Performance Stability and Regeneration Property of Catalytic Membranes Coupled with Advanced Oxidation Process: A Comprehensive Review
Abstract
:1. Introduction
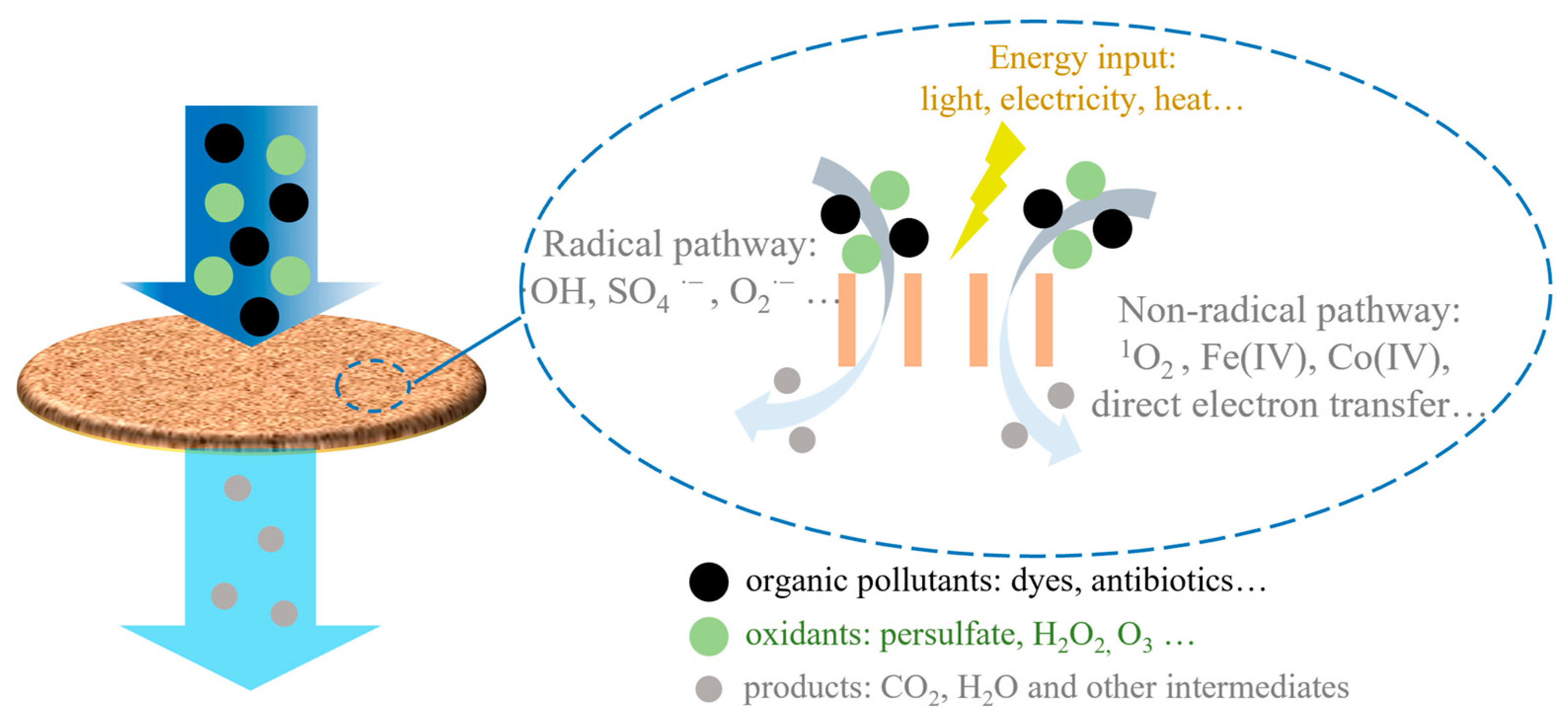
2. Reaction Mechanisms and Impacting Factors of Catalytic Membranes Coupled with Advanced Oxidation Process
- (a)
- the radical pathway, where various radicals are produced and then react with the pollutants;
- (b)
- the non-radical pathway, where the oxidation is achieved by direct electron transfer or with the assistance of other reactive species other than radicals.
3. Performance Stability of Catalytic Membranes Coupled with Advanced Oxidation Process
3.1. Performance Stability of Catalytic Membranes Tested by Multi-Cycle Experiments
3.1.1. Multi-Cycle Performance of Catalytic Membranes Coupled with Persulfate Oxidation
3.1.2. Multi-Cycle Performance of Catalytic Membranes Coupled with H2O2 Oxidation
3.1.3. Multi-Cycle Performance of Catalytic Membranes Coupled with Ozone Oxidation
3.1.4. Multi-Cycle Performance of Catalytic Membranes Coupled with Photocatalysis
3.1.5. Multi-Cycle Performance of Catalytic Membranes Coupled with Electrocatalysis
3.2. Performance Stability of Catalytic Membranes Tested by Long-Time Operation
| AOP Type | Substrate Membrane | Catalyst | Fabrication Method | Membrane Type | Filtration Mode | Target Pollutant | C0 | Flux (L/(m2·h)) | R0 | Operation Time (h) | η′ | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMS | PVDF | β-FeOOH | impregnation and in situ mineralization | flat-sheet | cross flow | RhB | 10 mg/L | 4097.9 | 99% | 12 | 99% | [63] |
| PMS | PVDF | MoS2 | filtration | flat-sheet | dead-end | BPA | 2 mg/L | 30.8 | 100% | 6 | 90% | [64] |
| PMS | PVDF | Co-CuOx | filtration | flat-sheet | dead-end | RA | 5 mg/L | 357 | 100% | 120 | ~100% | [65] |
| PMS | PVDF | ZIF-67-derived Co-carbon | flash freezing | flat-sheet | cross-flow | TC | 10 mg/L | 400 | 97% | 84 | 95% | [66] |
| PMS | PVDF | CoAl-LDH | filtration | flat | dead-end | RA | 2.5 mg/L | 80.3 | 91% | 29 | ~100% | [11] |
| PMS | PES | Co3O4/C@SiO2 | phase inversion | flat-sheet | dead-end | BPA | 10 mg/L | 229 | 95% | 42 | 90% | [67] |
| PMS | cellulose acetate | Fe-doped graphitic carbon nitride | filtration | flat-sheet | dead-end | BPA | 20 mg/L | 28.66 | 100% | 170 | 90% | [68] |
| PMS | cellulose acetate | Co3O4 | filtration | flat-sheet | dead-end | RA | 5 mg/L | 158.4 | 100% | 13 | 90% | [69] |
| PMS | mixed cellulose ester | Co-TiOx | filtration | flat-sheet | dead-end | RA | 5 mg/L | 130 | 100% | 100 | ~100% | [70] |
| PMS | nylon | Co@N CNT | filtration | flat-sheet | dead-end | TC | 10 mg/L | 40 | 100% | 24 | 90% | [71] |
| PMS | nylon | Fe2O3@CNT | filtration | flat-sheet | dead-end | TC | 0.04 mmol/L | 16.3 | 96.1% | 48 | 89% | [22] |
| PDS | nylon | rGO/NCNT | filtration | flat-sheet | dead-end | NSMX | 0.5 mg/L | 46.15 | 90% | 50 | 79% | [72] |
| PDS | nylon | NG/rGO/CNT | filtration | flat-sheet | dead-end | SMX | 0.5 mg/L | 46.15 | 99.8% | 24 | 99.8% | [23] |
| PMS | ceramic membrane | nitrogen doped carbon | dip-coating and pyrolysis | flat-sheet | dead-end | phenol | 0.1 mmol/L | 689.76 | 100% | 48 | 100% | [73] |
| PMS | ceramic membrane | Mn2O3 | solid state sintering and calcination | flat-sheet | dead-end | BPA EE2 NP | 0.1 mg/L 0.1 mg/L 0.1 mg/L | 60 | 92.2% 97.6% 97.3% | 8 | 96% 94% 95% | [74] |
| PMS | ceramic membrane | Mn2O3 | spraying and calcinating | flat-sheet | dead-end | acetaminophen | 1 mg/L | 60 | 95% | 24 | 92% | [28] |
| PMS | carbon nanofibrous membrane | CoOx | impregnation and pyrolysis | flat-sheet | dead-end | TC | 30 mg/L | 30 | 98% | 10 | 90% | [75] |
| H2O2 | PVDF | Prussian blue | in situ growth | flat-sheet | cross-flow | MB | 30 mg/L | 300 | 99% | 24 | 100% | [76] |
| H2O2 | PVDF | Fe3O4 | phase inversion | hollow fiber | cross-flow | MB | 100 mg/L | 175.8 | 97% | 12 | 100% | [77] |
| H2O2 | PVDF | BiOI | filtration | flat-sheet | dead-end | paracetamol | 10 mg/L | 120 | 96% | 100 | 88% | [78] |
| H2O2 | PP | Prussian blue/GO | filtration | flat-sheet | dead-end | MB | 10 mg/L | 27 | 98% | 24 | 100% | [79] |
| H2O2 | ceramic membrane | MoSx | hydrothermal | tubular | dead-end | clothianidin | 10 mg/L | 25 | 99.9% | 60 | 70% | [80] |
| H2O2 | ceramic membrane | FeOCl | impregnation and calcination | flat-sheet | dead-end | pCBA | 50 μmol/L | 100 | 100% | 120 | 80% | [81] |
| H2O2 | ceramic membrane | Co3O4/MCM-41 | packing | tubular | dead-end | AO7 | 50 mg/L | 11.25 | 96% | 40 | 103% | [82] |
| Photo | PVDF | ZnIn2S4 | filtration | flat-sheet | dead-end | TC | 100 μg/L | 84.06 | 90% | 36 | 100% | [83] |
| Photo | PVDF | Ag2CO3@UiO-66-NH2 | filtration | flat-sheet | dead-end | MB | 20 mg/L | 50 | 100% | 3.3 | 100% | [84] |
| Photo | PES | B doped- TiO2-SiO2/CoFe2O4 | phase inversion | flat-sheet | cross-flow | COD of biologically treated palm oil mill effluent | 1000 mg/L | 38.1 | 100% | 12 | 100% | [85] |
| Photo | hydrogel membrane | ZnCeOx/graphitic carbon nitride | crosslinking | flat-sheet | cross-flow | MB | 20 mg/L | 640 | 95% | 4 | 97% | [86] |
| Photo | ceramic membrane | TiO2 | atomic layer deposition and calcination | flat-sheet | dead-end | MB | 1 mg/L | 150 | 40% | 33 | 75% | [87] |
| Electro | Ti membrane (anode) stainless steel mesh (cathode) | Co3O4 (anode) –(cathode) | hydrothermal and calcination | tubular | dead-end | phenol | 5 mmol/L | 3.22 | 99% (COD removal) | 240 | 91% | [88] |
| Electro | coal-based carbon membrane (anode) titanium plate (cathode) | –(anode) –(cathode) | – | tubular | dead-end | BPA | 50 mg/L | 43.73 | 97% | 24 | 90% | [89] |
| Electro | carbon nanofiber membrane (anode) titanium mesh (cathode) | RuO2/TiO2 (anode) –(cathode) | hydrothermal and calcination | flat-sheet | dead-end | BPA SMX | 500 μg/L 500 μg/L | 360 | 98% 98% | 72 | 97% 97% | [90] |
| Electro | ceramic membrane/Ti mesh (anode) Ti mesh (cathode) | TiO2-SnO2-Sb (anode) –(cathode) | adhesive assemble | flat-sheet | dead-end | PCA | 1.28 mg/L | 17.4 | 85.5% | 72 | 97% | [91] |
| Electro | ceramic membrane/Ti mesh (anode) titanium mesh (cathode) | TiO2/SnO2-Sb (anode) –(cathode) | adhesive assemble | flat-sheet | dead-end | 2,4-D | 1 mg/L | 278 | 62.4% | 84 | 100% | [58] |
| Electro | Pt foil (anode) PTFE (cathode) | –(anode) GO-COOFe2+ (cathode) | filtration | flat-sheet | dead-end | florfenicol | 1 mg/L | 21.5 | 95% | 72 | 95% | [92] |
| Electro + O3 | titanium mesh (anode) PTFE (cathode) | –(anode) CNT (cathode) | filtration | flat-sheet | dead-end | ibuprofen | 2 mg/L | 140 | 95.19% | 10 | ~100% | [93] |
| Electro + PMS | perforated titanium plate (anode) PTFE (cathode) | –(anode) zero valence copper-CNT (cathode) | filtration | flat-sheet | dead-end | CR | 10.4 mg/L | 34.6 | 100% | 2.5 | 100% | [94] |
3.2.1. Long-Time Operation Performance of Catalytic Membranes Coupled with Persulfate Oxidation
3.2.2. Long-Time Operation Performance of Catalytic Membranes Coupled with H2O2 Oxidation
3.2.3. Long-Time Operation Performance of Catalytic Membranes Coupled with Photocatalysis
3.2.4. Long-Time Operation Performance of Catalytic Membranes Coupled with Electrocatalysis
4. Regeneration Performance of Catalytic Membranes Coupled with Advanced Oxidation Process
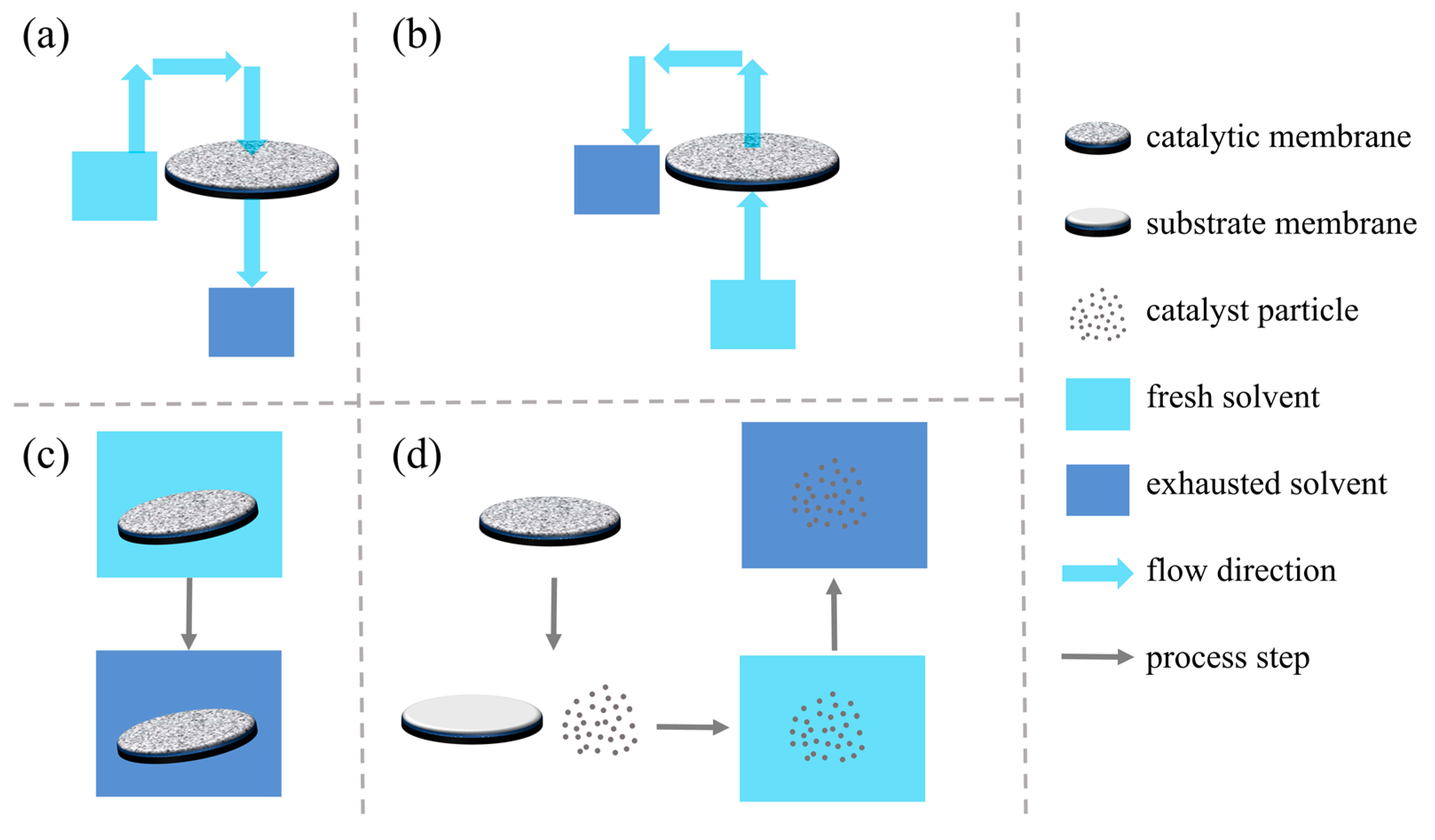
4.1. Regeneration Performance of Catalytic Membranes by Washing
- (a)
- The used membrane remains in the filtration apparatus, and filtration with the washing solvent is conducted. This method is applicable to most of the catalytic membranes.
