Prokaryotic Communities from Pristine Cave Environments: Biotechnological Potential with Sustainable Production
Abstract
:1. Introduction
2. Methodology
3. Pristine Cave Environments
4. Microorganisms in Pristine Cave Environments
4.1. Identification of Microorganisms
4.2. Microorganisms with Bioactivity
4.2.1. Proteobacteria
4.2.2. Bacteroidetes
4.2.3. Firmicutes
4.2.4. Actinobacteria
| Phylum | Microorganism | Activity | Source | Reference |
|---|---|---|---|---|
| Actinobacteria | Actinocorallia aurantiaca | Antibacterial (Paenibacillus lavae) | Phanangkoi Cave, Thailand | [69] |
| Actinoplanes brasiliensis | Antibacterial (Staphylococcus aureus) | Shuanghe Cave, China | [30] | |
| Actinoplanes friuliensis | Antibacterial (Escherichia coli and S. aureus) and Antifungal (Botrytis cinerea) | Shuanghe Cave, China | [30] | |
| Agromyces subbeticus | Antibacterial (E. coli and S. aureus) and Antifungal (B. Cinerea) | Shuanghe Cave, China | [30] | |
| Arthrobacter psychrolactophilus B7 | Antibacterial (S. aureus, E. coli, Enterobacter cloacae, Pseudomonas CN11, Pseudomonas aeruginosa, and MRSA) | Scarisoara Ice Cave, Romania | [70] | |
| Arthrobacter sp. R-36193 | Antibacterial (P. aeruginosa) and Antifungal (Rhodotorula. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Arthrobacter sp. R4 | Antibacterial (P. Aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Crossiella sp. | Antibacterial (B. cereus, S. aureus, E. coli, P. aeruginosa, and Acinetobacter baumannii) and Antifungal (Aspergillus versicolor, Penicillium chrysogenum, Cladosporium cladosporioides, Fusarium solani, and Ochroconis lascauxensis) | Altamira Cave, Spain | [42] | |
| Dietzia natronolimnaea 44860 | Antibacterial (S. aureus, E. coli, E. cloacae, Psudomonas CN11, MRSA, Enterococcus falcium, and Klebsiella 19094) | Scarisoara Ice Cave, Romania | [70] | |
| Microbacterium ginsengiterrae DCY37 | Antibacterial (S. aureus, E. coli, E. cloacae, Psudomonas CN11, MRSA, and E. falcium) | Scarisoara Ice Cave, Romania | [70] | |
| Microbacterium pygmaeum KV-490 | Antibacterial (S. aureus, E. coli, E. cloacae, Psudomonas CN11, MRSA, E. falcium, and Klebsiella 19094) | Scarisoara Ice Cave, Romania | [70] | |
| Micrococcus luteus CJ-G-TSA7 | Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Micromonospora carbonacea | Antibacterial (E. coli and S. aureus) and Antifungal (B. cinerea) | Shuanghe Cave, China | [30] | |
| Micromonospora chersinia | Antibacterial (B. cereus and P. lavae) and Anticancer (MCF7 and NCI-H187 cell lines) | Phanangkoi Cave, Thailand | [69] | |
| Micromonospora sagamiensis | Antibacterial (E. coli and S. aureus) | Shuanghe Cave, China | [30] | |
| Nocardia sungurluensis | Antifungal (B. cinerea) | Shuanghe Cave, China | [30] | |
| Nocardioides albus | Antibacterial (S. aureus) | Shuanghe Cave, China | [30] | |
| Nonomuraea roseola | Antibacterial (B cereus, MRSA, and P. lavae) and Anticancer (NCI-H187 and KB cell lines) | Phanangkoi Cave, Thailand | [69] | |
| Pseudarthrobacter polychromogenes 20136 | Antibacterial (S. aureus, E. coli, E. cloacae, P. aeruginosa, Pseudomonas CN11, and MRSA) | Scarisoara Ice Cave, Romania | [70] | |
| Saccharothrix texasensis | Anticancer (NCI-H187 and KB cell lines) | Phanangkoi Cave, Thailand | [69] | |
| Spirillospora albida | Antibacterial (B. cereus, MRSA, and P. lavae) and Anticancer (NCI-H187 cell line) | Phanangkoi Cave, Thailand | [69] | |
| Streptomyces alboflavus | Antifungal (B. cinerea) | Shuanghe Cave, China | [30] | |
| Streptomyces albogriseolus | Antibacterial (S. aureus) | Shuanghe Cave, China | [30] | |
| Streptomyces albus | Antibacterial (E. coli) | Shuanghe Cave, China | [30] | |
| Streptomyces anulatus | Antibacterial (S. aureus) | Shuanghe Cave, China | [30] | |
| Streptomyces aurantiacus | Antibacterial (E.coli, S. aureus, and P. aeruginosa) | Kotumsar Cave, India | [71] | |
| Streptomyces avidinii | Antibacterial (Salmonella typhimurium, S. aureus, E. coli, P. aeruginosa, Listeria monocytogenes, and Listeria innocua) | 12 Portuguese volcanic caves, Terceira Island, Azores | [72] | |
| Streptomyces flavofungini | Antibacterial (S. aureus) and Antifungal (B. cinerea) | Shuanghe Cave, China | [30] | |
| Streptomyces longisporoflavus | Antibacterial (E. coli, S. aureus, and P. aeruginosa) | Kotumsar Cave, India | [71] | |
| Streptomyces luridus | Antibacterial (E. coli and S. aureus) | Kotumsar Cave, India | [71] | |
| Streptomyces mauvecolor | Antibacterial (Proteus sp., S. typhimurium, S. aureus, E. coli, P. aeruginosa, L. monocytogenes, and L. innocua) | 12 Portuguese volcanic caves, Terceira Island, Azores | [72] | |
| Streptomyces nojiriensis | Antibacterial (Proteus sp. and E. coli) | 12 Portuguese volcanic caves, Terceira Island, Azores | [72] | |
| Streptomyces olivaceus | Antibacterial (E. coli and S. aureus) and Antifungal (B. cinerea) | Shuanghe Cave, China | [30] | |
