Evaluation of Biochar as an Additive in the Co-Composting of Green Waste and Food Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates, Additives, and Biochar
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Process Monitoring
2.5. End-Product Quality
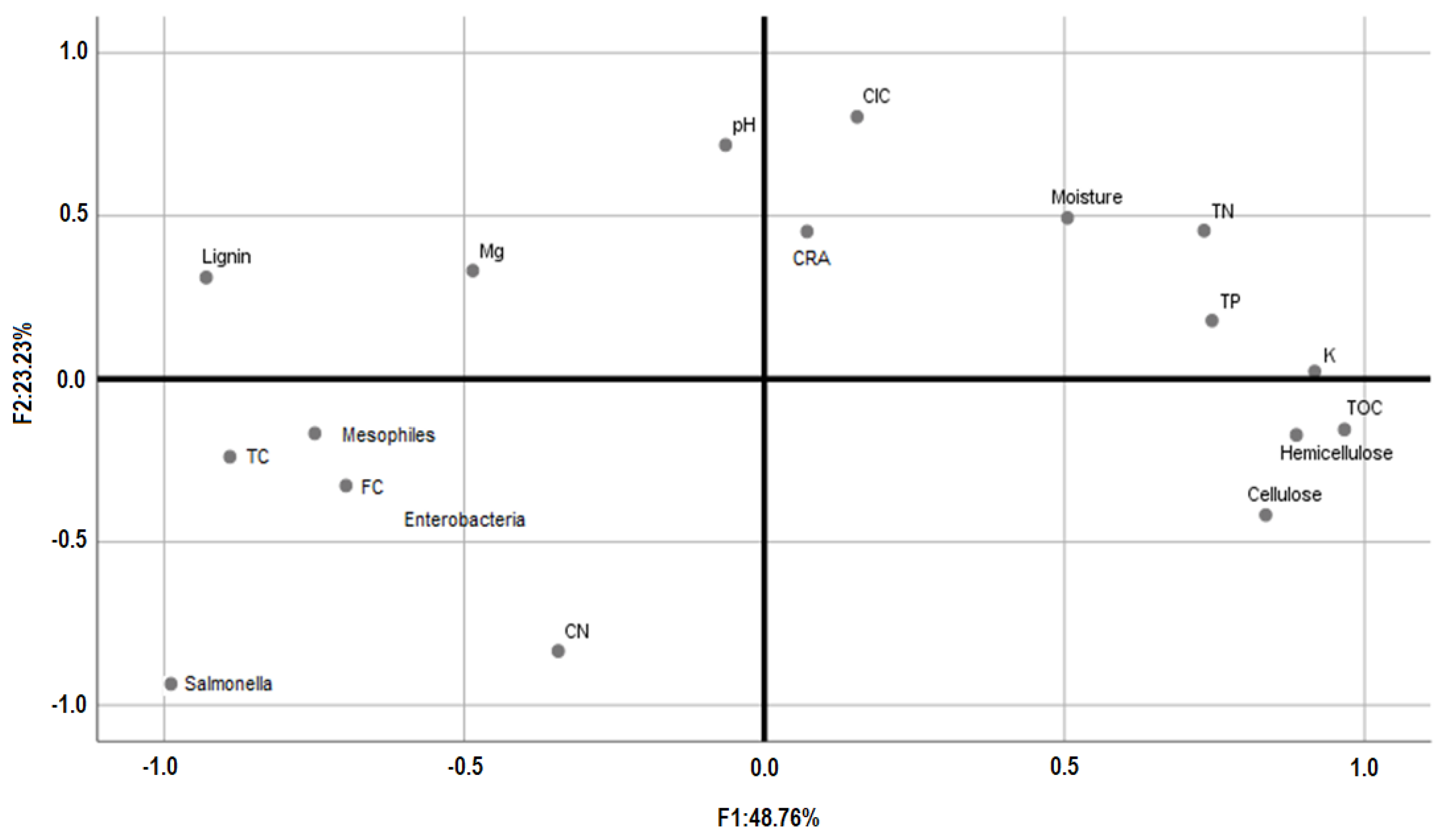
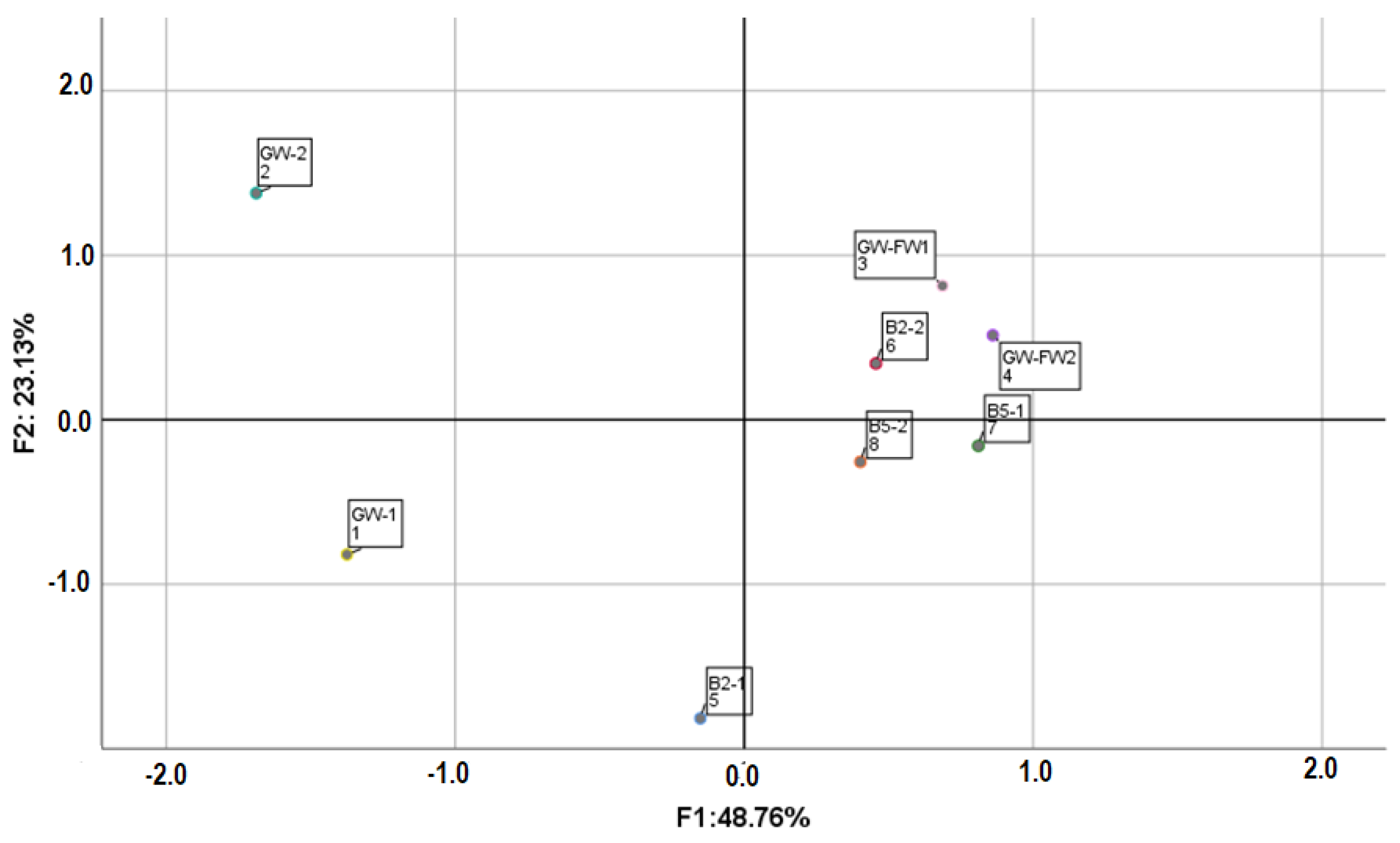
2.6. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Substrates and Additive
3.2. Co-Composting Process Monitoring
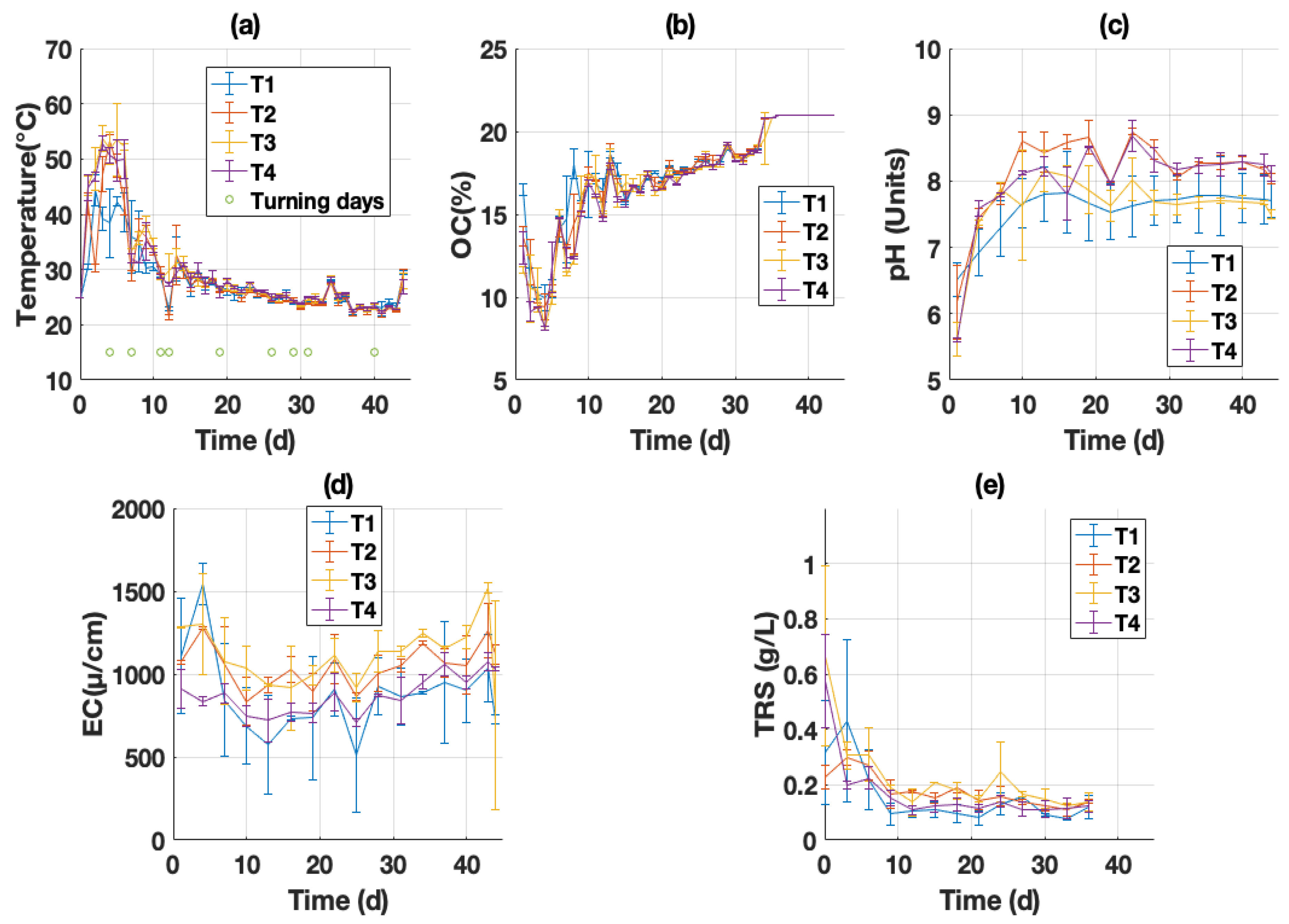
3.2.1. Monitoring of Operational Parameters
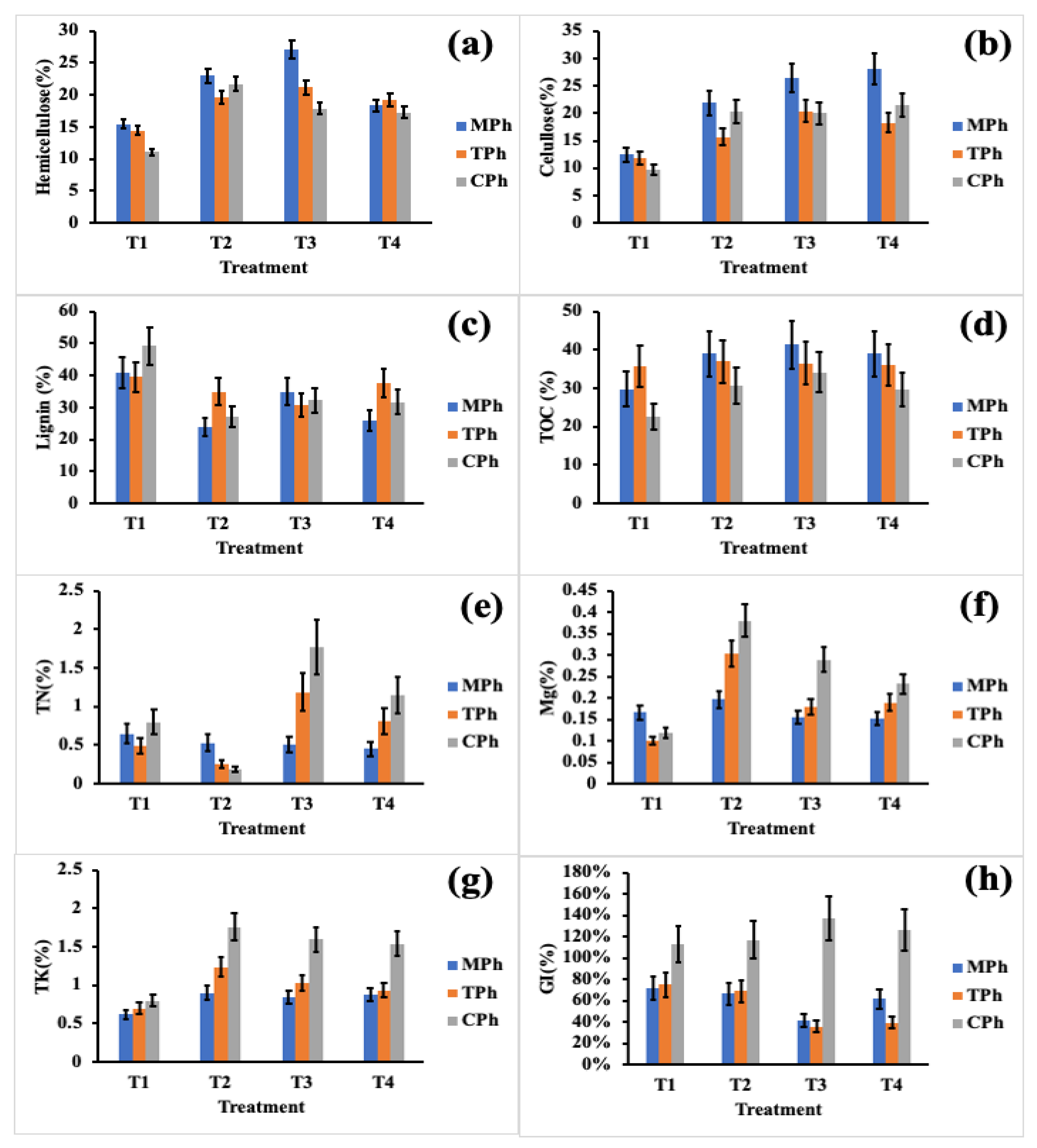
3.2.2. Monitoring of the Lignocellulosic Fraction, Total Organic Carbon, Total Nitrogen, and Micronutrients through the Process Phases
3.3. End-Product Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaviria-Cuevas, J.F.; Soto-Paz, J.; Manyoma-Velasquez, P.C.; Torres-Lozada, P. Tendencias de Investigación en la Cadena de Suministro de Residuos Sólidos Municipales. Inf. Tecnol. 2019, 30, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Mayes-Ramírez, M.M.; Gálvez-Sánchez, F.J.; Ramos-Ridao, Á.F.; Molina-Moreno, V. Urban Waste: Visualizing the Academic Literature through Bibliometric Analysis and Systematic Review. Sustainability 2023, 15, 1846. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Y.; Sheng, H. Green waste characteristics and sustainable recycling options. Resour. Environ. Sustain. 2023, 11, 100095. [Google Scholar] [CrossRef]
- Langsdorf, A.; Volkmar, M.; Holtmann, D.; Ulber, R. Material utilization of green waste: A review on potential valorization methods. Bioresour. Bioprocess. 2021, 8, 19. [Google Scholar] [CrossRef]
- Mandpe, A.; Yadav, N.; Paliya, S.; Tyagi, L.; Yadav, B.R.; Singh, L.; Kumar, S.; Kumar, R. Exploring the synergic effect of fly ash and garbage enzymes on biotransformation of organic wastes in in-vessel composting system. Bioresour. Technol. 2020, 322, 124557. [Google Scholar] [CrossRef]
- Soto-Paz, J.; Oviedo-Ocaña, E.R.; Angarita-Rangel, M.A.; Rodríguez-Flórez, L.V.; Castellanos-Suarez, L.J.; Nabarlatz, D.; Sanchez-Torres, V. Optimization of lignocellulolytic bacterial inoculum and substrate mix for lignocellulose degradation and product quality on co-composting of green waste with food waste. Bioresour. Technol. 2022, 359, 127452. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Effects of brown sugar and calcium superphosphate on the secondary fermentation of green waste. Bioresour. Technol. 2013, 131, 68–75. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Effects of earthworm casts and zeolite on the two-stage composting of green waste. Waste Manag. 2015, 39, 119–129. [Google Scholar] [CrossRef]
