The Application of an Electrocoagulation Process to the Sustainable Treatment of Initial Rainwater and the Simulation of a Flow Pattern in an Experimental Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Initial Rainwater Characterization and Analytical Methods
2.2. Reactor Setup
2.2.1. Experiment Equipment of the EC Process and Experimental Pilot-Scale Equipment for the ECF Process
2.2.2. Principle of the EC Process
2.3. Model Construction
2.3.1. Introduction to the COMSOL Software Model
2.3.2. Establishment of the COMSOL Model
3. Results
3.1. Single-Factor Experiments
3.1.1. Material Selection and Combinations of Electrode Plates
3.1.2. Electrolysis Time
3.1.3. Static Settling Time
3.1.4. Spacing of the Electrode Plates
3.1.5. Current Density and Number of Plates
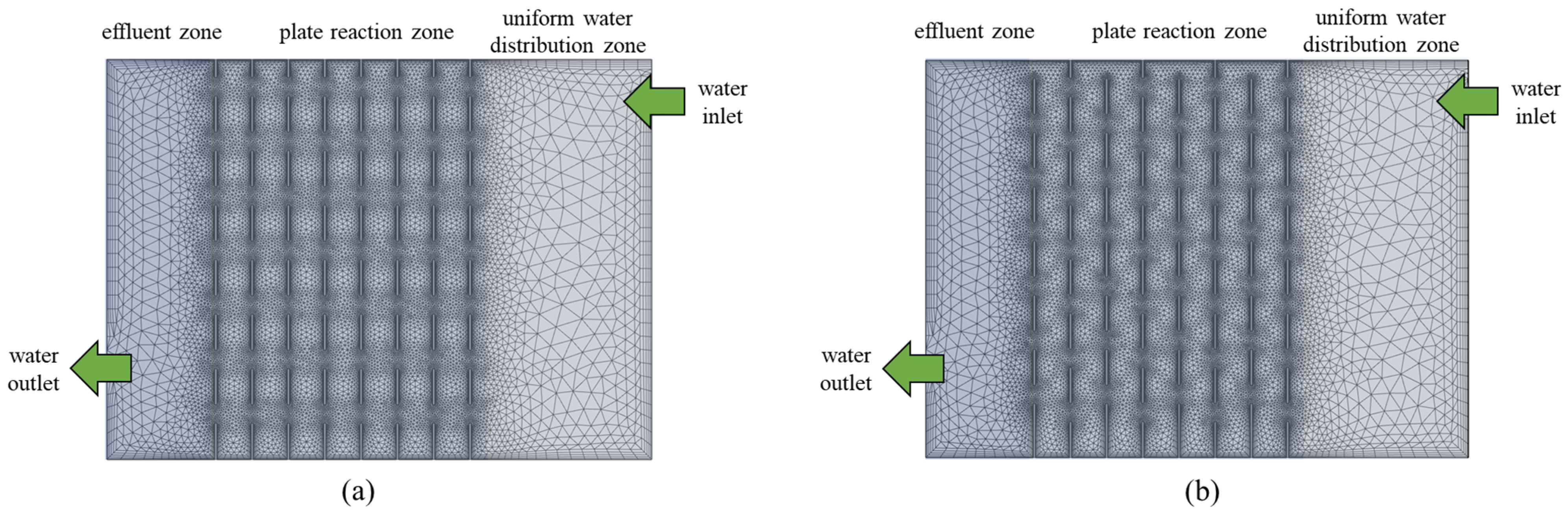
3.2. Flow Simulation Analysis
3.2.1. Flow Field Analysis
3.2.2. Kinetic Energy Analysis
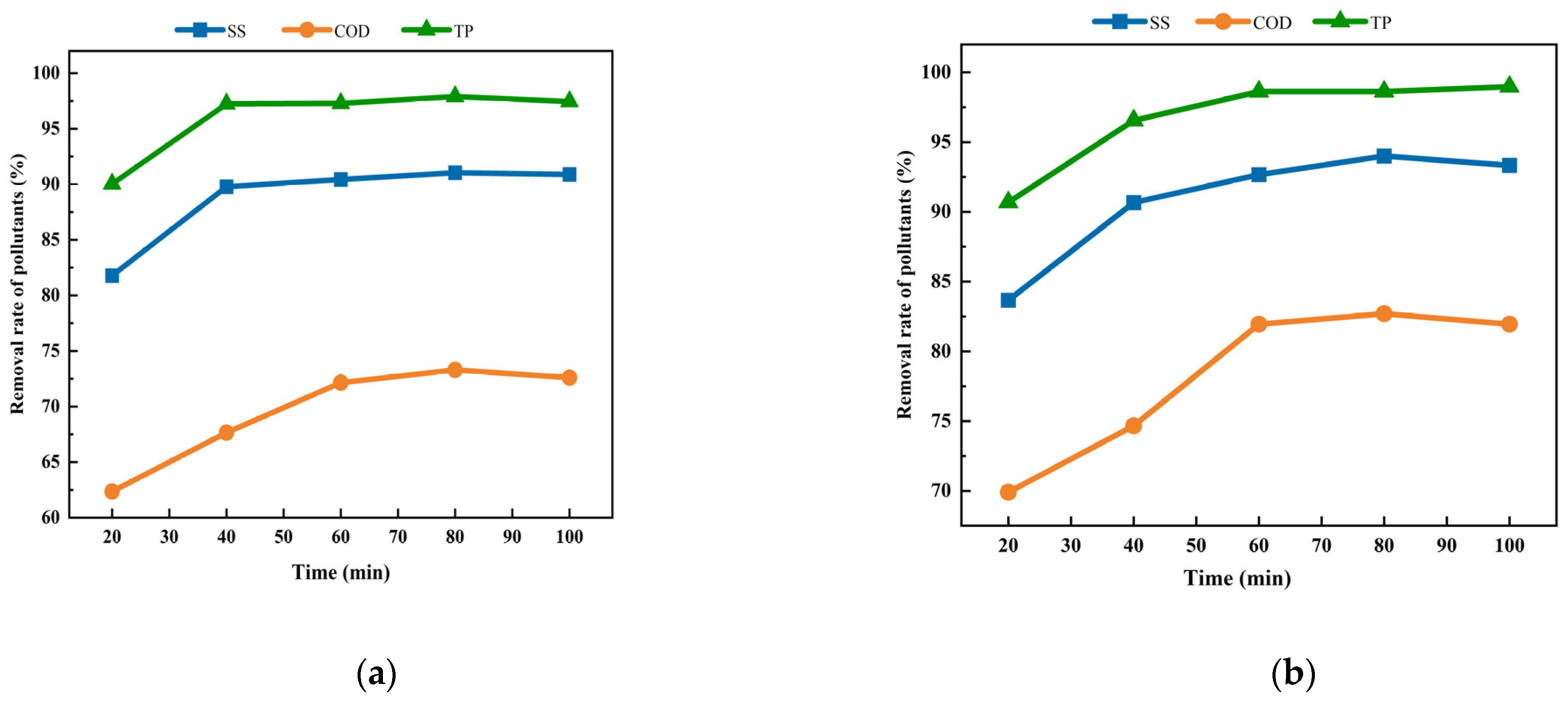
3.3. Analysis of the Pollutant Removal Effect for Different Flow Patterns
3.4. Pilot-Scale ECF Process Performance Analysis for Simulated Rainwater Treatment
3.4.1. Analysis of the Trial Operation Results of the Pilot Experiment under Four Working Conditions
3.4.2. Analysis of the Results from the Continuous Operation Experiment of the Pilot Plant
4. Conclusions
- (1)
- The analysis of fluid flowcharts and velocity cloud images showed that for the dislocation perforated flow pattern, the fluid flow condition was more complex, and the turbulent effect of the fluid was more substantial. The actual experimental verification of parallel and dislocation perforation flows was performed, and the results showed that under the same experimental conditions, the removal rates of pollutants by the EC process were more significant in the case of dislocation perforation flow, which verified the results of the flow pattern simulations. The SS, COD, and TP removal rates were 94.00%, 81.95%, and 98.97%, respectively.
