Modelling and Design of Habitat Features: Will Manufactured Poles Replace Living Trees as Perch Sites for Birds?
Abstract
:1. Introduction
1.1. Key Gaps
1.1.1. No Integration of ‘Natural’ and ‘Artificial’
1.1.2. The Lack of Fidelity, Range, and Dynamics
1.1.3. Limitations of Current Design Strategies
1.2. The Potential for Imaging and Computational Modelling of Habitat Structures
2. Materials and Methods
- Use a real-world case to confine the scope of consideration;
- Define the concept of integrated supply, which includes biological and industrial processes; and
- Model supply of habitat features and illustrate implications for design.
2.1. Use a Real-World Case
2.2. Define Integrated Supply of Habitat Features
2.2.1. Define ‘Supply’ in Terms of Three Scales: Spatial, Organisational, and Temporal
2.2.2. Define ‘Trees’, ‘Snags’, and ‘Poles’ as Three Modes of Supply
- Mode 1—Trees: increasing numbers of key engineers (by planting trees that can supply branches for perching);
- Mode 2—Snags: increasing activities of key engineers (by reusing dead trees known as snags);
- Mode 3—Poles: introducing artificially engineered products (by installing utility poles supplied via human manufacturing).
2.3. Describe the Model
2.3.1. Purpose and Patterns
2.3.2. Entities, State Variables, and Scales
2.3.3. Process Overview and Scheduling
- Agents execute ‘Survive’;
- Tree agents execute ‘Grow’;
- Tree Agents execute ‘Supply’;
- Newly instantiated Snag and Pole Agents execute ‘Supply’;
- Environment executes ‘Terminate’;
- Environment executes ‘Assess Supply’;
- At some years, Environment executes ‘Renew’.
2.4. Model Supply
2.4.1. Constrain Supply
2.4.2. Controlling Supply through Strategies
- Allocate budget for each supply mode;
- Assign weights to probability ranges used for ‘Terminate’, ‘Supply’, and ‘Renew’ agent actions, as well as to the ‘unit cost’ variable;
- Choose to run or disable the ‘Renew’ action.
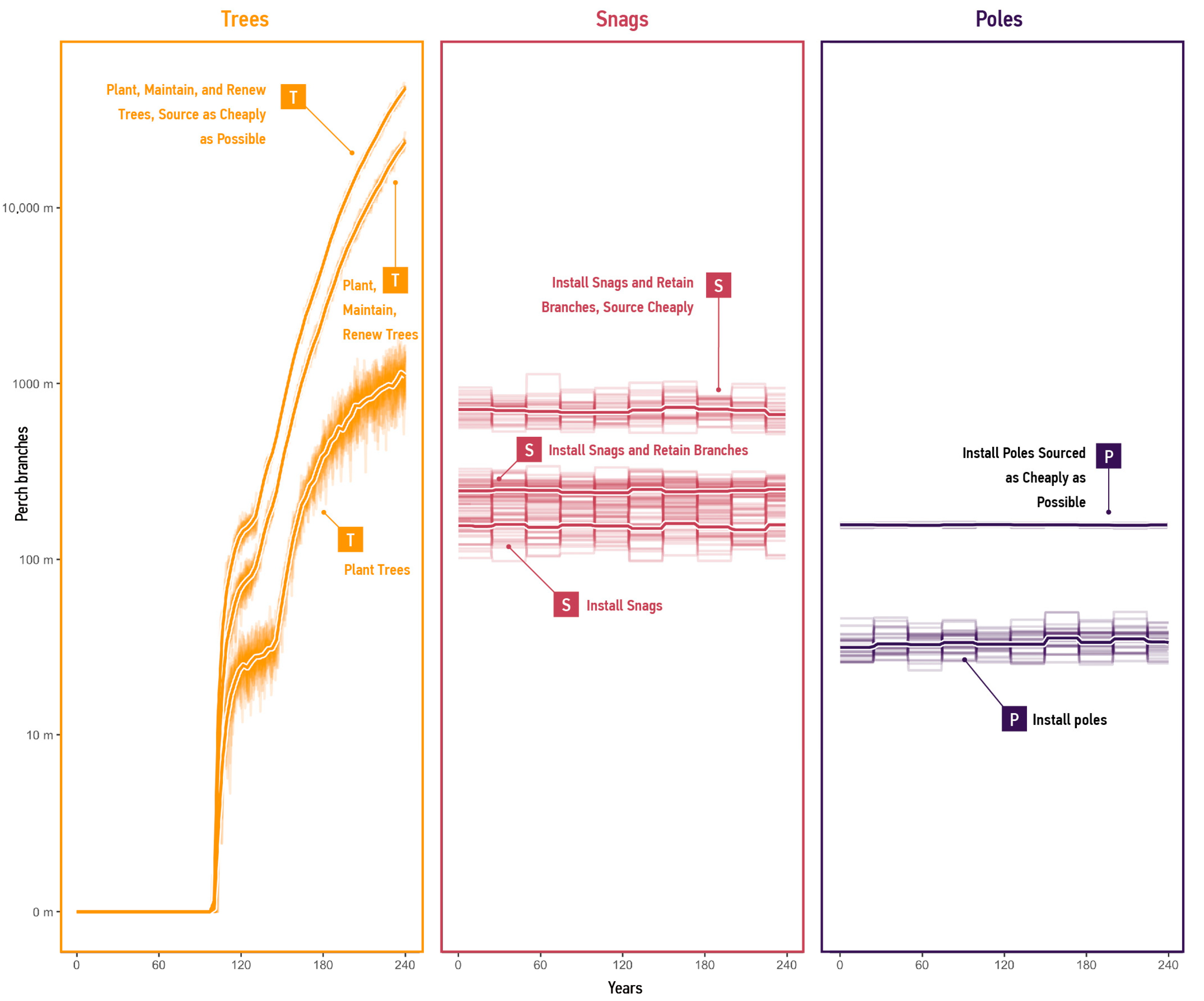
- The ‘Plant Trees’ strategy uses only the Tree mode, with default probability ranges for agents and the ‘Renew’ process disabled.
- ‘Plant, Maintain, and Renew Trees’ lowers the ‘Terminate’ probability range for agents and enables ‘Renew’, considering conservation guidelines from Gibbons et al. [23].
- ‘Plant, Maintain, and Renew Trees; Source as Cheaply as Possible’ lowers the ‘Terminate’ probability range, enables ‘Renew’, and increases agent populations by using lower probable ‘unit cost’ values.
- ‘Plant, Maintain and Renew Trees; Install Poles’ uses Tree and Pole modes (see Table 5 budget allocations), with default probability ranges for pole agents and tree agents parameterised as in ‘Plant, Maintain and Renew Trees’.
- ‘Plant, Maintain, and Renew Trees; Install Poles Sourced as Cheaply as Possible’ uses Tree and Pole modes while accounting for potential supply effects from economies of scale by lowering probable ‘unit cost’ values for pole agents.
- ‘Plant, Maintain, and Renew Trees; Install Snags’ uses Tree and Snag modes, with default probability ranges for snag agents and parameters from ‘Plant, Maintain and Renew trees’ for tree agents.
- ‘Plant, Maintain, and Renew Trees; Install Snags; Retain Branches’ uses Snag and Pole modes, increasing the ‘Supply’ probability range for snag agents to simulate potential supply improvements through possible retention of tree limbs during removal and transportation.‘Plant, Maintain, and Renew Trees; Install Snags; Retain Branches; Source Snags Cheaply’ is a marginal case that implements trees and high-performing snags by increasing the ‘Supply’ probability range and lowering the ‘unit cost’ probability ranges in snag agents.
3. Results
- Unify heterogenous modes of supply in one model;
- Capture differences between modes of supply;
- Predict consequences of design decisions.