- (b)
- The used membrane remains in the filtration apparatus, and back washing with the washing solvent is conducted. This method is not applicable to the catalytic membranes formed by filtration.
- (c)
- The used membrane is removed from the filtration apparatus and then immersed in the solvent, and sonication, stirring or another mechanical force is introduced to facilitate the desorption/detachment of organic pollutants on the membrane. This method is not applicable to the catalytic membranes formed by filtration and those with weak mechanical strength.
- (d)
- The used membrane is removed from the filtration apparatus, and the catalytic particles on the membrane were removed from the substrate membrane. After that, the separated catalytic particles were washed with the solvent with the assistance of sonication or stirring. After washing, the regenerated catalytic particles were reloaded on the substrate membrane. This method is only applicable to the catalytic membranes formed by filtration.
| AOP Type | Substrate Membrane | Catalyst | Fabrication Method | Membrane Type | Filtration Mode | Operation Mode | Target Pollutant | C0 | Flux (L/(m2·h)) | R0 | Washing Solvent | Number of Washing Cycles | η″ | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMS | ceramic membrane | MnO2 | ball-milling and calcination | flat-sheet | cross-flow | single pass | 4-hydroxylbenzoic acid | 80 mg/L | 19.50 | 100% | water | 6 | 91% | [95] |
| PMS | ceramic membrane | CoFe2O4 | impregnation and calcination | tubular | dead-end | single pass | MB | 25.0 mg·L−1 | 150 | 98% | water | 4 | 92% | [96] |
| PMS | ceramic membrane | Fe-doped CoTiO3 | impregnation and calcination | flat-sheet | dead-end | single pass | nimesulide | 10 mg/L | 300 | 96.3% | water | 5 | 88% | [97] |
| PMS | ceramic membrane | CoOx granular activated carbon | dipcoating and calcination; filling the channels | flat-sheet | dead-end | single pass | BPA | 10 mg/L | 35 | 95% | water | 5 | 95% | [98] |
| PMS | Ti membrane | Co3O4-Bi2O3 | impregnation and calcination | flat-sheet | dead-end | single pass | MB | 20 μmol/L | 76.8 | 98.7% | water | 4 | ~100% | [99] |
| PMS | Ti membrane | ZIF-67 | electro-deposition and calcination | flat-sheet | dead-end | single pass | SMX | 0.04 mmol/L | 56.9 | 96.3% | water | 5 | 93% | [100] |
| H2O2 | bacterial cellulose membrane | tetra-amino cobalt (II) phthalocyanine | impregnation and in situ reaction | flat-sheet | dead-end | recirculation | red X-3B | 100 µmol/L | 1146.50 | 95% | water | 5 | 97% | [101] |
| H2O2 | ceramic membrane | PDA-β-FeOOH | impregnation and in situ reaction | tubular | cross-flow | single pass | MO | 2 mg/L | 38 | 98% | water | 6 | ~100% | [102] |
| H2O2 | CNT membrane | Fe0 | wet-spinning and calcination | hollow fiber | dead-end | single pass | BPA | 10 mg/L | 545.9 | 97.8% | water | 5 | 97% | [103] |
| H2O2 | ceramic membrane | Cu-UiO-66 Mn-UiO-66 | solvothermal and impregnation | tubular | dead-end | single pass | phenol | 100 mg/L | 3.18 | 100% 90% | water | 5 | 90% 11% | [104] |
| O3 | ceramic membrane | Mn/FeOx | co-precipitation and calcination | flat-sheet | dead-end | single pass | trimethoprim | 5 mg/L | 10 | 98.6% | water | 5 | 91% | [105] |
| O3 | ceramic membrane | N-rGO | pneumatic method | tubular | cross-flow | single pass | benzotriazole | 0.084 mol/L | 660.86 | 100% | water | 1 | 95% | [35] |
| Photo | ceramic membrane | TiO2 | dip-coating and calcination | tubular | cross-flow | recirculation | OTC | 5 mg/L | 960 | 99% | water | 3 | ~100% | [106] |
| Electro | ceramic membrane | NH2-MIL-88B(Fe) | hydrothermal | flat-sheet | dead-end | recirculation | naproxen | 0.060 mmol/L | 15.9 | 97% | water | 5 | 89% | [107] |
| Electro | coal-based carbon membrane (anode) the titanium plate (cathode) | polyaniline (anode) –(cathode) | electrochemical polymerization deposition | tubular | dead-end | single pass | phenol | 50 mg/L | 77.42 | 99% | water | 10 | 97% | [108] |
| Electro | coal-based carbon membrane (anode) the titanium plate (cathode) | polyaniline (anode) –(cathode) | electrochemical polymerization deposition | tubular | dead-end | single pass | phenol | 50 mg/L | – | 99.3% | water | 6 | 96% | [109] |
| Electro | titanium (anode) carbon felt (cathode) | RuO2-IrO2 (anode) carbon black and polyaniline (cathode) | phase inversion | flat-sheet | dead-end | recirculation | TC | 50 mg/L | 3857.14 | 92.9% | water | 15 | 95% | [110] |
| Electro + PMS | titanium plate (anode) PVDF (cathode) | –(anode) CNT doping polypyrrole (cathode) | phase inversion | flat-sheet | cross-flow | single pass | CBZ | 2 mg/L | 142.5 | 95% | water | 20 | 95% | [111] |
| PMS | PTFE | Mn3O4/CNNS | filtration | flat-sheet | dead-end | recirculation | 4-chlorophenol | 0.1 mM | 15.92 | 90% | ethanol | 6 | 98% | [112] |
| PMS | PTFE | NiCo@NCNT | filtration | flat-sheet | dead-end | single pass | ibuprofen | 20 mg/L | 305.73 | 100% | ethanol | 3 | 95% | [113] |
| PMS | PVDF | Fe-Co@NCNT | phase inversion | flat-sheet | cross-flow | single pass | BPA | 30 mg/L | 45.5 | 100% | ethanol | 10 | 60% | [114] |
| PMS | PES | CoCu-LDH/PEG/calotropis gigantean fiber | filtration | flat-sheet | dead-end | single pass | SMX | 10 mg/L | 124.78 | 93% | ethanol | 10 | 100% | [115] |
| PMS | nylon | MnOOH | filtration | flat-sheet | dead-end | recirculation | 2,4-Dichlorophenol | 25 mg/L | 238.8 | 97.9% | ethanol | 4 | 99% | [116] |
| Electro | coal-based carbon membrane (anode) Ti plate (cathode) | –(anode) –(cathode) | – | tubular | dead-end | single pass | RhB | 250 mg/L | 88.7 | 100% | ethanol | 6 | ~100% | [117] |
| Electro | coal-based carbon membrane (anode) Ti plate (cathode) | CuO (anode) –(cathode) | electrodeposition | tubular | dead-end | single pass | RhB | 300 mg/L | 66.51 | 99.9% | ethanol | 4 | ~100% | [118] |
| PMS | Ag-La0.8Ca0.2Fe0.94O3−δ | – | phase inversion and calcination | hollow fiber | dead-end | recirculation | MB | 10 mg/L | 2228.57 | 90% | H2SO4 | 5 | 78% | [119] |
| H2O2 | ceramic membrane | FeOCl | impregnation and calcination | flat-sheet | dead-end | single pass | pCBA | 50 μmol/L | 100 | 100% | HCl | 4 | 80% | [81] |
| Photo | PVDF | PDA/BiOCl0.875Br0.125 | pneumatic method | flat-sheet | dead-end | recirculation | roxarsone | 17.5 mg/L | 380.4 | 100% | NaOH | 4 | 79% | [120] |
| Photo +H2O2 | ceramic membrane | FeOCl | cross-linking | flat-sheet | dead-end | single pass | nitrobenzene | 10 μmol/L | 69.2 | 100% | NaOH | 5 | 100% | [121] |
| Electro | Ti sheet (anode) PTFE (cathode) | – (anode) CNT@MIL-101(Fe) (cathode) | filtration | flat-sheet | dead-end | recirculation | TC | 17.8 mg/L | 25.35 | 94.3% | NaOH | 4 | 88% | [122] |
| Electro + PMS | PTFE | CNT-Fe3O4 | filtration and hydrothermal | flat-sheet | dead-emd | recirculation | roxarsone | 1.5 mg/L | – | 91.8% | NaOH | 4 | 90% | [123] |