- Reyes-Torres, M.; Oviedo-Ocaña, E.; Dominguez, I.; Komilis, D.; Sánchez, A. A systematic review on the composting of green waste: Feedstock quality and optimization strategies. Waste Manag. 2018, 77, 486–499. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. The use of coal fly ash and vinegar residue as additives in the two-stage composting of green waste. Environ. Sci. Pollut. Res. 2019, 26, 28173–28187. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manag. 2016, 48, 115–126. [Google Scholar] [CrossRef]
- Hernández-Gómez, A.; Calderón, A.; Medina, C.; Sanchez-Torres, V.; Oviedo-Ocaña, E.R. Implementation of strategies to optimize the co-composting of green waste and food waste in developing countries. A case study: Colombia. Environ. Sci. Pollut. Res. 2021, 28, 24321–24327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X. Effects of bean dregs and crab shell powder additives on the composting of green waste. Bioresour. Technol. 2018, 260, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X. Addition of fish pond sediment and rock phosphate enhances the composting of green waste. Bioresour. Technol. 2017, 233, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Ocaña, E.R.; Hernández-Gómez, A.; Dominguez, I.; Parra-Orobio, B.A.; Soto-Paz, J.; Sánchez, A. Evaluation of Co-Composting as an Alternative for the Use of Agricultural Waste of Spring Onions, Chicken Manure and Bio-Waste Produced in Moorland Ecosystems. Sustainability 2022, 14, 8720. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Duan, Y.; Liu, T.; Awasthi, S.K.; Zhang, Z. Relevance of biochar to influence the bacterial succession during pig manure composting. Bioresour. Technol. 2020, 304, 122962. [Google Scholar] [CrossRef] [PubMed]
- Rombel, A.; Krasucka, P.; Oleszczuk, P. Sustainable biochar-based soil fertilizers and amendments as a new trend in biochar research. Sci. Total. Environ. 2022, 816, 151588. [Google Scholar] [CrossRef]
- Chen, H.; Awasthi, M.K.; Liu, T.; Zhao, J.; Ren, X.; Wang, M.; Duan, Y.; Awasthi, S.K.; Zhang, Z. Influence of clay as additive on greenhouse gases emission and maturity evaluation during chicken manure composting. Bioresour. Technol. 2018, 266, 82–88. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Liu, T.; Awasthi, M.K.; Zhang, Z. Evaluation of biochar amendment on heavy metal resistant bacteria abundance in biosolids compost. Bioresour. Technol. 2020, 306, 123114. [Google Scholar] [CrossRef]
- Cui, L.; Yan, J.; Yang, Y.; Li, L.; Quan, G.; Ding, C.; Chen, T.; Fu, Q.; Chang, A. Influence of Biochar on Microbial Activities of Heavy Metals Contaminated Paddy Fields. Bioresources 2013, 8, 5536–5548. [Google Scholar] [CrossRef] [Green Version]