3.1. Model of Supply across Modes
3.1.1. Spatial Scale
3.1.2. Organisational Scale
3.1.3. Temporal Scale
3.2. Supply Predictions
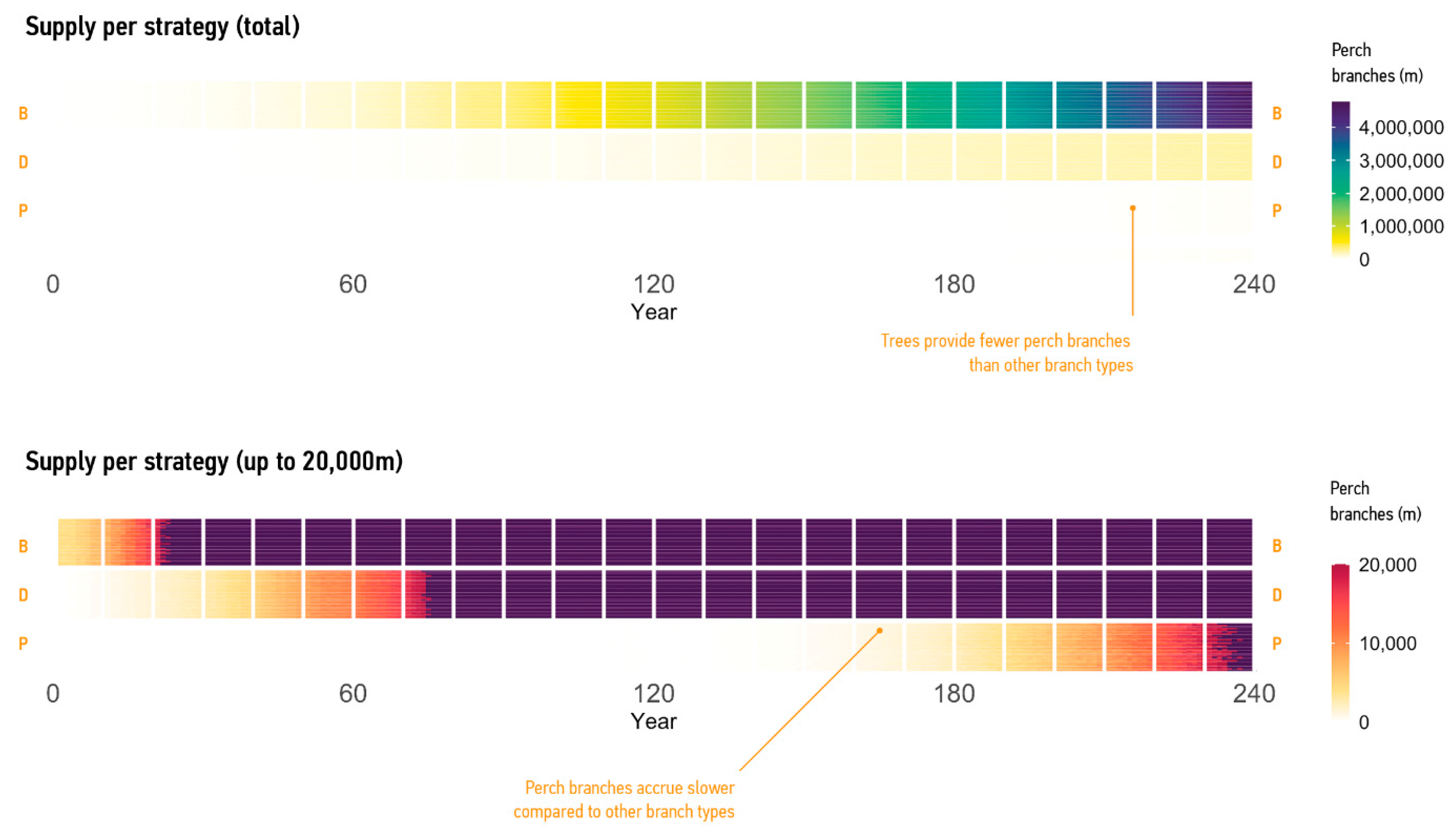
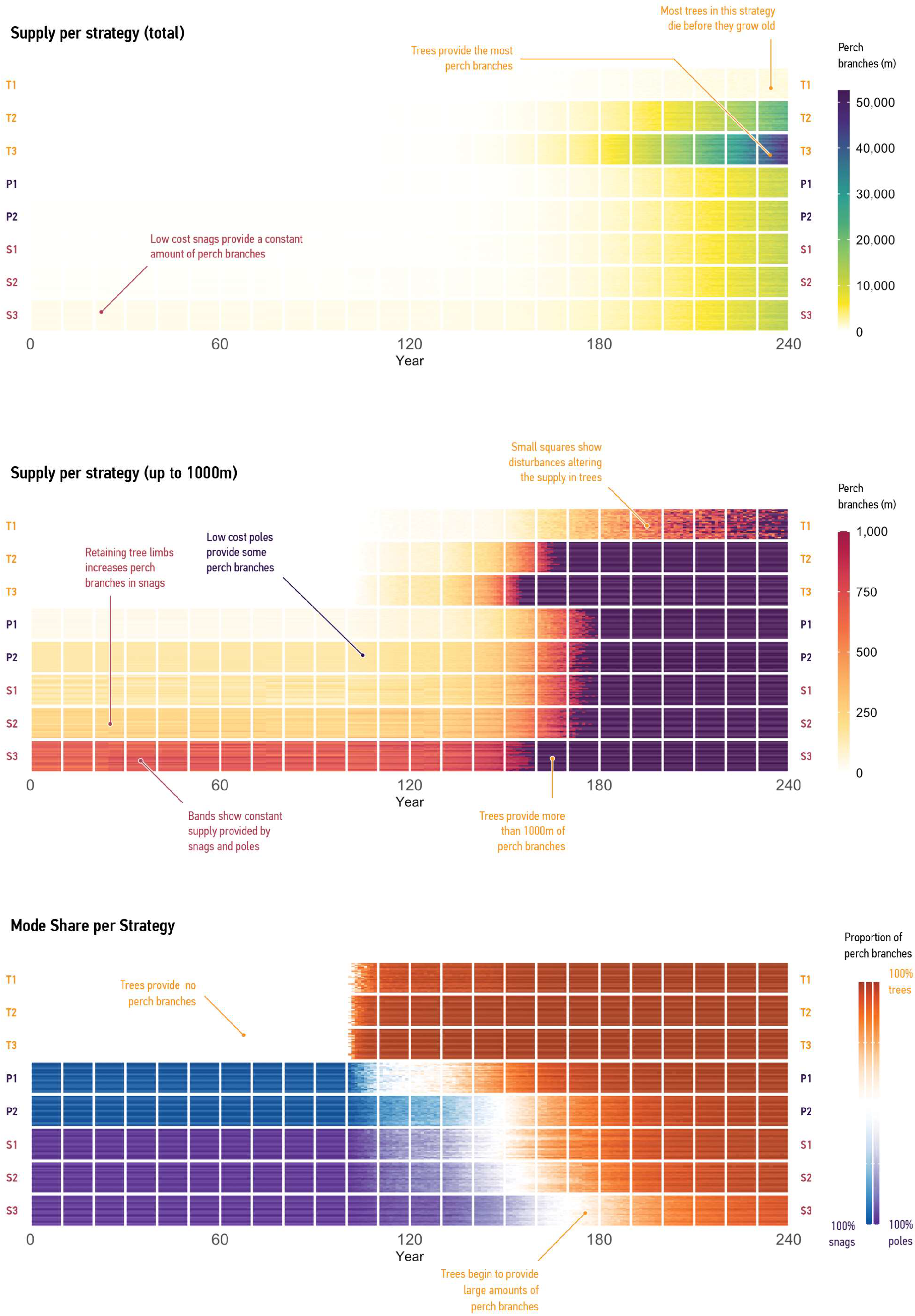
3.2.1. Supply of Branches
3.2.2. Supply per Mode
4. Discussion
4.1. Benefits of Integrated Supply Models
4.2. Current and Possible Predictions
4.3. Potential to Support Design Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prevedello, J.A.; Almeida-Gomes, M.; Lindenmayer, D.B. The Importance of Scattered Trees for Biodiversity Conservation: A Global Meta-Analysis. J. Appl. Ecol. 2018, 55, 205–214. [Google Scholar] [CrossRef]
- Lewandowski, P.; Przepióra, F.; Ciach, M. Single Dead Trees Matter: Small-Scale Canopy Gaps Increase the Species Richness, Diversity and Abundance of Birds Breeding in a Temperate Deciduous Forest. For. Ecol. Manag. 2021, 481, 118693. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Laurance, W.F.; Franklin, J.F. Global Decline in Large Old Trees. Science 2012, 338, 1305–1306. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Laurance, W.F. The Ecology, Distribution, Conservation and Management of Large Old Trees. Biol. Rev. 2016, 92, 1434–1458. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, P.; Boak, M. The Value of Paddock Trees for Regional Conservation in an Agricultural Landscape. Ecol. Manag. Restor. 2002, 3, 205–210. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Manning, A.D.; Gibbons, P. The Future of Large Old Trees in Urban Landscapes. PLoS ONE 2014, 9, e99403. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.D.; Gibbons, P.; Fischer, J.; Oliver, D.; Lindenmayer, D.B. Hollow Futures? Tree Decline, Lag Effects and Hollow-Dependent Species. Anim. Conserv. 2012, 16, 395–405. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Prober, S.; Crane, M.; Michael, D.; Okada, S.; Kay, G.; Keith, D.; Montague-Drake, R.; Burns, E. Temperate Eucalypt Woodlands. In Biodiversity and Environmental Change: Monitoring, Challenges and Direction; Lindenmayer, D.B., Burns, E., Thurgate, N., Lowe, A., Eds.; CSIRO: Colingwood, Australia, 2014; pp. 283–334. ISBN 978-0-643-10857-8. [Google Scholar]
- Ibarra, J.T.; Novoa, F.J.; Jaillard, H.; Altamirano, T.A. Large Trees and Decay: Suppliers of a Keystone Resource for Cavity-Using Wildlife in Old-Growth and Secondary Andean Temperate Forests. Austral Ecol. 2020, 45, 1135–1144. [Google Scholar] [CrossRef]
- Beyer, G.L.; Goldingay, R.L. The Value of Nest Boxes in the Research and Management of Australian Hollow-Using Arboreal Marsupials. Wildl. Res. 2006, 33, 161–174. [Google Scholar] [CrossRef]
- Hannan, L.; Le Roux, D.S.; Milner, R.N.C.; Gibbons, P. Erecting Dead Trees and Utility Poles to Offset the Loss of Mature Trees. Biol. Conserv. 2019, 236, 340–346. [Google Scholar] [CrossRef]
- Watchorn, D.J.; Cowan, M.A.; Driscoll, D.A.; Nimmo, D.G.; Ashman, K.R.; Garkaklis, M.J.; Wilson, B.A.; Doherty, T.S. Artificial Habitat Structures for Animal Conservation: Design and Implementation, Risks and Opportunities. Front. Ecol. Environ. 2022, 20, 301–309. [Google Scholar] [CrossRef]
- Schnell, S.; Kleinn, C.; Ståhl, G. Monitoring Trees Outside Forests: A Review. Environ. Monit. Assess. 2015, 187, 600. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Kern, C.C.; Kabrick, J.M. Can Assisted Tree Migration Today Sustain Forest Ecosystem Goods and Services for the Future? For. Ecol. Manag. 2023, 529, 120723. [Google Scholar] [CrossRef]
- Lindenmayer, D.B. Conserving Large Old Trees as Small Natural Features. Biol. Conserv. 2017, 211, 51–59. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Bistricer, G.; Manning, A.D.; Gibbons, P. Enriching Small Trees with Artificial Nest Boxes Cannot Mimic the Value of Large Trees for Hollow-Nesting Birds. Restor. Ecol. 2015, 24, 252–258. [Google Scholar] [CrossRef]
- Gibbons, P.; Lindenmayer, D.B. Offsets for Land Clearing: No Net Loss or the Tail Wagging the Dog? Ecol. Manag. Restor. 2007, 8, 26–31. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global Synthesis of Conservation Studies Reveals the Importance of Small Habitat Patches for Biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B. Small Patches Make Critical Contributions to Biodiversity Conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 717–719. [Google Scholar] [CrossRef]
- Pretzsch, H. Forest Dynamics, Growth, and Yield; Springer: Berlin, Germany, 2009; ISBN 978-3-540-88306-7. [Google Scholar]
