Chemical, Anatomical, and Productivity Responses of Cowpea (Vigna unguiculata L.) to Integrated Biofertilizer Applications with PGPR, Cyanobacteria, and Yeast
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
2.1.2. Plant Growth-Promoting Rhizobacterial, Yeast, and Cyanobacteria Strains
2.1.3. Soil
2.2. Methods
2.2.1. Soil Analysis
2.2.2. Field Experiment
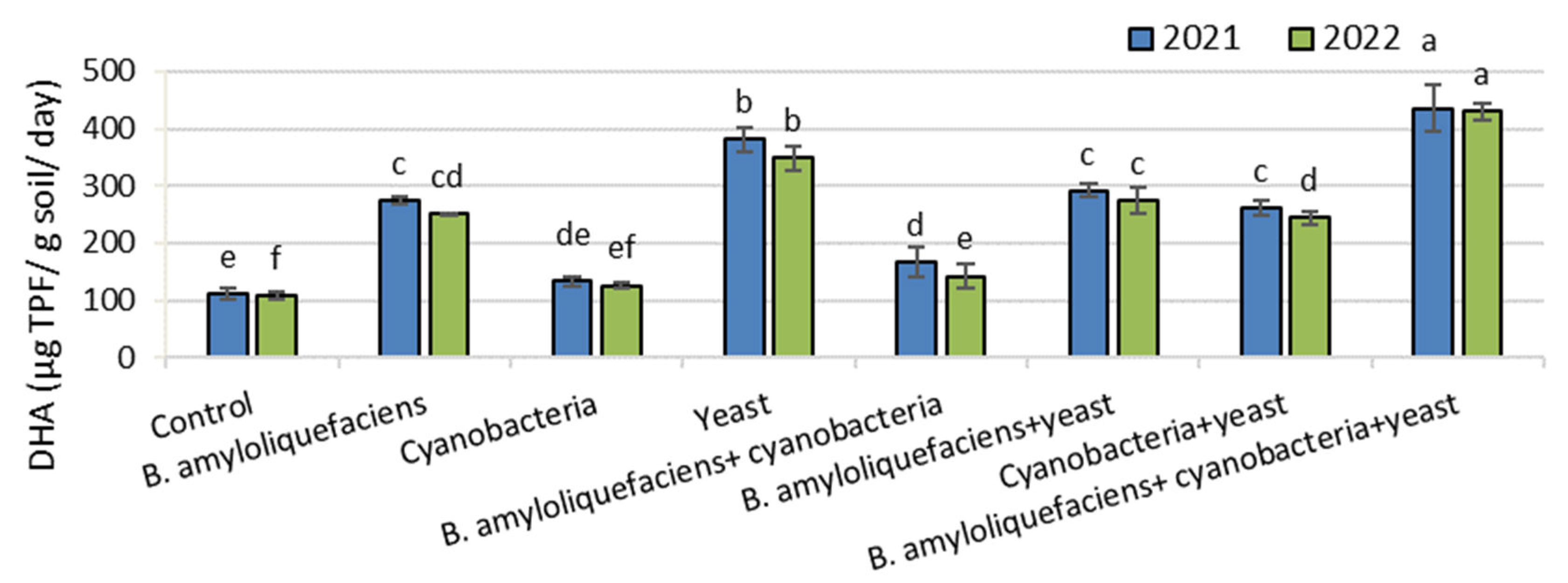
2.2.3. Analysis of Soil: Dehydrogenase Enzyme Activity (DHA)
2.2.4. Analysis of Plant
- Pigments of Photosynthesis:
- b.
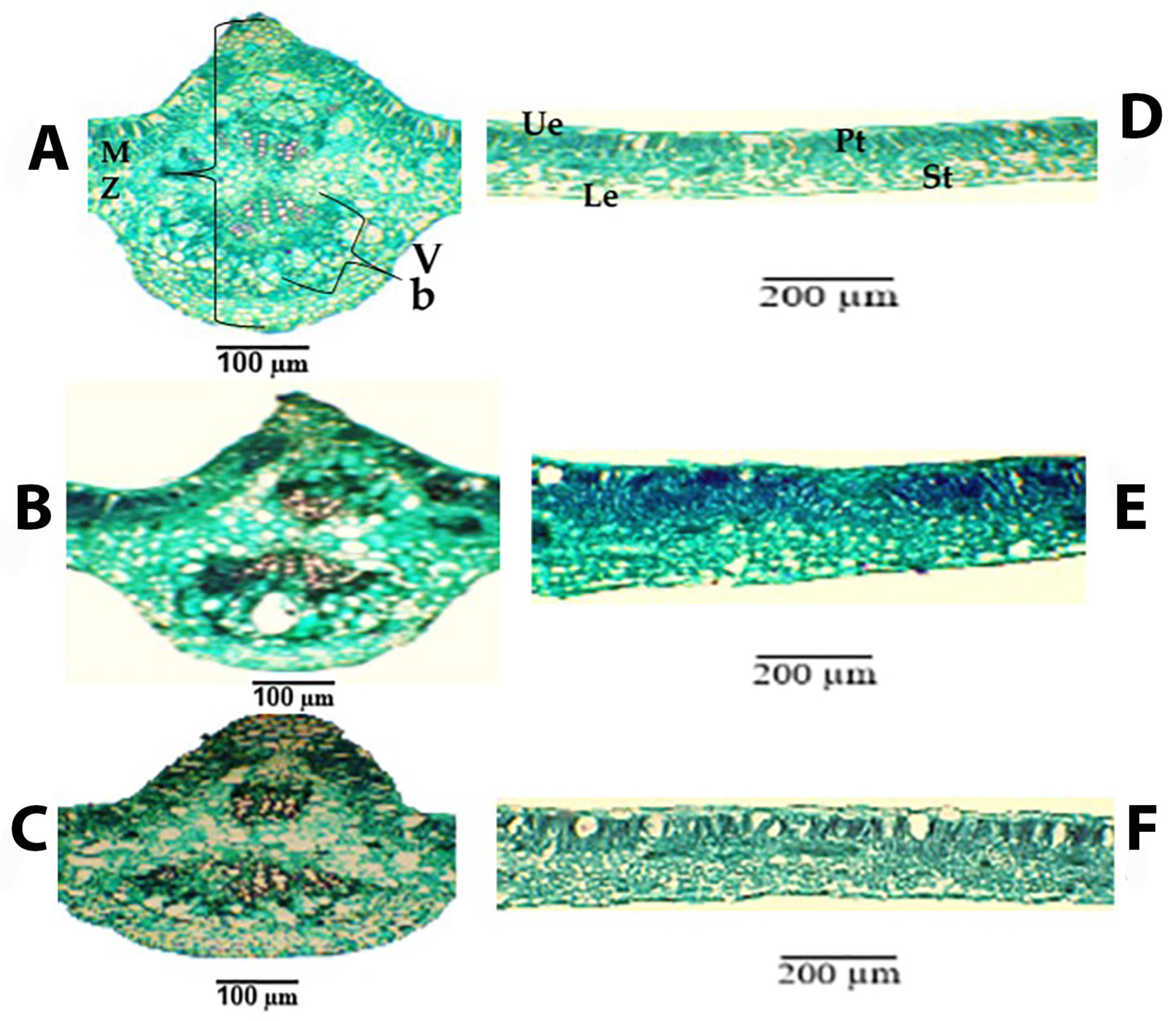
- Anatomical Studies:
2.2.5. Yield Parameters
- Productivity Criteria
- b.
