Eco-Efficient Artificial Stones Produced Using Quartzite Quarry Waste and Vegetable Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Characterization
3.2. Simplex Lattice Mix Design Analysis
3.3. Apparent Density, Water Absorption, and Apparent Porosity
3.4. Three-Point Flexural Strength
3.5. Microstructural Analysis
3.6. Petrographic Analysis
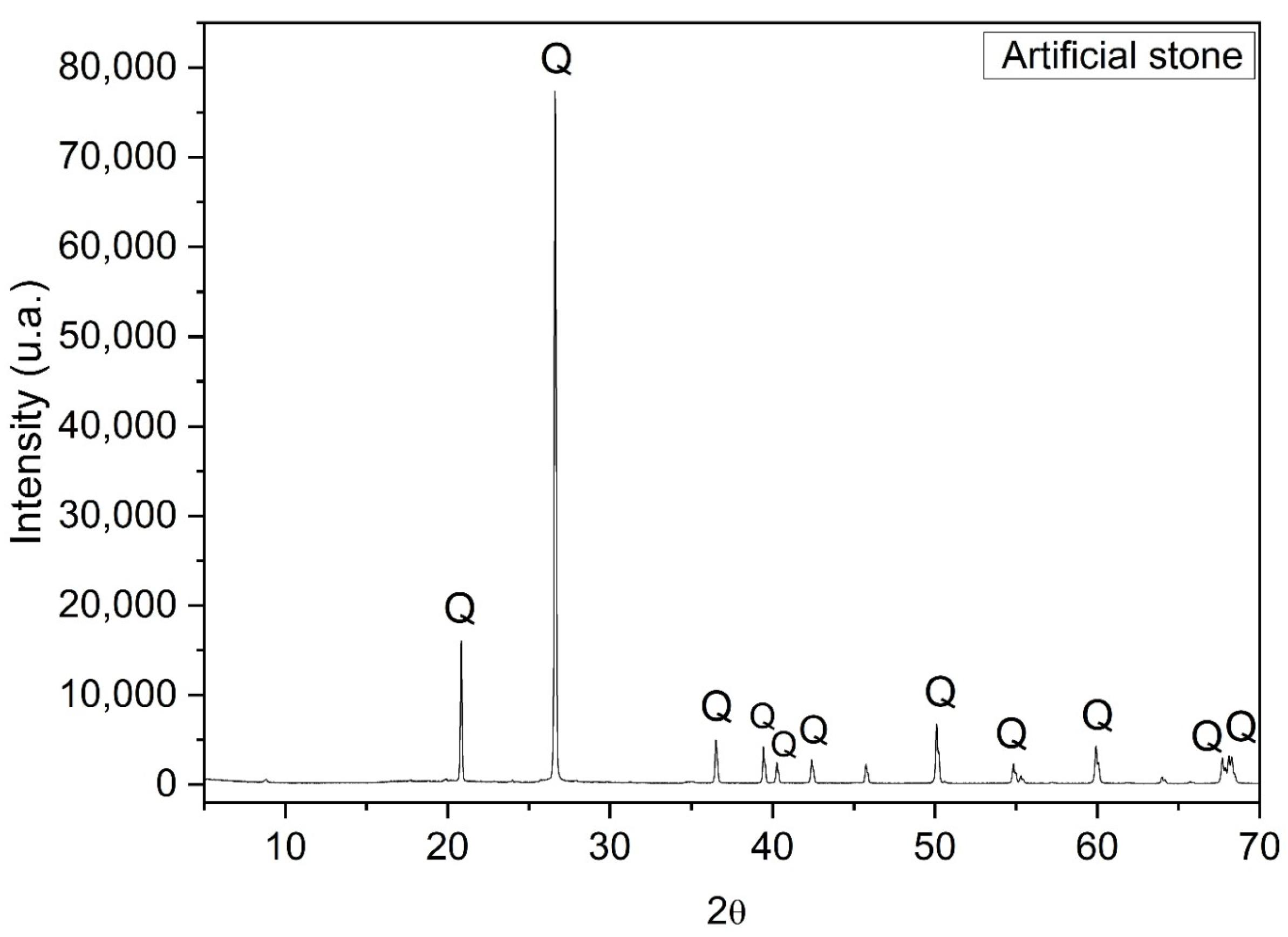
3.7. X-ray Diffractogram of the Slab Produced
4. Conclusions
- The characterization of the raw materials resulted in information that was fundamental for understanding the characteristics of the artificial stone. The quartzite wastes 1, 2, and 3 are formed by more than 90% of silicon oxide (SiO2), and the results are consistent with those in the consulted literature. Regarding the chemical structure of the resin under study, it was possible to observe the presence of functional groups, such as hydroxyls, alkanes, and esters. In addition, through thermal analysis, it was verified that the polyurethane resin has good thermal stability, that is, it is a resin resistant to a wide temperature range without suffering degradation, which makes its use in the production of artificial stones feasible;
- A simplex lattice mix design was used to assist in the execution of the experiment and to help in determining which mixture has the best apparent density. The composition that showed the highest apparent density was combination 7, with 66.7% large particles, 16.7% medium particles, and 16.7% fine particles;
- The artificial stones produced had good physical properties, with porosity and water absorption values lower than the minimum required by the natural stone standard. The three-point flexural strength test showed that the resistance strain value obtained is higher than that indicated by standard NBR ABNT 15844 (2015). Therefore, they can be classified as high-quality materials;
- SEM micrographs and petrographic analysis showed little evidence of pores in addition to how the quartzite ornamental stone waste is distributed in relation to the castor oil vegetable polyurethane resin matrix. The pores are related to an unsatisfactory adhesion of the load in the resin grains. The technique of vacuum vibro-thermo-compression used in the manufacture of artificial stones contributed to better compaction of the material, generating a smaller porosity in the stone produced.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montani, C. XXXII Rapporto Marmo e Pietre nel Mondo 2021, Marble and Stones in the World XXXII Report. Aldus. Casa di Edizioni in Carrara. 2021. Available online: https://www.abirochas.com.br (accessed on 10 September 2023).