4.2. Regeneration Performance of Catalytic Membranes by Heat Treatment
| AOP Type | Substrate Membrane | Catalyst | Fabrication Method | Membrane Type | Filtration Mode | Operation Mode | Target Pollutant | C0 | Flux (L/(m2·h)) | R0 | Treatment Temperature/°C | Atmosphere | Number of Heat Treatment Cycles | η″ | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMS | PTFE | NiCo@NCNT | filtration | flat-sheet | dead-end | single pass | ibuprofen | 20 mg/L | 305.73 | 100% | 350 | Ar | 1 | 100% | [113] |
| PDS | CNT | – | wet-spinning and pyrolyzation | hollow fiber | dead-end | single pass | phenol | 10 mg/L | – | 97% | 800 | N2 | 1 | 100% | [125] |
| PMS | ceramic membrane | CNT@nitrogen-doped carbon | filtration | flat-sheet | dead-end | single pass | SMX | 20 mg/L | 6.8 | 65% | 250 | air | 1 | 100% | [126] |
| PMS | nylon | nitrogen-doped rGO/CNT | filtration | flat-sheet | dead-end | single pass | 4-chlorophenol | 0.16 mM | 72.55 | 100% | 90 | air | 1 | 94% | [127] |
| PMS | PVDF | CoxFe3−xO4 | filtration | flat-sheet | dead-end | single pass | BPA | 10 mg/L | 100 | 99.9% | 100 | air | 1 | ~100% | [128] |
| PMS | steel mesh/non-woven fabric | LaFexCo1−xO3−λ/SiO2 | filtartion | flat-sheet | dead-end | recirculation | TC | 20 mg/L | 1064.5 | 99% | 450 | air | 1 | ~100% | [129] |
| PMS | stainless steel mesh | CoFe2O4/diatomite | filtration | flat-sheet | dead-end | recirculation | RhB | 50 mg/L | 98.68 | 99.9% | 200 | air | 5 | 87% | [130] |
| Air | ceramic membrane | Mo | hydrothermal and calcination | flat-sheet | dead-end | single pass | safranine O | 10 mg/L | 324 | 96% | 300 | air | 3 | 94% | [131] |
4.3. Regeneration Performance of Catalytic Membranes by Other Methods
| AOP Type | Substrate Membrane | Catalyst | Fabrication Method | Membrane Type | Filtration Mode | Operation Mode | Target Pollutant | C0 | Flux (L/(m2·h)) | R0 | Regeneration Method | Number of Regeneration Cycles | η″ | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMS | ceramic membrane | CoFe2O4 | hydrothermal | flat-sheet | dead-end | single pass | ofloxacin | 40 μmol/L | 98 | 100% | PMS | 8 | 95% | [132] |
| PMS | ceramic membrane | CoFe2O4 | impregnation and calcination | flat-sheet | dead-end | single pass | SMX | 10 mg/L | 236 | 50% | PMS | 3 | 95% | [133] |
| PMS | ceramic membrane | Co3O4 | impregnation and calcination | flat-sheet | dead-end | single pass | SMX | 0.08 mmol/L | 236 | 46% | PMS | 3 | 54% | [134] |
| PMS | ceramic membrane | Co3O4 | in situ reaction and calcination | flat-sheet | dead-end | recirculation | SMX | 10 mg/L | 211.68 | 75% | PMS | 1 | 95% | [135] |
| O3 | ceramic membrane | Mn2O3 | impregnation and calcination | flat-sheet | dead-end | single pass | SMX | 20 mg/L | 35 | 81.3% | O3 | 5 | ~100% | [136] |
| Photo | PVDF | TiO2/PSS | layer-by-layer deposition | flat-sheet | cross-flow | recirculation | Lanasol Blue 3R | 30 mg/L | 300 | 91.42% | photo | 5 | ~100% | [137] |
| PMS | Ni-foam | ZIF-67 | electrodeposion and calcination | flat-sheet | dead-end | recirculation | BPA | 0.044 mmol/L | 12.68 | 100% | photo | 5 | ~100% | [138] |
| Electro | porous Ti plate (anode) porous Ti plate (cathode) | blue TiO2 (anode) blue TiO2 (cathode) | electrooxidation, calcination and electroreduction | flat-sheet | dead-end | recirculation | CBZ | 10 mg/L | 11464.97 | 95.3% | electrochemical reduction | 4 | 99% | [139] |
| Electro | Ti membrane (anode) Ti mesh (cathode) | blue TiO2 (anode) –(cathode) | electrooxidation, calcination and electroreduction | flat-sheet | dead-end | single pass | MB | 10 mg/L | – | 99.5% | electrochemical reduction | 2 | ~100% | [140] |
| Electron | ceramic membrane (anode) stainless steel mesh (cathode) | CNT (anode) –(cathode) | filtration and pyrolyzation | tubular | cross-flow | single pass | TOC of water from Lingshui Reservoir | 3.281 mg/L | 347.84 | 90% | electrochemical back washing | 4 | 95% | [141] |
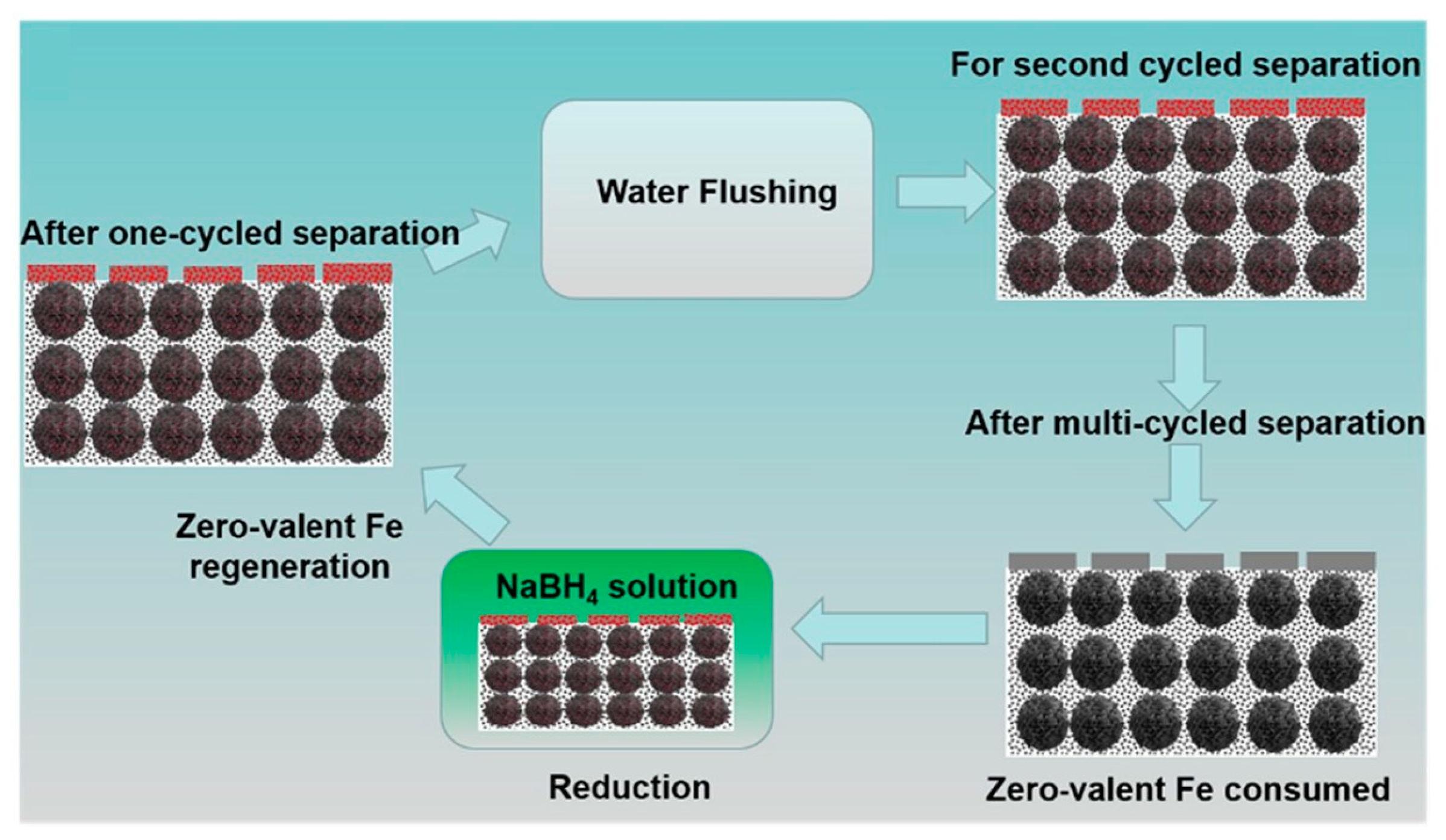
| H2O2 | PVDF | Fe/PDA/PEI | deposition, filtration and reduction | flat-sheet | cross-flow | single pass | RhB | 10 mg/L | 400 | 95% | chemical reduciton with NaBH4 | 1 | ~100% | [142] |
5. Recommendations for Future Works
- In future works, it is suggested to always include the performance stability or regeneration property of catalytic membranes when evaluating their abilities for pollutant removal. Catalytic membranes with high activity but poor stability and regeneration property are far away from practical applications.
- When evaluating the stability and regeneration property, it is suggested to clearly describe the process with all the details provided. Besides the removal efficiency of pollutants, other factors including change in kinetic constant, TMP, flux, and TOC/COD removal should also be paid attention to present a more comprehensive profile.
- Although the effects of inorganic ions and natural organic matter for the activity of fresh catalytic membranes are generally reported, their effects on the performance stability and regeneration property have been rarely investigated. In future works, more attention should be paid to this issue because these species may lead to serious membrane fouling, weakening the performance stability and making the current regeneration methods less effective.
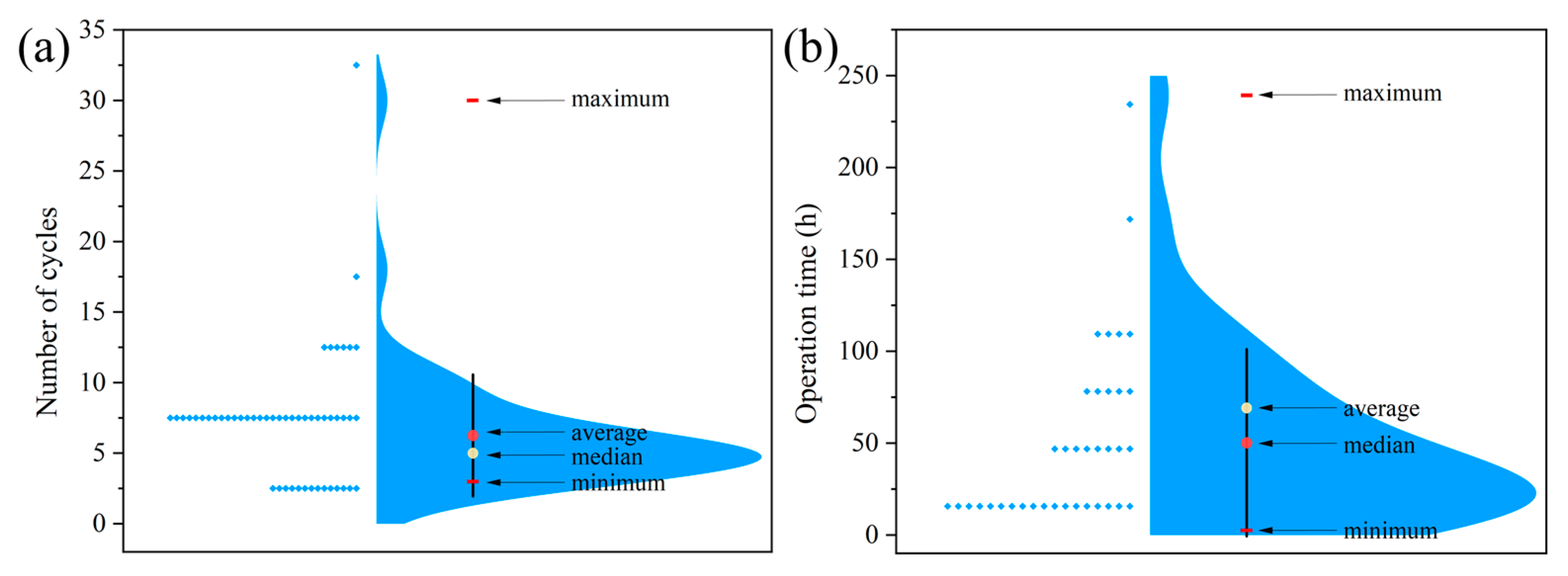
- The number of cycles in the multi-cycle run and the operation time in the long-time run summarized in Table 1 and Table 2 have been statistically analyzed. As shown in Figure 7a, for the number of cycles, most of the data were in the range of 5–10 cycles, and the median and average values were 5 and 6.3 cycles (n = 52). The operation time mostly fell in the range of 0–25 h with a median value of 33 h and an average value of 50.2 h (n = 37) (Figure 7b). For future works, it is suggested to select suitable cycle numbers and operation times based on these statistics. When possible, a large cycle number and a longer operation time are suggested, which can provide better references for potential practical applications of the investigated catalytic membranes.
- The types and distributions of organic pollutants on the fouled membrane are different from those in the polluted water, and the utilization of another AOP for its regeneration may be more efficient. In other words, a certain AOP may be suitable to regenerate the fouled membrane, although it is not suitable to treat the polluted water. Through the combination of different AOPs, better process optimization and lower whole process cost may be achieved.
- Faced with the decreased activity of metal-based membranes caused by metal-leaching, re-loading of metal species may be a possible solution, which is more economic than simply discarding the used membrane. However, the organic pollutants on the used membrane may impact the metal loading process, and more research on this issue is still in demand.