- Gámez, S.; Harris, N.C. Conceptualizing the 3D Niche and Vertical Space Use. Trends Ecol. Evol. 2022, 37, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.M.; Jones, C.G.; Turner, M.G.; Weathers, K.C. Ecosystem Function in Heterogeneous Landscapes. In Ecosystem Function in Heterogeneous Landscapes; Lovett, G.M., Jones, C.G., Turner, M.G., Weathers, K.C., Eds.; Springer: New York, NY, USA, 2005; pp. 1–4. ISBN 978-0-387-24089-3. [Google Scholar]
- Gibbons, P.; Lindenmayer, D.B.; Fischer, J.; Manning, A.D.; Weinberg, A.; Seddon, J.A.; Ryan, P.R.; Barrett, G. The Future of Scattered Trees in Agricultural Landscapes. Conserv. Biol. 2008, 22, 1309–1319. [Google Scholar] [CrossRef]
- LaRue, E.A.; Fahey, R.T.; Alveshere, B.C.; Atkins, J.W.; Bhatt, P.; Buma, B.; Chen, A.; Cousins, S.; Elliott, J.M.; Elmore, A.J.; et al. A Theoretical Framework for the Ecological Role of Three-Dimensional Structural Diversity. Front. Ecol. Environ. 2023, 21, 4–13. [Google Scholar] [CrossRef]
- Seidel, D.; Ehbrecht, M.; Dorji, Y.; Jambay, J.; Ammer, C.; Annighöfer, P. Identifying Architectural Characteristics That Determine Tree Structural Complexity. Trees 2019, 33, 911–919. [Google Scholar] [CrossRef]
- Eichhorn, M.; Johst, K.; Seppelt, R.; Drechsler, M. Model-Based Estimation of Collision Risks of Predatory Birds with Wind Turbines. Ecol. Soc. 2012, 17, 1. [Google Scholar] [CrossRef]
- Winter, L.; Lehmann, A.; Finogenova, N.; Finkbeiner, M. Including Biodiversity in Life Cycle Assessment—State of the Art, Gaps and Research Needs. Environ. Impact Assess. Rev. 2017, 67, 88–100. [Google Scholar] [CrossRef]
- Campos, M.B.; Litkey, P.; Wang, Y.; Chen, Y.; Hyyti, H.; Hyyppä, J.; Puttonen, E. A Long-Term Terrestrial Laser Scanning Measurement Station to Continuously Monitor Structural and Phenological Dynamics of Boreal Forest Canopy. Front. Plant Sci. 2021, 11. [Google Scholar] [CrossRef]
- Strain, E.M.A.; Olabarria, C.; Mayer-Pinto, M.; Cumbo, V.; Morris, R.L.; Bugnot, A.B.; Dafforn, K.A.; Heery, E.; Firth, L.B.; Brooks, P.R.; et al. Eco-Engineering Urban Infrastructure for Marine and Coastal Biodiversity: Which Interventions Have the Greatest Ecological Benefit? J. Appl. Ecol. 2018, 55, 426–441. [Google Scholar] [CrossRef]
- Morris, R.; Chapman, M.G.; Firth, L.; Coleman, R. Increasing Habitat Complexity on Seawalls: Investigating Large-and Small-Scale Effects on Fish Assemblages. Ecol. Evol. 2017, 7, 9567–9579. [Google Scholar] [CrossRef] [PubMed]
- Suzdaleva, A.L.; Beznosov, V.N. Artificial Reef: Status, Life Cycle, and Environmental Impact Assessment. Power Technol. Eng. 2021, 55, 558–561. [Google Scholar] [CrossRef]
- Bishop, M.J.; Vozzo, M.L.; Mayer-Pinto, M.; Dafforn, K.A. Complexity–Biodiversity Relationships on Marine Urban Structures: Reintroducing Habitat Heterogeneity Through Eco-Engineering. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210393. [Google Scholar] [CrossRef]
- Holland, A.; Roudavski, S. Participatory Design for Multispecies Cohabitation: By Trees, for Birds, with Humans. In Designing More-than-Human Smart Cities: Beyond Sustainability, towards Cohabitation; Heitlinger, S., Foth, M., Clarke, R., Eds.; Oxford University Press: Oxford, UK, 2023; in press. [Google Scholar]
- Lindenmayer, D.B.; Welsh, A.; Donnelly, C.; Crane, M.; Michael, D.; Macgregor, C.; McBurney, L.; Montague-Drake, R.; Gibbons, P. Are Nest Boxes a Viable Alternative Source of Cavities for Hollow-Dependent Animals? Long-Term Monitoring of Nest Box Occupancy, Pest Use and Attrition. Biol. Conserv. 2009, 142, 33–42. [Google Scholar] [CrossRef]
- Schoonees, T.; Mancheño, A.G.; Scheres, B.; Bouma, T.J.; Silva, R.; Schlurmann, T.; Schüttrumpf, H. Hard Structures for Coastal Protection, Towards Greener Designs. Estuaries Coasts 2019, 42, 1709–1729. [Google Scholar] [CrossRef]
- Figueiredo, L.; Krauss, J.; Steffan-Dewenter, I.; Sarmento Cabral, J. Understanding Extinction Debts: Spatio–Temporal Scales, Mechanisms and a Roadmap for Future Research. Ecography 2019, 42, 1973–1990. [Google Scholar] [CrossRef]
- Best, K.; Haslem, A.; Maisey, A.C.; Semmens, K.; Griffiths, S.R. Occupancy of Chainsaw-Carved Hollows by an Australian Arboreal Mammal Is Influenced by Cavity Attributes and Surrounding Habitat. For. Ecol. Manag. 2022, 503, 119747. [Google Scholar] [CrossRef]
- Hunter, M.L.; Acuña, V.; Bauer, D.M.; Bell, K.P.; Calhoun, A.J.K.; Felipe-Lucia, M.R.; Fitzsimons, J.A.; González, E.; Kinnison, M.; Lindenmayer, D.; et al. Conserving Small Natural Features with Large Ecological Roles: A Synthetic Overview. Biol. Conserv. 2017, 211, 88–95. [Google Scholar] [CrossRef]