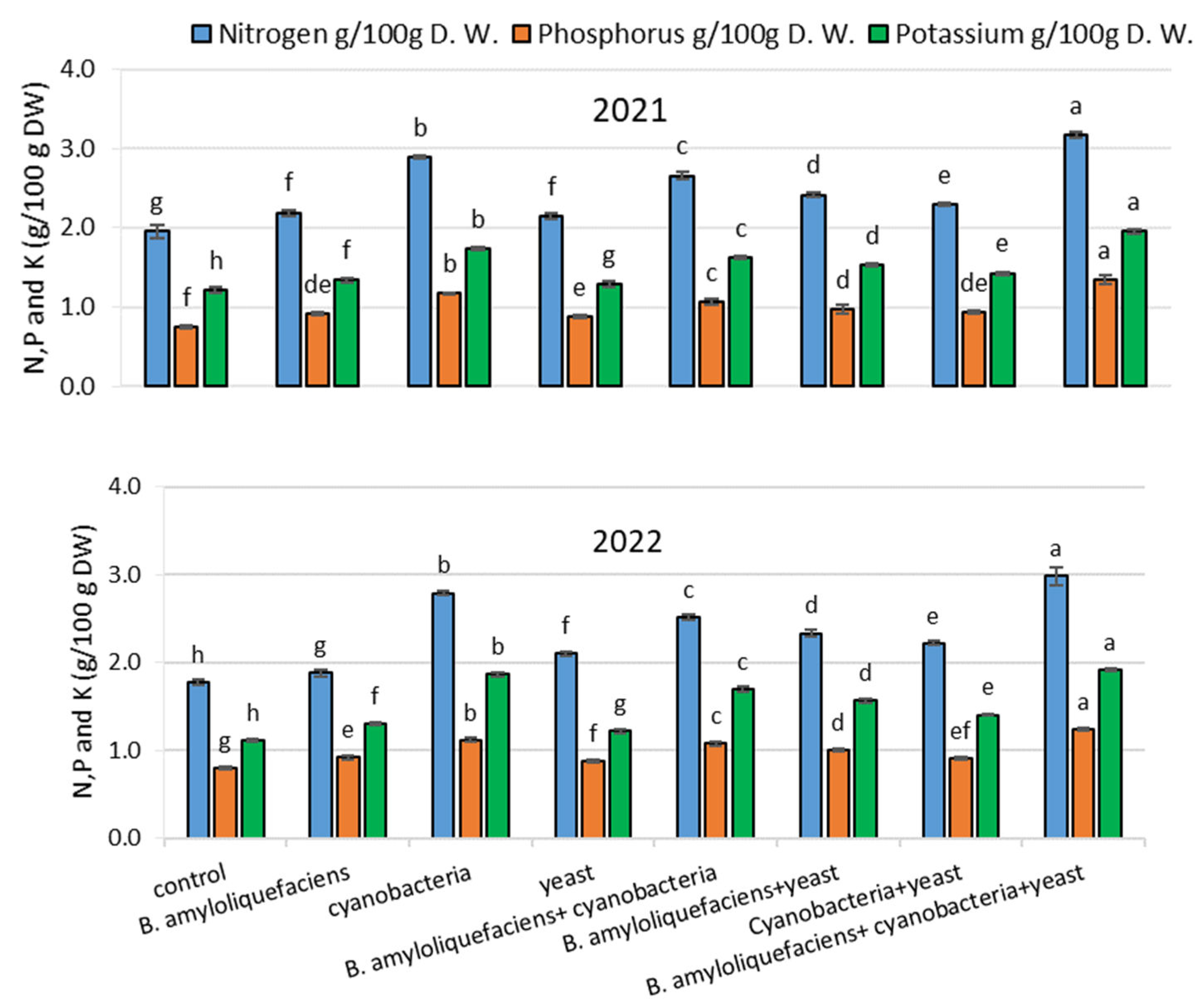
- Chemical Contents of Seed
- c.
- Total Carbohydrates Contents
2.3. Statistical Analysis
3. Results
3.1. Analysis of Soil (Dehydrogenase Enzyme Activity (DHA))
3.2. Plant Analysis
3.2.1. Photosynthetic Pigments
3.2.2. Anatomical Studies
3.3. Yield Parameters
- Productivity Criteria
- b.
- Pod Features Parameters
- c.
- Seed Chemical Content
- d.