- Haddad, S.A.; Mowrer, J.; Thapa, B. Biochar and compost from cotton residues inconsistently affect water use efficiency, nodulation, and growth of legumes under arid conditions. J. Environ. Manag. 2022, 307, 114558. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Sanderson, P.; Dang, B.T.; Bui, X.T.; Nguyen, N.S.H.; Vo, D.-V.N.; Tran, H.T. Evaluate the role of biochar during the organic waste composting process: A critical review. Chemosphere 2022, 299, 134488. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.K.; Lin, C.; Hoang, H.G.; Bui, X.T.; Ngo, H.H.; Le, V.G.; Tran, H.-T. Investigation of biochar amendments on odor reduction and their characteristics during food waste co-composting. Sci. Total Environ. 2023, 865, 161128. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Shim, J.; Chang, S.W.; Ravindran, B. Effect of Biochar Amendments on the Co-Composting of Food Waste and Livestock Manure. Agronomy 2023, 13, 35. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Yang, T.; Liu, Y.; Zheng, T.; Zheng, C. Effects of biochar carried microbial agent on compost quality, greenhouse gas emission and bacterial community during sheep manure composting. Biochar 2023, 5, 3. [Google Scholar] [CrossRef]
- Ravindran, B.; Karmegam, N.; Awasthi, M.K.; Chang, S.W.; Selvi, P.; Balachandar, R.; Chinnappan, S.; Azelee, N.I.W.; Munuswamy-Ramanujam, G. Valorization of food waste and poultry manure through co-composting amending saw dust, biochar and mineral salts for value-added compost production. Bioresour. Technol. 2022, 346, 126442. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z. Influence of bamboo biochar on mitigating greenhouse gas emissions and nitrogen loss during poultry manure composting. Bioresour. Technol. 2020, 303, 122952. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef]
- Wang, S.-P.; Wang, L.; Sun, Z.-Y.; Wang, S.-T.; Shen, C.-H.; Tang, Y.-Q.; Kida, K. Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour. Technol. 2021, 337, 125492. [Google Scholar] [CrossRef]
- Malinowski, M.; Wolny-Koładka, K.; Vaverková, M.D. Effect of biochar addition on the OFMSW composting process under real conditions. Waste Manag. 2019, 84, 364–372. [Google Scholar] [CrossRef]
- Jindo, K.; Sánchez-Monedero, M.A.; Hernández, T.; García, C.; Furukawa, T.; Matsumoto, K.; Sonoki, T.; Bastida, F. Biochar influences the microbial community structure during manure composting with agricultural wastes. Sci. Total Environ. 2012, 416, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Ocaña, E.R.; Soto-Paz, J.; Torres, V.S.; Castellanos-Suarez, L.J.; Komilis, D. Effect of the addition of the Bacillus sp., Paenibacillus sp. bacterial strains on the co-composting of green and food waste. J. Environ. Chem. Eng. 2022, 10, 107816. [Google Scholar] [CrossRef]
- Oviedo-Ocaña, E.R.; Dominguez, I.; Komilis, D.; Sánchez, A. Co-composting of Green Waste Mixed with Unprocessed and Processed Food Waste: Influence on the Composting Process and Product Quality. Waste Biomass-Valorization 2019, 10, 63–74. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association: Washington, DC, USA, 2005. [Google Scholar]