- Pustkowiak, S.; Kwieciński, Z.; Lenda, M.; Żmihorski, M.; Rosin, Z.M.; Tryjanowski, P.; Skórka, P. Small Things Are Important: The Value of Singular Point Elements for Birds in Agricultural Landscapes. Biol. Rev. 2021, 96, 1386–1403. [Google Scholar] [CrossRef]
- Austern, G.; Capeluto, I.G.; Grobman, Y.J. Rationalization Methods in Computer Aided Fabrication: A Critical Review. Autom. Constr. 2018, 90, 281–293. [Google Scholar] [CrossRef]
- Pottmann, H.; Eigensatz, M.; Vaxman, A.; Wallner, J. Architectural Geometry. Comput. Graph. 2015, 47, 145–164. [Google Scholar] [CrossRef]
- Parker, D.; Roudavski, S.; Jones, T.M.; Bradsworth, N.; Isaac, B.; Lockett, M.T.; Soanes, K. A Framework for Computer-Aided Design and Manufacturing of Habitat Structures for Cavity-Dependent Animals. Methods Ecol. Evol. 2022, 13, 826–841. [Google Scholar] [CrossRef]
- Mirra, G.; Holland, A.; Roudavski, S.; Wijnands, J.; Pugnale, A. An Artificial Intelligence Agent That Synthesises Visual Abstractions of Natural Forms to Support the Design of Human-Made Habitat Structures. Front. Ecol. Evol. 2022, 10, 806453. [Google Scholar] [CrossRef]
- Loke, L.H.L.; Ladle, R.J.; Bouma, T.J.; Todd, P.A. Creating Complex Habitats for Restoration and Reconciliation. Ecol. Eng. 2015, 77, 307–313. [Google Scholar] [CrossRef]
- Camarretta, N.; Harrison, P.A.; Bailey, T.; Potts, B.; Lucieer, A.; Davidson, N.; Hunt, M. Monitoring Forest Structure to Guide Adaptive Management of Forest Restoration: A Review of Remote Sensing Approaches. New For. 2019, 51, 573–596. [Google Scholar] [CrossRef]
- Roudavski, S.; Parker, D. Modelling Workflows for More-than-Human Design: Prosthetic Habitats for the Powerful Owl (Ninox strenua). In Impact—Design with All Senses: Proceedings of the Design Modelling Symposium, Berlin 2019; Christoph, G., Olivier, B., Jane, B., Ramsgaard, T.M., Stefan, W., Eds.; Springer: Cham, Germany, 2020; pp. 554–564. ISBN 978-3-030-29828-9. [Google Scholar]
- Varin, M.; Chalghaf, B.; Joanisse, G. Object-Based Approach Using Very High Spatial Resolution 16-Band WorldView-3 and LiDAR Data for Tree Species Classification in a Broadleaf Forest in Quebec, Canada. Remote Sens. 2020, 12, 3092. [Google Scholar] [CrossRef]
- Glad, A.; Reineking, B.; Montadert, M.; Depraz, A.; Monnet, J.-M. Assessing the Performance of Object-Oriented Lidar Predictors for Forest Bird Habitat Suitability Modeling. Remote Sens. Ecol. Conserv. 2020, 6, 5–19. [Google Scholar] [CrossRef]
- Beland, M.; Parker, G.; Sparrow, B.; Harding, D.; Chasmer, L.; Phinn, S.; Antonarakis, A.; Strahler, A. On Promoting the Use of LiDAR Systems in Forest Ecosystem Research. For. Ecol. Manag. 2019, 450, 117484. [Google Scholar] [CrossRef]
- Shugart, H.H.; Asner, G.P.; Fischer, R.; Huth, A.; Knapp, N.; Le Toan, T.; Shuman, J.K. Computer and Remote-Sensing Infrastructure to Enhance Large-Scale Testing of Individual-Based Forest Models. Front. Ecol. Environ. 2015, 13, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Department of Environment, Climate Change and Water NSW. National Recovery Plan for White Box—Yellow Box—Blakely’s Red Gum Grassy Woodland and Derived Native Grassland: A Critically Endangered Ecological Community; Department of Environment, Climate Change and Water New South Wales: Sydney, Australia, 2011; ISBN 978-1-74232-311-4. Available online: https://www.dcceew.gov.au/sites/default/files/documents/white-and-yellow-box.pdf (accessed on 1 April 2023).
- Flapper, T.; Cook, T.; Farrelly, S.; Dickson, K.; Auty, K. Independent Audit of the Molonglo Valley Strategic Assessment; Australian Capital Territory Government: Canberra, Australia, 2018.
- Collins, A.; Joseph, D.; Bielaczyc, K. Design Research: Theoretical and Methodological Issues. J. Learn. Sci. 2004, 13, 15–42. [Google Scholar] [CrossRef]
- Rawlings, K.; Freudenberger, D.; Carr, D. A Guide to Managing Box Gum Grassy Woodlands; Department of the Environment, Water, Heritage and the Arts: Canberra, Australia, 2010.
- Manning, A.D.; Fischer, J.; Lindenmayer, D.B. Scattered Trees Are Keystone Structures: Implications for Conservation. Biol. Conserv. 2006, 132, 311–321. [Google Scholar] [CrossRef]
- ACT Planning and Land Authority Molongolo Valley Plan for the Protection of Matters of National Environmental Significance; Australian Capital Territory Government: Canberra, Australia, 2011.