- Total Carbohydrate Contents
3.4. Multivariate Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes, A.M.F.; Draper, D.; Nhantumbo, N.; Massinga, R.; Ramalho, J.C.; Marques, I.; Ribeiro-Barros, A.I. Diversity of cowpea [Vigna unguiculata (L.) Walp] landraces in Mozambique: New opportunities for crop improvement and future breeding programs. Agronomy 2021, 11, 991. [Google Scholar] [CrossRef]
- Owade, J.O.; Abong, G.O.; Okoth, M.W.; Mwang’ombe, A.W. Trends and constraints in the production and utilization of cowpea leaves in the arid and semi-arid lands of Kenya. Open Agric. 2020, 5, 325–334. [Google Scholar] [CrossRef]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.T.; Mafongoya, P. The potential role of neglected and underutilized crop species as future crops under water scarce conditions in sub-Saharan Africa. Int. J. Environ. Res. Public. Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O. Cowpea (Vigna unguiculata): Genetics, genomics and breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef][Green Version]
- Langyintuo, A.S.; Lowenberg-DeBoer, J.; Faye, M.; Lambert, D.; Ibro, G.; Moussa, B.; Kergna, A.; Kushwaha, S.; Musa, S.; Ntoukam, G. Cowpea supply and demand in west and central Africa. Field Crop. Res. 2003, 82, 215–231. [Google Scholar] [CrossRef]
- Singh, B.B. Recent genetic studies in cowpea. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production, Proceedings of the World Cowpea Conference III held at the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria, 4–8 September 2000; Fatokun, C.A., Tarawali, S., Singh, B., Kormawa, P., Tamo, M., Eds.; ITTA: Ibadan, Nigeria, 2002; pp. 3–13. [Google Scholar]
- Carsky, R.J.; Vanlauwe, B.; Lyasse, O. Cowpea rotation as a resource management technology for cereal-based systems in the savannas of west Africa. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production; Fatokun, C.A., Tarawali, S.A., Singh, B.B., Kormawa, P.M., Tamo, M., Eds.; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2002; pp. 252–266. [Google Scholar]
- Sanginga, N.; Dashiell, K.E.; Diels, J.; Vanlauwe, B.; Lyasse, O.; Carsky, R.J.; Tarawali, S.; Asafo-Adjei, B.; Menkir, A.; Schulz, S. Sustainable resource management coupled to resilient germplasm to provide new intensive cereal–grain–legume–livestock systems in the dry savanna. Agric. Ecosyst. Environ. 2003, 100, 305–314. [Google Scholar] [CrossRef]
- Tarawali, S.A.; Singh, B.B.; Gupta, S.C.; Tabo, R.; Harris, F.; Nokoe, S.; Ferandez-Rivera, S.; Bationo, A.; Manyong, V.M.; Makinde, K.; et al. Cowpea as a key factor for a new approach to integrated crop-livestock systems research in the dry savannas of West Africa. In Challenges and Opportunities for Enhancing Sustainable Cowpea Production; Fatokun, C.A., Tarawali, S.A., Singh, B.B., Kormawa, P.M., Tamo, M., Eds.; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2000; pp. 233–251. [Google Scholar]
- Timko, M.P.; Ehlers, J.D.; Roberts, P.A. Cowpea. In Pulses, Sugar and Tuber Crops; Genome mapping and molecular breeding in plants; Kole, C., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2007; Volume 3, pp. 49–67. ISBN 978-3-540-34515-2. [Google Scholar] [CrossRef]
- Enyiukwu, D.N.; Amadioha, A.; Ononuju, C. Nutritional significance of cowpea leaves for human consumption. Greener Trends Food Sci. Nutr. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An overview on its nutritional facts and health benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef]
- Kirigia, D.; Winkelmann, T.; Kasili, R.; Mibus, H. Development stage, storage temperature and storage duration influence phytonutrient content in cowpea (Vigna unguiculata L. Walp.). Heliyon 2018, 4, e00656. [Google Scholar] [CrossRef]
- Bado, B.V.; Bationo, A.; Cescas, M.P. Assessment of cowpea and groundnut contributions to soil fertility and succeeding Sorghum yields in the Guinean savannah zone of Burkina Faso (West Africa). Biol. Fertil. Soils 2006, 43, 171–176. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Roy, T.; Bandopadhyay, A.; Paul, C.; Majumdar, S.; Das, N. Role of plasmid in pesticide degradation and metal tolerance in two plant growth-promoting rhizobacteria Bacillus cereus (NCIM 5557) and Bacillus safensis (NCIM 5558). Curr. Microbiol. 2022, 79, 106. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Johnson, K.B.; Sugar, D.; Loper, J.E. Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology 2011, 101, 113–123. [Google Scholar] [CrossRef][Green Version]
- Singh, H.; Khattar, J.S.; Ahluwalia, A.S. Cyanobacteria and agricultural crops. Vegetos 2014, 27, 37–44. [Google Scholar] [CrossRef]
- Ashmawi, A.; Salem, G.; Ghazal, M.; Elemshaty, A.; El, A. Effect of some indigenous Bacilli and Cyanobacteria strains inoculants on growth characteristics and productivity of sweet pepper (Capsicum frutescens). Aust. J. Basic Appl. Sci. 2022, 16, 1–11. [Google Scholar]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic Effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef][Green Version]
- Balasubramanian, A. Soil Microorganisms; Technical Report; University of Mysore: Mysore, India, 2017. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Zahir, Z.A. Screening plant growth-promoting Rhizobacteria for improving growth and yield of wheat. J. Appl. Microbiol. 2004, 96, 473–480. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Kloepper, J.W. Plant growth-promoting Rhizobacteria as biological control agents. In Soil Microbial Ecology Applications in Agricultural and Environmental Management; Metting, F.B., Jr., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1992. [Google Scholar]