- Associação Brasileira da Indústria de Rochas Ornamentais—ABIROCHAS. Balanço das Exportações e Importações Brasileiras de Rochas Ornamentais no 1º Trimestre de 2023. Informe: 01/2023. 2023. Available online: http://www.abirochas.com.br (accessed on 10 September 2023).
- Silveira, L.L.; Gomes, J.C.; Castilho, E.D.; Almeida, P.F. Estudo comparativo da resistência à flexão quatro pontos entre granitos ornamentais telados com resina poliuretana de mamona e epóxi. In Proceedings of the XXVII Encontro Nacional de Tratamento de Minérios e Metalurgia Extrativa, Belém, Brazil, 23–27 October 2017. [Google Scholar]
- Vidal, F.W.H.; Azevedo, H.C.A.; Castro, N.F. Tecnologia de Rochas Ornamentais: Pesquisa, Lavra e Beneficiamento; CETEM/MCTI; Centro de Tecnologia Mineral: Rio de Janeiro, Brazil, 2014; 700p. [Google Scholar]
- Xavier, G.C.; Azevedo, A.R.G.; Alexandre, J.; Monteiro, S.N.; Pedroti, L.G. Determination of Useful Life of Red Ceramic Parts Incorporated with Ornamental Stone Waste. J. Mater. Civ. Eng. 2018, 31, 1–3. [Google Scholar] [CrossRef]
- Aguiar, M.C.; Gadioli, M.C.B.; Sant’Ana, M.A.K.; Almeida, K.M.; Vidal, F.W.H.; Vieira, C.M.F. Red Ceramics Produced with Primary Processing Fine Waste of Ornamental Stones According to the Circular Economy Model. Sustainability 2022, 14, 12887. [Google Scholar] [CrossRef]
- Coelho, A.M.R.; Saggioro, F.G.; Sales Junior, J.C.C.; Andrade, J.C.S.; Cavalcante, R.H. Evaluation of the potential use of granite waste in products of the red ceramic industry in the state of Amazonas. Matéria 2022, 27, e13209. [Google Scholar] [CrossRef]
- Nascimento, A.S.; Santos, C.P.; Melo, F.M.C.; Oliveira, V.G.A.; Oliveira, R.M.P.B.; Macedo, Z.S.; Oliveira, H.A. Production of plaster mortar with incorporation of granite cutting wastes. J. Clean. Prod. 2020, 265, 121808. [Google Scholar] [CrossRef]
- Gomes, V.R.; Babisk, M.P.; Vieira, C.M.F.; Sampaio, J.A.; Vidal, F.W.H.; Gadioli, M.C.B. Ornamental stone wastes as an alternate raw material for soda-lime glass manufacturing. Mater. Lett. 2020, 269, 127579. [Google Scholar] [CrossRef]
- Lima, A.F.; Picanço, M.S.; Pompeu Neto, B.B.; Coelho, G.T.F. Resíduos de rochas ornamentais como agregado miúdo para a constituição de concretos estruturais (Ornamental stone wastes as fine aggregate for the constitution of structural concrete). Braz. J. Dev. 2021, 7, 66500–66512. [Google Scholar]
- Callister, W.D., Jr.; Rethwisch, D.G. Ciência e Engenharia de Materiais: Uma Introdução, 8th ed.; LTC: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Associação Brasileira da Indústria de Rochas Ornamentais—ABIROCHAS. Balanço das Exportações e Importações Brasileiras de Materiais Rochosos Naturais e Artificiais de Revestimento no Período Janeiro-Maio de 2022. Informe: 03/2022. 2022. Available online: https://www.worldstonereport.com/ (accessed on 10 September 2023).