| Streptomyces prasinosporus | Antibacterial (E. coli and P. aeruginosa) | Kotumsar Cave, India | [71] | |
| Streptomyces roseus | Antibacterial (E. coli, S. aureus, and P. aeruginosa) | Kotumsar Cave, India | [71] | |
| Streptomyces sp. 82293 | Antibacterial (M. luteus and S. aureus) | Volcanic cave, Canada | [73] | |
| Streptomyces spiroverticillatus | Antibacterial (S. typhimurium, S. aureus, E. coli, P. aeruginosa, L. monocytogenes, and L. innocua) | 12 Portuguese volcanic caves, Terceira Island, Azores | [72] | |
| Streptomyces yanii | Antibacterial (S. aureus) and Antifungal (B. cinerea) | Shuanghe Cave, China | [30] | |
| Proteobacterias | Acinetobacter sp. CJ-S-PYD4 | Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] |
| Candidimonas bauzanensis BZ59 | Antibacterial (S. aureus, E. coli, E. cloacae, Pseudomonas CN11, P. aeruginosa, and MRSA) | Scarisoara Ice Cave, Romania | [70] | |
| Caulobacter henricii 15253 | Antibacterial (S. aureus, E. coli, E. cloacae, Pseudomonas CN11, P. aeruginosa, and MRSA) | Scarisoara Ice Cave, Romania | [70] | |
| Comamonas sp. BM-9_6 | Antibacterial (B. subtilis, X. oryzae, and P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Delftia acidovorans 14950 | Antibacterial (S. aureus, E. coli, E. cloacae, Pseudomonas CN11, P. aeruginosa, MRSA, and Klebsiella 19094) | Scarisoara Ice Cave, Romania | [70] | |
| Micrococcus luteus | Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Obesumbacterium proteus | Antibacterial (P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas brenneri 97-391 | Antibacterial (S. aureus, E. cloacae, Pseudomonas CN11, P. aeruginosa, and MRSA) | Scarisoara Ice Cave, Romania | [70] | |
| Pseudomonas fluorescens | Antibacterial (P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas fluorescens 15834 | Antibacterial (Xanthomonas oryzae and P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas fluorescens LMG 14576 | Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas fragi | Antibacterial (P. aeruginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas grimontii 97-514 | Antibacterial (S. aureus, E. coli, E. cloacae, Pseudomonas CN11, P. aeruginosa, MRSA, Klebsiella 19094, and E. falcium) | Scarisoara Ice Cave, Romania | [70] | |
| Pseudomonas kilonensis DSM 13647 | Antibacterial (B. subtilis) | Yumugi River cave, New Guinea | [61] | |
| Pseudomonas migulae NBRC 103157 | Antibacterial (B. subtilis and P. aeruginosa) | Yumugi River cave, New Guinea | [61] | |
| Pseudomonas plecoglossicida | Antibacterial (B. subtilis, X. oryzae, and P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas putida | Antibacteral (B. subtilis and X. oryzae) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas resinovorans ATCC 14235 | Antibacterial (B. subtilis) | Yumugi River cave, New Guinea | [61] | |
| Pseudomonas sp. | Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Serratia proteamaculans | Antibacterial (P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Serratia sp. | Antibacterial (B. subtilis, X. oryzae, and P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Serratia sp. 136-2 | Antibacterial (P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Serratia sp. L0305 | Antibacterial (P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Stenotrophomonas sp. | Antibacterial (P. aeruginosa) | Magura Cave, Bulgaria | [19] | |
| Stenotrophomonas sp. DIC6JA | Antibacterial (P. aeruginosa) | Magura Cave, Bulgaria | [19] | |
| Pseudomonas sp. | Antibacterial (S. aureus) | Kadıini Cave, Turkey | [13] | |
| Bacteroidetes | Myroides sp. IT-2012 | Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] |
| Sphingobacterium sp. | Antibacterial (X. oryzae and P. aeruginosa) and Antifungal (R. mucilaginosa) | Magura Cave, Bulgaria | [19] | |
| Sphingobacterium sp. Ag8 | Antibacterial (P. aeruginosa) | Magura Cave, Bulgaria | [19] | |
| Firmicutes | Bacillus amyloliquefaciens | Antibacterial (P. aeruginosa) | Magura Cave, Bulgaria | [19] |
| Bacillus cereus | Antibacterial (S. epidermidis, B. subtilis) | Kadıini Cave, Turkey | [13] | |
| Bacillus eiseniae | Antibacterial (S. aureus) | Cave in the Hindu Kush Mountain, Pakistain | [39] | |
| Bacillus humi | Antibacterial (S. typhi) | Cave in the Hindu Kush Mountain, Pakistain | [39] | |
| Bacillus sp. | Antibacterial (S. aureus JE2 and S. aureus SH1000) | Rogers Belmont Cave, USA | [74] | |
| Bacillus sp. | Antibacterial (B. subtilis) | Kadıini Cave, Turkey | [13] | |
| Bacillus thuringiensis | Antibacterial (S. epidermidis and B. subtilis) | Kadıini Cave, Turkey | [13] | |
| Bacillus toyonensis BCT-7112 | Antibacterial (S. aureus, E. cloacae, Pseudomonas CN11, P. aeruginosa, and MRSA) | Scarisoara Ice Cave, Romania | [70] | |
| Bacillus weihenstephanensis | Antibacterial (S. epidermidis and B. subtilis) | Kadıini Cave, Turkey | [13] | |