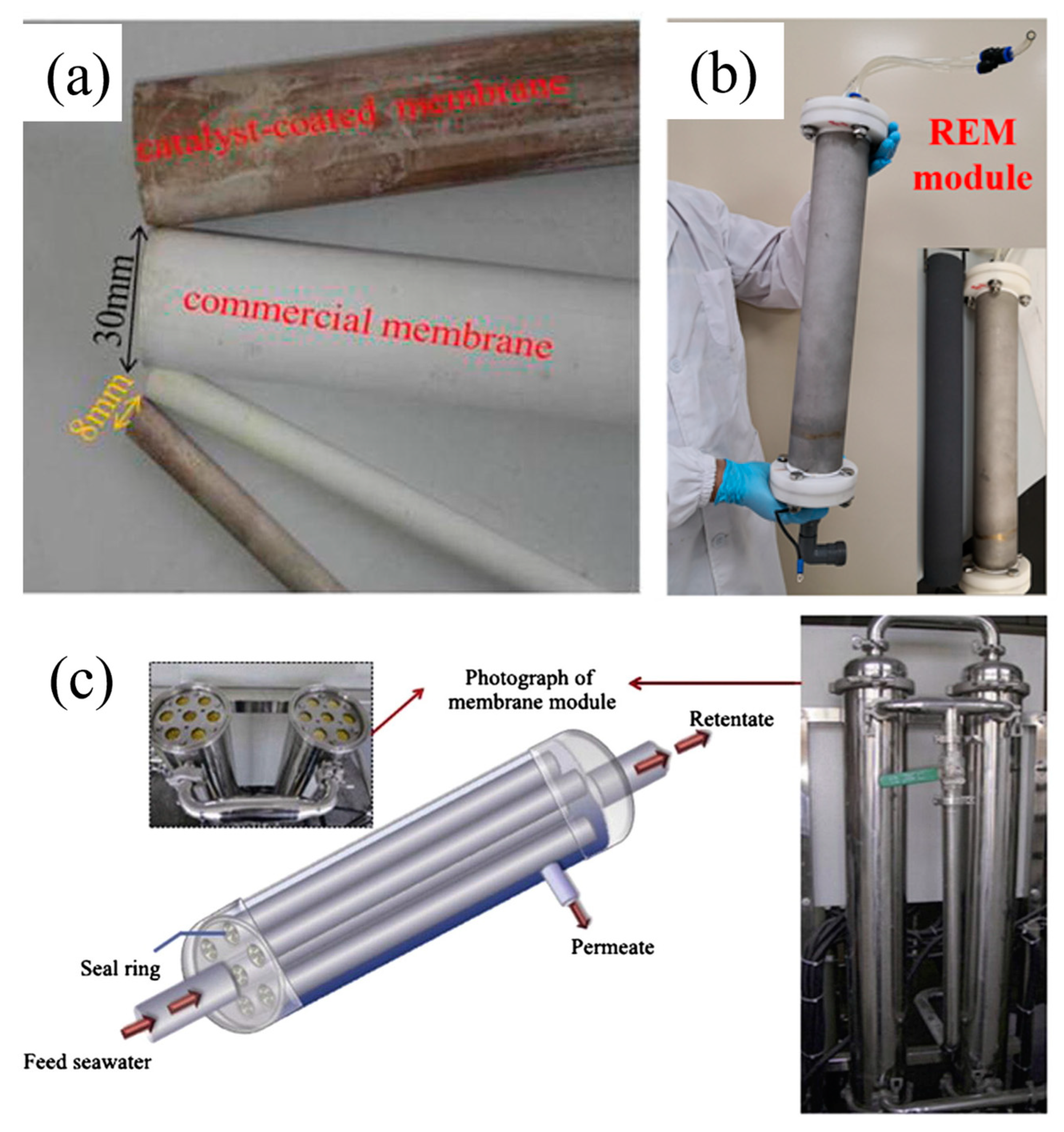
- Almost all of the reports summarized and discussed above were conducted at bench-scale. To the best of our knowledge, there is no industrial scale application of this new technology at the current stage. Only several pilot-scale studies are available, where catalytic membranes were coupled with ozone oxidation [147,148,149], electrochemical oxidation [150,151], and electro-Fenton [152] processes. In these studies, to increase the throughput of the catalytic filtration process, two strategies have been utilized. One is to build catalytic membranes with larger sizes, as in the cases of Ti-Mn/ceramic membrane (Figure 8a) [147] and PbO2/Ti membrane (Figure 8b) [151]. Another is the numbering up strategy where multiple bench-scale membranes are integrated, such as in the cases of Ti-Mn/TiO2/ceramic membrane (Figure 8c) [148]. In these pilot studies, natural water sources with complex compositions were treated, leading to membrane fouling in the long run and making membrane regeneration inevitable. From a practical viewpoint, the large-sized membranes are difficult to fabricate and difficult to regenerate by some of the above-mentioned methods such as the heat treatment method, during which large-sized furnaces are required to fit the large-sized membranes, increasing the equipment investment. The numbering up strategy is convenient for membrane fabrication, but complex pipe systems would be required, making it difficult to maintain, disassemble, and regenerate the integrated multiple membranes. In future works, more research is required regarding the stability and regeneration property of pilot-scale catalytic membranes.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process. Saf. Environ. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total. Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Kang, H.; Zhang, Z. Gravity-driven layered double hydroxide nanosheet membrane activated peroxymonosulfate system for micropollutant degradation. J. Hazard. Mater. 2022, 425, 127988. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Sun, X. A Self-cleaning, catalytic titanium carbide (MXene) membrane for efficient tetracycline degradation through peroxymonosulfate activation: Performance evaluation and mechanism study. Sep. Purif. Technol. 2021, 279, 119796. [Google Scholar] [CrossRef]
- Pan, Z.; Song, C.; Li, L.; Wang, H.; Pan, Y.; Wang, C.; Li, J.; Wang, T.; Feng, X. Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: Recent advances and future prospects. Chem. Eng. J. 2019, 376, 120909. [Google Scholar] [CrossRef]
- Zhu, X.; Jassby, D. Electroactive Membranes for Water Treatment: Enhanced Treatment Functionalities, Energy Considerations, and Future Challenges. Acc. Chem. Res. 2019, 52, 1177–1186. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Hu, X.; Hua, T. Electrochemical advanced oxidation processes coupled with membrane filtration for degrading antibiotic residues: A review on its potential applications, advances, and challenges. Sci. Total. Environ. 2021, 784, 146912. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Wang, D.; Liu, S.; Li, X.; Zhou, X.; Yi, H.; Li, B.; Zhang, M.; Li, L.; et al. In situ chemical oxidation: Peroxide or persulfate coupled with membrane technology for wastewater treatment. J. Mater. Chem. A 2021, 9, 11944–11960. [Google Scholar] [CrossRef]
- Yu, C.; Xiong, Z.; Zhou, H.; Zhou, P.; Zhang, H.; Huang, R.; Yao, G.; Lai, B. Marriage of membrane filtration and sulfate radical-advanced oxidation processes (SR-AOPs) for water purification: Current developments, challenges and prospects. Chem. Eng. J. 2021, 433, 133802. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Yu, S.; Chen, B.; Chen, C.; Shen, L.; Li, B.; Lin, H. The coupling of persulfate activation and membrane separation for the effective pollutant degradation and membrane fouling alleviation. Chem. Eng. J. 2023, 451, 139009. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef] [PubMed]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.E.; Akbour, R.A.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process.-Process. Intensif. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, H.; Jiang, S.; Zhu, M.; Zhou, Y.; Wang, D.; Fan, Y.; Zhang, D.; Zhang, L. Fabrication of FeOCl/MoS2 catalytic membranes for pollutant degradation and alleviating membrane fouling with peroxymonosulfate activation. J. Environ. Chem. Eng. 2022, 10, 107717. [Google Scholar] [CrossRef]
- Chen, B.; Hu, X.; Wang, J.; Li, R.; Shen, L.; Xu, Y.; Zhang, M.; Hong, H.; Lin, H. Novel catalytic self-cleaning membrane with peroxymonosulfate activation for dual-function wastewater purification: Performance and mechanism. J. Clean. Prod. 2022, 355, 131858. [Google Scholar] [CrossRef]
- Ye, J.; Dai, J.; Yang, D.; Li, C.; Yan, Y.; Wang, Y. 2D/2D confinement graphene-supported bimetallic Sulfides/g-C3N4 composites with abundant sulfur vacancies as highly active catalytic self-cleaning membranes for organic contaminants degradation. Chem. Eng. J. 2021, 418, 129383. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, B.; Zhang, C.; Zhang, L.; Zhang, L.; Sun, Y.; Yang, N. Co@N-C nanocatalysts anchored in confined membrane pores for instantaneous pollutants degradation and antifouling via peroxymonosulfate activation. J. Water Process Eng. 2022, 47, 102639. [Google Scholar] [CrossRef]
- Ye, J.; Dai, J.; Li, C.; Yan, Y. Lawn-like Co3O4@N-doped carbon-based catalytic self-cleaning membrane with peroxymonosulfate activation: A highly efficient singlet oxygen dominated process for sulfamethoxazole degradation. Chem. Eng. J. 2021, 421, 102639. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Li, Z.; Yang, D.; Li, C.; Yan, Y.; Dai, J. 2D confinement freestanding graphene oxide composite membranes with enriched oxygen vacancies for enhanced organic contaminants removal via peroxymonosulfate activation. J. Hazard. Mater. 2021, 417, 126028. [Google Scholar] [CrossRef]
- Pedrosa, M.; Drazic, G.; Tavares, P.B.; Figueiredo, J.L.; Silva, A.M.T. Metal-free graphene-based catalytic membrane for degradation of organic contaminants by persulfate activation. Chem. Eng. J. 2019, 369, 223–232. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Han, Y.; Wang, X.; Liu, S.; Zhang, L.; Sun, Y.; Jiang, B. Development of polyacrylonitrile/perovskite catalytic membrane with abundant channel-assisted reaction sites for organic pollutant removal. Chem. Eng. J. 2022, 437, 135163. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.Q.; Zhang, L.F.; Guo, J.Q.; Wei, J.H. Polysulfone Ultrafiltration Membrane Promoted by Brownmillerite SrCuxCo1−xO3−λ-Deposited MCM-41 for Industrial Wastewater Decontamination: Catalytic Oxidation and Antifouling Properties. Ind. Eng. Chem. Res. 2020, 59, 7805–7815. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Feng, G.; Ren, B.; Pan, Z.; Shi, Y.; Xu, R.; Wang, P.; Liu, Y.; Wang, G.; et al. Carbon nanotube membrane armed with confined iron for peroxymonosulfate activation towards efficient tetracycline removal. Sep. Purif. Technol. 2023, 312, 123319. [Google Scholar] [CrossRef]
- Qian, F.; Luo, J.; Yin, H.; Liu, F.; Gao, S.; Gu, X. Carbonaceous composite membranes for peroxydisulfate activation to remove sulfamethoxazole in a real water matrix. Chemosphere 2022, 288, 132597. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, J.; Wang, Q.; Zhao, Z.; Cui, F.; Li, G. Low-temperature sintered high-strength CuO doped ceramic hollow fiber membrane: Preparation, characterization and catalytic activity. J. Membr. Sci. 2019, 570–571, 333–342. [Google Scholar] [CrossRef]
- Wang, S.X.; Tian, J.Y.; Wang, Z.H.; Wang, Q.; Jia, J.L.; Hao, X.J.; Gao, S.S.; Cui, F.Y. Integrated process for membrane fouling mitigation and organic pollutants removal using copper oxide modified ceramic hollow fiber membrane with in-situ peroxymonosulfate activation. Chem. Eng. J. 2020, 396, 11. [Google Scholar] [CrossRef]
- Wang, S.; Tian, J.; Wang, Q.; Xiao, F.; Gao, S.; Shi, W.; Cui, F. Development of CuO coated ceramic hollow fiber membrane for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for bisphenol a degradation. Appl. Catal. B Environ. 2019, 256, 117783. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Fan, Q.; Wang, L.; Shi, S.; Luo, X.; Zhu, X.; Wu, D.; Liang, H. Preparation of Co3O4@carbon nanotubes modified ceramic membrane for simultaneous catalytic oxidation and filtration of secondary effluent. Chem. Eng. J. 2023, 454, 140450. [Google Scholar] [CrossRef]
- Chen, L.; Maqbool, T.; Fu, W.; Yang, Y.; Hou, C.; Guo, J.; Zhang, X. Highly efficient manganese (III) oxide submerged catalytic ceramic membrane for nonradical degradation of emerging organic compounds. Sep. Purif. Technol. 2022, 295, 121272. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Q.; Yan, B.; Guo, Y.; Xia, W.; Li, J.; Cui, F.; Tian, J. A novel integrated process of ceramic membrane filtration coupled with peroxymonosulfate activation and adsorption for water treatment. Sep. Purif. Technol. 2022, 291, 120874. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Huang, Q.; Xiao, C. Poly(tetrafluoroethylene-co-hexafluoropropylene)/Ferric Oxide Hybrid Membranes for High Concentration of Dye Wastewater Treatment by Heterogeneous Fenton-Like Catalysis. Catal. Lett. 2021, 151, 3020–3029. [Google Scholar] [CrossRef]
- Karimnezhad, H.; Navarchian, A.H.; Tavakoli Gheinani, T.; Zinadini, S. Amoxicillin removal by Fe-based nanoparticles immobilized on polyacrylonitrile membrane: Individual nanofiltration or Fenton reaction, vs. engineered combined process. Chem. Eng. Res. Des. 2020, 153, 187–200. [Google Scholar] [CrossRef]
- Scaratti, G.; De Noni Junior, A.; Jose, H.J.; Peralta Muniz Moreira, R.D.F. 1,4-Dioxane removal from water and membrane fouling elimination using CuO-coated ceramic membrane coupled with ozone. Environ. Sci. Pollut. Res. 2020, 27, 22144–22154. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, L.; Chen, Z.; Huang, X.; Wang, X.; Zhang, X.; Wen, X. Novel catalytic ceramic membranes anchored with MnMe oxide and their catalytic ozonation performance towards atrazine degradation. J. Membr. Sci. 2022, 648, 120362. [Google Scholar] [CrossRef]