- Ashmawi, A.E.; Elemshaty, A.M.; Salem, G.M.; Ghazal, M.F. Enhancing Cucurbita pepo growth, productivity, and fruit quality using Bacilli strains and cyanobacteria treatments. J. Adv. Biol. Biotechnol. 2021, 24, 1–11. [Google Scholar] [CrossRef]
- Rezki, M.A.; Kouadri, F.; Bekki, A. Evaluation of plant growth-promoting yeasts and their effect on chickpea plant growth. S. Asian J. Exp. Biol. 2022, 12, 547–556. [Google Scholar] [CrossRef]
- Omran, Y.A. Studies on Histophysiological Effect of Hydrogen Cyanamide (Dormex) and Yeast Application on Bud Fertility, Vegetative Growth and Yield of “Roumi Red” Grape Cultivar. Ph.D. Thesis, Assiut University, Asyut, Egypt, 2000. [Google Scholar]
- Karajeh, M.R. Enhancement of tomato growth, yield and resistance to the root-knot nematode (Meloidogyne javanica) after the field application of Saccharomyces cerevisiae. Hell. Plant Prot. J. 2014, 7, 35–41. [Google Scholar]
- Mekki, B.B.; Ahmed, A.G. Growth, yield and seed quality of soybean (Glycine max L.) as affected by organic, biofertilizer and yeast application. Res. J. Agric. Biol. Sci. 2005, 1, 320–324. [Google Scholar]
- Azzam, S.A.; Karam, N.S.; Hameed, K.M.; Goussous, S.J.; Maraqa, A.D.; Makhadmeh, I.M. Investigation of indigenous plant root associated bacteria and yeast isolates for plant growth promoting ability. Jordan J. Agric. Sci. 2012, 8, 1–14. [Google Scholar]
- Karajeh, M.R. Efficacy of Saccharomyces cerevisiae on controlling the root-knot Nematode (Meloidogyne javanica) infection and promoting Cucumber growth and yield under laboratory and field conditions. Arch. Phytopathol. Plant Prot. 2013, 46, 2492–2500. [Google Scholar] [CrossRef]
- Singh, H.; Ahluwalia, A.S.; Khattar, J.I.S. Induction of sporulation by different nitrogen sources in Anabaena naviculoides, a diazotrophic strain capable of colonizing Paddy Field Soil of Punjab (India). Vegetos 2013, 26, 283–292. [Google Scholar] [CrossRef]
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2013, 63, 1–19. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis (Part. 2): Chemical and Microbiological Properties, 2nd ed.; The American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Bashour, I.I.; Sayegh, A.H. Methods of Analysis for Soils of Arid and Semi-Arid Regions; FAO: Rome, Italy, 2007. [Google Scholar]
- Veihmeyer, F.J.; Hendrickson, A.H. Methods of measuring field capacity and permanent wilting percentage of soils. Soil Sci. 1949, 68, 75–94. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall, Inc.: Hoboken, NJ, USA, 1967. [Google Scholar]
- Allison, L. Organic carbon. In Methods of Soil Analysis. Part 2; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1367–1378. ISBN 978-0-8911-8374-7. [Google Scholar]
- Markus, D.K.; Mckinnon, J.P.; Buccasuri, A.S. Automated Analysis of Nitrate and Ammonium Nitrogen in Soils; New Jersey Agricultural Experiment Station: New Brunswick, NJ, USA, 1982; Publication No. 15117–84. [Google Scholar]
- Soltanpour, P.N. Determination of Nutrient Availability and Elemental Toxicity by AB-DTPA Soil Test and ICPS. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1991; Volume 16, pp. 165–190. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Interscience Publishers, Inc.: New York, NY, USA, 1950. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Allen, M.M.; Stanier, R.Y.Y. Growth and division of some unicellular blue-green algae. Microbiology 1968, 51, 199–202. [Google Scholar] [CrossRef][Green Version]
- Glathe, H.; Thalmann, A. Uber die mikrobielle aktivitat und ihre beziehungen zu fruchtbarkeitsmerkmalen einiger ackerboden unter besonderer berucksichtigung der dehydrogenaseaktivitat (TTC-Reduktion). III. Mikrobiologische untersuchungen an proben von freilandversuchen auf boden mit unterschiedlichen fruchtbarkeitsmerkmalen. Zent. Bakteriol Parasitenk Infekt. Hyg II Abt. Bd. 1970, 124, 37–55. (In German) [Google Scholar]
- Moran, R. Formulae for Determination of chlorophyllous pigments extracted with N, N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef][Green Version]
- Nassar, M.A.; El-Sahhar, K.F. Botanical Preparations and Microscopy (Microtechnique); Academic Bookshop: Giza, Egypt, 1998; 219p. [Google Scholar]
- Chapman, N.; Pratt, P. Methods of Soil Analysis for Soils, Plant and Water; Division of Agricultural Sciences, University of California: Riverside, CA, USA, 1961. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis—Advanced Course: A Manual of Methods Useful for Instruction and Research in Soil Chemistry, Physical Chemistry of Soils, Soil Fertility and Soil Genesis; Department of Soil Sciences, University of Wisconsin: Madison, WI, USA, 1979. [Google Scholar]
- Herbert, D.; Phipps, P.J.; Strange, R.E. Chapter III Chemical analysis of microbial cells. In Methods in Microbiology; Norris, J.R., Ribbons, D.W., Eds.; Academic Press: Cambridge, MA, USA, 1971; Volume 5, pp. 209–344. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]
- White, P.J.; Brown, P. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef][Green Version]
- Gourley, C.J.; Dougherty, W.J.; Weaver, D.M.; Aarons, S.R.; Awty, I.M.; Gibson, D.M.; Hannah, M.C.; Smith, A.P.; Peverill, K.I. Farm-scale nitrogen, phosphorus, potassium and sulfur balances and use efficiencies on Australian dairy farms. Anim. Prod. Sci. 2012, 52, 929–944. [Google Scholar] [CrossRef][Green Version]