- ICONTEC. Norma Técnica Colombiana 5167. Productos para la Industria Agrícola. Productos Orgáncios Usados como Abonos o Fertilizantes y Enmiendas de Suelo; ICONTEC: Bogota, Colombia, 2011. [Google Scholar]
- Van Soest, P.J. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. AOAC Int. 1990, 73, 491–497. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- USEPA. Process Design Manual, Land Application of Sewage Sludge and Domestic Septage; EPA; United States Environmental Protection Agency: Washington, DC, USA, 1995; 302p.
- Boost, M.; Poon, C.S. The effect of a modified method of lime-stabilisation sewage treatment on enteric pathogens. Environ. Int. 1998, 24, 783–788. [Google Scholar] [CrossRef]
- Issarakraisila, M.; Ma, Q.; Turner, D.W. Photosynthetic and growth responses of juvenile Chinese kale (Brassica oleracea var. alboglabra) and Caisin (Brassica rapa subsp. parachinensis) to waterlogging and water deficit. Sci. Hortic. 2007, 111, 107–113. [Google Scholar] [CrossRef]
- Brinton, W.; Evans, E.; Droffner, M.L.; Brinton, R. Standardized test for evaluation of compost self-heating. Biocycle 1995, 36, 64–69. [Google Scholar]
- Saha, J.; Panwar, N.; Singh, M. An assessment of municipal solid waste compost quality produced in different cities of India in the perspective of developing quality control indices. Waste Manag. 2010, 30, 192–201. [Google Scholar] [CrossRef]
- Montgomery, D.C. Diseño y Análisi de Experimentos; John Wiley & Sons: México City, Mexico, 2004. [Google Scholar]
- Chai, L.; Han, Z.; Liang, Y.; Su, Y.; Huang, G. Understanding the blue water footprint of households in China from a perspective of consumption expenditure. J. Clean. Prod. 2020, 262, 121321. [Google Scholar] [CrossRef]
- Gil, A.; Toledo, M.; Siles, J.A.; Martín, M.A. Multivariate analysis and biodegradability test to evaluate different organic wastes for biological treatments: Anaerobic co-digestion and co-composting. Waste Manag. 2018, 78, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, B.; Boogaerts, C.; Vandaele, E. Combining woody biomass for combustion with green waste composting: Effect of removal of woody biomass on compost quality. Waste Manag. 2016, 58, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Foronda-Zapata, K.; Arias-Giraldo, C.; Soto-Paz, J.; Marmolejo-Rebellón, L.F.; Torres-Lozada, P. Effect of Grass Star Incorporation on the Composting Biowaste Process and on the Quality of the Product. Rev. EIA 2020, 17, 1–11. [Google Scholar]
- Molina-Montes, M.E.; Martín-Islán, Á.P. La fibra dietética procesada como alimento funcional. Offarm 2007, 26, 70–77. [Google Scholar]