- Guo, Y.; Song, Z.; Xu, B.; Li, Y.; Qi, F.; Croue, J.P.; Yuan, D. A novel catalytic ceramic membrane fabricated with CuMn2O4 particles for emerging UV absorbers degradation from aqueous and membrane fouling elimination. J. Hazard. Mater. 2018, 344, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Sun, J.; Wang, W.; Wang, Z.; Zhang, Y.; Xu, B.; Qi, F. Stable synergistic decontamination and self-cleaning performance of powerful N-rGO catalytic ozonation membrane: Clustering effect of free electrons and role of interface properties. Appl. Catal. B Environ. 2021, 283, 119662. [Google Scholar] [CrossRef]
- Dekkouche, S.; Morales-Torres, S.; Ribeiro, A.R.; Faria, J.L.; Fontàs, C.; Kebiche-Senhadji, O.; Silva, A.M.T. In situ growth and crystallization of TiO2 on polymeric membranes for the photocatalytic degradation of diclofenac and 17α-ethinylestradiol. Chem. Eng. J. 2022, 427, 131476. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Meringolo, C.; Poerio, T.; Scarpelli, F.; Godbert, N.; Di Profio, G.; Fontananova, E. Multistimuli Activation of TiO2/alpha-Alumina Membranes for Degradation of Methylene Blue. Ind. Eng. Chem. Res. 2017, 56, 11049–11057. [Google Scholar] [CrossRef]
- Li, C.; Lu, Z.; Ao, X.; Sun, W.; Huang, X. Degradation kinetics and removal efficiencies of pharmaceuticals by photocatalytic ceramic membranes using ultraviolet light-emitting diodes. Chem. Eng. J. 2022, 427, 130828. [Google Scholar] [CrossRef]
- Nair, A.K.; JagadeeshBabu, P.E. TiO2 nanosheet-graphene oxide based photocatalytic hierarchical membrane for water purification. Surf. Coat. Technol. 2017, 320, 259–262. [Google Scholar] [CrossRef]
- Chen, W.; Ye, T.; Xu, H.; Chen, T.; Geng, N.; Gao, X. An ultrafiltration membrane with enhanced photocatalytic performance from grafted N–TiO2/graphene oxide. RSC Adv. 2017, 7, 9880–9887. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, N.; Jiang, C.; Xu, C.; Yu, S.; Liang, P.; Zhang, X.; Liang, S.; Huang, X. Filtration-enhanced highly efficient photocatalytic degradation with a novel electrospun rGO@TiO2 nanofibrous membrane: Implication for improving photocatalytic efficiency. Appl. Catal. B Environ. 2020, 268, 118737. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Liu, X.; Sun, C.; Lv, Y.; Miao, R.; Wang, X. Dynamic photocatalytic membrane coated with ZnIn2S4 for enhanced photocatalytic performance and antifouling property. Chem. Eng. J. 2020, 379, 122379. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, Z.; Wei, J.; Xu, Q.; Zhang, X.; Wei, Z. Efficient degradation of antibiotics by photo-Fenton reactive ceramic membrane with high flux by a facile spraying method under visible LED light. J. Clean. Prod. 2022, 366, 132849. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.J.; Wu, G.P.; Darling, S.B.; Xu, Z.K. Photocatalytic Nanofiltration Membranes with Self-Cleaning Property for Wastewater Treatment. Adv. Funct. Mater. 2017, 27, 8. [Google Scholar] [CrossRef]
- Deng, R.; Xia, X.-Z.; Han, J.-C.; Wu, Q.-Y.; Yang, H.-C. Siphon-driven interfacial photocatalytic reactors enhanced by capillary flow for continuous wastewater treatment. Sep. Purif. Technol. 2022, 300, 121835. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Chen, J.; Wen, S.; Li, D.; Wang, B.; Zhang, Q. Multifunctional ferrocene-based photo-Fenton membrane: An efficient integration of rejection and catalytic process. Sep. Purif. Technol. 2022, 298, 121557. [Google Scholar] [CrossRef]
- Li, N.; Chen, G.Y.; Zhao, J.H.; Yan, B.B.; Cheng, Z.J.; Meng, L.J.; Chen, V. Self-cleaning PDA/ZIF-67@PP membrane for dye wastewater remediation with peroxymonosulfate and visible light activation. J. Membr. Sci. 2019, 591, 9. [Google Scholar] [CrossRef]
- Zhu, Z.S.; Qu, J.; Hao, S.M.; Han, S.; Jia, K.L.; Yu, Z.Z. alpha-Fe2O3 Nanodisk/Bacterial Cellulose Hybrid Membranes as High-Performance Sulfate-Radical-Based Visible Light Photocatalysts under Stirring/Flowing States. ACS Appl. Mater. Interfaces 2018, 10, 30670–30679. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Chen, Q.Y.; Liu, L.F.; Wang, Y.; Leung, M.K.H. Activation of peroxymonosulfate and recycled effluent filtration over cathode membrane CNFs-CoFe2O4/PVDF in a photocatalytic fuel cell for water pollution control. Chem. Eng. J. 2020, 399, 125731. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Dong, X.; Zhang, X.; Ma, H. Sodium persulfate based PVDF membrane for concurrent advanced oxidation and ultrafiltration of ofloxacin in water. Chem. Eng. J. 2017, 315, 509–515. [Google Scholar] [CrossRef]
- Ren, L.; Chen, M.; Ma, J.; Li, Y.; Wang, Z. Pd–O2 interaction and singlet oxygen formation in a novel reactive electrochemical membrane for ultrafast sulfamethoxazole oxidation. Chem. Eng. J. 2022, 428, 131194. [Google Scholar] [CrossRef]
- Yu, S.; Gao, Y.; Khan, R.; Liang, P.; Zhang, X.; Huang, X. Electrospun PAN-based graphene/SnO2 carbon nanofibers as anodic electrocatalysis microfiltration membrane for sulfamethoxazole degradation. J. Membr. Sci. 2020, 614, 118368. [Google Scholar] [CrossRef]
- Qian, X.; Xu, L.; Zhu, Y.; Yu, H.; Niu, J. Removal of aqueous triclosan using TiO2 nanotube arrays reactive membrane by sequential adsorption and electrochemical degradation. Chem. Eng. J. 2021, 420, 127615. [Google Scholar] [CrossRef]
- Tan, T.-Y.; Zeng, Z.-T.; Zeng, G.-M.; Gong, J.-L.; Xiao, R.; Zhang, P.; Song, B.; Tang, W.-W.; Ren, X.-Y. Electrochemically enhanced simultaneous degradation of sulfamethoxazole, ciprofloxacin and amoxicillin from aqueous solution by multi-walled carbon nanotube filter. Sep. Purif. Technol. 2020, 235, 116167. [Google Scholar] [CrossRef]
- Pan, M.; Wang, J.; Gao, G.; Chew, J.W. Incorporation of single cobalt active sites onto N-doped graphene for superior conductive membranes in electrochemical filtration. J. Membr. Sci. 2020, 602, 117966. [Google Scholar] [CrossRef]
- Malekabadi, F.K.; Yousefi, F.; Karimi, R.; Ghaedi, M.; Dashtian, K. Electrocatalytic membrane containing CuFeO2/nanoporous carbon for organic dye removal application. Chem. Eng. Res. Des. 2022, 183, 345–356. [Google Scholar] [CrossRef]
- Wei, G.; Quan, X.; Fan, X.; Chen, S.; Zhang, Y. Carbon-nanotube-based sandwich-like hollow fiber membranes for expanded microcystin-LR removal applications. Chem. Eng. J. 2017, 319, 212–218. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, J.; Dai, R.; Wu, Z.; Wang, Z. Preferential removal of 2,4-dichlorophenoxyacetic acid from contaminated waters using an electrocatalytic ceramic membrane filtration system: Mechanisms and implications. Chem. Eng. J. 2020, 387, 124132. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Li, F.; Hu, X.; Xie, Z.; Hua, T. Activation of peroxymonosulfate in an electrochemical filter by MnFe2O4-rGO electro-assisted catalytic membrane for the degradation of oxytetracycline. J. Environ. Chem. Eng. 2022, 10, 107008. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, M.; Zhao, Y.; Wang, L.; Lu, D.; Ma, J. Electrified ceramic membrane actuates non-radical mediated peroxymonosulfate activation for highly efficient water decontamination. Water Res. 2022, 225, 119140. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Conlan, X.A.; Zeng, X.; Kong, L.; O’Dell, L.A.; Sadek, A.; Merenda, A.; Dumée, L.F. Stimuli-responsive heterojunctions based photo-electrocatalytic membrane reactors for reactive filtration of persistent organic pollutants. Chem. Eng. J. 2023, 452, 139374. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Chen, S.; Fan, X.; Quan, X.; Yu, H. Integration of membrane filtration and photoelectrocatalysis on g-C3N4/CNTs/Al2O3 membrane with visible-light response for enhanced water treatment. J. Membr. Sci. 2017, 541, 153–161. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Han, Y.; Wang, X.; Zhang, L.; Sun, Y.; Jiang, B. Highly dispersed beta-FeOOH nanocatalysts anchored in confined membrane pores for simultaneously improving catalytic and separation performance. Sep. Purif. Technol. 2021, 279, 119684. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Liu, H.; Qu, J. Confining Free Radicals in Close Vicinity to Contaminants Enables Ultrafast Fenton-like Processes in the Interspacing of MoS2 Membranes. Angew. Chem. Int. Ed. Engl. 2019, 58, 8134–8138. [Google Scholar] [CrossRef]
- Meng, C.; Wang, Z.; Zhang, W.; Cui, L.; Yang, B.; Xie, H.; Zhang, Z. Laminar membranes assembled by ultrathin cobalt-copper oxide nanosheets for nanoconfined catalytic degradation of contaminants. Chem. Eng. J. 2022, 449, 137811. [Google Scholar] [CrossRef]
- Lu, N.; Lin, H.; Xu, S.; Wang, J.; Han, Q.; Liu, F. Bone/muscle-inspired polymer porous matrix toughened carbon nanofibrous catalytic membranes for robust emerging contaminants removal. Chem. Eng. J. 2022, 442, 136069. [Google Scholar] [CrossRef]
- Xie, J.; Liao, Z.P.; Zhang, M.; Ni, L.H.; Qi, J.W.; Wang, C.H.; Sun, X.Y.; Wang, L.J.; Wang, S.B.; Li, J.S. Sequential Ultrafiltration-Catalysis Membrane for Excellent Removal of Multiple Pollutants in Water. Environ. Sci. Technol. 2021, 55, 2652–2661. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Liang, C.; Yin, L.; Yang, Y. Reconstructing the coordination environment of single atomic Fe-catalysts for boosting the Fenton-like degradation activities. Appl. Catal. B Environ. 2022, 315, 121536. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, C.; Zhang, W.; Zhang, S.; Yang, B.; Zhang, Z. Honeycomb-like holey Co3O4 membrane triggered peroxymonosulfate activation for rapid degradation of organic contaminants. Sci. Total Environ. 2022, 814, 152698. [Google Scholar] [CrossRef]
- Meng, C.; Ding, B.; Zhang, S.; Cui, L.; Ostrikov, K.K.; Huang, Z.; Yang, B.; Kim, J.H.; Zhang, Z. Angstrom-confined catalytic water purification within Co-TiOx laminar membrane nanochannels. Nat. Commun. 2022, 13, 4010. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Zhuang, J.; Shi, H. Hierarchical microsphere encapsulated in graphene oxide composite for durable synergetic membrane separation and Fenton-like degradation. Chem. Eng. J. 2022, 430, 133124. [Google Scholar] [CrossRef]
- Luo, J.; Liu, T.; Qian, F.; Xia, X.; Zhou, X.; Zhu, Y. Boosting non-radical oxidation in peroxydisulfate activation with carbonaceous catalytic membranes by coupling structural defects and nitrogen doping sites. J. Environ. Chem. Eng. 2022, 10, 108101. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, G.; Gao, Y.; Ma, H.; Dong, X.; Zhang, X. Surface and pore co-functionalized ceramic membrane with nitrogen doped carbon for enhanced water treatment through coupling peroxymonosulfate activation. Sep. Purif. Technol. 2022, 292, 120996. [Google Scholar] [CrossRef]