- Nada, R.S.; Ashmawi, A.E.; Mady, E.; Randhir, T.O.; Elateeq, A.A. Effect of organic manure and plant growth promoting microbes on yield, quality and essential oil constituents of fennel bulb (Foeniculum vulgare Mill.). J. Ecol. Eng. 2022, 23, 149–164. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef][Green Version]
- Pandey, V.; Chandra, K. Agriculturally important microorganisms as biofertilizers: Commercialization and regulatory requirements in Asia. In Agriculturally Important Microorganisms; Springer: Berlin/Heidelberg, Germany, 2016; pp. 133–145. [Google Scholar] [CrossRef]
- Roldán, A.; Salinas-García, J.R.; Alguacil, M.M.; Díaz, G.; Caravaca, F. Changes in soil microbial activity following conservation tillage practices in a Sorghum field under subtropical conditions. In Proceedings of the ISCO 2004—13th International Soil Conservation Organisation Conference, Brisbane, Australia, 4–8 July 2004. [Google Scholar]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Plant growth-promoting traits in enterobacter cloacae Subsp. dissolvens MDSR9 Isolated from soybean rhizosphere and its impact on growth and nutrition of soybean and wheat upon inoculation. Agric. Res. 2014, 3, 53–66. [Google Scholar] [CrossRef]
- Gopinath, K.A.; Saha, S.; Mina, B.L.; Pande, H.; Kundu, S.; Gupta, H.S. Influence of organic amendments on growth, yield and quality of wheat and on soil properties during transition to organic production. Nutr. Cycl. Agroecosyst. 2008, 82, 51–60. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and Cyanobacteria. Eur. J. Soil Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ramesh, A.; Johri, B.N. Isolation and characterization of plant growth promoting Bacillus amyloliquefaciens strain Sks_bnj_1 and its influence on rhizosphere soil properties and nutrition of soybean (Glycine max L. Merrill). J. Virol. Microbiol. 2013, 2013, 446006. [Google Scholar] [CrossRef][Green Version]
- Tolba, H.I.; Morsy, E.M.; Ahmed, S.M.; EL-Sayed, G.A. Effect of Saccharomyces cerevisiae and humate substances application on maize (Zea mays) productivity under different levels of mineral fertilization. N. Egypt J. Microbiol. 2016, 43, 83–98. [Google Scholar]
- de Caire, G.Z.; de Cano, M.S.; Palma, R.M.; de Mulé, C.Z. Changes in soil enzyme activities following additions of cyanobacterial biomass and exopolysaccharide. Soil Biol. Biochem. 2000, 32, 1985–1987. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Gámez, B.Y.; Garruna, R.; Tun-Suarez, J.M.; Kantun-Can, J.; Reyes-Ramirez, A.; Cervantes-Diaz, L. Bacillus spp. Inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants. Chil. J. Agric. Res. 2016, 76, 409–416. [Google Scholar] [CrossRef][Green Version]
- Helaly, A.A.; Mady, E.; Salem, E.A.; Randhir, T.O. Stimulatory effects of growth-promoting bacteria on growth, nutritional composition, and yield of kale plants. J. Plant Nutr. 2022, 45, 2465–2477. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Burjus, S.J.; Jawad, A.M.; Al-Ani, N.K. Effect of two species of Cyanobacteria as biofertilizers on characteristics and yield of chickpea plant. Iraqi J. Sci. 2014, 55, 685–696. [Google Scholar] [CrossRef]
- Younis, M.; Hasaneen, N.A.; Tourky, S.M. Plant growth, metabolism and adaptation in relation to stress conditions. XXIV. salinity-bio fertility interactive effects on proline, glycine and various antioxidants in Lactuca sativa L. J. Plant Prod. 2009, 2, 197–205. [Google Scholar] [CrossRef]
- Hayat, A.H. Physiological Studies on Hibiscus sabdariffa L. Production in New Reclamated Soils. Master’s Thesis, Faculty of Agriculture, Zagazig University, Zagazig, Egypt, 2007. [Google Scholar]
- Stino, R.G.; Mohsen, A.T.; Maksoud, M.A.; Abd El-Migeed, M.M.M.; Gomaa, A.M.A.; Ibrahim, A.Y. Bio-organic fertilization and its impact on apricot young trees in newly reclaimed soil. Am.-Eurasian J. Agric. Environ. Sci. 2009, 6, 62–69. [Google Scholar]
- Hussain, T.; Anjum, A.D.; Tahir, J. Technology of beneficial microorganisms. Nat. Farm Environ. 2002, 3, 1–14. [Google Scholar]
- Paradiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front. Plant Sci. 2017, 8, 674. [Google Scholar] [CrossRef][Green Version]
- Pieruschka, R.; Schurr, U.; Jahnke, S. Lateral gas diffusion inside leaves. J. Exp. Bot. 2005, 56, 857–864. [Google Scholar] [CrossRef][Green Version]
- Gashash, E.A.; Osman, N.A.; Alsahli, A.A.; Hewait, H.M.; Ashmawi, A.E.; Alshallash, K.S.; El-Taher, A.M.; Azab, E.S.; Abd El-Raouf, H.S.; Ibrahim, M.F. Effects of plant-growth-promoting Rhizobacteria (PGPR) and Cyanobacteria on botanical characteristics of tomato (Solanum lycopersicon L.) plants. Plants 2022, 11, 2732. [Google Scholar] [CrossRef]
- Maslenkova, L.; Peeva, V.; Stojnova, Z.; Popova, L. Salicylic acid-induced changes in photosystem II reactions in barley plants. Biotechnol. Equip. 2009, 23, 297–300. [Google Scholar] [CrossRef]
- Farouk, S.; Osman, M.A. The Effect of plant defense elicitors on common bean (Tetranychus urtica Koch) infestation. J. Stress Physiol. Biochem. 2011, 7, 5. [Google Scholar]
- Nour, K.A.M.; Mansour, N.T.S.; Eisa, G.S.A. Effect of some antioxidants on some physiological and anatomical characters of snap bean plants under sandy soil conditions. N. Y. Sci. J. 2012, 5, 1–9. [Google Scholar]
- Ali, Z.A.; Hussein, M.M.; El-Taher, A.M. Effect of antioxidants on some morphological and anatomical features of maize grown under salinity conditions. Int. J. Chem. Tech. Res. 2015, 8, 389–400. [Google Scholar]