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Manning, A.D.; Gibbons, P. The Value of Scattered Trees for Wildlife: Contrasting Effects of Landscape Context and Tree Size. Divers. Distrib. 2018, 24, 69–81. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Manning, A.D.; Gibbons, P. Single Large or Several Small? Applying Biogeographic Principles to Tree-Level Conservation and Biodiversity Offsets. Biol. Conserv. 2015, 191, 558–566. [Google Scholar] [CrossRef]
- Seidel, D.; Fleck, S.; Leuschner, C.; Hammett, T. Review of Ground-Based Methods to Measure the Distribution of Biomass in Forest Canopies. Ann. For. Sci. 2011, 68, 225–244. [Google Scholar] [CrossRef]
- Whitelaw, M.; Hwang, J.; Le Roux, D. Design Collaboration and Exaptation in a Habitat Restoration Project. She Ji J. Des. Econ. Innov. 2021, 7, 223–241. [Google Scholar] [CrossRef]
- Gibbons, P.; Lindenmayer, D.B. Issues Associated with the Retention of Hollow-Bearing Trees Within Eucalypt Forests Managed for Wood Production. For. Ecol. Manag. 1996, 83, 245–279. [Google Scholar] [CrossRef]
- Ball, I.R.; Lindenmayer, D.B.; Possingham, H.P. A Tree Hollow Dynamics Simulation Model. For. Ecol. Manag. 1999, 123, 179–194. [Google Scholar] [CrossRef]
- Vesk, P.A.; Nolan, R.; Thomson, J.R.; Dorrough, J.W.; Nally, R.M. Time Lags in Provision of Habitat Resources Through Revegetation. Biol. Conserv. 2008, 141, 174–186. [Google Scholar] [CrossRef]
- Dykstra, P.R. Thresholds in Habitat Supply: A Review of the Literature; Ministry of Sustainable Resource Management: British Columbia, VA, Canada, 2004.
- Forests as Complex Social and Ecological Systems: A Festschrift for Chadwick D. Oliver; Baker, P.J.; Larsen, D.R.; Saxena, A. (Eds.) Springer: Cham, Germany, 2022; ISBN 978-3-030-88554-0. [Google Scholar]
- Burkhard, B.; Kroll, F.; Nedkov, S.; Müller, F. Mapping Ecosystem Service Supply, Demand and Budgets. Ecol. Indic. 2012, 21, 17–29. [Google Scholar] [CrossRef]
- Rullens, V.; Townsend, M.; Lohrer, A.M.; Stephenson, F.; Pilditch, C.A. Who Is Contributing Where? Predicting Ecosystem Service Multifunctionality for Shellfish Species Through Ecological Principles. Sci. Total Environ. 2022, 808, 152147. [Google Scholar] [CrossRef]
- Blattner, C.E. Animal Labor, Ecosystem Services. Anim. Nat. Resour. Law Rev. 2020, 16, 1–40. [Google Scholar]
- Williams, J.W.; Ordonez, A.; Svenning, J.-C. A Unifying Framework for Studying and Managing Climate-Driven Rates of Ecological Change. Nat. Ecol. Evol. 2021, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wolkovich, E.M.; Cook, B.I.; McLauchlan, K.K.; Davies, T.J. Temporal Ecology in the Anthropocene. Ecol. Lett. 2014, 17, 1365–1379. [Google Scholar] [CrossRef]
- Blonder, B.; Moulton, D.E.; Blois, J.; Enquist, B.J.; Graae, B.J.; Macias-Fauria, M.; McGill, B.; Nogué, S.; Ordonez, A.; Sandel, B.; et al. Predictability in Community Dynamics. Ecol. Lett. 2017, 20, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Scaling and Uncertainty Analysis in Ecology: Methods and Applications; Wu, J. (Ed.) Springer: Dordrecht, The Netherlands, 2006; ISBN 978-1-4020-4664-3. [Google Scholar]
- Becker, M.E.; Bednekoff, P.A.; Janis, M.W.; Ruthven, D.C. Characteristics of Foraging Perch-Sites Used by Loggerhead Shrikes. Wilson J. Ornithol. 2009, 121, 104–111. [Google Scholar] [CrossRef]
- Lu, H.R.; El Hanandeh, A. Environmental and Economic Assessment of Utility Poles Using Life Cycle Approach. Clean Technol. Environ. Policy 2017, 19, 1047–1066. [Google Scholar] [CrossRef]
- Crawford, R.H. Life Cycle Energy and Greenhouse Emissions Analysis of Wind Turbines and the Effect of Size on Energy Yield. Renew. Sustain. Energy Rev. 2009, 13, 2653–2660. [Google Scholar] [CrossRef]
- Boogert, N.J.; Paterson, D.M.; Laland, K.N. The Implications of Niche Construction and Ecosystem Engineering for Conservation Biology. BioScience 2006, 56, 570–578. [Google Scholar] [CrossRef]
- Grimm, V.; Railsback, S.F.; Vincenot, C.E.; Berger, U.; Gallagher, C.; DeAngelis, D.L.; Edmonds, B.; Ge, J.; Giske, J.; Groeneveld, J.; et al. The ODD Protocol for Describing Agent-Based and Other Simulation Models: A Second Update to Improve Clarity, Replication, and Structural Realism. J. Artif. Soc. Soc. Simul. 2020, 23, 7. [Google Scholar] [CrossRef]
- Belton, D.; Moncrieff, S.; Chapman, J. Processing Tree Point Clouds Using Gaussian Mixture Models. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2013, II-5/W2, 43–48. [Google Scholar] [CrossRef]
- Hackenberg, J.; Spiecker, H.; Calders, K.; Disney, M.; Raumonen, P. SimpleTree: An Efficient Open Source Tool to Build Tree Models from TLS Clouds. Forests 2015, 6, 4245–4294. [Google Scholar] [CrossRef]
- Zanini, L.; Ganade, G. Restoration of Araucaria Forest: The Role of Perches, Pioneer Vegetation, and Soil Fertility. Restor. Ecol. 2005, 13, 507–514. [Google Scholar] [CrossRef]
- La Mantia, T.; Rühl, J.; Massa, B.; Pipitone, S.; Lo Verde, G.; Bueno, R.S. Vertebrate-Mediated Seed Rain and Artificial Perches Contribute to Overcome Seed Dispersal Limitation in a Mediterranean Old Field. Restor. Ecol. 2019, 27, 1393–1400. [Google Scholar] [CrossRef]
- Fraixedas, S.; Lindén, A.; Piha, M.; Cabeza, M.; Gregory, R.; Lehikoinen, A. A State-of-the-Art Review on Birds as Indicators of Biodiversity: Advances, Challenges, and Future Directions. Ecol. Indic. 2020, 118, 106728. [Google Scholar] [CrossRef]
- Evans, K.L.; Newson, S.E.; Gaston, K.J. Habitat Influences on Urban Avian Assemblages. Ibis 2009, 151, 19–39. [Google Scholar] [CrossRef]
- Basile, M.; Asbeck, T.; Jonker, M.; Knuff, A.K.; Bauhus, J.; Braunisch, V.; Mikusiński, G.; Storch, I. What Do Tree-Related Microhabitats Tell Us About the Abundance of Forest-Dwelling Bats, Birds, and Insects? J. Environ. Manage. 2020, 264, 110401. [Google Scholar] [CrossRef]
- Banks, J.C.G. Tree Ages and Ageing in Yellow Box. In The Coming of Age. Forest Age and Heritage Values; Dargavel, J., Ed.; Environment Australia: Canberra, Australia, 1997; pp. 17–28. [Google Scholar]
- Le Roux, D.S.; Waas, J.R. Do Long-Tailed Bats Alter Their Evening Activity in Response to Aircraft Noise? Acta Chiropterologica 2012, 14, 111–120. [Google Scholar] [CrossRef]
- Kuhn, M.; Wickham, H. Tidymodels (version 1.1.10). R Programming Language. 2020. Available online: https://www.tidymodels.org (accessed on 1 April 2023).
- Grasselli, F.; Airoldi, L. How and to What Degree Does Physical Structure Differ Between Natural and Artificial Habitats? A Multi-Scale Assessment in Marine Intertidal Systems. Front. Mar. Sci. 2021, 8, 766903. [Google Scholar] [CrossRef]
- Gibbons, P.; Lindenmayer, D.B. Tree Hollows and Wildlife Conservation in Australia; CSIRO: Collingwood, Australia, 2002.