- Gomes, M.L.M.; Carvalho, E.A.S.; Barreto, G.N.S.; Rodriguez, R.J.S.; Monteiro, S.N.; Vieira, C.M. Development of Sustainable Artificial Stone Using Granite Waste and Biodegradable Polyurethane from Castor Oil. Sustainability 2022, 14, 6380. [Google Scholar] [CrossRef]
- Agrizzi, C.P.; Carvalho, E.A.S.; Gadioli, M.C.B.; Barreto, G.N.S.; Azevedo, A.R.G.; Monteiro, S.N.; Vieira, C.M.F. Comparison between Synthetic and Biodegradable Polymer Matrices on the Development of Quartzite Waste-Based Artificial Stone. Sustainability 2022, 14, 6388. [Google Scholar] [CrossRef]
- Barreto, G.N.S.; Carvalho, E.A.S.; Souza, V.S.; Gomes, M.L.P.M.; Azevedo, A.G.; Monteiro, S.N.; Vieira, C.M.F. engineered stones Produced with Glass Packaging Waste, Quartz Powder, and Epoxy Resin. Sustainability 2022, 14, 7227. [Google Scholar] [CrossRef]
- Gomes, M.L.P.M.; Carvalho, E.A.S.; Dermatini, T.J.C.; Carvalho, E.A.; Colorado, H.A.; Vieira, C.M.F. Mechanical and physical investigation of an artificial stone produced with granite residue and epoxy resin. J. Compos. Mater. 2021, 55, 1247–1254. [Google Scholar] [CrossRef]
- Peixoto, J.; Carvalho, E.A.S.; Gomes, M.L.P.M.; Da Silva Guimarães, R.; Monteiro, S.N.; De Azevedo, A.R.G.; Vieira, C.M.F. Incorporation of Industrial Glass Waste into Polymeric Resin to Develop Artificial Stones for Civil Construction. Arab. J. Sci. Eng. 2021, 46, 56. [Google Scholar] [CrossRef]
- Carvalho, E.A.S.; Vilela, N.F.; Monteiro, S.N.; Vieira, C.M.F.; Silva, L.C. Novel Artificial Ornamental Stone Developed with Quarry Waste in Epoxy Composite. Mater. Res. 2018, 21, 1–6. [Google Scholar] [CrossRef]
- UNE-EN 14617-1; Piedra Aglomerada: Determinación de la Densidad Aparente y la Absorción de Agua. Spanish Association for Standardization and Certification: Madrid, Spain, 2013.
- UNE-EN 14617-2; Piedra Aglomerada: Determinación de la Resistencia a Flexión. Spanish Association for Standardization and Certification: Madrid, Spain, 2008.
- ABNT NBR–15845. Rocks of Cladding—Test Methods. Associação Brasileira de Normas Técnicas: Sao Paulo, Brazil, 2010.