| Brevibacillus borstelensis | Antifungal (C. albicans) | Cave in the Hindu Kush Mountain, Pakistain | [39] | |
| Brevibacterium frigoritolerans | Antibacterial (S. epidermidis and B. subtilis) | Kadıini Cave, Turkey | [13] | |
| Fictibacillus nanhaiensis | Antibacterial (S. typhi and S. aureus) | Cave in the Hindu Kush Mountain, Pakistain | [39] |
5. Potential for Bioactive Compounds Production
| Compound | Isolation and Identification Technique | Activity | Microorganism | Source | Reference |
|---|---|---|---|---|---|
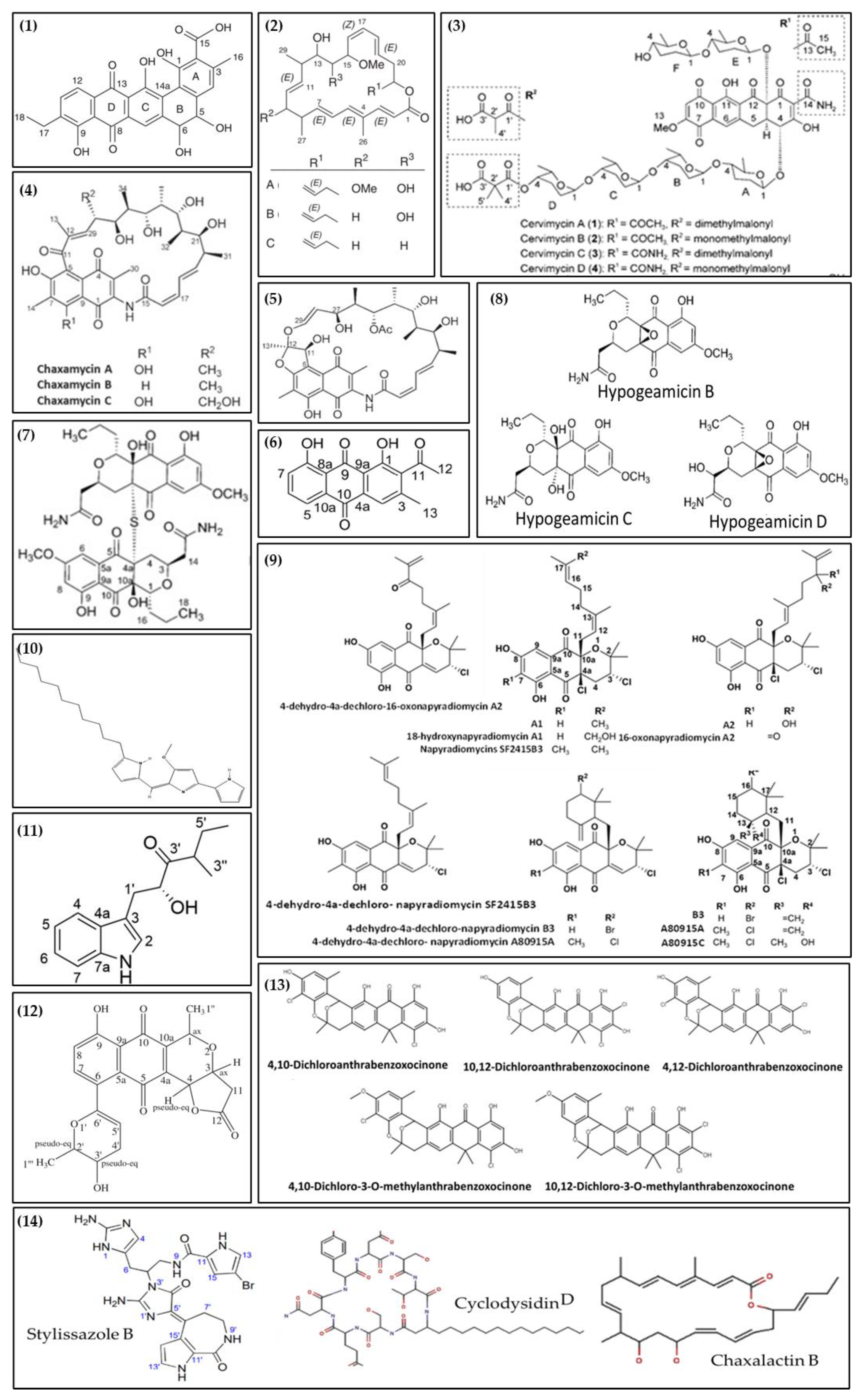
| Antibiotic R2 (1) | Exclusion chromatography; RP-HPLC NMR HMBC | Antibacterial (Bacillus subtilis, Escherichia coli, Listeria monocytogens, Micrococcus luteus, Mycobacterium smegmatis, Pseudomonas fluorescens, and Staphylococcus aureus) and Antifungal (Aspergillus carbonarius, Candida albicans, Mucor ramannianus, and Saccharomyces cerevisiae) | Streptosporangium sp. Sg3 | Saharan soil sample collected from Béni-Abbès, Béchar [3] | [82] |
| Atacamycins A-C (2) | HPLC-UV/Vis RP-HPLC LC-MS MS NMR | Anticancer (BXF 1218L, DIFI, LXFL 529L, MAXF 401 NL, MEXF 462NL, 22Rv1, UXF 1138L, and RKO cell lines) | Streptomyces sp. C38 | Hyper-arid soil collected from Atacama Desert, Chile | [83] |
| Cervimycin A-D (3) | RP-HPLC NMR HPLC-UV/Vis HRMS | Antibacterial (B. Subtilis, S. aureus, and Enterococcus faecalis) | Streptomyces tendae | Cave Grotta dei Cervi, Italy | [84] |
| Chaxamycins A-C (4) | NMR HR-MS HPLC-MS | Antibacterial (B. Subtilis, L. monocytogenes, and S. aureus) | Streptomyces sp. C34 | Hyper-arid soil collected from Atacama Desert, Chile | [85] |
| Chaxamycin D (5) | NMR UV/vis spectrometer HPLC-MS RP-HPLC X-ray | Antibacterial (E. coli and S. aureus) | Streptomyces sp. C34 | Hyper-arid soil collected from Atacama Desert, Chile | [86] |
| Huanglongmycin A (6) | HRMS NMR RP-HPLC HPLC-UV/Vis | Anticancer (SKOV3, HeLa, and Caco-2 cell lines) | Streptomyces sp. CB09001 | Soil of karstic cave in Xiangxi, China. | [87] |
| Hypogeamicin A (7) | NMR HRMS RP-HPLC X-ray | Anticancer (TCT-1 cell line) | Nonomuraea specus | Soil of Hardin’s cave, Ashland, Tennessee | [88] |
| Hypogeamicins B−D (8) | NMR HRMS RP-HPLC X-ray | Antibacterial (B. subtilis) | Nonomuraea specus | Soil of Hardin’s cave, Ashland, Tennessee | [88] |
| Napyradiomycins (A1, 18-hydroxynapyradiomycin A1; A2; 16-oxonapyradiomycin A2; 4-dehydro-4a-dechloro-16-oxonapyradiomycin A2; B3; 4-dehydro-4a-dechloro-napyradiomycin B3) (9) | RP-HPLC HR-MS NMR | Antibacterial (Cobetia marina, Phaeobacter inhibens, Pseusooceanicola batsensis, and M. luteus.) | Streptomyces aculeolatus PTM-420 | Desertas Island in Madeira, Portugal | [89] |
| Napyradiomycins (SF2415B3, 4-dehydro-4a-dechloro- napyradiomycin SF2415B3; A80915A; A80915C; 4-dehydro-4a-dechloro- napyradiomycin A80915A) (9) | RP-HPLC HR-MS NMR | Antibiofilm (Marinobacter hydrocarbonoclasticus, and C. marina) | Streptomyces aculeolatus PTM-029 | Desertas Island in Madeira, Portugal | [89] |
| Undecylprodigiosin (10) | LC-MS HPLC- | Antimicrobial (M. luteus, B. subtilis, and C. albicans) and Antioxidant | Streptomyces sp. JS520 | Soil Cave on mountain Miroc in Serbia. | [90] |
| Xenocylion B (11) | NMR X-ray | Antioxidant | Streptomyces sp. CB09001 | Karstic cave in Xiangxi, China | [91] |
| Xiakemycin A (12) | HR-ESI-MS NMR HPLC-UV/Vis | Antibacterial (S. aureus (MSSA and MRSA), Staphylococcus epidermidis (MSSE and MRSE), and E. faecalis (VSE and VRE)), and Anticancer (A549, MCF-7, HepG-2, HeLa, HCT-116, SHSY5Y, and PC-3 cell lines) | Streptomyces sp. CC8-201 | Soil of Karst cave, Chongqing, China | [92] |
| Mixture of compounds (4,10-dichloroanthrabenzoxocinone; 10,12-dichloroanthrabenzoxocinone; 4,12-dichloroanthrabenzoxocinone; 4,10-dichloro-3-O-methylanthrabenzoxocinone; and 10,12-dichloro-3-O-methylanthrabenzoxocinone) (13) | LC-MS HPLC | Antibacterial (B. subtilis, Bacillus megaterium, Bacillus cereus, E. coli, Pseudomonas aeruginosa, S. aureus (MRSA), and Salmonella enterica), Antifungal (Candida glabrata, Candida dubliniensis, C. albicans, and Candida guilliermondii), and Anticancer (T47D cell line) | Streptomyces sp. M4_24, and M5_8 | Caves Tatra Mountains, Poland | [93] |
| Mixture of compounds (Cyclodysiden D; Chaxalactin B 14-Deoxy; Stylissazole B; Gyrophoric acid (4-Me ether; L-alanine amide)) (14) | HPLC LC-MS | Antibacterial (B. subtilis, E. coli, and Pseudomonas putida) and Antifungal (C. albicans) | Streptomyces sp. IB 2014/I/78-8 | Moonmilk from Karstic Cave in Siberia, Russia | [20] |
New Technological Advances