- Killey, P.; Mcelhinny, C.; Rayner, I.; Wood, J. Modelling Fallen Branch Volumes in a Temperate Eucalypt Woodland: Implications for Large Senescent Trees and Benchmark Loads of Coarse Woody Debris. Austral Ecol. 2010, 35, 956–968. [Google Scholar] [CrossRef]
- Schwartz, T.; Genouville, A.; Besnard, A. Increased Microclimatic Variation in Artificial Nests Does Not Create Ecological Traps for a Secondary Cavity Breeder, the European Roller. Ecol. Evol. 2020, 10, 13649–13663. [Google Scholar] [CrossRef] [PubMed]
- Cardullo, P.; Kitchin, R. Being a ‘Citizen’ in the Smart City: Up and down the Scaffold of Smart Citizen Participation in Dublin, Ireland. GeoJournal 2019, 84, 1–24. [Google Scholar] [CrossRef]
- Ruhlandt, R.W.S. The Governance of Smart Cities: A Systematic Literature Review. Cities 2018, 81, 1–23. [Google Scholar] [CrossRef]
- Grimm, V.; Ayllón, D.; Railsback, S.F. Next-Generation Individual-Based Models Integrate Biodiversity and Ecosystems: Yes We Can, and Yes We Must. Ecosystems 2017, 20, 229–236. [Google Scholar] [CrossRef]
- Evers, J.B. Simulating Crop Growth and Development Using Functional-Structural Plant Modeling. In Canopy Photosynthesis: From Basics to Applications; Hikosaka, K., Niinemets, Ü., Anten, N.P.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 219–236. [Google Scholar]
- Petter, G.; Kreft, H.; Ong, Y.; Zotz, G.; Cabral, J.S. Modelling the Long-Term Dynamics of Tropical Forests: From Leaf Traits to Whole-Tree Growth Patterns. Ecol. Model. 2021, 460, 109735. [Google Scholar] [CrossRef]
- Loke, L.H.L.; Chisholm, R.A. Measuring Habitat Complexity and Spatial Heterogeneity in Ecology. Ecol. Lett. 2022, 25, 2269–2288. [Google Scholar] [CrossRef] [PubMed]
- Bjørn, A.; Chandrakumar, C.; Boulay, A.-M.; Doka, G.; Fang, K.; Gondran, N.; Hauschild, M.Z.; Kerkhof, A.; King, H.; Margni, M.; et al. Review of Life-Cycle Based Methods for Absolute Environmental Sustainability Assessment and Their Applications. Environ. Res. Lett. 2020, 15, 083001. [Google Scholar] [CrossRef]
- Lauver, C.L.; Busby, W.H.; Whistler, J.L. Testing a GIS Model of Habitat Suitability for a Declining Grassland Bird. Environ. Manage. 2002, 30, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Jobin, B.; Falardeau, G. Habitat Associations of Grasshopper Sparrows in Southern Québec. Northeast. Nat. 2010, 17, 135–146. [Google Scholar] [CrossRef]
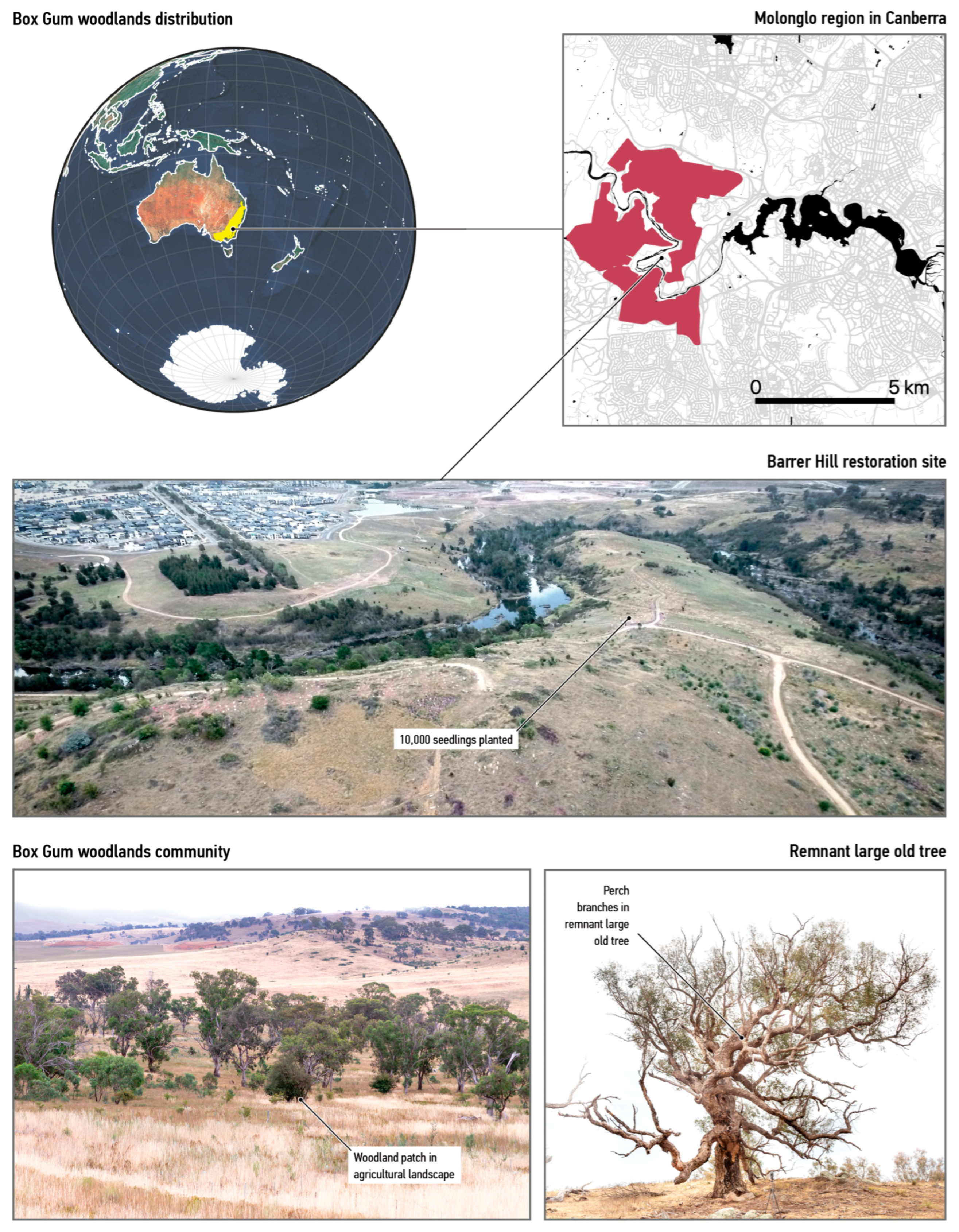

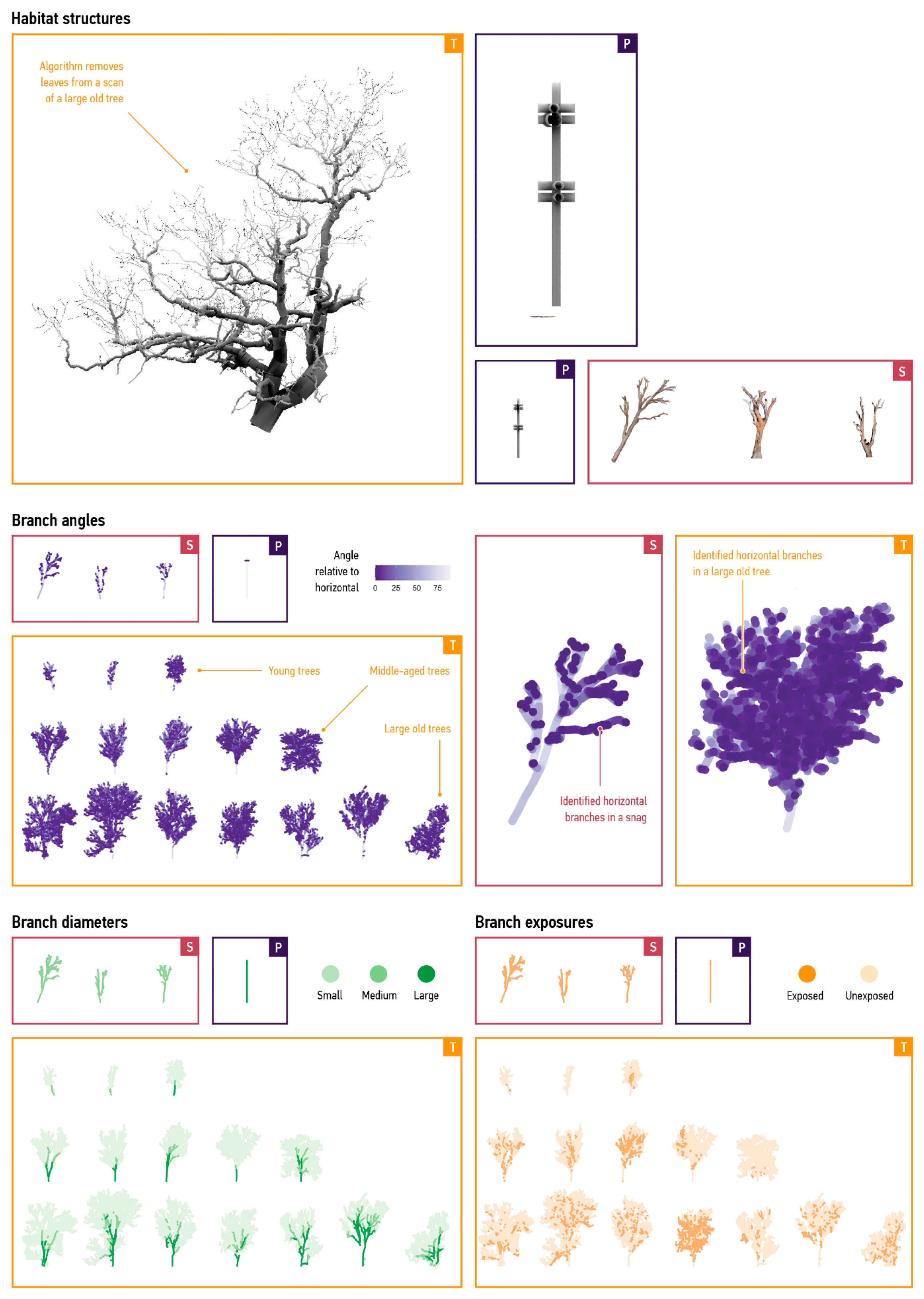

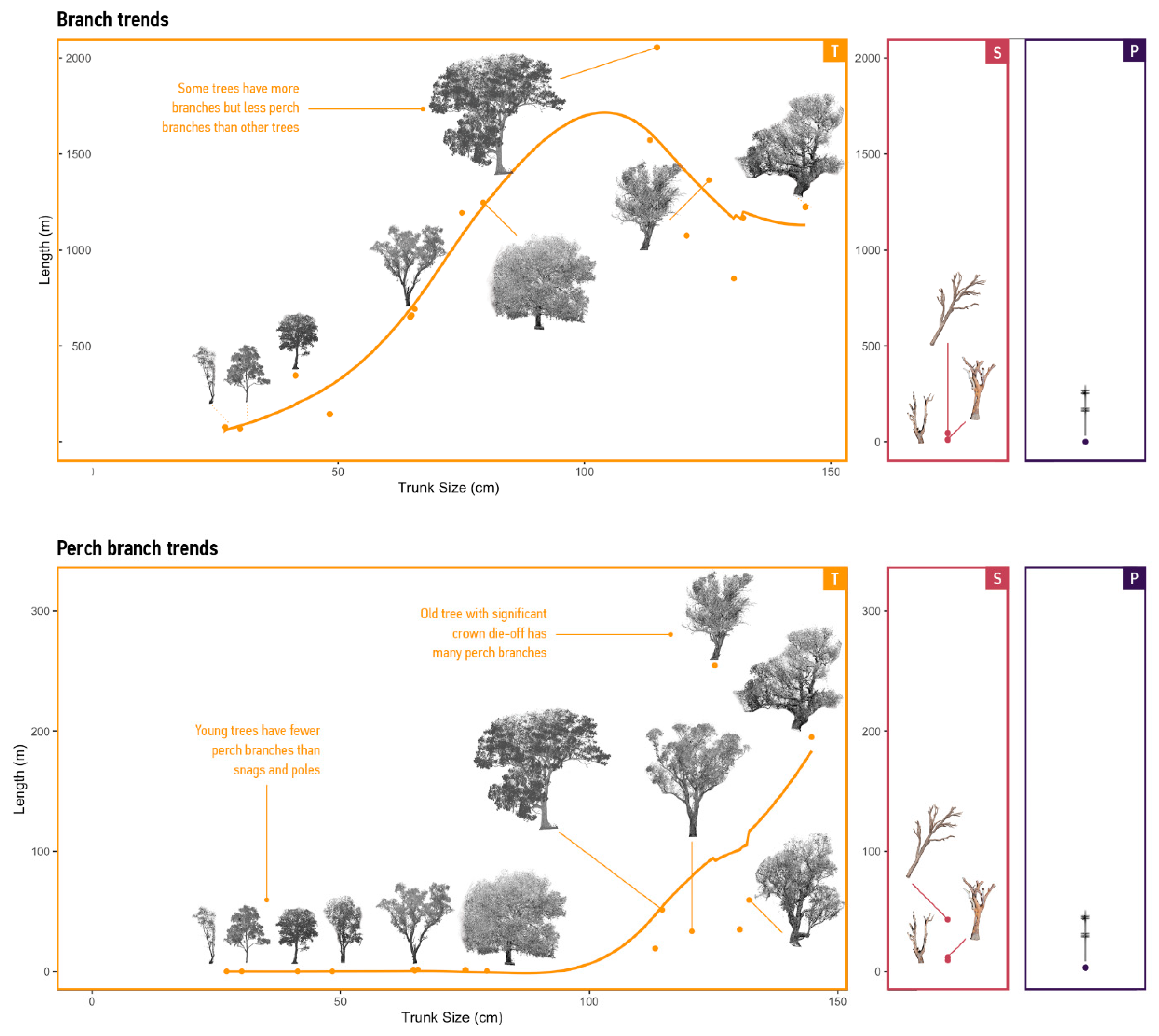
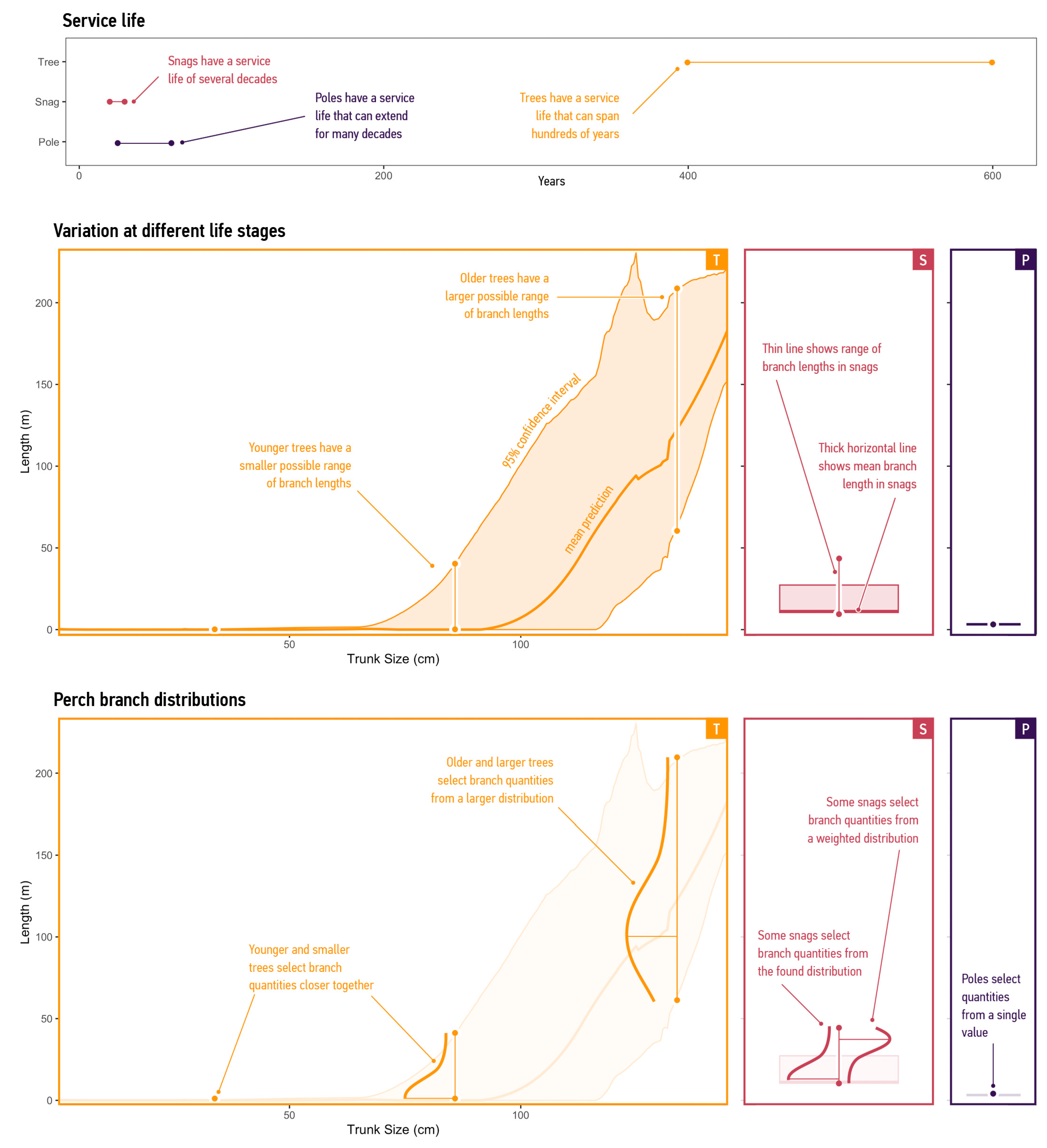

| Scale | Process | Data |
|---|---|---|
| Spatial | Physical structures exhibit characteristic shapes, sizes, and features. | Custom workflow extracting branch information from manufactured and living habitat structures Restoration plans [52] |
| Organisational | Birds recognise structures as habitat features. | Observations of bird–branch interactions and structural properties of branches obtained via computational-feature extractions (see Organisational Scale Results in Section 3.1.2) Evidence of bird preferences for branches [5,73] |
| Temporal | Habitat structures exhibit characteristic development patterns, renewal cycles, and disturbance events. | Life-cycle analysis estimating lifespans and costs of built structures [74,75]. Ecological studies quantifying the density of trees in landscapes, mortality rates, renewal processes, and other dynamics [11,23] |