- (2)
- The pilot-scale ECF experiment effectively treated initial rainwater, producing high-quality effluent water. The final effluent had an SS of 15.00 mg/L, a COD of 21.06 mg/L, and a TP of 0.11 mg/L, making it suitable for use as urban industrial water. In addition, the energy consumption during operation was low, with an electricity consumption of approximately 0.57 kWh per ton of water. These results confirmed the reliability of the EC process for initial rainwater treatment and demonstrated the stability of continuous long-term operation. Furthermore, it highlighted the practical applicability of the EC process for rainwater treatment in real-world projects.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yang, Z.; Zou, L.; Xia, J.; Qiao, Y.; Bai, F.; Wang, Q.; Cai, D. Spatiotemporal variation characteristics and source identification of water pollution: Insights from urban water system. Ecol. Indic. 2022, 139, 108892. [Google Scholar] [CrossRef]
- Huang, L.; Han, X.; Wang, X.; Zhang, Y.; Yang, J.; Feng, A.; Li, J.; Zhu, N. Coupling with high-resolution remote sensing data to evaluate urban non-point source pollution in Tongzhou, China. Sci. Total Environ. 2022, 831, 154632. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Hui, E.; Wang, N.; Zhou, X.; Gan, F. Study on the Concept and Characteristics of Stormwater First Flush. Environ. Impact Assess. 2022, 44, 58–62. [Google Scholar] [CrossRef]
- Qionghua, Z.H.; Qian, W.A.; Xiaochang, W.A.; Fei, C.O. Discussion on road runoff pollution and its utilization standard in typical cities. Chin. J. Environ. Eng. 2016, 10, 3451–3456. [Google Scholar] [CrossRef]
- Zheng, Z.; Duan, X.; Lu, S. The application research of rainwater wetland based on the Sponge City. Sci. Total Environ. 2021, 771, 144475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lan, Y.; Cheng, Y.; Dang, W. Testing and analysis of rainwater quality in Shenyang. J. Southeast Univ. 2014, 30, 246–250. [Google Scholar]
- Zhang, Q.; Wang, X.; Hou, P.; Wan, W.; Ren, Y.; Ouyang, Z.; Yang, L. The temporal changes in road stormwater runoff quality and the implications to first flush control in Chongqing, China. Environ. Monit. Assess. 2013, 185, 9763–9775. [Google Scholar] [CrossRef]
- Lu, Y. Design Strategy of Emergency Pools at Source Water Protection Areas. J. Archit. Res. Dev. 2022, 6, 35–40. Available online: https://go.exlibris.link/dtypPrjl (accessed on 5 August 2022). [CrossRef]
- Yang, L.; Li, J.; Zhou, K.; Feng, P.; Dong, L. The effects of surface pollution on urban river water quality under rainfall events in Wuqing district, Tianjin, China. J. Clean. Prod. 2021, 293, 126136. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, J.; Du, Z.; Zhang, Y.; Lai, X. Simulation of interception capacity of Nanhe initial rain storage tanks. IOP Conf. Ser. Ear. Environ. Sci. 2021, 787, 012138. [Google Scholar] [CrossRef]
- Selbig, W.R.; Buer, N.; Danz, M.E. Stormwater-quality performance of lined permeable pavement systems. J. Environ. Manag. 2019, 251, 109510. [Google Scholar] [CrossRef] [PubMed]
- Molineux, C.J.; Gange, A.C.; Newport, D.J. Using soil microbial inoculations to enhance substrate performance on extensive green roofs. Sci. Total Environ. 2017, 580, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Anwar Baig, M. Characterization of First Flush in Urban Highway Runoffs. Environ. Eng. Manag. J. 2015, 14, 45–50. [Google Scholar] [CrossRef]
- He, S.; Chen, W.; Mu, X.; Cui, W. Constrained optimization model of the volume of initial rainwater storage tank based on ANN and PSO. Environ. Sci. Pollut. Res. 2020, 27, 21057–21070. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; You, X.-Y.; Wang, F.; Ji, M. On Regulation of Urban Runoff Pollution Abatement. In Advances in Computer Science, Intelligent System and Environment; Springer: Berlin/Heidelberg, Germany, 2011; pp. 645–650. [Google Scholar]