- Torres, P.; Manjate, S.R.; Quaresma, S.; Fernandes, R.H.; Ferreira, F.M.J. Development of ceramic floor tile compositions based on quartzite and granite sludges. J. Eur. Ceram. Soc. 2007, 27, 4649–4655. [Google Scholar] [CrossRef]
- Carreiro, A.E.M.; Santos, R.C.; Silva, J.V.; Lira, L.H.; Neves, A.G.; Menezes, R.R.; Santana, L.N.L. Resíduo de quartzito—Matéria-prima alternativa para uso em massas de cerâmica estrutural. Cerâmica 2016, 62, 170–178. [Google Scholar] [CrossRef][Green Version]
- Barros, A.V.S.; Marciano, A.E.J.; Romualdo, F.C.H.; Menezes, R.; Neves, A.G. Addition of quartzite residues on mortars: Analysis of the alkali aggregate reaction and the mechanical behavior. Constr. Build. Mater. 2016, 118, 344–351. [Google Scholar] [CrossRef]
- Medeiros, S.S.P.; Lira, L.H.; Rodriguez, A.M.; Menezes, R.R.; Neves, A.G.; Santana, L.N.L. Incorporation of quartzite waste in mixtures used to prepare sanitary ware. J. Mater. Res. Technol. 2019, 8, 2148–2156. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef]
- Coura, J.C.; Profeti, D.; Profeti, L.P.R. Eco-friendly chitosan/quartzite composite as adsorbent for dye removal. Mater. Chem. Phys. 2020, 256, 123711. [Google Scholar] [CrossRef]
- Maradini, G.S.; Oliveira, M.P.; Carreira, L.G.; Guimaraes, D.; Profeti, D.; Dias Júnior, A.F.; Boschetti, W.T.N.; Oliveira, B.F.; Pereira, A.C.; Monteiro, S.N. Impact and Tensile Properties of Polyester Nanocomposites Reiforced with Conifer Fiber Cellulose Nanocrystal: A Previous Study Extension. Polymers 2021, 13, 1878. [Google Scholar] [CrossRef]
- Rodrigues, J.M.E. Preparação de Poliuretana à Base de Óleo de Mamona. Ph.D. Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2005. [Google Scholar]
- Vazquéz, R.A.; Delgado, S.R.; Hernandèz, G.E.; Martinez, M.M.A. Síntesis, caracterización y análisis matemático de materiales compuestos de poliuretano/polianilina (pu/pani). Rev. Cuba Química 2010, 22, 90–99. [Google Scholar]
- SIGMAALDRICH. Tabela de Espectro iv por Faixa de Frequência. Available online: https://www.sigmaaldrich.com/BR/pt/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table (accessed on 28 September 2023).
- Ristić, I.S.; Bjelović, Z.D.; Holló, B.; Mészáros Szécsényi, K.; Budinski-Simendić, J.; Lazić, N.; Kićanović, M. Thermal stability of polyurethane materials based on castor oil as polyol component. J. Therm. Calorim. 2013, 111, 1083–1091. [Google Scholar] [CrossRef]
- Neto, F.N.M.; Fialho, A.C.V.; Moura, W.L.; Rosa, A.G.F.; Matos, J.E.; Reis, F.S.; Mendes, M.T.A.; Sales, E.S.D. Castor polyurethane used as osteosynthesis plates: Microstructural and thermal analysis. Polímeros 2019, 29, e2019029. [Google Scholar] [CrossRef]
- Sathiskumar, P.S.; Madras, G. Synthesis, characterization, degradation of biodegradable castor oil based polyesters. Polym. Degrad. Stab. 2011, 96, 1695–1704. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols. Ind. Crops Prod. 2014, 52, 380–388. [Google Scholar] [CrossRef]
- Bortoletto-Santos, R.; Ribeiro, C.; Polito, W.L. Controlled release of nitrogen-source fertilizers by natural-oil-based poly (urethane) coatings: The kinetic aspects of urea release. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Pinto, E.R.P.; Gameiro, F.A.; Santos, F.A.; Trindade, W.; Polito, L.W.; Messadeq, Y.; Ribeiro, L.J.S. Caracterização da resina poliuretanas produzida a partir de oléos vegetais. Anais do 9° Congresso Brasileiro de Polímeros. 2008, p. 1. Available online: https://www.ipen.br/biblioteca/cd/cbpol/2007/PDF/469.pdf (accessed on 10 September 2023).