- Oviedo-Ocaña, E.; Torres-Lozada, P.; Marmolejo-Rebellon, L.F.; Torres-López, W.A.; Dominguez, I.; Komilis, D.; Sánchez, A. A systematic approach to evaluate parameter consistency in the inlet stream of source separated biowaste composting facilities: A case study in Colombia. Waste Manag. 2017, 62, 24–32. [Google Scholar] [CrossRef]
- Parra-Orobio, B.A.; Donoso-Bravo, A.; Ruiz-Sánchez, J.C.; Valencia-Molina, K.J.; Torres-Lozada, P. Effect of inoculum on the anaerobic digestion of food waste accounting for the concentration of trace elements. Waste Manag. 2018, 71, 342–349. [Google Scholar] [CrossRef]
- Sailer, G.; Eichermüller, J.; Poetsch, J.; Paczkowski, S.; Pelz, S.; Oechsner, H.; Müller, J. Characterization of the separately collected organic fraction of municipal solid waste (OFMSW) from rural and urban districts for a one-year period in Germany. Waste Manag. 2021, 131, 471–482. [Google Scholar] [CrossRef]
- Castro-Herrera, D.; Prost, K.; Schäfer, Y.; Kim, D.G.; Yimer, F.; Tadesse, M.; Gebrehiwot, M.; Brüggemann, N. Nutrient dynamics during composting of human excreta, cattle manure, and organic waste affected by biochar. J. Environ. Qual. 2022, 51, 19–32. [Google Scholar] [CrossRef]
- Akter, B.; Shammi, M.; Akbor, M.A.; Yasmin, S.; Nahar, A.; Akhter, S.; Jolly, Y.N.; Uddin, K. Preparation and characterization of biochar: A case study on textile and food industry sludge management. Case Stud. Chem. Environ. Eng. 2023, 7, 100282. [Google Scholar] [CrossRef]
- Duborská, E.; Šebesta, M.; Matulová, M.; Zvěřina, O.; Urík, M. Current Strategies for Selenium and Iodine Biofortification in Crop Plants. Nutrients 2022, 14, 4717. [Google Scholar] [CrossRef]
- Colpas, F.; Tarón, A.; González, R. Surface area of activated and modified charcoals obtained from agricultural resources Saccharum officinarum. Rev. Cienc. Agric. 2017, 34, 62–72. [Google Scholar]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef] [PubMed]
- De Guardia, A.; Mallard, P.; Teglia, C.; Marin, A.; Le Pape, C.; Launay, M.; Benoist, J.; Petiot, C. Comparison of five organic wastes regarding their behaviour during composting: Part 1, biodegradability, stabilization kinetics and temperature rise. Waste Manag. 2010, 30, 402–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soobhany, N. Assessing the physicochemical properties and quality parameters during composting of different organic constituents of Municipal Solid Waste. J. Environ. Chem. Eng. 2018, 6, 1979–1988. [Google Scholar] [CrossRef]
- López-Cano, I.; Roig, A.; Cayuela, M.L.; Alburquerque, J.A.; Sánchez-Monedero, M.A. Biochar improves N cycling during composting of olive mill wastes and sheep manure. Waste Manag. 2016, 49, 553–559. [Google Scholar] [CrossRef]
- Soto-Paz, J.; Oviedo-Ocaña, E.R.; Manyoma, P.C.; Marmolejo-Rebellón, L.F.; Torres-Lozada, P.; Barrena, R.; Sánchez, A.; Komilis, D. Influence of mixing ratio and turning frequency on the co-composting of biowaste with sugarcane filter cake: A mixture experimental design. Waste Biomass-Valorization 2020, 11, 2475–2489. [Google Scholar] [CrossRef]
