates. In Agronomy Monographs; Black, C.A., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 2015; pp. 499–510. ISBN 978-0-89118-203-0. [Google Scholar]
- Tyler, S.W.; Wheatcraft, S.W. Fractal Scaling of Soil Particle-Size Distributions: Analysis and Limitations. Soil Sci. Soc. Am. J. 1992, 56, 362–369. [Google Scholar] [CrossRef]
- Xu, L.; He, N.; Yu, G. Methods of Evaluating Soil Bulk Density: Impact on Estimating Large Scale Soil Organic Carbon Storage. CATENA 2016, 144, 94–101. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-Term Effects of Biochar on Soil Physical Properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Cai, L. Effects of Biochar on the Soil Carbon Cycle in Agroecosystems: An Promising Way to Increase the Carbon Pool in Dryland. IOP Conf. Ser. Earth Environ. Sci. 2021, 693, 012082. [Google Scholar] [CrossRef]
- El-Naggar, A.; Awad, Y.M.; Tang, X.-Y.; Liu, C.; Niazi, N.K.; Jien, S.-H.; Tsang, D.C.W.; Song, H.; Ok, Y.S.; Lee, S.S. Biochar Influences Soil Carbon Pools and Facilitates Interactions with Soil: A Field Investigation. Land Degrad. Dev. 2018, 29, 2162–2171. [Google Scholar] [CrossRef]
- Obia, A.; Mulder, J.; Martinsen, V.; Cornelissen, G.; Børresen, T. In Situ Effects of Biochar on Aggregation, Water Retention and Porosity in Light-Textured Tropical Soils. Soil Tillage Res. 2016, 155, 35–44. [Google Scholar] [CrossRef]
- Nan, H.; Yin, J.; Yang, F.; Luo, Y.; Zhao, L.; Cao, X. Pyrolysis Temperature-Dependent Carbon Retention and Stability of Biochar with Participation of Calcium: Implications to Carbon Sequestration. Environ. Pollut. 2021, 287, 117566. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar Applications Influence Soil Physical and Chemical Properties, Microbial Diversity, and Crop Productivity: A Meta-Analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A Review of Biochar in Anaerobic Digestion to Improve Biogas Production: Performances, Mechanisms and Economic Assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Liang, C.; Wei, X.; Yao, Y. Soil Erosion Significantly Decreases Aggregate-Associated OC and N in Agricultural Soils of Northeast China. Agric. Ecosyst. Environ. 2022, 323, 107677. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.; Ye, Y.; Zhao, J.; Wang, K. Soil Aggregate Mediates the Impacts of Land Uses on Organic Carbon, Total Nitrogen, and Microbial Activity in a Karst Ecosystem. Sci. Rep. 2017, 7, 41402. [Google Scholar] [CrossRef] [PubMed]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of Biochar on Carbon Fractions and Enzyme Activity of Red Soil. CATENA 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Grunwald, D.; Kaiser, M.; Ludwig, B. Effect of Biochar and Organic Fertilizers on C Mineralization and Macro-Aggregate Dynamics under Different Incubation Temperatures. Soil Tillage Res. 2016, 164, 11–17. [Google Scholar] [CrossRef]
- Fungo, B.; Lehmann, J.; Kalbitz, K.; Thionģo, M.; Okeyo, I.; Tenywa, M.; Neufeldt, H. Aggregate Size Distribution in a Biochar-Amended Tropical Ultisol under Conventional Hand-Hoe Tillage. Soil Tillage Res. 2017, 165, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar Additions Can Enhance Soil Structure and the Physical Stabilization of C in Aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Grunwald, D.; Kaiser, M.; Junker, S.; Marhan, S.; Piepho, H.-P.; Poll, C.; Bamminger, C.; Ludwig, B. Influence of Elevated Soil Temperature and Biochar Application on Organic Matter Associated with Aggregate-Size and Density Fractions in an Arable Soil. Agric. Ecosyst. Environ. 2017, 241, 79–87. [Google Scholar] [CrossRef]
- Xu, G.; Li, Z.; Li, P. Fractal Features of Soil Particle-Size Distribution and Total Soil Nitrogen Distribution in a Typical Watershed in the Source Area of the Middle Dan River, China. CATENA 2013, 101, 17–23. [Google Scholar] [CrossRef]