| Variable | Units | Description |
|---|---|---|
| Agent Variables | ||
| Mode | Type | Supply mode (Snags, Poles, or Trees) |
| Age | Years | Variables that are common to all agent types |
| Location | X, Y coordinates | |
| Unit cost | AUD | |
| Service life | Years | |
| Has been terminated | Boolean | |
| Length of all branches in an agent | Metres | Habitat resources supplied by individual agents |
| Length of all dead branches | Metres | |
| Length of all lateral branches | Metres | |
| Length of all perch branches in an agent | Metres | |
| Girth diameter | Metres | An equivalent to trunk diameter at breast height for trees. This variable differs between modes: tree agents can grow, snags and poles cannot. |
| Environment Variables | ||
| Boundary | GIS polygon | Site boundary that yields the area |
| Budget | AUD | Total funds for agents in one simulation |
| Strategy | List of routines that manage agent populations and a list of statistical weightings that influence probability rang | Management actions |
| Supply | Metres/year | Cumulative length of total branches and perch branches |
| Process | Description | Run Time |
|---|---|---|
| Agent Actions | ||
| Survive | Increase ‘age’ by one Check if ‘age’ is less than ‘service life’, terminate if not. | Once per loop |
| Grow | Increase ‘girth diameter’ in tree agents based on a probability range. | Once per loop |
| Supply | Calculate the lengths of four branch types. Agents select resources randomly from a resource distribution calculated for each mode. | Snags and Poles run this process once when the environment runs ‘Initialise’ or ‘Renew’. Tree agents run this process after calling their ‘Grow’ process. |
| Environment Processes | ||
| Initialise | Define a strategy by setting variable values and renewal capabilities. Create the first generation of agents and set their variables. | At model initialisation |
| Renew | Create additional generations of agents. | Periodically, as defined at initialisation |
| Terminate | Terminate tree agents based on a probability range. | Once per loop |
| Update | Run agent actions. | Once per loop |
| Assess supply | Calculate lengths of branches by summing the lengths of all agents per mode. Remove terminated agents. | Once per loop |
| Operation | Resulting Data |
|---|---|
| Scan and model the shapes of habitat features. | Point clouds and polygonal meshes |
| Use feature-recognition algorithms to extract structural attributes. | Connected lines and their attributes |
| Use observational data to develop statistical models that estimate perch branches in sample structures. | Lengths of branches as extracted from scanned data |
| Link individual measures to define ranges of possible quantities of perch branches per mode. | Probability distributions representing likely perch branches |
| Make agents select values from within probability distributions. | Predicted branch lengths per agent |
| Budget Allowances | Distribution Weightings | ||||||
|---|---|---|---|---|---|---|---|
| Strategies | Trees | Snags | Poles | Unit Cost | Supply | Terminate | Renew |