- Wu, Z.-Y. Synthesis and properties of moisture-cured reactive polyurethane containing castor oil and oxime compounds. Polymers 2020, 12, 1838. [Google Scholar] [CrossRef]
- Gomes, M.L.P.M.; Carvalho, E.A.S.; Sobrinho, L.N.; Monteiro, R.J.S.R.; Vieira, C.M.F. Production and characterization of a novel artificial stone using brick residue and quarry dust in epoxy matrix. J. Mater. Res. Technol. 2018, 7, 492–498. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kob, C.H.; Lo, S.L.; Lin, J.D.; Shan, M.Y.; Lee, J.C. Artificial stone slab production using waste glass, stone fragments and vacuum vibratory compaction. Cem. Concr. Compos. 2008, 30, 583–587. [Google Scholar] [CrossRef]
- ABNT NBR–15844; Rocks of Cladding—Requiremens for Granite. Associação Brasileira de Normas Técnicas: Sao Paulo, Brazil, 2015.
- Chiodi Filho, C.; de Paula Rodrigues, E. Guide Application of Stone Coverings; ABIROCHAS: São Paulo, Brazil, 2009. [Google Scholar]
- Gadioli, M.C.B.; Agrizzi, C.P.; Aguiar, M.C.; Lima, R.M.; Pedruzzi, A.D.; Ribeiro, C.E.G. Evaluation of the contents of ornamentals stones wastes and vegetable polyurethane resin in the production of engineered stones. J. Build. Eng. 2023, 78, 107594. [Google Scholar] [CrossRef]
- Gomes, M.L.; Carvalho, E.A.S.; Vieira, C. Physical and mechanical properties of artificial stone produced with granite waste and vegetable polyurethane. In Green Materials Engineering, the Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2019; pp. 23–29. [Google Scholar]
- Debnath, S.; Ranade, R.; Wunder, S.L.; Mccool, J.; Boberick, K.; Baran, G. Interface effects on mechanical properties of particlereinforced composites. Dent. Mater. 2004, 20, 677–686. [Google Scholar] [CrossRef]







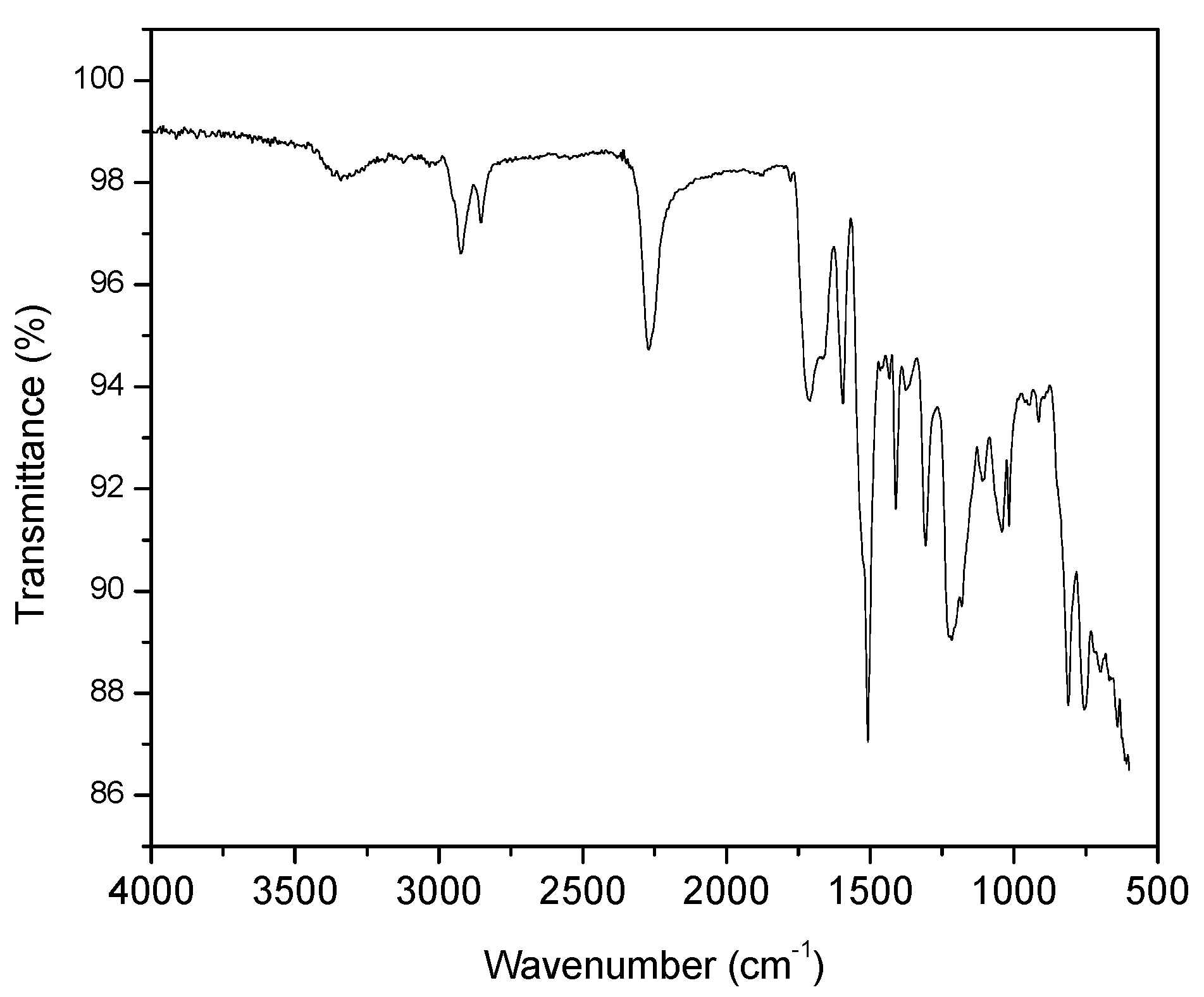
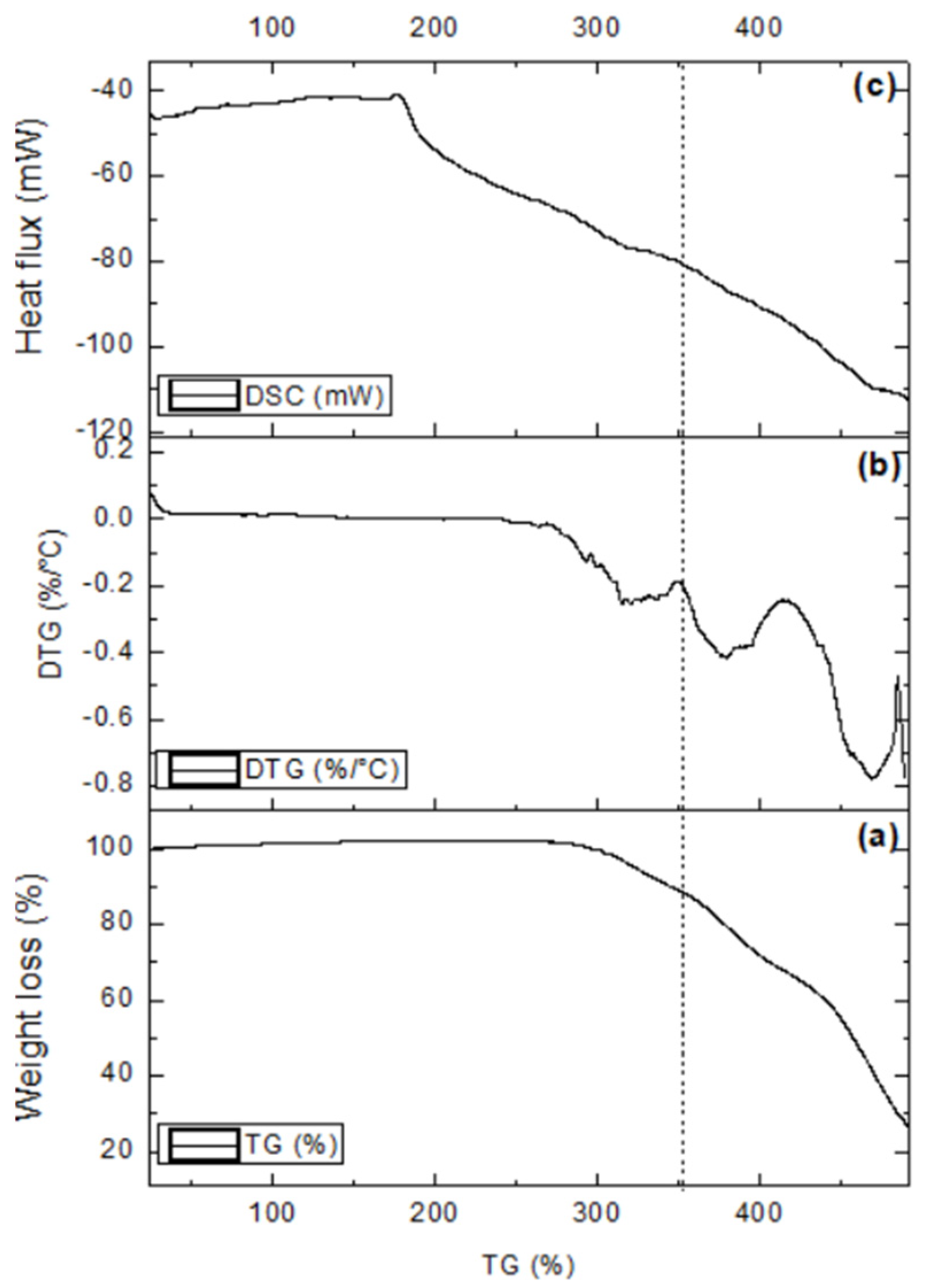
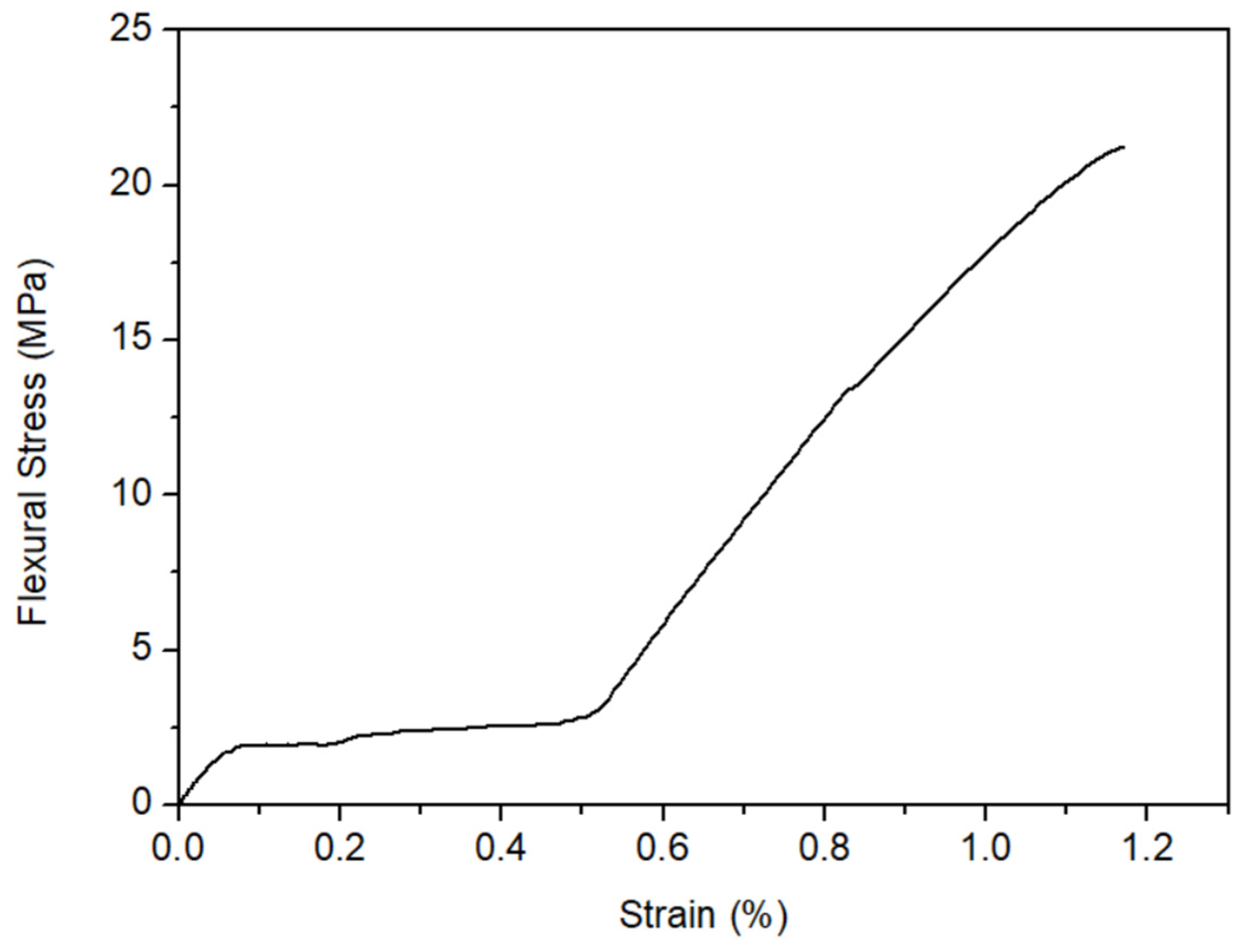


| Quartzite 1 | Quartzite 2 | Quartzite 3 | |
|---|---|---|---|
| SiO2 | 90.60 | 93.60 | 93.30 |
| Al2O3 | 5.10 | 4.50 | 5.10 |
| MgO | 1.30 | 0.43 | 0.17 |
| K2O | 0.88 | 0.78 | 0.80 |
| Fe2O3 | 0.30 | 0.22 | 0.13 |
| * LOI | 1.40 | 0.51 | 0.50 |