- Gomaa, E.F.; Nassar, R.M.; Madkour, M.A. Effect of foliar spray with salicylic acid on vegetative growth, stem and leaf anatomy, photosynthetic pigments and productivity of egyptian lupine plant (Lupinus termis Forssk.). Int. J. Adv. Res. 2015, 3, 803–813. [Google Scholar]
- Gashash, E.A.; Ashmawi, A.E.; El-Taher, A.M.; Omar, M.A.; Osman, N.A.; Taha, N.M.; Elkelish, A. Effect of fertilizing with different levels of phosphorous and zinc on the botanical characteristics of table beet (Beta Vulgaris L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12579. [Google Scholar] [CrossRef]
- El-Desouky, S.A.; Ismaeil, F.H.; Wanas, A.L.; Fathy, E.S.L.; AbdEl-All, M.M.; Abd, M.M. Effect of yeast extract, amino acids and citric acid on physioanatomical aspects and productivity of tomato plants grown in late summer season. Minufiya J. Agric. Res. 2011, 36, 859–884. [Google Scholar] [CrossRef][Green Version]
- Malusà, E.; Pinzari, F.; Canfora, L. Efficacy of biofertilizers: Challenges to improve crop production. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 17–40. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Growth and nutrition of cowpea (Vigna unguiculata) under water deficit as influenced by microbial inoculation via seed coating. J. Agron. Crop Sci. 2019, 205, 447–459. [Google Scholar] [CrossRef]
- Prasanna, R.; Nain, L.; Ancha, R.; Srikrishna, J.; Joshi, M.; Kaushik, B.D. Rhizosphere dynamics of inoculated Cyanobacteria and their growth-promoting role in rice crop. Egypt. J. Biol. 2009, 11, 26–36. [Google Scholar]
- Mäder, P.; Kaiser, F.; Adholeya, A.; Singh, R.; Uppal, H.S.; Sharma, A.K.; Srivastava, R.; Sahai, V.; Aragno, M.; Wiemken, A. Inoculation of root microorganisms for sustainable wheat–rice and wheat–black gram rotations in India. Soil Biol. Biochem. 2011, 43, 609–619. [Google Scholar] [CrossRef]
- Prasanna, R.; Chaudhary, V.; Gupta, V.; Babu, S.; Kumar, A.; Singh, R.; Shivay, Y.S.; Nain, L. Cyanobacteria mediated plant growth promotion and bioprotection against Fusarium wilt in tomato. Eur. J. Plant Pathol. 2013, 136, 337–353. [Google Scholar] [CrossRef]
- Nayak, S.; Prasanna, R.; Pabby, A.; Dominic, T.K.; Singh, P.K. Effect of urea, blue green algae and azolla on nitrogen fixation and chlorophyll accumulation in soil under rice. Biol. Fertil. Soils 2004, 40, 67–72. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting Rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus Subtilis/Amyloliquefaciens. Microb. Biotechnol. 2015, 8, 281–295. [Google Scholar] [CrossRef]
- Salvatierra-Martinez, R.; Arancibia, W.; Araya, M.; Aguilera, S.; Olalde, V.; Bravo, J.; Stoll, A. Colonization ability as an indicator of enhanced biocontrol capacity—An example using two Bacillus amyloliquefaciens strains and Botrytis cinerea infection of tomatos. J. Phytopathol. 2018, 166, 601–612. [Google Scholar] [CrossRef]
- Kuklinsky-Sobral, J.; Araújo, W.L.; Mendes, R.; Geraldi, I.O.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004, 6, 1244–1251. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef][Green Version]
- Salim, H.A.; Kadhum, A.A.; Ali, A.F.; Saleh, U.N.; Jassim, N.H.; Hamad, A.R.; Attia, J.A.; Darwish, J.J.; Hassan, A.F. Response of cucumber plants to PGPR bacteria (Azospirillum brasilense, Pseudomonas fluorescens and Bacillus megaterium) and bread yeast (Saccharomyces cerevisiae). Syst. Rev. Pharm. 2021, 12, 969–975. [Google Scholar]
- Sarhan, T.Z. Effect of Biological Fertilizers, Animal Residues and Urea on Growth and Yield of Potato Plant cv Desiree (Solanum tuberosum L.). Horticulture Sciences and Landscape Design (Vegetable). Ph.D. Thesis, College of Agriculture and Forestry, University of Mosul, Mosul, Iraq, 2008. [Google Scholar]
- Bevilacqua, A.; Corbo, M.R.; Mastromatteo, M.; Sinigaglia, M. Combined effects of pH, yeast extract, carbohydrates and di-ammonium hydrogen citrate on the biomass production and acidifying ability of a probiotic Lactobacillus plantarum strain, isolated from table olives, in a batch system. World J. Microbiol. Biotechnol. 2008, 24, 1721–1729. [Google Scholar] [CrossRef]
- Nain, L.; Rana, A.; Joshi, M.; Jadhav, S.D.; Kumar, D.; Shivay, Y.S.; Paul, S.; Prasanna, R. Evaluation of synergistic effects of bacterial and cyanobacterial strains as biofertilizers for wheat. Plant Soil 2010, 331, 217–230. [Google Scholar] [CrossRef]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Schmidt, S.; Paungfoo-Lonhienne, C. Yeast as a biofertilizer alters plant growth and morphology. Crop Sci. 2014, 54, 785–790. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Helaly, A.A.; Hassan, S.M.; Craker, L.E.; Mady, E. Effects of growth-promoting bacteria on growth, yield and nutritional value of collard plants. Ann. Agric. Sci. 2020, 65, 77–82. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Nada, R.S.; Mady, E.; Ashmawi, A.E.; Gashash, E.A.; Elateeq, A.A.; Suliman, A.A.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Zarad, M.M.; et al. Effect of organic and bio-fertilization on fruit yield, bioactive constituents, and estragole content in fennel fruits. Agronomy 2023, 13, 1189. [Google Scholar] [CrossRef]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Azcon, R.; Medina, A. Application of free and Ca-Alginate-entrapped Glomus deserticola and Yarowia lipolytica in a soil–plant system. J. Biotechnol. 2001, 91, 237–242. [Google Scholar] [CrossRef]
- Al-Falih, A.M. Phosphate solubilization in vitro by some soil yeasts. Qatar Univ. Sci. J. 2005, 25, 119–125. Available online: http://hdl.handle.net/10576/9742 (accessed on 7 December 2022).