| Plant Trees | 100% | 0 | 0 | Average | Average | Low | No |
| Plant, Maintain, and Renew Trees | 100% | 0 | 0 | Average | Average | High | Yes |
| Plant, Maintain, and Renew Trees; Source as Cheaply as Possible | 100% | 0 | 0 | Low | Average | High | Yes |
| Plant, Maintain, and Renew Trees; Install Poles | 50% | 0 | 50% | Average | Average | Average | Yes |
| Plant, Maintain, and Renew Trees; Install Poles Sourced Cheaply as Possible | 50% | 0 | 50% | Low | Average | Average | Yes |
| Plant, Maintain, and Renew Trees; Install Snags | 50% | 50% | 0 | Average | Average | Average | Yes |
| Plant, Maintain, and Renew Trees; Install Snags; Retain Snag Branches | 50% | 50% | 0 | Average | High | Average | Yes |
| Plant, Maintain, and Renew Trees; Install Snags; Retain Snag Branches; Source Snags Cheaply | 50% | 50% | 0 | Low | High | Average | Yes |
| Aspect | State of the Art | Benefits of Our Approach | Opportunity for Further Work |
|---|---|---|---|
| Detail | Informed by coarse resolution the aerial-lidar and other remote-sensing methods [50]. Assesses generic habitat features and typical shapes [11,23]. | Informed by higher resolutions of the terrestrial lidar. Assesses individual branch-like structures of any kind. | Includes additional habitat features such as hollows, peeling bark, and coarse woody debris. |
| Range | Can represent trees as simple shapes over extended periods [94] or represent detailed tree structures over short time spans [95,96]. Uses limited spatial and temporal ranges to assess artificial structures [29]. | Can represent detailed habitat features over time. Compares across extended ranges. | Models in response to geography and interactions within groups of habitat structures. |
| Dynamics | Evaluates strategies providing diverse habitat structures using opportunistic tools [97]. Considers individual events or strategies with limited scope for comparison [98]. | Evaluates strategies offering varied habitat structures in relation to bird utilization of features. Can assess and compare long-term trends. | Consider embodied energy and other properties of materials. Consider constructability and adaptability of structures. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holland, A.; Gibbons, P.; Thompson, J.; Roudavski, S. Modelling and Design of Habitat Features: Will Manufactured Poles Replace Living Trees as Perch Sites for Birds? Sustainability 2023, 15, 7588. https://doi.org/10.3390/su15097588
Holland A, Gibbons P, Thompson J, Roudavski S. Modelling and Design of Habitat Features: Will Manufactured Poles Replace Living Trees as Perch Sites for Birds? Sustainability. 2023; 15(9):7588. https://doi.org/10.3390/su15097588
Chicago/Turabian StyleHolland, Alexander, Philip Gibbons, Jason Thompson, and Stanislav Roudavski. 2023. "Modelling and Design of Habitat Features: Will Manufactured Poles Replace Living Trees as Perch Sites for Birds?" Sustainability 15, no. 9: 7588. https://doi.org/10.3390/su15097588
APA StyleHolland, A., Gibbons, P., Thompson, J., & Roudavski, S. (2023). Modelling and Design of Habitat Features: Will Manufactured Poles Replace Living Trees as Perch Sites for Birds? Sustainability, 15(9), 7588. https://doi.org/10.3390/su15097588