- Chen, L.; Maqbool, T.; Nazir, G.; Hou, C.; Yang, Y.; Guo, J.; Zhang, X. Developing the large-area manganese-based catalytic ceramic membrane for peroxymonosulfate activation: Applications in degradation of endocrine disrupting compounds in drinking water. J. Membr. Sci. 2022, 655, 120602. [Google Scholar] [CrossRef]
- Lu, N.; Lin, H.; Li, G.; Wang, J.; Han, Q.; Liu, F. ZIF-67 derived nanofibrous catalytic membranes for ultrafast removal of antibiotics under flow-through filtration via non-radical dominated pathway. J. Membr. Sci. 2021, 639, 119782. [Google Scholar] [CrossRef]
- Lin, H.; Fang, Q.; Wang, W.; Li, G.; Guan, J.; Shen, Y.; Ye, J.; Liu, F. Prussian blue/PVDF catalytic membrane with exceptional and stable Fenton oxidation performance for organic pollutants removal. Appl. Catal. B Environ. 2020, 273, 119047. [Google Scholar] [CrossRef]
- Huang, Z.H.; Zhang, X.; Wang, Y.X.; Sun, J.Y.; Zhang, H.; Liu, W.L.; Li, M.P.; Ma, X.H.; Xu, Z.L. Fe3O4/PVDF catalytic membrane treatment organic wastewater with simultaneously improved permeability, catalytic property and anti-fouling. Environ. Res. 2020, 187, 8. [Google Scholar] [CrossRef]
- Qu, W.; Chen, C.; Tang, Z.; Xia, D.; Ma, D.; Huang, Y.; Lian, Q.; He, C.; Shu, D.; Han, B. Electron-rich/poor reaction sites enable ultrafast confining Fenton-like processes in facet-engineered BiOI membranes for water purification. Appl. Catal. B Environ. 2022, 304, 120970. [Google Scholar] [CrossRef]
- Song, H.-M.; Zhu, L.-J.; Wang, Y.; Wang, G.; Zeng, Z.-X. Fe-based Prussian blue cubes confined in graphene oxide nanosheets for the catalytic degradation of dyes in wastewater. Sep. Purif. Technol. 2022, 288, 120676. [Google Scholar] [CrossRef]
- Yi, Q.; Li, Y.; Dai, R.; Li, X.; Li, Z.; Wang, Z. Efficient removal of neonicotinoid by singlet oxygen dominated MoSx/ceramic membrane-integrated Fenton-like process. J. Hazard. Mater. 2022, 439, 129672. [Google Scholar] [CrossRef]
- Zhang, S.; Hedtke, T.; Zhu, Q.; Sun, M.; Weon, S.; Zhao, Y.; Stavitski, E.; Elimelech, M.; Kim, J.H. Membrane-Confined Iron Oxychloride Nanocatalysts for Highly Efficient Heterogeneous Fenton Water Treatment. Environ. Sci. Technol. 2021, 55, 9266–9275. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Sarrigani, G.V.; Qu, J.; Ebrahimi, A.; Zhong, X.; Hou, W.-C.; Cairney, J.M.; Huang, J.; Wiley, D.E.; Wang, D.K. Designing Co3O4/silica catalysts and intensified ultrafiltration membrane-catalysis process for wastewater treatment. Chem. Eng. J. 2021, 419, 129465. [Google Scholar] [CrossRef]
- Gao, B.; Chen, W.; Liu, J.; An, J.; Wang, L.; Zhu, Y.; Sillanpää, M. Continuous removal of tetracycline in a photocatalytic membrane reactor (PMR) with ZnIn2S4 as adsorption and photocatalytic coating layer on PVDF membrane. J. Photochem. Photobiol. A Chem. 2018, 364, 732–739. [Google Scholar] [CrossRef]
- Zeng, H.J.; Yu, Z.X.; Shao, L.Y.; Li, X.H.; Zhu, M.; Liu, Y.C.; Feng, X.F.; Zhu, X.M. Ag2CO3@UiO-66-NH2 embedding graphene oxide sheets photocatalytic membrane for enhancing the removal performance of Cr(VI) and dyes based on filtration. Desalination 2020, 491, 11. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. Preparation and characterization of a novel photocatalytic self-cleaning PES nanofiltration membrane by embedding a visible-driven photocatalyst boron doped-TiO2-SiO2/CoFe2O4 nanoparticles. Sep. Purif. Technol. 2019, 209, 764–775. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, H. Preparation and decontamination performance of a flexible self-standing hydrogel photocatalytic membrane. J. Membr. Sci. 2022, 644, 119979. [Google Scholar] [CrossRef]
- Berger, T.E.; Regmi, C.; Schäfer, A.I.; Richards, B.S. Photocatalytic degradation of organic dye via atomic layer deposited TiO2 on ceramic membranes in single-pass flow-through operation. J. Membr. Sci. 2020, 604, 118015. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, K.; Ma, N.; Liu, X.; Yin, Z.; Wang, H.; Yang, X.; Wang, Y.; Qin, X.; Cheng, D.; et al. Catalytic membrane electrode with Co3O4 nanoarrays for simultaneous recovery of water and generation of hydrogen from wastewater. Sci. China Mater. 2022, 66, 651–663. [Google Scholar] [CrossRef]
- Pan, Z.L.; Yu, F.P.; Li, L.; Song, C.W.; Yang, J.W.; Wang, C.L.; Pan, Y.Q.; Wang, T.H. Electrochemical microfiltration treatment of bisphenol A wastewater using coal-based carbon membrane. Sep. Purif. Technol. 2019, 227, 8. [Google Scholar] [CrossRef]
- Li, X.; Shao, S.; Yang, Y.; Mei, Y.; Qing, W.; Guo, H.; Peng, L.E.; Wang, P.; Tang, C.Y. Engineering Interface with a One-Dimensional RuO2/TiO2 Heteronanostructure in an Electrocatalytic Membrane Electrode: Toward Highly Efficient Micropollutant Decomposition. Acs Appl. Mater. Interfaces 2020, 12, 21596–21604. [Google Scholar] [CrossRef]
- Zheng, J.J.; Wang, Z.W.; Ma, J.X.; Xu, S.P.; Wu, Z.C. Development of an Electrochemical Ceramic Membrane Filtration System for Efficient Contaminant Removal from Waters. Environ. Sci. Technol. 2018, 52, 4117–4126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.L.; Xia, X.; Han, J.L.; Ding, Y.C.; Haider, M.R.; Wang, A.J. Graphene Modified Electro-Fenton Catalytic Membrane for in Situ Degradation of Antibiotic Florfenicol. Environ. Sci. Technol. 2018, 52, 9972–9982. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, M.; Wang, J.; Huang, H. Synergistic electro-catalytic oxidation of ibuprofen in electro-peroxone system with flow-through carbon nanotube membrane cathode. Chem. Eng. J. 2022, 435, 135180. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Liu, W.; Ji, H.; Li, F.; Shen, C.; Fang, X.; Li, X.; Duan, X. A novel electrocatalytic filtration system with carbon nanotube supported nanoscale zerovalent copper toward ultrafast oxidation of organic pollutants. Water Res. 2021, 194, 116961. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, X.; Shi, L.; Yin, Y.; Zhang, L.-C.; Wu, Z.; Duan, X.; Wang, S.; Sun, H. Manganese oxide integrated catalytic ceramic membrane for degradation of organic pollutants using sulfate radicals. Water Res. 2019, 167, 115110. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Yu, H.; Yang, F.; Tang, C.Y.; Quan, X.; Dong, Y. High-flux robust ceramic membranes functionally decorated with nano-catalyst for emerging micro-pollutant removal from water. J. Membr. Sci. 2020, 611, 118281. [Google Scholar] [CrossRef]
- Wei, J.; Bi, J.; Zhang, L.; Han, D.; Gong, J. Gravity-driven Fe-doped CoTiO3/SiO2 fiber membrane with open catalytic network: Activation of peroxymonosulfate and efficient pollutants removal. Sep. Purif. Technol. 2022, 280, 119975. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Q.; Huang, R.; Guo, Y.; Yan, B.; Hao, X.; Li, J.; Xia, W.; Tian, J. Catalytic ceramic membrane integrated with granular activated carbon for efficient removal of organic pollutants. J. Water Process Eng. 2022, 47, 102751. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Shi, Y.; Zhu, J.; Zhao, B.; Zhang, Z.; Ding, G.; Zhang, H. Fabrication of Co3O4-Bi2O3-Ti catalytic membrane for efficient degradation of organic pollutants in water by peroxymonosulfate activation. J. Colloid Interface Sci. 2022, 607, 451–461. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Pan, Z.; Xu, R.; Wang, P.; Li, H.; Shi, Y.; Fan, X.; Song, C. MOF derivative functionalized titanium-based catalytic membrane for efficient sulfamethoxazole removal via peroxymonosulfate activation. J. Membr. Sci. 2022, 661, 120924. [Google Scholar] [CrossRef]
- Chen, S.; Teng, Q. Quantitative Immobilization of Phthalocyanine onto Bacterial Cellulose for Construction of a High-Performance Catalytic Membrane Reactor. Materials 2017, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Liang, F.; Wang, X.; Li, B. Loose composite nanofiltration membrane with in-situ immobilized β-FeOOH film for effective dyes degradation and separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130115. [Google Scholar] [CrossRef]
- Sun, M.H.; Zou, L.Z.; Wang, P.C.; Fan, X.F.; Pan, Z.L.; Liu, Y.M.; Song, C.W. Nano valent zero iron (NZVI) immobilized CNTs hollow fiber membrane for flow-through heterogeneous Fenton process. J. Environ. Chem. Eng. 2022, 10, 9. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, Z.; Cui, K.; Tang, Y.; Du, X.; He, B.; Li, M.; Feng, J.; Yu, B.; Xiong, W. Catalytic wet peroxide oxidation of phenolic wastewater on novel Cu/Mn-UiO-66@Al2O3 ceramic tube membrane catalysts. Chem. Eng. J. 2022, 430, 132787. [Google Scholar] [CrossRef]
- Li, M.; Yang, K.; Huang, X.; Liu, S.; Jia, Y.; Gu, P.; Miao, H. Efficient degradation of trimethoprim by catalytic ozonation coupled with Mn/FeOx-functionalized ceramic membrane: Synergic catalytic effect and enhanced anti-fouling performance. J. Colloid Interface Sci. 2022, 616, 440–452. [Google Scholar] [CrossRef]
- Espindola, J.C.; Cristovao, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Ye, Z.; Oriol, R.; Yang, C.; Sirés, I.; Li, X.-Y. A novel NH2-MIL-88B(Fe)-modified ceramic membrane for the integration of electro-Fenton and filtration processes: A case study on naproxen degradation. Chem. Eng. J. 2022, 433, 133547. [Google Scholar] [CrossRef]
- Pan, Z.L.; Xin, H.; Xu, S.; Xu, R.S.; Wang, P.C.; Yuan, Y.; Fan, X.F.; Song, Y.X.; Song, C.W.; Wang, T.H. Preparation and performance of polyaniline modified coal-based carbon membrane for electrochemical filtration treatment of organic wastewater. Sep. Purif. Technol. 2022, 287, 13. [Google Scholar] [CrossRef]
- Pan, Z.; Xu, S.; Xin, H.; Yuan, Y.; Xu, R.; Wang, P.; Yan, X.; Fan, X.; Song, C.; Wang, T. High performance polypyrrole coated carbon-based electrocatalytic membrane for organic contaminants removal from aqueous solution. J. Colloid Interface Sci. 2022, 626, 283–295. [Google Scholar] [CrossRef]
- Song, X.; Jo, C.; Zhou, M. Enhanced tetracycline removal using membrane-like air-cathode with high flux and anti-fouling performance in flow-through electro-filtration system. Water Res. 2022, 224, 119054. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Wang, Y.; Song, Y.; Zhao, C.; Han, L. Synergistic oxidation-filtration process of electroactive peroxydisulfate with a cathodic composite CNT-PPy/PVDF ultrafiltration membrane. Water Res. 2022, 210, 117971. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Xie, M.; Kong, L.S.; Lu, W.H.; Feng, Z.Y.; Zhan, J.H. Mn3O4 nanodots loaded g-C3N4 nanosheets for catalytic membrane degradation of organic contaminants. J. Hazard. Mater. 2020, 390, 122146. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, H.Y.; Duan, X.G.; Sun, H.Q.; Tan, X.Y.; Liu, S.M.; Wang, S.B. Magnetic Ni-Co alloy encapsulated N-doped carbon nanotubes for catalytic membrane degradation of emerging contaminants. Chem. Eng. J. 2019, 362, 251–261. [Google Scholar] [CrossRef]