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atashgahi, S.; Häggblom, M.M.; Smidt, H. Organohalide respiration in pristine environments: Implications for the natural halogen cycle. Environ. Microbiol. 2018, 20, 934–948. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D. Bioactive molecules from extreme environments. Mar. Drugs 2020, 19, 642. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, F.C.; Bouras, N.; Mokrane, S.; Zitouni, A.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Streptosporangium becharense sp. Nov., an actinobacterium isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.Z.; Luis, J.; Pimentel, G.; Caldeira, A.T.; Morillo, N.T.J.; Laiz, L.; Rosa, J.M.D.E.L.A.; Candeias, A.; Eichinger, C.M.; Waele, J.D.E. Search of life in a Mars analogue site microbes and associated biosignatures in the deep and completely dark salt caves of the Atacama Desert. In Proceedings of the 18th International Congress of Speleology—Savoie, Mont Blanc, France, 24–31 July 2022; Volume 1, pp. 309–312. [Google Scholar]
- Junge, K.; Eicken, H.; Deming, J.W. Bacterial Activity at −2 to −20 °C in Arctic Wintertime Sea Ice. Appl. Environ. Microbiol. 2004, 70, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yu, Y.; Li, H.R.; Dong, N.; Zhang, X.H. Phylogenetic diversity and biological activity of actinobacteria isolated from the chukchi shelf marine sediments in the arctic ocean. Mar. Drugs 2014, 12, 1281–1297. [Google Scholar] [CrossRef]
- Bhadra, B.; Raghukumar, C.; Pindi, P.K.; Shivaji, S. Brevibacterium oceani sp. nov., isolated from deep-sea sediment of the Chagos Trench, Indian Ocean. Int. J. Syst. Evol. Microbiol. 2008, 58, 57–60. [Google Scholar] [CrossRef]
- Pei, S.; Xie, F.; Niu, S.; Ma, L.; Zhang, R.; Zhang, G. Brevibacterium profundi sp. nov., isolated from deep-sea sediment of the Western Pacific Ocean. Int. J. Syst. Evol. Microbiol. 2020, 70, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Zada, S.; Sajjad, W.; Rafiq, M.; Ali, S.; Hu, Z.; Wang, H.; Cai, R. Cave Microbes as a Potential Source of Drugs Development in the Modern Era. Microb. Ecol. 2021, 84, 676–687. [Google Scholar] [CrossRef]
- Abdelghani, Z.; Hourani, N.; Zaidan, Z.; Dbaibo, G.; Mrad, M.; Hage-Sleiman, R. Therapeutic applications and biological activities of bacterial bioactive extracts. Arch. Microbiol. 2021, 203, 4755–4776. [Google Scholar] [CrossRef]
- Cheeptham, N.; Sadoway, T.; Rule, D.; Watson, K.; Moote, P.; Soliman, L.C.; Azad, N.; Donkor, K.K.; Horne, D. Cure from the cave: Volcanic cave actinomycetes and their potential in drug discovery. Int. J. Speleol. 2013, 42, 35–47. [Google Scholar] [CrossRef]
- Schabereiter-Gurtner, C.; Saiz-Jimenez, C.; Piñar, G.; Lubitz, W.; Rölleke, S. Altamira cave Paleolithic paintings harbor partly unknown bacterial communities. FEMS Microbiol. Lett. 2002, 211, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Doğruöz-Güngör, N.; Çandıroğlu, B.; Altuğ, G. Enzyme profiles and antimicrobial activities of bacteria isolated from the Kadiini cave, Alanya, Turkey. J. Cave Karst Stud. 2020, 82, 106–115. [Google Scholar] [CrossRef]
- White, W.B.; Culver, D.C. Cave, Definition of. In Encyclopedia of Caves; White, W.B., Culver, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 81–85. ISBN 0121986519. [Google Scholar]
- Ghosh, S.; Kuisiene, N.; Cheeptham, N. The cave microbiome as a source for drug discovery: Reality or pipe dream? Biochem. Pharmacol. 2017, 134, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Adetutu, E.M.; Ball, A.S. Microbial diversity and activity in caves. Microbiol. Aust. 2014, 35, 192. [Google Scholar] [CrossRef]
- Kathrin, H. Adaptation to Low Food. In Encyclopedia of Caves; White, W.B., Culver, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 4–10. [Google Scholar]
- Thomas, L.P. Food Sources. In Encyclopedia of Caves; White, W.B., Culver, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 255–264. [Google Scholar]
- Tomova, I.; Lazarkevich, I.; Tomova, A.; Kambourova, M.; Vasileva-Tonkova, E. Diversity and biosynthetic potential of culturable aerobic heterotrophic bacteria isolated from Magura Cave, Bulgaria. Int. J. Speleol. 2013, 42, 65–76. [Google Scholar] [CrossRef]
- Axenov-Gribanov, D.V.; Voytsekhovskaya, I.V.; Tokovenko, B.T.; Protasov, E.S.; Gamaiunov, S.V.; Rebets, Y.V.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria isolated from an underground lake and moonmilk speleothem from the biggest conglomeratic karstic cave in Siberia as sources of novel biologically active compounds. PLoS ONE 2016, 11, e0149216. [Google Scholar] [CrossRef]
- Pipite, A.; Lockhart, P.J.; McLenachan, P.A.; Christi, K.; Kumar, D.; Prasad, S.; Subramani, R. Isolation, antibacterial screening, and identification of bioactive cave dwelling bacteria in Fiji. Front. Microbiol. 2022, 13, 1012867. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Bianchi, C.N. Mediterranean Marine Caves: A Synthesis of Current Knowledge. In Oceanography and Marine Biology: An Annual Review; Hawkins, S.J., Lemasson, A.J., Allcock, A.L., Bates, A.E., Byrne, M., Evans, A.J., Firth, L.B., Marzinelli, E.M., Russell, B.D., Smith, I.P., Swearer, S.E., Todd, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; Volume 59, ISBN 9780367685225. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Voultsiadou, E. Marine caves of the mediterranean sea: A sponge biodiversity reservoir within a biodiversity hotspot. PLoS ONE 2012, 7, e39873. [Google Scholar] [CrossRef]