- Cloete, K.J.; Valentine, A.J.; Stander, M.A.; Blomerus, L.M.; Botha, A. Evidence of symbiosis between the soil yeast cryptococcus Laurentii and Sclerophyllous medicinal shrub, Agathosma betulina (Berg.) Pillans. Microbiol. Ecol. 2009, 57, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Prakash, S.; Luthra, S.K.; Singh, B.; Chand, P.; Kumar, V.; Singh, R.; Alam, K. Analysis of correlation and path coefficient among the yield and yield attributes characters in potato (Solanum tuberosum L.). Biol. Forum. 2022, 14, 916–922. [Google Scholar]



| Property | Value | Property | Value |
|---|---|---|---|
| pH (in suspension 1:2.5) | 7.81 | N (ppm) | 25 |
| P (ppm) | 9 | ||
| Saturation Percentage (SP%) | 21.12 | K (ppm) | 87 |
| Fe (ppm) | 12.4 | ||
| EC (ds/m) | 0.59 | Mn (ppm) | 9.8 |
| Organic Carbon (O. C. %) | 0.314 | Zn (ppm) | 1.6 |
| Cu (ppm) | 0.87 | ||
| Soluble cations (mmole kg−1) | Soil Type | Sandy clay loam | |
| Ca++ | 5.51 | Particle size distribution (%) | |
| Mg++ | 2.75 | Fine sand% | 34.07 |
| Na+ | 10.69 | Coarse sand% | 18.68 |
| K+ | 1.03 | Silt% | 9.0 |
| Soluble anions (mmole kg−1) | Clay% | 27.3 | |
| CO3− | 7.7 | Physical properties | |
| HCO3− | 3.2 | % Field Capacity | 40 |
| CI− | 14.9 | (v/v) % Wilting point (v/v) | 32 |
| SO4− | 2.9 | Hydraulic conductivity (cmh−1) | 1.35 |
| Available water (% v/v) | 8.4 |
| Height of Plant (cm) | Leaf Number | Branches Number/Plant | Fresh Weight of Plant (g) | Dry Weight of Plant (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seasons | ||||||||||
| Treatment | 2021 * | 2022 * | 2021 * | 2022 * | 2021 * | 2022 * | 2021 * | 2022 * | 2021 * | 2022 * |
| Control | 169 ± 6 h | 162 ± 2.5 h | 42 ± 1.5 h | 38 ± 1.0 h | 3 ± 0.58 f | 4 ± 0.10 h | 327 ± 5.7 h | 319 ± 2.1 h | 82.5 ± 1.4 h | 78.2 ± 1.2 h |
| B. amyloliquefaciens | 181 ± 1.7 g | 176 ± 2.5 g | 50 ± 1.0 g | 44 ± 0.6 g | 4 ± 0.29 f | 4 ± 0.06 g | 340 ± 1.5 g | 327 ± 0.7 g | 85.9 ± 1.5 g | 80.8 ± 1.2 g |
| Cyanobacteria | 186 ± 0.9 f | 180 ± 0.9 f | 56 ± 1.0 f | 49 ± 1.5 f | 4 ± 0.06 e | 4 ± 0.06 f | 351 ± 1.5 f | 333 ± 1.6 f | 89.8 ± 1.1 f | 83.1 ± 1 f |
| Yeast | 195 ± 2.7 e | 185 ± 2.5 e | 61 ± 1.0 e | 55 ± 0.6 e | 5 ± 0.10 d | 5 ± 0.06 e | 359 ± 0.7 e | 343 ± 1.5 e | 95.4 ± 1.1 e | 87.5 ± 0.6 e |
| B. amyloliquefaciens + cyanobacteria | 204 ± 2.5 d | 194 ± 1.8 d | 64 ± 0.6 d | 61 ± 1.0 d | 5 ± 0.12 c | 5 ± 0.06 d | 367 ± 3.7 d | 350 ± 1.6 d | 98.5 ± 0.4 d | 92.2 ± 1 d |
| B. amyloliquefaciens + yeast | 213 ± 2.9 c | 199 ± 1.7 c | 68 ± 1.0 c | 64 ± 0.6 c | 6 ± 0.12 b c | 5 ± 0.06 c | 375 ± 0.8 c | 355 ± 0.7 c | 101.5 ± 0.5 c | 95.8 ± 0.3 c |
| Cyanobacteria + yeast | 221 ± 0.8 b | 209 ± 1.9 b | 75 ± 1.0 b | 72 ± 0.6 b | 6 ± 0.17 b | 6 ± 0.17 b | 383 ± 2.5 b | 361 ± 2.0 b | 103.9 ± 0.8 b | 99.7 ± 0.5 b |
| B. amyloliquefaciens + cyanobacteria + yeast | 231 ± 1.7 a | 220 ± 1.7 a | 80 ± 1.0 a | 78 ± 1.5 a | 6 ± 0.06 a | 6 ± 0.10 a | 390 ± 1.4 a | 369 ± 1.0 a | 105.7 ± 0.8 a | 103.1 ± 0.8 a |
| LSD at 0.05 | 3.506 | 3.507 | 1.802 | 1.731 | 0.428 | 0.158 | 4.735 | 2.592 | 1.758 | 1.522 |
| Length of Pods (cm) | Diameter of Pods (cm) | Fresh Weight of Pod (g) | Dry Weight of Pod (g) | |||||
|---|---|---|---|---|---|---|---|---|
| Seasons | ||||||||
| Treatment | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2022 |
| Control * | 17.1 ± 0.69 h | 14.7 ± 0.48 g | 0.62 ± 0.01 h | 0.59 ± 0.01 h | 8.3 ± 0.2 f | 7.7 ± 0.06 h | 2.2 ± 0.05 h | 2.4 ± 0.02 h |
| B. amyloliquefaciens | 18.3 ± 0.46 e | 16.6 ± 0.52 f | 0.66 ± 0.02 g | 0.63 ± 0.01 g | 8.9 ± 0.03 e | 8.5 ± 0.12 g | 2.8 ± 0.16 g | 2.9 ± 0.02 g |
| Cyanobacteria | 19.4 ± 0.32 d | 18.2 ± 0.5 e | 0.71 ± 0.01 f | 0.69 ± 0.01 f | 9.1 ± 0.07 d e | 9 ± 0.11 f | 3.1 ± 0.08 f | 3.1 ± 0.04 f |
| Yeast | 20.8 ± 0.06 c | 19.5 ± 0.59 d | 0.74 ± 0.01 e | 0.72 ± 0.02 e | 9.4 ± 0.19 d | 9.3 ± 0.08 e | 3.5 ± 0.18 e | 3.3 ± 0.05 e |
| B. amyloliquefaciens + cyanobacteria | 21.2 ± 0.06 c | 20.5 ± 0.39 c | 0.8 ± 0.01 d | 0.77 ± 0.01 d | 10.1 ± 0.04 c | 9.8 ± 0.07 d | 4 ± 0.08 d | 3.7 ± 0.06 d |