- Ye, J.; Dai, J.; Wang, L.; Li, C.; Yan, Y.; Yang, G. Investigation of catalytic self-cleaning process of multiple active species decorated macroporous PVDF membranes through peroxymonosulfate activation. J. Colloid Interface Sci. 2021, 586, 178–189. [Google Scholar] [CrossRef]
- Guo, R.N.; Li, Y.H.; Chen, Y.; Liu, Y.; Niu, B.H.; Gou, J.F.; Cheng, X.W. Efficient degradation of sulfamethoxazole by CoCu LDH composite membrane activating peroxymonosulfate with decreased metal ion leaching. Chem. Eng. J. 2021, 417, 127887. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wang, X.S.; Li, Y.C.; Zuo, K.C.; Lyu, C. A novel MnOOH coated nylon membrane for efficient removal of 2,4-dichlorophenol through peroxymonosulfate activation. J. Hazard. Mater. 2021, 414, 125526. [Google Scholar] [CrossRef]
- Sun, M.H.; An, J.S.; Pan, Z.L.; Feng, G.Q.; Fan, X.F.; Song, C.W.; Wang, T.H. Enhanced organic wastewater treatment performance in electrochemical filtration process of coal-based carbon membrane via the simple Fe2+ addition. Sep. Purif. Technol. 2021, 276, 10. [Google Scholar] [CrossRef]
- Li, C.; Feng, G.Q.; Pan, Z.L.; Song, C.W.; Fan, X.F.; Tao, P.; Wang, T.H.; Shao, M.H.; Zhao, S.F. High-performance electrocatalytic microfiltration CuO/Carbon membrane by facile dynamic electrodeposition for small-sized organic pollutants removal. J. Membr. Sci. 2020, 601, 13. [Google Scholar] [CrossRef]
- Ma, T.; Liu, L.; Meng, B.; Gao, J.; Wang, S.; Liu, S. Heterogeneous activation of peroxymonosulfate via a Ag-La0.8Ca0.2Fe0.94O3−δ perovskite hollow fibre membrane reactor for dye degradation. Sep. Purif. Technol. 2019, 211, 298–302. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Z.; Wang, Y.; Ding, Z.; Xu, X.; Peng, W.; Fan, J.; Zhou, X.; Liu, J. BiOCl0.875Br0.125/polydopamine functionalized PVDF membrane for highly efficient visible-light-driven photocatalytic degradation of roxarsone and simultaneous arsenic immobilization. Chem. Eng. J. 2020, 402, 126048. [Google Scholar] [CrossRef]
- Liu, F.; Yao, H.; Sun, S.; Tao, W.; Wei, T.; Sun, P. Photo-Fenton activation mechanism and antifouling performance of an FeOCl-coated ceramic membrane. Chem. Eng. J. 2020, 402, 125477. [Google Scholar] [CrossRef]
- Dai, Y.; Yao, Y.; Li, M.; Fang, X.; Shen, C.; Li, F.; Liu, Y. Carbon nanotube filter functionalized with MIL-101(Fe) for enhanced flow-through electro-Fenton. Environ. Res. 2022, 204, 112117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zheng, W.; Jin, L.; Zhang, S.; You, S.; Liu, Y. Effective peroxymonosulfate activation using electrified nanohybrid filter towards one-step decontamination of roxarsone: Performance and mechanism. J. Environ. Chem. Eng. 2022, 10, 108643. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Fu, W.; Hua, L.; Zhang, G.; Zhang, W. Reactive Photo-Fenton ceramic membranes: Synthesis, characterization and antifouling performance. Water Res. 2018, 144, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Yu, Y.L.; Yang, Y.; Sun, T.J.; Dong, S.J.; Yang, H.L.; Liu, Y.M.; Fan, X.F.; Song, C.W. Improved separation performance of carbon nanotube hollow fiber membrane by peroxydisulfate activation. Sep. Purif. Technol. 2021, 276, 8. [Google Scholar] [CrossRef]
- Ma, H.; Wang, G.; Miao, Z.; Dong, X.; Zhang, X. Integration of membrane filtration and peroxymonosulfate activation on CNT@nitrogen doped carbon/Al2O3 membrane for enhanced water treatment: Insight into the synergistic mechanism. Sep. Purif. Technol. 2020, 252, 117479. [Google Scholar] [CrossRef]
- Ma, H.; Wang, G.; Xu, Z.; Dong, X.; Zhang, X. Confining peroxymonosulfate activation in carbon nanotube intercalated nitrogen doped reduced graphene oxide membrane for enhanced water treatment: The role of nanoconfinement effect. J. Colloid Interface Sci. 2022, 608, 2740–2751. [Google Scholar] [CrossRef]
- Qu, W.; Wen, H.; Qu, X.; Guo, Y.; Hu, L.; Liu, W.; Tian, S.; He, C.; Shu, D. Enhanced Fenton-like catalysis for pollutants removal via MOF-derived CoxFe3-xO4 membrane: Oxygen vacancy-mediated mechanism. Chemosphere 2022, 303, 135301. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhang, Y.Q.; Wei, J.H.; Liu, W. Perovskite LaFexCo1−xO3−λ deposited SiO2 catalytic membrane for deeply cleaning wastewater. Chem. Eng. J. 2021, 403, 126386. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.Q.; Wang, J.Q. Nano spinel CoFe2O4 deposited diatomite catalytic separation membrane for efficiently cleaning wastewater. J. Membr. Sci. 2020, 615, 118559. [Google Scholar] [CrossRef]
- Bao, Y.; Lee, W.J.; Wang, P.; Xing, J.; Liang, Y.N.; Lim, T.-T.; Hu, X. A novel molybdenum-based nanocrystal decorated ceramic membrane for organics degradation via catalytic wet air oxidation (CWAO) at ambient conditions. Catal. Today 2021, 364, 276–284. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Y.; Feng, Y.; Wang, P.; Li, X.; Shih, K. Fabrication of reactive flat-sheet ceramic membranes for oxidative degradation of ofloxacin by peroxymonosulfate. J. Membr. Sci. 2020, 611, 118302. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Urea-assisted one-step synthesis of cobalt ferrite impregnated ceramic membrane for sulfamethoxazole degradation via peroxymonosulfate activation. Chem. Eng. J. 2018, 343, 737–747. [Google Scholar] [CrossRef]
- Bao, Y.; Lee, W.J.; Lim, T.-T.; Wang, R.; Hu, X. Pore-functionalized ceramic membrane with isotropically impregnated cobalt oxide for sulfamethoxazole degradation and membrane fouling elimination: Synergistic effect between catalytic oxidation and membrane separation. Appl. Catal. B Environ. 2019, 254, 37–46. [Google Scholar] [CrossRef]
- Bao, Y.; Oh, W.-D.; Lim, T.-T.; Wang, R.; Webster, R.D.; Hu, X. Surface-nucleated heterogeneous growth of zeolitic imidazolate framework—A unique precursor towards catalytic ceramic membranes: Synthesis, characterization and organics degradation. Chem. Eng. J. 2018, 353, 69–79. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, W.; Chen, X.; Chen, L.; Hou, C.; Tang, T.; Zhang, X. Ceramic nanofiber membrane anchoring nanosized Mn2O3 catalytic ozonation of sulfamethoxazole in water. J. Hazard. Mater. 2022, 436, 129168. [Google Scholar] [CrossRef]
- Luo, J.; Chen, W.W.; Song, H.W.; Liu, J.R. Fabrication of hierarchical layer-by-layer membrane as the photocatalytic degradation of foulants and effective mitigation of membrane fouling for wastewater treatment. Sci. Total. Environ. 2020, 699, 12. [Google Scholar] [CrossRef]
- Li, M.; You, S.; Duan, X.; Liu, Y. Selective formation of reactive oxygen species in peroxymonosulfate activation by metal-organic framework-derived membranes: A defect engineering-dependent study. Appl. Catal. B Environ. 2022, 312, 121419. [Google Scholar] [CrossRef]
- Xu, L.; Niu, J.; Xie, H.; Ma, X.; Zhu, Y.; Crittenden, J. Effective degradation of aqueous carbamazepine on a novel blue-colored TiO2 nanotube arrays membrane filter anode. J. Hazard. Mater. 2021, 402, 123530. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Shi, Y.; Zhao, B.; Zhang, Z.; Ding, G.; Zhang, H. Blue TiO2 nanotube electrocatalytic membrane electrode for efficiency electrochemical degradation of organic pollutants. Chemosphere 2022, 306, 135628. [Google Scholar] [CrossRef]
- Sun, M.H.; Cui, M.R.; Wang, Y.B.; Fan, X.F.; Song, C.W. Enhanced Permeability and Removal Efficiency for Phenol and Perfluorooctane Sulphonate by a Multifunctional CNT/Al2O3 Membrane with Electrochemical Assistance. J. Nanosci. Nanotechnol. 2020, 20, 5951–5958. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Mahmud, S.; Yang, Y.; Zhu, L.; Zhao, Y.; Zeng, Q.; Xiong, Z.; Zhao, S. Polyvinylidene fluoride membrane functionalized with zero valent iron for highly efficient degradation of organic contaminants. Sep. Purif. Technol. 2020, 250, 117266. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, W.; Chen, Z.; Zhai, X.; Ma, J.; Zhang, T. Selective oxidation of water pollutants by surface-complexed peroxymonosulfate during filtration with highly dispersed Co(II)-doped ceramic membrane. Chem. Eng. J. 2022, 448, 137686. [Google Scholar] [CrossRef]
- Sun, M.; Zucker, I.; Davenport, D.M.; Zhou, X.C.; Qu, J.H.; Elimelech, M. Reactive, Self-Cleaning Ultrafiltration Membrane Functionalized with Iron Oxychloride Nanocatalysts. Environ. Sci. Technol. 2018, 52, 8674–8683. [Google Scholar] [CrossRef]
- Li, C.; Feng, G.Q.; Pan, Z.L.; Sun, M.H.; Fan, X.F.; Song, C.W.; Wang, T.H. Facile morphology-controlled synthesis of ZnO electrocatalysts on coal-based carbon membrane for antibiotics wastewater treatment. J. Membr. Sci. 2021, 639, 12. [Google Scholar] [CrossRef]
- Aher, A.; Papp, J.; Colburn, A.; Wan, H.; Hatakeyama, E.; Prakash, P.; Weaver, B.; Bhattacharyya, D. Naphthenic acids removal from high TDS produced water by persulfate mediated iron oxide functionalized catalytic membrane, and by nanofiltration. Chem. Eng. J. 2017, 327, 573–583. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y. Ceramic membrane separation coupled with catalytic ozonation for tertiary treatment of dyestuff wastewater in a pilot-scale study. Chem. Eng. J. 2016, 301, 19–26. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Wang, H.; Yu, H.; Quan, X. A pilot-scale coupling catalytic ozonation–membrane filtration system for recirculating aquaculture wastewater treatment. Desalination 2015, 363, 37–43. [Google Scholar] [CrossRef]
- Hu, J.; Fu, W.; Ni, F.; Zhang, X.; Yang, C.; Sang, J. An integrated process for the advanced treatment of hypersaline petrochemical wastewater: A pilot study. Water Res. 2020, 182, 116019. [Google Scholar] [CrossRef]
- Pei, S.; Wang, Y.; You, S.; Li, Z.; Ren, N. Electrochemical Removal of Chlorophenol Pollutants by Reactive Electrode Membranes: Scale-Up Strategy for Engineered Applications. Engineering 2022, 9, 77–84. [Google Scholar] [CrossRef]
- Lin, H.; Peng, H.; Feng, X.; Li, X.; Zhao, J.; Yang, K.; Liao, J.; Cheng, D.; Liu, X.; Lv, S.; et al. Energy-efficient for advanced oxidation of bio-treated landfill leachate effluent by reactive electrochemical membranes (REMs): Laboratory and pilot scale studies. Water Res. 2021, 190, 116790. [Google Scholar] [CrossRef] [PubMed]