- Mammola, S. Finding answers in the dark: Caves as models in ecology fifty years after Poulson and White. Ecography 2019, 42, 1331–1351. [Google Scholar] [CrossRef]
- Moulds, T.A. The Seasonality, Diversity and Ecology of Cavernicolous Guano Dependent Arthropod Ecosystems in Southern Australia. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2006. [Google Scholar]
- Mazzoli, R.; Riedel, K.; Pessione, E. Editorial: Bioactive Compounds fom Microbes. Front. Microbiol. 2017, 8, 392. [Google Scholar] [CrossRef]
- Tomczyk-Żak, K.; Zielenkiewicz, U. Microbial Diversity in Caves. Geomicrobiol. J. 2016, 33, 20–38. [Google Scholar] [CrossRef]
- Martin-Pozas, T.; Gonzalez-Pimentel, J.L.; Jurado, V.; Cuezva, S.; Dominguez-Moñino, I.; Fernandez-Cortes, A.; Cañaveras, J.C.; Sanchez-Moral, S.; Saiz-Jimenez, C. Microbial activity in subterranean ecosystems: Recent advances. Appl. Sci. 2020, 10, 8130. [Google Scholar] [CrossRef]
- Jones, A.A.; Bennett, P.C. Mineral ecology: Surface specific colonization and geochemical drivers of biofilm accumulation, composition, and phylogeny. Front. Microbiol. 2017, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Jiang, J.; Hu, X.; Zhou, J.; Hu, J.; Zhou, S. Actinobacterial community in Shuanghe Cave using culture-dependent and -independent approaches. World J. Microbiol. Biotechnol. 2019, 35, 153. [Google Scholar] [CrossRef]
- González, J.M.; Saiz-Jiménez, C. Application of molecular nucleic acid-based techniques for the study of microbial communities in monuments and artworks. Int. Microbiol. 2005, 8, 189–194. [Google Scholar]
- Long, R.A.; Qureshi, A.; Faulkner, D.J.; Azam, F. 2-n-pentyl-4-quinolinol produced by a marine Alteromonas sp. and its potential ecological and biogeochemical roles. Appl. Environ. Microbiol. 2003, 69, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S. Methods for Characterizing Microbial Communities in Caves and Karst: A Review. In Microbial Life in Cave Systems; Engel, A.S., Ed.; Walter de Gruyter: Berlin, Germany, 2016; pp. 23–46. [Google Scholar] [CrossRef]
- Lanoot, B.; Vancanneyt, M.; Hoste, B.; Vandemeulebroecke, K.; Cnockaert, M.C.; Dawyndt, P.; Liu, Z.; Huang, Y.; Swings, J. Grouping of streptomycetes using 16S-ITS RFLP fingerprinting. Res. Microbiol. 2005, 156, 755–762. [Google Scholar] [CrossRef]
- Gray, S.M.; Akob, D.M.; Green, S.J.; Kostka, J.E. The Bacterial Composition within the Sarracenia purpurea Model System: Local Scale Differences and the Relationship with the Other Members of the Food Web. PLoS ONE 2012, 7, 50969. [Google Scholar] [CrossRef]
- Kittelmann, S.; Friedrich, M.W. Identification of novel perchloroethene-respiring microorganisms in anoxic river sediment by RNA-based stable isotope probing. Environ. Microbiol. 2008, 10, 31–46. [Google Scholar] [CrossRef]
- Hutchens, E.; Radajewski, S.; Dumont, M.G.; McDonald, I.R.; Murrell, J.C. Analysis of methanotrophic bacteria in Movile Cave by stable isotope probing. Environ. Microbiol. 2004, 6, 111–120. [Google Scholar] [CrossRef]
- Rosado, T.; Dias, L.; Lança, M.; Nogueira, C.; Santos, R.; Martins, M.R.; Candeias, A.; Mirão, J.; Caldeira, A.T. Assessment of microbiota present on a Portuguese historical stone convent using high-throughput sequencing approaches. Microbiologyopen 2020, 9, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M. Analysis of bacterial communities and characterization of antimicrobial strains from cave microbiota. Braz. J. Microbiol. 2018, 49, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Adam, D.; Martinet, L.; Naômé, A.; Całusińska, M.; Delfosse, P.; Carnol, M.; Barton, H.A.; Hayette, M.P.; Smargiasso, N.; et al. A phenotypic and genotypic analysis of the antimicrobial potential of cultivable Streptomyces isolated from cave moonmilk deposits. Front. Microbiol. 2016, 7, 1455. [Google Scholar] [CrossRef]
- Rausch, P.; Rühlemann, M.; Hermes, B.M.; Doms, S.; Dagan, T.; Dierking, K.; Domin, H.; Fraune, S.; Von Frieling, J.; Hentschel, U.; et al. Comparative analysis of amplicon and metagenomic sequencing methods reveals key features in the evolution of animal metaorganisms. Microbiome 2019, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pimentel, J.L.; Dominguez-Moñino, I.; Jurado, V.; Laiz, L.; Caldeira, A.T.; Saiz-Jimenez, C. The Rare Actinobacterium Crossiella sp. Is a Potential Source of New Bioactive Compounds with Activity against Bacteria and Fungi. Microorganisms 2022, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- Wiseschart, A.; Mhuantong, W.; Tangphatsornruang, S.; Chantasingh, D.; Pootanakit, K. Shotgun metagenomic sequencing from Manao-Pee cave, Thailand, reveals insight into the microbial community structure and its metabolic potential. BMC Microbiol. 2019, 19, 144. [Google Scholar] [CrossRef]