- Zuo, X.; Zhang, H.; Yu, J. Microbial diversity for the improvement of nitrogen removal in stormwater bioretention cells with three aquatic plants. Chemosphere 2020, 244, 125626. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yue, X.; Duan, Y.; Zhou, A.; Gao, Y.; Zhang, X. A bilayer media bioretention system for enhanced nitrogen removal from road runoff. Sci. Total Environ. 2020, 705, 135893. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Xing, Y.; Zhu, F.; An, Y.; Zhou, Z. Research Progress of Urban Initial Stormwater Pollution and Treatment Measures. Water Purif. Technol. 2022, 41, 17–26. [Google Scholar] [CrossRef]
- Qin, P.; Zhu, D.; Jia, X.; Ding, Y.; Feng, H. Flocculation-Magnetic Separation Technology for the Rapid Treatment of First Flush Rain. Environ. Sci. Technol. 2019, 42, 012085. [Google Scholar]
- Chen, Y.; Zhao, J.; Hu, B. Study on the pollution load of the urban trunkroad runoff in Xi’an. J. Saf. Environ. 2011, 31, 781–788. [Google Scholar]
- Lee, J.Y.; Kim, H.; Kim, Y.; Han, M.Y. Characteristics of the event mean concentration (EMC) from rainfall runoff on an urban highway. Environ. Pollut. 2011, 159, 884–888. [Google Scholar] [CrossRef]
- Zhong, D.; Zhang, H.; Li, L.; Xu, Y. Pollution and treatment measures of urban initial rainwater: A review. Environ. Pollut. Control 2019, 41, 224–230. [Google Scholar]
- Yang, L.; Wang, Y.; Wang, Y.; Wang, S.; Yue, J.; Guan, G.; Guo, Y.; Zhang, Y.; Zhang, Q. Water quality improvement project for initial rainwater pollution and its performance evaluation. Environ. Res. 2023, 237 Pt 1, 116987. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y. Design proposal of treatment process for the inital rainwater treatment plant in old town of Feixi County of Hefei City. Water Purif. Technol. 2019, 38, 47–51. [Google Scholar] [CrossRef]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Balouchi, H.; Baziar, M.; Dehghan, A.; Alidadi, H.; Shams, M. Combination of electrocoagulation and MOF adsorption systems for EBT removal from water. Int. J. Environ. Anal. Chem. 2020, 102, 1307–1317. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process- a systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, X.; Zhao, W.; Wang, Z.; Lu, X.; Zhu, L.; Wang, Z.; Liang, H. Roofing rainwater cleaner production using pilot-scale electrocoagulation coupled with a gravity-driven membrane bioreactor (EC-GDMBR): Water treatment and energy efficiency. J. Clean. Prod. 2021, 314, 128055. [Google Scholar] [CrossRef]
- Juan, M.-R.; Belkis, S.-R.; Kelly Joel, G.-T.; Jorge, d.R.-O.; Virgilio, Z.-G. Modeling and Optimization of COD Removal from Cold Meat Industry Wastewater by Electrocoagulation Using Computational Techniques. Processes 2020, 8, 1139. [Google Scholar] [CrossRef]
- Alkhatib, A.M.; Hawari, A.H.; Hafiz, M.A.; Benamor, A. A novel cylindrical electrode configuration for inducing dielectrophoretic forces during electrocoagulation. J. Water Process Eng. 2020, 35, 101195. [Google Scholar] [CrossRef]
- Ano, J.; Assémian, A.S.; Yobouet, Y.A.; Adouby, K.; Drogui, P. Electrochemical removal of phosphate from synthetic effluent: A comparative study between iron and aluminum by using experimental design methodology. Process Saf. Environ. Prot. 2019, 129, 184–195. [Google Scholar] [CrossRef]
- Yilmaz, S.; Gerek, E.E.; Yavuz, Y.; Koparal, A.S. Treatment of vinegar industry wastewater by electrocoagulation with monopolar aluminum and iron electrodes and toxicity evaluation. Water Sci. Technol. 2018, 78, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186 Pt 1, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, H.; Wang, J.; Pan, G. Rainwater utilization and storm pollution control based on urban runoff characterization. J. Environ. Sci. 2010, 22, 40–46. [Google Scholar] [CrossRef] [PubMed]
- You, X.-Y.; Li, Y.-W.; Ji, M.; Wang, X.-D.; Zhao, L.-J.; Pan, L.-M. On the Control Strategies of Initial Period Rainwater Pollution in Tianjin City. In Proceedings of the 2010 International Conference on Challenges in Environmental Science and Computer Engineering, Wuhan, China, 6–7 March 2010; pp. 232–235. [Google Scholar] [CrossRef]
- SZDB/Z 49-2011; Technical Specifications for Shenzhen Rainwater Utilization Engineering. SMSA: Abu Dhabi, United Arab Emirates, 2011.