| Granulometry | Mesh | Grain Size (mm) |
|---|---|---|
| Coarse | −8 Ø + 25 | 2.38 > Ø > 2.00 |
| Medium | −25 Ø + 230 | 2.00 > Ø > 0.63 |
| Fine | −230 Ø | 0.63 > Ø |
| Experiment | Coarse (%) | Medium (%) | Fine (%) |
|---|---|---|---|
| 1 | 100.0 | 0.0 | 0.0 |
| 2 | 0.0 | 100.0 | 0.0 |
| 3 | 0.0 | 0.0 | 100.0 |
| 4 | 50.0 | 50.0 | 0.0 |
| 5 | 50.0 | 0.0 | 50.0 |
| 6 | 0.0 | 50.0 | 50.0 |
| 7 | 66.7 | 16.7 | 16.7 |
| 8 | 16.7 | 66.7 | 16.7 |
| 9 | 16.7 | 16.7 | 66.7 |
| 10 | 33.3 | 33.3 | 33.3 |
| Experiment | Apparent Density (g/m3) | Standard Deviation |
|---|---|---|
| 1 | 1.75 | 0.04 |
| 2 | 1.83 | 0.03 |
| 3 | 1.60 | 0.02 |
| 4 | 1.89 | 0.15 |
| 5 | 2.08 | 0.05 |
| 6 | 1.82 | 0.10 |
| 7 | 2.16 | 0.09 |
| 8 | 1.97 | 0.03 |
| 9 | 1.80 | 0.02 |
| 10 | 2.08 | 0.05 |
| Density (g/cm3) | Water Absorption (%) | Porosity (%) | |
|---|---|---|---|
| Experiment 7 | 2.16 ± 0.09 | 0.15 ± 0.03 | 0.33 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, M.C.d.; Fernandes, M.C.S.; Sant’Ana, M.A.K.; Sagrillo, V.P.D.; Anastácio, A.d.S.; Gadioli, M.C.B. Eco-Efficient Artificial Stones Produced Using Quartzite Quarry Waste and Vegetable Resin. Sustainability 2024, 16, 247. https://doi.org/10.3390/su16010247
Aguiar MCd, Fernandes MCS, Sant’Ana MAK, Sagrillo VPD, Anastácio AdS, Gadioli MCB. Eco-Efficient Artificial Stones Produced Using Quartzite Quarry Waste and Vegetable Resin. Sustainability. 2024; 16(1):247. https://doi.org/10.3390/su16010247
Chicago/Turabian StyleAguiar, Mariane Costalonga de, Maria Carolyna Sopeletti Fernandes, Maria Angelica Kramer Sant’Ana, Viviana Possamai Della Sagrillo, Alexandre dos Santos Anastácio, and Monica Castoldi Borlini Gadioli. 2024. "Eco-Efficient Artificial Stones Produced Using Quartzite Quarry Waste and Vegetable Resin" Sustainability 16, no. 1: 247. https://doi.org/10.3390/su16010247
APA StyleAguiar, M. C. d., Fernandes, M. C. S., Sant’Ana, M. A. K., Sagrillo, V. P. D., Anastácio, A. d. S., & Gadioli, M. C. B. (2024). Eco-Efficient Artificial Stones Produced Using Quartzite Quarry Waste and Vegetable Resin. Sustainability, 16(1), 247. https://doi.org/10.3390/su16010247