- Frey, K.G.; Herrera-Galeano, J.E.; Redden, C.L.; Luu, T.V.; Servetas, S.L.; Mateczun, A.J.; Mokashi, V.P.; Bishop-Lilly, K.A. Comparison of three next-generation sequencing platforms for metagenomic sequencing and identification of pathogens in blood. BMC Genom. 2014, 15, 96. [Google Scholar] [CrossRef]
- Cardenas, E.; Tiedje, J.M. New tools for discovering and characterizing microbial diversity. Curr. Opin. Biotechnol. 2008, 19, 544–549. [Google Scholar] [CrossRef]
- Riesenfeld, C.S.; Schloss, P.D.; Handelsman, J. Metagenomics: Genomic analysis of microbial communities. Annu. Rev. Genet. 2004, 38, 525–552. [Google Scholar] [CrossRef]
- Teeling, H.; Glöckner, F.O. Current opportunities and challenges in microbial metagenome analysis-A bioinformatic perspective. Brief. Bioinform. 2012, 13, 728–742. [Google Scholar] [CrossRef]
- Mantri, S.S.; Negri, T.; Sales-Ortells, H.; Angelov, A.; Peter, S.; Neidhardt, H.; Oelmann, Y.; Ziemert, N. Metagenomic Sequencing of Multiple Soil Horizons and Sites in Close Vicinity Revealed Novel Secondary Metabolite Diversity. mSystems 2021, 6, e01018-21. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, A.T.; Schiavon, N.; Mauran, G.; Salvador, C.; Rosado, T.; Mirão, J.; Candeias, A. On the biodiversity and biodeteriogenic activity of microbial communities present in the hypogenic environment of the Escoural Cave, Alentejo, Portugal. Coatings 2021, 11, 209. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, X.; Wu, Q.; Li, S.; Liu, Q.; Tan, L.; Weng, Q. Arthrobacter cavernae sp. nov., a novel actinobacterium isolated from sediment of karst cave. Int. J. Syst. Evol. Microbiol. 2022, 72, 5445. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Pessi, I.S.; Arguelles-Arias, A.; Noirfalise, P.; Luis, G.; Ongena, M.; Barton, H.; Carnol, M.; Rigali, S. Streptomyces lunaelactis sp. nov., a novel ferroverdin A-producing Streptomyces species isolated from a moonmilk speleothem. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2015, 107, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Schumann, P.; Spröer, C.; Gounot, A.M. Arthrobacter psychrophenolicus sp. nov., isolated from an alpine ice cave. Int. J. Syst. Evol. Microbiol. 2004, 54, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Kim, E.S.; Roe, J.H.; Kim, J.H.; Kang, S.O.; Hah, Y.C. Saccharothrix violacea sp. nov., isolated from a gold mine cave, and Saccharothrix albidocapillata comb. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 1315–1323. [Google Scholar] [CrossRef]
- Nakaew, N.; Sungthong, R.; Yokota, A.; Lumyong, S. Nonomuraea monospora sp. nov., an actinomycete isolated from cave soil in Thailand, and emended description of the genus Nonomuraea. Int. J. Syst. Evol. Microbiol. 2012, 62, 3007–3012. [Google Scholar] [CrossRef]
- Nakaew, N.; Pathom-aree, W.; Lumyong, S. First Record of the Isolation, Identification and Biological Activity of a New Strain of Spirillospora albida from Thai Cave Soil. Actinomycetologica 2009, 23, 1–7. [Google Scholar] [CrossRef]
- Nimaichand, S.; Tamrihao, K.; Yang, L.L.; Zhu, W.Y.; Zhang, Y.G.; Li, L.; Tang, S.K.; Ningthoujam, D.S.; Li, W.J. Streptomyces hundungensis sp. nov., a novel actinomycete with antifungal activity and plant growth promoting traits. J. Antibiot. 2013, 66, 205–209. [Google Scholar] [CrossRef]
- Nimaichand, S.; Devi, A.M.; Tamreihao, K.; Ningthoujam, D.S.; Li, W.J. Actinobacterial diversity in limestone deposit sites in Hundung, Manipur (India) and their antimicrobial activities. Front. Microbiol. 2015, 6, 413. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pimentel, J.L.; Martin-Pozas, T.; Jurado, V.; Miller, A.Z.; Caldeira, A.T.; Fernandez-Lorenzo, O.; Sanchez-Moral, S.; Saiz-Jimenez, C. Prokaryotic communities from a lava tube cave in La Palma Island (Spain) are involved in the biogeochemical cycle of major elements. PeerJ 2021, 9, e11386. [Google Scholar] [CrossRef] [PubMed]
- Kersters, K.; De Vos, P.; Gillis, M.; Swings, J.; Vandamme, P.; Stackebrandt, E. Introduction to the Proteobacteria. In The Prokaryotes; Springer: New York, NY, USA, 2006; ISBN 978-0-387-25476-0. [Google Scholar] [CrossRef]
- Turrini, P.; Tescari, M.; Visaggio, D.; Pirolo, M.; Lugli, G.A.; Ventura, M.; Frangipani, E.; Visca, P. The microbial community of a biofilm lining the wall of a pristine cave in Western New Guinea. Microbiol. Res. 2020, 241, 126584. [Google Scholar] [CrossRef] [PubMed]