- Gong, X.; Li, S.; Sun, X.; Zhang, L.; Zhang, T.; Wei, L. Maturation of green waste compost as affected by inoculation with the white-rot fungi Trametes versicolor and Phanerochaete chrysosporium. Environ. Technol. 2016, 38, 872–879. [Google Scholar] [CrossRef]
- Wang, N.; Ren, L.; Zhang, J.; Awasthi, M.K.; Yan, B.; Zhang, L.; Wan, F.; Luo, L.; Huang, H.; Zhao, K. Activities of functional enzymes involved in C, N, and P conversion and their stoichiometry during agricultural waste composting with biochar and biogas residue amendments. Bioresour. Technol. 2022, 345, 126489. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, L.; Lin, H.; Zhou, S. Enhanced removal of antibiotic resistance genes during chicken manure composting after combined inoculation of Bacillus subtilis with biochar. J. Environ. Sci. 2024, 135, 274–284. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L. Combined addition of biochar, lactic acid, and pond sediment improves green waste composting. Sci. Total Environ. 2022, 852, 158326. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Wang, K.; Li, D. Biochar drives humus formation during composting by regulating the specialized metabolic features of microbiome. Chem. Eng. J. 2023, 458, 141380. [Google Scholar] [CrossRef]
- Yu, H.; Xie, B.; Khan, R.; Shen, G. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour. Technol. 2018, 271, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Mumivand, H.; Izadi, Z.; Amirizadeh, F.; Maggi, F.; Morshedloo, M.R. Biochar amendment improves growth and the essential oil quality and quantity of peppermint (Mentha × piperita L.) grown under waste water and reduces environmental contamination from waste water disposal. J. Hazard. Mater. 2023, 446, 130674. [Google Scholar] [CrossRef] [PubMed]
- Kalemelawa, F.; Nishihara, E.; Endo, T.; Ahmad, Z.; Yeasmin, R.; Tenywa, M.M.; Yamamoto, S. An evaluation of aerobic and anaerobic composting of banana peels treated with different inoculums for soil nutrient replenishment. Bioresour. Technol. 2012, 126, 375–382. [Google Scholar] [CrossRef]
- Yu, K.; Sun, X.; Li, S.; Cai, L.; Zhang, P.; Kang, Y.; Yu, Z.; Tong, J.; Wang, L. Application of quadratic regression orthogonal design to develop a composite inoculum for promoting lignocellulose degradation during green waste composting. Waste Manag. 2018, 79, 443–453. [Google Scholar] [CrossRef]
- Coelho, J.J.; Prieto, M.L.; Dowling, S.; Hennessy, A.; Casey, I.; Woodcock, T.; Kennedy, N. Physical-chemical traits, phytotoxicity and pathogen detection in liquid anaerobic digestates. Waste Manag. 2018, 78, 8–15. [Google Scholar] [CrossRef]