- Koyuncu, S.; Ariman, S. Domestic wastewater treatment by real-scale electrocoagulation process. Water Sci. Technol. 2020, 81, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Boinpally, S.; Kolla, A.; Kainthola, J.; Kodali, R.; Vemuri, J. A state-of-the-art review of the electrocoagulation technology for wastewater treatment. Water Cycle 2023, 4, 26–36. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Mitchell, C.A. The future for electrocoagulation as a localised water treatment technology. Chemosphere 2005, 59, 355–367. [Google Scholar] [CrossRef]
- Dickinson, E.J.; Ekström, H.; Fontes, E. COMSOL Multiphysics®: Finite element software for electrochemical analysis. A mini-review. Electrochem. Commun. 2014, 40, 71–74. [Google Scholar] [CrossRef]
- Qi, Z.; You, S.; Liu, R.; Chuah, C.J. Performance and mechanistic study on electrocoagulation process for municipal wastewater treatment based on horizontal bipolar electrodes. Front. Environ. Sci. Eng. 2020, 14, 2–9. [Google Scholar] [CrossRef]
- Hawari, A.H.; Alkhatib, A.M.; Hafiz, M.; Das, P. A novel electrocoagulation electrode configuration for the removal of total organic carbon from primary treated municipal wastewater. Environ. Sci. Pollut. Res. 2020, 27, 23888–23898. [Google Scholar] [CrossRef]
- Al-Barakat, H.S.; Matloub, F.K.; Ajjam, S.K.; Al-Hattab, T.A. Modeling and Simulation of Wastewater Electrocoagulation Reactor. Environ. Pollut. Control 2020, 87, 012002. [Google Scholar] [CrossRef]
- Di, Z.; Zhou, L.; Bai, Y.; Ma, K.; Cao, Z.; Zhang, Z. Study on the effect of electrode layout on the performance of electrocoagulation. Water Wastewater Eng. 2020, 56, 513–516+520. [Google Scholar]
- Jiang, T.-W.; Huang, Z.-W.; Li, J.-B.; Zhou, Y.-S. Internal flow mechanism of cone-straight nozzle. Pet. Sci. 2021, 18, 1507–1519. [Google Scholar] [CrossRef]
- Wei, W.; Li, P.; Hong, Y.; Liu, Y. Numerical simulation study on hydraulic characteristics of wall jets on corrugated beds with limited tailwater. Chin. J. Appl. Mech. 2016, 33, 7. [Google Scholar]
- Omwene, P.I.; Kobya, M. Treatment of domestic wastewater phosphate by electrocoagulation using Fe and Al electrodes: A comparative study. Process Saf. Environ. Prot. 2018, 116, 34–51. [Google Scholar] [CrossRef]
- Dang, Y.; Lan, S.; Hu, Y.; Luo, X. Influence Factors and Mechanisms of Ni-EDTA Wastewater Treatment by Electrocoagulation with Fe/Al. Sci. Total Environ. 2017, 17, 150–156. [Google Scholar]
- Dura, A.; Breslin, C.B. Electrocoagulation using aluminium anodes activated with Mg, In and Zn alloying elements. J. Hazard. Mater. 2019, 366, 39–45. [Google Scholar] [CrossRef]
- Dura, A.; Breslin, C.B. The removal of phosphates using electrocoagulation with Al−Mg anodes. J. Electroanal. Chem. 2019, 846, 113161. [Google Scholar] [CrossRef]
- Tian, Y.; He, W.; Zhu, X.; Yang, W.; Ren, N.; Logan, B.E. Improved electrocoagulation reactor for rapid removal of phosphate from wastewater. ACS Sustain. Chem. Eng. 2017, 5, 67–71. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, F.; Zhang, H.; Zhao, X.; Liu, J. Influence Mechanism of Cu2+ Removal in Wastewater by Electrocoagulation. Environ. Sci. Technol. 2014, 37, 53–56. [Google Scholar]
- Wu, D.; Tan, F. Study on flocculation kinetics of turbulence. Ind. Water Treat. 1999, 19, 6–7. [Google Scholar] [CrossRef]
- Xia, X.; Lan, S.; Li, X.; Xie, Y.; Liang, Y.; Yan, P.; Chen, Z.; Xing, Y. Characterization and coagulation-flocculation performance of a composite flocculant in high-turbidity drinking water treatment. Chemosphere 2018, 206, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Babayemi, K.A.; Onukwuli, D.O.; Okewale, O.A. Statistical Modeling and Optimization of Coag-Flocculation Process of Phosphate Removal from Aqueous Solution Using Ox-Eye Seed (Chrysanthemum leucanthemum) as Coagulant. Adv. Eng. Forum 2018, 28, 90–95. [Google Scholar] [CrossRef]
- GB 50400-2016; Technical Code for Rainwater Management and Utilization of Building and Sub-District. China Architecture & Building Press: Beijing, China, 2016.