- Seong, C.N.; Kang, J.W.; Lee, J.H.; Seo, S.Y.; Woo, J.J.; Park, C.; Bae, K.S.; Kim, M.S. Taxonomic hierarchy of the phylum Firmicutes and novel Firmicutes species originated from various environments in Korea. J. Microbiol. 2018, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Snchez, M.; Rocha, D.; Snchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Garrity, G.M.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W.B. Phylum XIII. Firmicutes Gibbons and Murray 1978. In Bergey’s Manual® of Systematic Bacteriology; Springer: New York, NY, USA, 2009; ISBN 9780387950419. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Nathalie Gaveau-Vaillant, C.J.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezeld, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Am. Soc. Microbiol. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Yücel, S.; Yamaç, M. Selection of streptomyces isolates from Turkish karstic caves against antibiotic resistant microorganisms. Pak. J. Pharm. Sci. 2010, 23, 1–6. [Google Scholar]
- Adam, D.; Maciejewska, M.; Naômé, A.; Martinet, L.; Coppieters, W.; Karim, L.; Baurain, D.; Rigali, S. Isolation, characterization, and antibacterial activity of hard-to-culture actinobacteria from cave moonmilk deposits. Antibiotics 2018, 7, 28. [Google Scholar] [CrossRef]
- Nakaew, N.; Pathom-aree, W.; Lumyong, S. Generic Diversity of Rare Actinomycetes from Thai Cave Soils and Their Possible Use as New Bioactive Compounds. Actinomycetologica 2009, 23, 21–26. [Google Scholar] [CrossRef]
- Paun, V.I.; Lavin, P.; Chifiriuc, M.C.; Purcarea, C. First report on antibiotic resistance and antimicrobial activity of bacterial isolates from 13,000-year old cave ice core. Sci. Rep. 2021, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Rajput, Y.; Biswas, J.; Rai, V. Potentiality test in antimicrobial activity and antibiotic sensitivity of subterranean streptomyces strains isolated from Kotumsar cave of India. Int. J. Biol. Chem. 2012, 6, 53–60. [Google Scholar] [CrossRef]
- Riquelme, C.; Enes Dapkevicius, M.d.L.; Miller, A.Z.; Charlop-Powers, Z.; Brady, S.; Mason, C.; Cheeptham, N. Biotechnological potential of Actinobacteria from Canadian and Azorean volcanic caves. Appl. Microbiol. Biotechnol. 2017, 101, 843–857. [Google Scholar] [CrossRef]
- Rule, D.; Cheeptham, N. The effects of UV light on the antimicrobial activities of cave actinomycetes. Int. J. Speleol. 2013, 42, 147–153. [Google Scholar] [CrossRef]
- Farmer, J.T.; Shimkevitch, A.V.; Reilly, P.S.; Mlynek, K.D.; Jensen, K.S.; Callahan, M.T.; Bushaw-Newton, K.L.; Kaplan, J.B. Environmental bacteria produce abundant and diverse antibiofilm compounds. J. Appl. Microbiol. 2014, 117, 1663–1673. [Google Scholar] [CrossRef]
- Candiroglu, B.; Dogruoz Gungor, N. Cave Ecosystems: Microbiological View. Eur. J. Biol. 2017, 76, 36–42. [Google Scholar] [CrossRef]
- Pompilio, A.; Scocchi, M.; Mangoni, M.L.; Shirooie, S.; Serio, A.; Ferreira Garcia da Costa, Y.; Alves, M.S.; Şeker Karatoprak, G.; Süntar, I.; Khan, H.; et al. Bioactive compounds: A goldmine for defining new strategies against pathogenic bacterial biofilms? Crit. Rev. Microbiol. 2022, 49, 117–149. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.D.; Patel, A.K.; Singh, M.; Vandana; Kumari, A. Secondary Metabolites from Bacteria and Viruses; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128206553. [Google Scholar] [CrossRef]
- O’Brien, J.; Wright, G.D. An ecological perspective of microbial secondary metabolism. Curr. Opin. Biotechnol. 2011, 22, 552–558. [Google Scholar] [CrossRef]
- Jaspars, M.; De Pascale, D.; Andersen, J.H.; Reyes, F.; Crawford, A.D.; Ianora, A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef]
- Sekurova, O.N.; Schneider, O.; Zotchev, S.B. Novel bioactive natural products from bacteria via bioprospecting, genome mining and metabolic engineering. Microb. Biotechnol. 2019, 12, 828–844. [Google Scholar] [CrossRef]
- Goodfellow, M.; Fiedler, H.P. A guide to successful bioprospecting: Informed by actinobacterial systematics. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2010, 98, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Boudjella, H.; Zitouni, A.; Coppel, Y.; Mathieu, F.; Monje, M.C.; Sabaou, N.; Lebrihi, A. Antibiotic R2, a new angucyclinone compound from Streptosporangium sp. Sg3. J. Antibiot. 2010, 63, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, J.; Kulik, A.; Helaly, S.; Bull, A.T.; Goodfellow, M.; Asenjo, J.A.; Maier, A.; Wiese, J.; Imhoff, J.F.; Süssmuth, R.D.; et al. Atacamycins A-C, 22-membered antitumor macrolactones produced by Streptomyces sp. C38. J. Antibiot. 2011, 64, 775–780. [Google Scholar] [CrossRef]