- Greenberg, I.; Kaiser, M.; Polifka, S.; Wiedner, K.; Glaser, B.; Ludwig, B. The effect of biochar with biogas digestate or mineral fertilizer on fertility, aggregation and organic carbon content of a sandy soil: Results of a temperate field experiment. J. Plant Nutr. Soil Sci. 2019, 182, 824–835. [Google Scholar] [CrossRef]
- Cantero-Flores, A.; Bailón-Morales, R.; Villanueva-Arce, R.; Calixto-Mosqueda, M.D.C.; Robles-Martínez, F. Composta elaborada con resi-duos verdes como mejorador de un suelo urbano. Ing. Agrícola Biosist. 2016, 8, 72–83. [Google Scholar]
- Zhang, L.; Sun, X. Food waste and montmorillonite contribute to the enhancement of green waste composting. Process. Saf. Environ. Prot. 2023, 170, 983–998. [Google Scholar] [CrossRef]
- Benedi, J. Antisépticos. Farm. Prof. 2005, 19, 58–61. [Google Scholar]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Qing, C.; Guoxue, L.; Michel, F.C. Chapter Three—Current Approaches and Future Trends in Compost Quality Criteria for Agronomic, Environmental, and Human Health Benefits. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Amsterdam, The Netherlands, 2017; pp. 143–233. [Google Scholar]


| Treatment | Mixture Composition (w/w) | Mass |
|---|---|---|
| T1 | 100% GW | 40 kg |
| T2 | M1: 50% GW + 32.5% FW * + 2.5% FW ** + 13% Sd + 2% Pr | 20 kg (GW) + 13 kg + (FW *) + 1 kg (FW **) + 5.2 kg (Sd) + 0.8 kg (Pr) |
| T3 | M2: (48% GW + 32.5%FW * + 2.5% FW ** + 13% Sd + 2% Pr) + 2% Bch | 19.2 kg (GW) + 13 kg + (FW *) + 1 kg (FW **) + 5.2 kg (Sd) + 0.8 kg (Pr) + 0.8 kg (Bch) |
| T4 | M3: (45% GW + 32.5% FW * + 2.5% FW ** + 13% Sd + 2% Pr) + 5% Bch | 18 kg (GW) + 13 kg + (FW *) + 1 kg (FW **) + 5.2 kg (Sd) + 0.8 kg (Pr) + 2 kg (Bch) |
| Parameter | FW | GW | Bch * | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| pH | 4.7 ± 0.2 | 7.5 ± 0.1 | 8.1 ± 0.2 | 6.2 ± 0.1 | 5.61 ± 0.3 | 5.62 ± 0.2 |
| Moisture (%) | 78 ± 1.5 | 63 ± 2.0 | N.D | 61 ± 3.2 | 62.1 ± 2.1 | 63.3 ± 1.4 |
| Mg (%) | 0.08 ± 0.01 | 0.17 ± 0.01 | 0.30 ± 0.01 | 0.20 ± 0.02 | 0.16 ± 0.01 | 0.15 ± 0.01 |
| TK (%) | 1.37 ± 0.3 | 0.62 ± 0.4 | 0.38 ± 0.02 | 0.9 ± 0.1 | 0.85 ± 0.1 | 0.88 ± 0.2 |
| Hemicellulose (%) | 74.80 ± 2.2 | 15.47 ± 3.5 | N.D | 23 ± 2.5 | 27.1 ± 2.0 | 18.4 ± 1.4 |
| Cellulose (%) | 3.07 ± 0.7 | 12.43 ± 2.4 | N.D | 21.9 ± 1.3 | 26.4 ± 1.7 | 28.1 ± 0.8 |
| Lignin (%) | 1.02 ± 0.3 | 40.80 ± 6.1 | N.D | 23.8 ± 3.7 | 35 ± 4.0 | 25.9 ± 1.3 |
| TOC (%) | 40.73 ± 4.2 | 29.87 ± 3.8 | 7.4 ± 0.5 | 39.1 ± 2.6 | 41.4 ± 3.1 | 39.1 ± 2.8 |
| TN (%) | 0.46 ± 0.03 | 0.65 ± 0.2 | 0.43 ± 0.02 | 0.53 ± 0.1 | 0.51 ± 0.1 | 0.45 ± 0.1 |
| TP (%) | 0.22 ± 0.12 | 0.25 ± 0.11 | N.D | 2.43 ± 0.21 | 1.34 ± 0.13 | 1.05 ± 0.12 |
| C/N | 88.54 ± 3.34 | 46.19 ± 4.43 | 17.21 ± 0.36 | 73.77 ± 3.52 | 81.18 ± 4.49 | 86.89 ± 4.29 |
| T | Added Water (L) | Initial pH | Start of the TPh (Days) | Maximum Temperature (°C) | Length of the TPh (Days) | Time to Reach Ambient Temperature (Days) |