- Plakas, K.V.; Sklari, S.D.; Yiankakis, D.A.; Sideropoulos, G.T.; Zaspalis, V.T.; Karabelas, A.J. Removal of organic micropollutants from drinking water by a novel electro-Fenton filter: Pilot-scale studies. Water Res. 2016, 91, 183–194. [Google Scholar] [CrossRef] [PubMed]


| AOP Type | Substrate Membrane | Catalyst | Fabrication Method | Membrane Type | Filtration Mode | Operation Mode | Target Pollutant | C0 | Flux (L/(m2·h)) | R1 | Number of Cycles | η | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMS | PVDF | CoAl-LDH | filtration | flat-sheet | dead-end | recirculation | RA | 2.5 mg/L | 80.3 | 94% | 10 | ~100% | [3] |
| PMS | PVDF | Co2+/Mxene | filtration | flat-sheet | dead-end | single pass | TC | 10 mg/L | 65.7 | 98.2% | 3 | 84% | [4] |
| PMS | PVDF | FeOCl/MoS2 | filtration | flat-sheet | dead-end | single pass | RhB | 10 mg/L | 400 | 70% | 5 | 87% | [13] |
| PMS | PVDF | MnO2/CNT | filtration | flat-sheet | dead-end | single pass | RhB | 50 mg/L | 248.51 | 100% | 8 | 100% | [14] |
| PMS | PVDF | FeCoS@N-rGO | filtration | flat-sheet | dead-end | single pass | SMX | 30 mg/L | 632.12 | 99.2% | 5 | 91% | [15] |
| PMS | PVDF | Co@N-C | phase inversion | flat-sheet | cross-flow | single pass | TC | 20 mg/L | 636.0 | 99.3% | 5 | 91% | [16] |
| PMS | PTFE | Co3O4@NCNTs/g-CN | filtration | flat-sheet | dead-end | recirculation | SMX | 10 mg/L | 276.86 | 98.9% | 5 | 89% | [17] |
| PMS | PTFE | FeCo@GCTs/GO | filtration | flat-sheet | dead-end | recirculation | SMX | 10 mg/L | 487.3 | 98.4% | 5 | 91% | [18] |
| PDS | PTFE | N-doped GO | filtration | flat-sheet | dead-end | recirculation | phenol oxalic acid | 50 mg/L 90 mg/L | 1125 | 94% 100% | 3 | 29% 80% | [19] |
| PMS | PAN | Fe-doped LaCoO3 | phase inversion | flat-sheet | cross flow | single pass | TC | 10 mg/L | 220.7 | 99% | 5 | 99% | [20] |
| PMS | PSF | SCCM | phase inversion | flat-sheet | cross-flow | single pass | RhB | 20 mg/L | 126.90 | 100% | 5 | ~100% | [21] |
| PMS | nylon | Fe2O3@CNT | filtration | flat-sheet | dead-end | single pass | TC | 0.04 mmol/L | 16.3 | 96.1% | 5 | 91% | [22] |
| PDS | nylon | NG/rGO/CNT | filtration | flat-sheet | dead-end | recirculation | SMX | 0.5 mg/L | 46.15 | 94.8% | 5 | 85% | [23] |
| PMS | ceramic membrane | CuO | phase-inversion and liquid-phase sintering | hollow fiber | cross-flow | single pass | RhB | 20 mg/L | 500 | 81.5% | 5 | 93% | [24] |
| PMS | ceramic membrane | CuO | impregnation and calcination | hollow fiber | cross-flow | single pass | SDZ | 1 mg/L | 70 | 91.7% | 5 | 93% | [25] |
| PMS | ceramic membrane | CuO | impregnation and calcination | hollow fiber | cross-flow | single pass | BPA | 10 mg/L | 70 | 91.4% | 5 | ~100% | [26] |
| PMS | ceramic membrane | Co3O4@CNT | filtration | flat-sheet | dead-end | single pass | MB | 15 mg/L | 224 | 84.4% | 6 | 83% | [27] |
| PMS | ceramic membrane | Mn2O3 | spraying and calcinating | flat-sheet | dead-end | single pass | acetaminophen | 1 mg/L | 60 | 99% | 10 | ~100% | [28] |
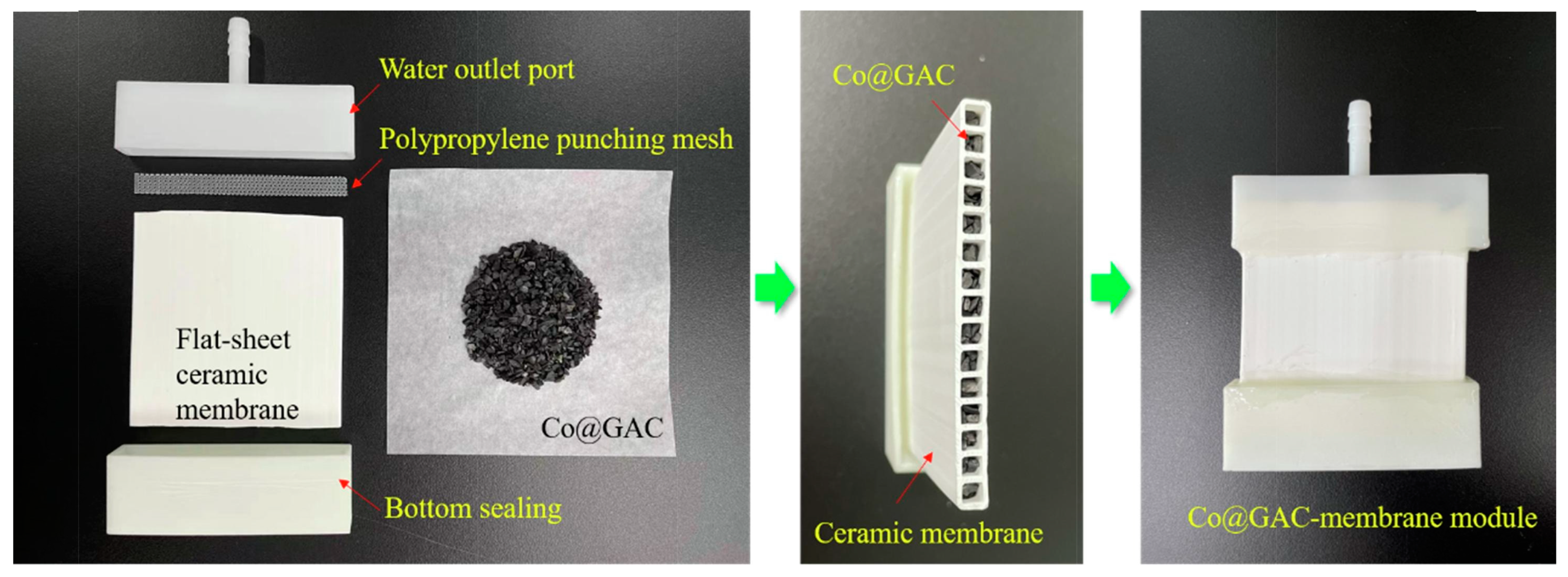
| PMS | ceramic membrane | Co@GAC | filling the channels | flat-sheet | dead-end | single pass | BPA | 10 mg/L | 35 | 92.8% | 6 | 97% | [29] |
| H2O2 | FEP | Fe2O3 | thermoforming method | flat-sheet | cross-flow | single pass | MB | 40 mg/L | 15 | 99.69% | 3 | 73% | [30] |
| H2O2 | PAN | goethite/maleate ferroxane | phase inversion | flat-sheet | dead-end | single pass | amoxicillin | 105 mg/L | 23.2 | 86.3%/92.3% | 4 | 97%/98% | [31] |
| O3 | ceramic membrane | CuO | filtration and calcination | tubular | dead-end | recirculation | 1,4-dioxane | 200 mg/L | 363.81 | 46% | 4 | 98% | [32] |
| O3 | ceramic membrane | MnCe oxide | filtration, in situ reaction and calcination | flat-sheet | dead-end | recirculation | atrazine | 1 mg/L | 60 | 90% | 5 | 94% | [33] |
| O3 | ceramic membrane | CuMn2O4 | impregnation and calcination | tubular | cross-flow | single pass | BP-3 | 2 mg/L | 63.157 | 75.3% | 30 | 91% | [34] |
| O3 | ceramic membrane | N-rGO | pneumatic method | tubular | cross-flow | single pass | benzotriazole | 0.084 mol/L | 660.86 | 100% | 18 | 92% | [35] |
| Photo | PVDF PTFE | TiO2 | impregnation and hydrothermal treatment | flat-sheet | dead-end | recirculation | diclofenac/ ethinylestradiol | 1.01 µmol/L | 1144/1331 | 92%, 94%; 89%, 92% | 3 | ~100%, 96%; 67%, 83% | [36] |
| Photo | ceramic membrane | TiO2 | impregnation and calcination | flat-sheet | cross-flow | recirculation | MB | 0.083 mmol/L | 125 | 80% | 4 | ~100% | [37] |
| Photo | ceramic membrane | GO–TiO2 | filtration and calcination | flat-sheet | dead-end cross-flow | recirculation recirculation | naproxen diclofenac CBZ | 50 ppb | 92.6 | 100%, 100%, 90%; 100%, 100%, 30% | 7 11 | – | [38] |
| Photo | cellulose acetate | GO–TiO2 | filtration | flat-sheet | dead-end | single pass | CR | 50 mg/L | 483.5 | 97% | 3 | 98% | [39] |
| Photo | PSF | GO–N-TiO2 | filtration | flat-sheet | dead-end | single pass | MB | 50 mg/L | 70 | 50% | 8 | 66% | [40] |
| Photo | None: free standing | rGO@TiO2 | electro-spinning and calcination | flat-sheet | cross-flow | recirculation | propranolol | 2 mg/L | 342.86 | 76.1% | 10 | ~100% | [41] |
| Photo | PVDF | ZnIn2S4 | filtration | flat-sheet | dead-end | recirculation | fluvastatin | 10 mg/L | 270 | 97.19% | 6 | 94% | [42] |
| Photo + H2O2 | ceramic membrane | α-Fe2O3 | spraying and calcinating | flat-sheet | dead-end | recirculation | TC | 20 mg/L | 158.73 | 82% | 5 | 88% | [43] |
| Photo + H2O2 | PAN | β-FeOOH | impregnation and in situ mineralization | flat-sheet | cross-flow | single pas | methyl blue | 20 mg/L | 11–13 | 99.8% | 5 | 97% | [44] |
| Photo + H2O2 | air-laid paper | β-FeOOH | impregnation and in situ mineralization | flat-sheet | dead-end | single pass | TC | 40 mg/L | 600 | 99.5% | 5 | 98% | [45] |
| Photo + H2O2 | PSF | ferrocene | layer-by-layer interfacial polymerization and ion-exchange | flat-sheet | dead-end | single pass | BPS | 20 mg/L | 4.2 | 99.5% | 3 | – | [46] |
| Photo + PMS | PP | ZIF-67 | in situ synthesis | flat-sheet | cross-flow | single pass | MB MO | 20 mg/L 20 mg/L | 216.8 | TOC removal: 74.2% 83.5% | 5 | 99% 98% | [47] |
| Photo + PMS | PTFE | α-Fe2O3/bacterial cellulose | filtration | flat-sheet | dead-end | single pass | RhB | 20 mg/L | 4.93 | 100% | 5 | 97% | [48] |
| Photo + PMS | PVDF | CoFe2O4/carbon nanofiber | phase inversion | flat-sheet | dead-end | recirculation | berberine | 10 mg/L | 368 | 93.2% | 4 | 97% | [49] |
| Photo + PDS | PVDF | – | – | flat-sheet | dead-end | single pass | ofloxacin | 5 mg/L | – | 54% | 10 | 26% | [50] |
| Electro | porous titanium plate (anode) titanium mesh (cathode) | Pd (anode) –(cathode) | spray coating and calcination | flat-sheet | dead-end | single pass | SMX | 100 μg/L | 5000 | 96.3% | 10 | ~100% | [51] |
| Electro | RuO2 plated titanic mesh (anode) RuO2 plated titanic mesh (cathode) | graphene/SnO2/carbon nanofibers (anode) –(cathode) | electrospinning and carbonization | flat-sheet | dead-end | recirculation | SMX | 15 mg/L | 216.4 | 85% | 6 | 94% | [52] |
| Electro | porous Ti plate (anode) steel mesh (cathode) | blue TiO2 (anode) –(cathode) | electrooxidation, calcination and electroreduction | flat-sheet | dead-end | recirculation | triclosan | 10 mg/L | 3582 | 98.5% | 4 | ~100% | [53] |
| Electro | stainless steel network/PTFE (anode) stainless steel network (cathode) | CNT (anode) –(cathode) | filtration | flat-sheet | dead-end | single pass | SMX ciprofloxacin amoxicillin | 50 mg/L | 127.48 | 90% 76% 98% | 4 | 72% 79% 92% | [54] |
| Electro | titanium ring/PTFE (anode) stainless steel (cathode) | single cobalt atom and nitrogen atom co-doped graphene (anode) –(cathode) | filtration | flat-sheet | dead-end | single pass | MB | 5 mg/L | 15.29 | 99.5% | 5 | 90% | [55] |
| Electro | stainless-steel mesh/PVDF (anode) titanium foil (cathode) | nano porous carbon/CuFeO2 (anode) –(cathode) | phase inversion | flat-sheet | dead-end | single pass | MO | 10 mg/L | 125 | 98.72% | 4 | 69% | [56] |
| Electro | outer CNT layer (anode) inner CNT layer (cathode) | –(anode) –(cathode) | layer-by-layer coating | hollow fiber | dead-end | single pass | MC-LR | 0.5 mg/L | 500 | 99.8% | 3 | ~100% | [57] |
| Electro | ceramic membrane (anode)/Ti mesh titanium mesh (cathode) | TiO2/SnO2-Sb (anode) –(cathode) | adhesive assemble | flat-sheet | dead-end | single pass | 2,4-D | 1 mg/L | 278 | 62.4% | 8 | ~100% | [58] |
| Electro + PMS | titanium mesh (anode) PTFE (cathode) | –(anode) MnFe2O4-rGO (cathode) | filtration | flat-sheet | dead-end | recirculation | OTC | 10 mg/L | 553.6 | 88.7% | 5 | 86.1% | [59] |
| Electro + PMS | ceramic membrane (anode and cathode) | Pd (anode and cathode) | confocal magnetron co−sputtering | flat-sheet | cross flow | single pass | MB | 10 μmol/L | 461.34 | 94.5% | 8 | 95% | [60] |
| Electro + Photo + PMS | stainless steel (anode) titanium plate (cathode) | TiO2-ZnO (anode) –(cathode) | atomic layer deposition | flat-sheet | cross flow | single pass | atrazine MB | 5 mg/L 5 mg/L | 59.9 | 100% | 5 | 90% 85% | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhang, T.; Chang, Q.; Ma, C.; Yang, Y.; Wang, S.; Pan, Z.; Sun, Y.; Ding, G. Performance Stability and Regeneration Property of Catalytic Membranes Coupled with Advanced Oxidation Process: A Comprehensive Review. Sustainability 2023, 15, 7556. https://doi.org/10.3390/su15097556
Shi Y, Zhang T, Chang Q, Ma C, Yang Y, Wang S, Pan Z, Sun Y, Ding G. Performance Stability and Regeneration Property of Catalytic Membranes Coupled with Advanced Oxidation Process: A Comprehensive Review. Sustainability. 2023; 15(9):7556. https://doi.org/10.3390/su15097556
Chicago/Turabian StyleShi, Yawei, Tongwen Zhang, Qian Chang, Chang Ma, Yao Yang, Songbo Wang, Zonglin Pan, Ya Sun, and Guanghui Ding. 2023. "Performance Stability and Regeneration Property of Catalytic Membranes Coupled with Advanced Oxidation Process: A Comprehensive Review" Sustainability 15, no. 9: 7556. https://doi.org/10.3390/su15097556
APA StyleShi, Y., Zhang, T., Chang, Q., Ma, C., Yang, Y., Wang, S., Pan, Z., Sun, Y., & Ding, G. (2023). Performance Stability and Regeneration Property of Catalytic Membranes Coupled with Advanced Oxidation Process: A Comprehensive Review. Sustainability, 15(9), 7556. https://doi.org/10.3390/su15097556