| B. amyloliquefaciens + yeast | 21.8 ± 0.15 b | 21.1 ± 0.09 b c | 0.82 ± 0.01 c | 0.8 ± 0.01 c | 10.9 ± 0.08 b | 10.2 ± 0.1 c | 4.3 ± 0.02 c | 3.9 ± 0.03 c |
| Cyanobacteria + yeast | 22.3 ± 0.15 b | 21.8 ± 0.15 a b | 0.86 ± 0.01 b | 0.83 ± 0.01 b | 11.2 ± 0.17 b | 10.6 ± 0.06 b | 4.6 ± 0.1 b | 4.2 ± 0.06 b |
| B. amyloliquefaciens + cyanobacteria + yeast | 23.0 ± 0.17 a | 22.4 ± 0.25 a | 0.9 ± 0.01 a | 0.88 ± 0.02 a | 12.1 ± 0.36 a | 11.1 ± 0.12 a | 4.8 ± 0.03 a | 4.5 ± 0.04 a |
| LSD at 0.05 | 0.573 | 0.707 | 0.014 | 0.019 | 0.308 | 0.162 | 0.178 | 0.072 |
| Plant Height | No. of Leaves | No. of Branches/Plant | Pod Length | Pod Diameter | Pod Fresh Weight | Pod dry Weight | Plant Fresh Weight | Plant Dry Weight | Carbohydrates | DHA | Nitrogen | Phosphorus | Potassium | Chl A | Chl B | Carotenoids | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 1.00 | ||||||||||||||||
| No. of leaves | 0.92 | 1.00 | |||||||||||||||
| No of. branch/plant | 0.87 | 0.94 | 1.00 | ||||||||||||||
| Pod length | 0.84 | 0.96 | 0.86 | 1.00 | |||||||||||||
| Pod diameter | 0.91 | 0.99 | 0.94 | 0.96 | 1.00 | ||||||||||||
| Pod fresh weight | 0.92 | 0.97 | 0.92 | 0.93 | 0.97 | 1.00 | |||||||||||
| Pod dry weight | 0.89 | 0.97 | 0.95 | 0.93 | 0.98 | 0.97 | 1.00 | ||||||||||
| Plant fresh weight | 0.85 | 0.95 | 0.86 | 0.93 | 0.93 | 0.95 | 0.94 | 1.00 | |||||||||
| Plant dry weight | 0.86 | 0.98 | 0.90 | 0.96 | 0.97 | 0.96 | 0.95 | 0.97 | 1.00 | ||||||||
| Carbohydrates | 0.86 | 0.87 | 0.93 | 0.78 | 0.89 | 0.85 | 0.87 | 0.71 | 0.78 | 1.00 | |||||||
| DHA | 0.63 | 0.64 | 0.62 | 0.60 | 0.60 | 0.63 | 0.60 | 0.59 | 0.60 | 0.60 | 1.00 | ||||||
| N | 0.60 | 0.63 | 0.58 | 0.61 | 0.63 | 0.63 | 0.59 | 0.60 | 0.57 | 0.57 | 0.27 | 1.00 | |||||
| P | 0.62 | 0.59 | 0.59 | 0.55 | 0.60 | 0.62 | 0.58 | 0.55 | 0.52 | 0.60 | 0.34 | 0.95 | 1.00 | ||||
| K | 0.58 | 0.58 | 0.58 | 0.56 | 0.60 | 0.60 | 0.58 | 0.52 | 0.51 | 0.61 | 0.20 | 0.96 | 0.94 | 1.00 | |||
| Chl A | 0.60 | 0.62 | 0.55 | 0.60 | 0.61 | 0.65 | 0.61 | 0.68 | 0.60 | 0.45 | 0.29 | 0.91 | 0.89 | 0.84 | 1.00 | ||
| Chl B | 0.61 | 0.63 | 0.55 | 0.63 | 0.62 | 0.65 | 0.60 | 0.69 | 0.61 | 0.44 | 0.33 | 0.91 | 0.89 | 0.85 | 0.96 | 1.00 | |
| Carotenoids | 0.55 | 0.59 | 0.55 | 0.60 | 0.60 | 0.62 | 0.58 | 0.62 | 0.58 | 0.47 | 0.19 | 0.95 | 0.90 | 0.92 | 0.94 | 0.93 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omer, R.M.; Hewait, H.M.; Mady, E.; Yousif, S.K.M.; Gashash, E.A.; Randhir, R.; Ashmawi, A.E.; El-Taher, A.M.; Al-Harbi, N.A.; Randhir, T.O. Chemical, Anatomical, and Productivity Responses of Cowpea (Vigna unguiculata L.) to Integrated Biofertilizer Applications with PGPR, Cyanobacteria, and Yeast. Sustainability 2023, 15, 7599. https://doi.org/10.3390/su15097599
Omer RM, Hewait HM, Mady E, Yousif SKM, Gashash EA, Randhir R, Ashmawi AE, El-Taher AM, Al-Harbi NA, Randhir TO. Chemical, Anatomical, and Productivity Responses of Cowpea (Vigna unguiculata L.) to Integrated Biofertilizer Applications with PGPR, Cyanobacteria, and Yeast. Sustainability. 2023; 15(9):7599. https://doi.org/10.3390/su15097599
Chicago/Turabian StyleOmer, Rihab M., Heba M. Hewait, Emad Mady, Sawsan K. M. Yousif, Ebtesam A. Gashash, Reena Randhir, Ashmawi E. Ashmawi, Ahmed M. El-Taher, Nadi A. Al-Harbi, and Timothy O. Randhir. 2023. "Chemical, Anatomical, and Productivity Responses of Cowpea (Vigna unguiculata L.) to Integrated Biofertilizer Applications with PGPR, Cyanobacteria, and Yeast" Sustainability 15, no. 9: 7599. https://doi.org/10.3390/su15097599
APA StyleOmer, R. M., Hewait, H. M., Mady, E., Yousif, S. K. M., Gashash, E. A., Randhir, R., Ashmawi, A. E., El-Taher, A. M., Al-Harbi, N. A., & Randhir, T. O. (2023). Chemical, Anatomical, and Productivity Responses of Cowpea (Vigna unguiculata L.) to Integrated Biofertilizer Applications with PGPR, Cyanobacteria, and Yeast. Sustainability, 15(9), 7599. https://doi.org/10.3390/su15097599