- Albanese, L.; Baronti, S.; Liguori, F.; Meneguzzo, F.; Barbaro, P.; Vaccari, F.P. Hydrodynamic Cavitation as an Energy Efficient Process to Increase Biochar Surface Area and Porosity: A Case Study. J. Clean. Prod. 2019, 210, 159–169. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for Soil Applications-Sustainability Aspects, Challenges and Future Prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of Biochar Effects on Soil Hydrological Properties Using Meta-Analysis of Literature Data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Lu, S.; Zong, Y. Pore Structure and Environmental Serves of Biochars Derived from Different Feedstocks and Pyrolysis Conditions. Environ. Sci. Pollut. Res. 2018, 25, 30401–30409. [Google Scholar] [CrossRef]



| Feedstocks/ Biochars | pH | CEC (cmol(+)kg−1) | C (%) | H (%) | N (%) | O (%) | SSA (m2 g−1) | TP (cm3 g−1) | BD (g cm−3) |
|---|---|---|---|---|---|---|---|---|---|
| Wheat straw | 7.01 ± 0.04 | 12.7 ± 1.34 | 77.1 ± 3.47 | 6.13 ± 0.36 | 2.53 ± 0.06 | 1.68 ± 0.21 | 35.16 ± 1.23 | 0.006 ± 0.00 | 0.76 ± 0.02 |
| Wood residue | 7.29 ± 0.03 | 9.08 ± 1.25 | 80.8 ± 3.11 | 5.24 ± 0.28 | 1.91 ± 0.06 | 2.13 ± 0.43 | 18.21 ± 1.01 | 0.003 ± 0.00 | 0.57 ± 0.01 |
| BWS350 | 7.04 ± 0.03 | 62.7 ± 5.12 | 55.4 ± 2.21 | 4.32 ± 0.24 | 2.31 ± 0.07 | 21.2 ± 1.03 | 103.9 ± 9.97 | 0.031 ± 0.00 | 0.42 ± 0.02 |
| BWS450 | 7.75 ± 0.02 | 51.8 ± 3.89 | 65.8 ± 3.52 | 3.04 ± 0.13 | 1.24 ± 0.05 | 15.1 ± 0.98 | 132.4 ± 12.1 | 0.037 ± 0.00 | 0.27 ± 0.01 |
| BWS550 | 9.02 ± 0.01 | 39.1 ± 3.72 | 66.9 ± 2.61 | 3.72 ± 0.32 | 0.81 ± 0.04 | 9.35 ± 0.53 | 166.5 ± 15.3 | 0.043 ± 0.00 | 0.21 ± 0.01 |
| BWR350 | 8.41 ± 0.04 | 45.7 ± 4.29 | 68.5 ± 3.82 | 4.23 ± 0.22 | 1.36 ± 0.06 | 18.3 ± 1.12 | 89.62 ± 3.13 | 0.012 ± 0.00 | 0.38 ± 0.02 |
| BWR450 | 8.89 ± 0.03 | 34.8 ± 4.11 | 72.8 ± 2.06 | 3.84 ± 0.41 | 0.84 ± 0.03 | 12.1 ± 0.95 | 96.81 ± 8.02 | 0.015 ± 0.00 | 0.33 ± 0.01 |
| BWR550 | 9.41 ± 0.05 | 25.3 ± 3.01 | 78.1 ± 2.14 | 3.27 ± 0.21 | 0.58 ± 0.03 | 6.87 ± 0.26 | 108.1 ± 9.84 | 0.022 ± 0.00 | 0.27 ± 0.01 |
| O-BWS350 | 4.22 ± 0.05 | 74.2 ± 3.52 | 52.2 ± 3.69 | 4.24 ± 0.36 | 1.38 ± 0.09 | 26.3 ± 2.31 | 136.1 ± 15.2 | 0.039 ± 0.00 | 0.26 ± 0.02 |
| O-BWS450 | 4.68 ± 0.06 | 68.4 ± 4.81 | 62.6 ± 5.21 | 3.15 ± 0.35 | 1.29 ± 0.08 | 17.2 ± 2.04 | 159.8 ± 10.1 | 0.045 ± 0.00 | 0.18 ± 0.01 |
| O-BWS550 | 4.89 ± 0.05 | 53.1 ± 2.98 | 68.9 ± 4.26 | 3.03 ± 0.24 | 1.12 ± 0.09 | 13.5 ± 1.68 | 191.5 ± 13.4 | 0.051 ± 0.00 | 0.12 ± 0.02 |
| O-BWR350 | 4.91 ± 0.03 | 51.7 ± 3.42 | 63.7 ± 5.12 | 4.17 ± 0.14 | 1.24 ± 0.09 | 22.1 ± 2.11 | 98.25 ± 6.24 | 0.019 ± 0.00 | 0.31 ± 0.01 |
| O-BWR450 | 5.34 ± 0.06 | 43.5 ± 5.28 | 66.8 ± 5.38 | 3.94 ± 0.23 | 0.95 ± 0.06 | 18.2 ± 1.28 | 113.7 ± 12.8 | 0.024 ± 0.00 | 0.25 ± 0.01 |
| O-BWR550 | 6.63 ± 0.04 | 34.6 ± 3.58 | 75.5 ± 5.14 | 3.18 ± 0.12 | 0.66 ± 0.04 | 10.3 ± 1.36 | 158.4 ± 14.7 | 0.036 ± 0.00 | 0.21 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbani, M.; Neugschwandtner, R.W.; Soja, G.; Konvalina, P.; Kopecký, M. Carbon Fixation and Soil Aggregation Affected by Biochar Oxidized with Hydrogen Peroxide: Considering the Efficiency of Pyrolysis Temperature. Sustainability 2023, 15, 7158. https://doi.org/10.3390/su15097158
Ghorbani M, Neugschwandtner RW, Soja G, Konvalina P, Kopecký M. Carbon Fixation and Soil Aggregation Affected by Biochar Oxidized with Hydrogen Peroxide: Considering the Efficiency of Pyrolysis Temperature. Sustainability. 2023; 15(9):7158. https://doi.org/10.3390/su15097158
Chicago/Turabian StyleGhorbani, Mohammad, Reinhard W. Neugschwandtner, Gerhard Soja, Petr Konvalina, and Marek Kopecký. 2023. "Carbon Fixation and Soil Aggregation Affected by Biochar Oxidized with Hydrogen Peroxide: Considering the Efficiency of Pyrolysis Temperature" Sustainability 15, no. 9: 7158. https://doi.org/10.3390/su15097158
APA StyleGhorbani, M., Neugschwandtner, R. W., Soja, G., Konvalina, P., & Kopecký, M. (2023). Carbon Fixation and Soil Aggregation Affected by Biochar Oxidized with Hydrogen Peroxide: Considering the Efficiency of Pyrolysis Temperature. Sustainability, 15(9), 7158. https://doi.org/10.3390/su15097158