- Herold, K.; Gollmick, F.A.; Groth, I.; Roth, M.; Menzel, K.D.; Möllmann, U.; Gräfe, U.; Hertweck, C. Cervimycin A-D: A polyketide glycoside complex from a cave bacterium can defeat vancomycin resistance. Chem. A Eur. J. 2005, 11, 5523–5530. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Houssen, W.E.; Harrison, W.T.A.; Deng, H.; Okoro, C.K.; Asenjo, J.A.; Andrews, B.A.; Bull, A.T.; Goodfellow, M.; Ebel, R.; et al. Diverse metabolic profiles of a Streptomyces strain isolated from a hyper-arid environment. J. Nat. Prod. 2011, 74, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Houssen, W.E.; Arnold, M.; Abdelrahman, M.H.; Deng, H.; Harrison, W.T.A.; Okoro, C.K.; Asenjo, J.A.; Andrews, B.A.; Ferguson, G.; et al. Chaxamycins A–D, bioactive ansamycins from a hyper-arid desert Streptomyces sp. J. Nat. Prod. 2011, 74, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Pu, H.; Xiang, J.; Su, M.; Yan, X.; Yang, D.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Huanglongmycin A-C, cytotoxic polyketides biosynthesized by a putative type II polyketide synthase from Streptomyces sp. CB09001. Front. Chem. 2018, 6, 254. [Google Scholar] [CrossRef]
- Derewacz, D.K.; McNees, C.R.; Scalmani, G.; Covington, C.L.; Shanmugam, G.; Marnett, L.J.; Polavarapu, P.L.; Bachmann, B.O. Structure and stereochemical determination of hypogeamicins from a cave-derived actinomycete. J. Nat. Prod. 2014, 77, 1759–1763. [Google Scholar] [CrossRef]
- Pereira, F.; Almeida, J.R.; Paulino, M.; Grilo, I.R.; Macedo, H.; Cunha, I.; Sobral, R.G.; Vasconcelos, V.; Gaudêncio, S.P. Antifouling napyradiomycins from marine-derived actinomycetes streptomyces aculeolatus. Mar. Drugs 2020, 18, 63. [Google Scholar] [CrossRef]
- Stankovic, N.; Radulovic, V.; Petkovic, M.; Vuckovic, I.; Jadranin, M.; Vasiljevic, B.; Nikodinovic-Runic, J. Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Appl. Microbiol. Biotechnol. 2012, 96, 1217–1231. [Google Scholar] [CrossRef]
- Jiang, L.; Pu, H.; Qin, X.; Liu, J.; Wen, Z.; Huang, Y.; Xiang, J.; Xiang, Y.; Ju, J.; Duan, Y.; et al. Syn-2, 3-diols and anti-inflammatory indole derivatives from Streptomyces sp. CB09001. Nat. Prod. Res. 2021, 35, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.K.; Guo, L.; Chen, C.; Liu, S.W.; Zhang, L.; Dai, S.J.; He, Q.Y.; You, X.F.; Hu, X.X.; Tuo, L.; et al. Xiakemycin A, a novel pyranonaphthoquinone antibiotic, produced by the Streptomyces sp. CC8-201 from the soil of a karst cave. J. Antibiot. 2015, 68, 771–774. [Google Scholar] [CrossRef]
- Jaroszewicz, W.; Bielańska, P.; Lubomska, D.; Kosznik-Kwaśnicka, K.; Golec, P.; Grabowski, Ł.; Wieczerzak, E.; Dróżdż, W.; Gaffke, L.; Pierzynowska, K.; et al. Antibacterial, Antifungal and Anticancer Activities of Compounds Produced by Newly Isolated Streptomyces Strains from the Szczelina Chochołowska Cave (Tatra Mountains, Poland). Antibiotics 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Compound Summary for CID 135433360, Undecylprodigiosin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Undecylprodigiosin (accessed on 8 April 2023).
- Patel, K.; Laville, R.; Martin, M.T.; Tilvi, S.; Moriou, C.; Gallard, J.F.; Ermolenko, L.; Debitus, C.; Al-Mourabit, A. Unprecedented stylissazoles A-C from sty lissa carteri: Another dimension for marine pyrrole-2-aminoimidazole metabolite diversity. Angew. Chemie Int. Ed. 2010, 49, 4775–4779. [Google Scholar] [CrossRef]
- Kodzius, R.; Gojobori, T. Marine metagenomics as a source for bioprospecting. Mar. Genom. 2015, 24, 21–30. [Google Scholar] [CrossRef]
- Liao, S.; Wang, Y.; Liu, H.; Fan, G.; Sahu, S.K.; Jin, T.; Chen, J.; Zhang, P.; Gram, L.; Strube, M.L.; et al. Deciphering the Microbial Taxonomy and Functionality of Two Diverse Mangrove Ecosystems and Their Potential Abilities To Produce Bioactive Compounds. mSystems 2020, 5, e00851-19. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M. Metaproteomics: Much More than Measuring Gene Expression in Microbial Communities. mSystems 2019, 4, e00115-19. [Google Scholar] [CrossRef]
- Raza, S.; Ameen, A. Metaproteomics approaches and techniques: A review. Int. J. Adv. Sci. Res. 2017, 3, 49–51. [Google Scholar]
- Wang, Y.; Zhou, Y.; Xiao, X.; Zheng, J.; Zhou, H. Metaproteomics: A strategy to study the taxonomy and functionality of the gut microbiota. J. Proteomics 2020, 219, 103737. [Google Scholar] [CrossRef]
- Kovacevic, V.; Simpson, M.J. Fundamentals of environmental metabolomics. In Environmental Metabolomics; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 9780128181966. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatinho, P.; Salvador, C.; Silva, A.M.; Caldeira, A.T. Prokaryotic Communities from Pristine Cave Environments: Biotechnological Potential with Sustainable Production. Sustainability 2023, 15, 7471. https://doi.org/10.3390/su15097471
Gatinho P, Salvador C, Silva AM, Caldeira AT. Prokaryotic Communities from Pristine Cave Environments: Biotechnological Potential with Sustainable Production. Sustainability. 2023; 15(9):7471. https://doi.org/10.3390/su15097471
Chicago/Turabian StyleGatinho, Patrícia, Cátia Salvador, Amélia M. Silva, and Ana Teresa Caldeira. 2023. "Prokaryotic Communities from Pristine Cave Environments: Biotechnological Potential with Sustainable Production" Sustainability 15, no. 9: 7471. https://doi.org/10.3390/su15097471