|---|---|---|---|---|---|---|
| T1 | 10 | 6.51 | 2 | 44.3 | 1 | 24 |
| T2 | 2 | 6.18 | 4 | 53.2 | 2 | 24 |
| T3 | 0.7 | 5.62 | 3 | 53.6 | 6 | 24 |
| T4 | 1.4 | 5.60 | 3 | 52.8 | 6 | 24 |
| Parameter | Unit | NTC 5167 | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| Physicochemical | ||||||
| pH * | Units | 7.0–9.0 | 7.13 ± 0.47 a | 7.16 ± 0.13 a | 6.93 ± 0.03 a | 7.06 ± 0.30 a |
| Moisture | % | >35.0 | 55.87 ± 5.22 a | 47.70 ± 8.2 b | 55.28 ± 1.19 a | 54.11 ± 2.82 a |
| CEC * | meq/100 g | ≥30.0 | 29.3 ± 2.97 a | 31.60 ± 0.99 a | 35 ± 0.66 b | 38.30 ± 2.97 b |
| EC | dS/m | <3.0 | 0.14 ± 0.01 a | 0.21 ± 0.01 a | 0.16 ± 0.06 a | 0.11 ± 0.02 a |
| WRC | % | R.D | 194.5 ± 62.01 a | 299.4 ± 27.7 b | 333.90 ± 25.03 c | 312.90 ± 56.4 c |
| TOC * | % | ≥15 | 18.15 ± 2.19 a | 33.30 ± 2.55 b | 31.35 ± 1.34 b | 34.40 ± 2.12 b |
| TN * | % | >1.0 | 0.35 ± 0.21 a | 1.05 ± 0.49 b | 1.30 ± 0.16 c | 1.32 ± 0.14 c |
| TP * | % | >1.0 | 0.47 ± 0.36 a | 2.85 ± 0.46 b | 2.14 ± 0.25 c | 1.20 ± 0.16 d |
| TK * | % | >1.0 | 1.3 ± 0.10 a | 1.8 ± 0.03 b | 1.52 ± 0.02 a | 1.61 ± 0.16 b |
| Microbiological | ||||||
| FC * | MPN/g | <1000 | >2400 | >2400 | 240 | >2400 |
| TC * | MPN/g | <1000 | >2400 | >2400 | 240 | >2400 |
| Salmonella sp. * | CFU/25 g | A | A | A | A | A |
| Enterobacteria * | CFU/g | <1000 | 0 | 490 ±650 | 11.5 ± 16.26 | 450 ± 63.64 |
| Mesophiles * | CFU/g | R.D | 4.3 × 109 | 3.41 × 109 | 1 × 108 | 7 × 105 |
| Agronomic | ||||||
| FI | - | 5.00 | 3.40 a | 4.25 b | 4.50 c | 4.40 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Orobio, B.A.; Soto-Paz, J.; Hernández-Cruz, J.A.; Gómez-Herreño, M.C.; Domínguez-Rivera, I.C.; Oviedo-Ocaña, E.R. Evaluation of Biochar as an Additive in the Co-Composting of Green Waste and Food Waste. Sustainability 2023, 15, 7437. https://doi.org/10.3390/su15097437
Parra-Orobio BA, Soto-Paz J, Hernández-Cruz JA, Gómez-Herreño MC, Domínguez-Rivera IC, Oviedo-Ocaña ER. Evaluation of Biochar as an Additive in the Co-Composting of Green Waste and Food Waste. Sustainability. 2023; 15(9):7437. https://doi.org/10.3390/su15097437
Chicago/Turabian StyleParra-Orobio, Brayan Alexis, Jonathan Soto-Paz, Jhon Alexander Hernández-Cruz, Martha Cecilia Gómez-Herreño, Isabel Cristina Domínguez-Rivera, and Edgar Ricardo Oviedo-Ocaña. 2023. "Evaluation of Biochar as an Additive in the Co-Composting of Green Waste and Food Waste" Sustainability 15, no. 9: 7437. https://doi.org/10.3390/su15097437
APA StyleParra-Orobio, B. A., Soto-Paz, J., Hernández-Cruz, J. A., Gómez-Herreño, M. C., Domínguez-Rivera, I. C., & Oviedo-Ocaña, E. R. (2023). Evaluation of Biochar as an Additive in the Co-Composting of Green Waste and Food Waste. Sustainability, 15(9), 7437. https://doi.org/10.3390/su15097437