- Carvalho, E.H.d.S.; Cuba, R.; Carvalho, R.V.d.; Marra, E. Application of eletrocoagulation/flotation process for rainwater reuse. Period. Tche Quim. 2019, 16, 89–94. [Google Scholar] [CrossRef]



| Pollutant | SS | COD | TP |
|---|---|---|---|
| Initial concentration | 300–310 mg/L | 190–210 mg/L | 5–6 mg/L |
| Effluent discharge Concentration | 30 mg/L | 40 mg/L | 0.2 mg/L |
| Target removal rate | 90% | 80% | 96% |
| Experimental Parameter | Experimental Value Range | Recommended Value Range | Optimal Value of This Time 1 |
|---|---|---|---|
| Reaction time | 10–60 min | 20–40 min | 30 min |
| Settling time | 10–60 min | 20–40 min | 30 min |
| Plate spacing | 1–5 cm | 2–3 cm | 2 cm |
| Number of plates | Four to eight pieces | Six to eight pieces | eight pieces |
| Current density | 20–100 A/m2 | 40–60 A/m2 | 40 A/m2 |
| Working Condition | Combinations of Electrode Plates | Number of Metal Anode Plates | Current Density (A/m2) | Residence Time in EC Reactor (min) | Spacing of the Electrode Plates (cm) |
|---|---|---|---|---|---|
| I | Fe-Al | 16 | 13.89 | 30 | 2 |
| II | Fe-Al | 16 | 13.89 | 45 | 2 |
| III | Fe-Al | 10 | 22.22 | 30 | 2 |
| IV | Fe-Al | 10 | 22.22 | 45 | 2 |
| Working Condition | Water Treatment Reactor | Time (min) | Removal Rate of Pollutant SS (%) | Removal Rate of Pollutant COD (%) | Removal Rate of Pollutant SS (%) |
|---|---|---|---|---|---|
| I | EC | 240 | 62.12 | 58.57 | 92.58 |
| filtration | 240 | 69.70 | 65.50 | 93.96 | |
| II | EC | 300 | 77.04 | 65.66 | 89.49 |
| filtration | 300 | 88.89 | 73.46 | 93.64 | |
| III | EC | 300 | 54.73 | 53.48 | 79.12 |
| filtration | 300 | 83.78 | 78.22 | 96.47 | |
| IV | EC | 300 | 74.89 | 74.02 | 94.03 |
| filtration | 300 | 93.01 | 80.51 | 95.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, Z.; Fu, K.; Luo, Q. The Application of an Electrocoagulation Process to the Sustainable Treatment of Initial Rainwater and the Simulation of a Flow Pattern in an Experimental Device. Sustainability 2024, 16, 161. https://doi.org/10.3390/su16010161
Yang H, Wang Z, Fu K, Luo Q. The Application of an Electrocoagulation Process to the Sustainable Treatment of Initial Rainwater and the Simulation of a Flow Pattern in an Experimental Device. Sustainability. 2024; 16(1):161. https://doi.org/10.3390/su16010161
Chicago/Turabian StyleYang, Haiyan, Zhe Wang, Kai Fu, and Qingda Luo. 2024. "The Application of an Electrocoagulation Process to the Sustainable Treatment of Initial Rainwater and the Simulation of a Flow Pattern in an Experimental Device" Sustainability 16, no. 1: 161. https://doi.org/10.3390/su16010161





