Activated Carbon Fabricated from Biomass for Adsorption/Bio-Adsorption of 2,4-D and MCPA: Kinetics, Isotherms, and Artificial Neural Network Modeling
Abstract
:1. Introduction
2. Materials and Methods
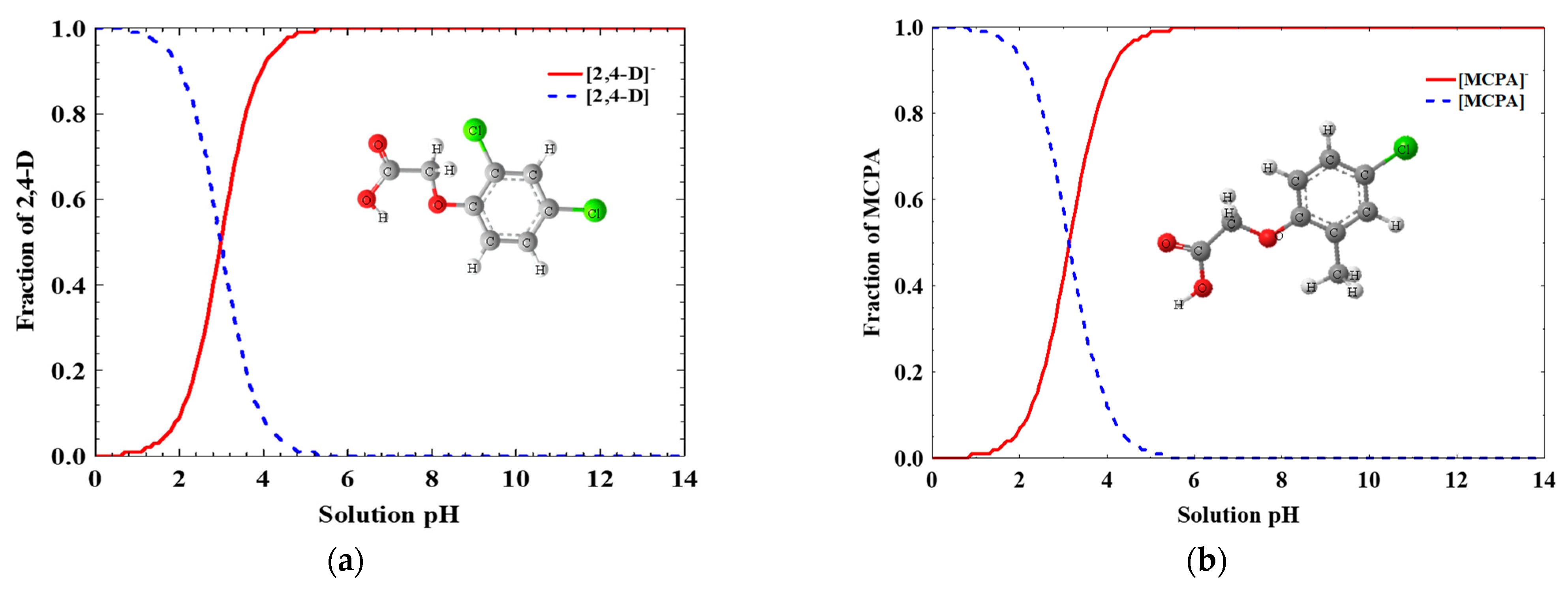
2.1. Reagents
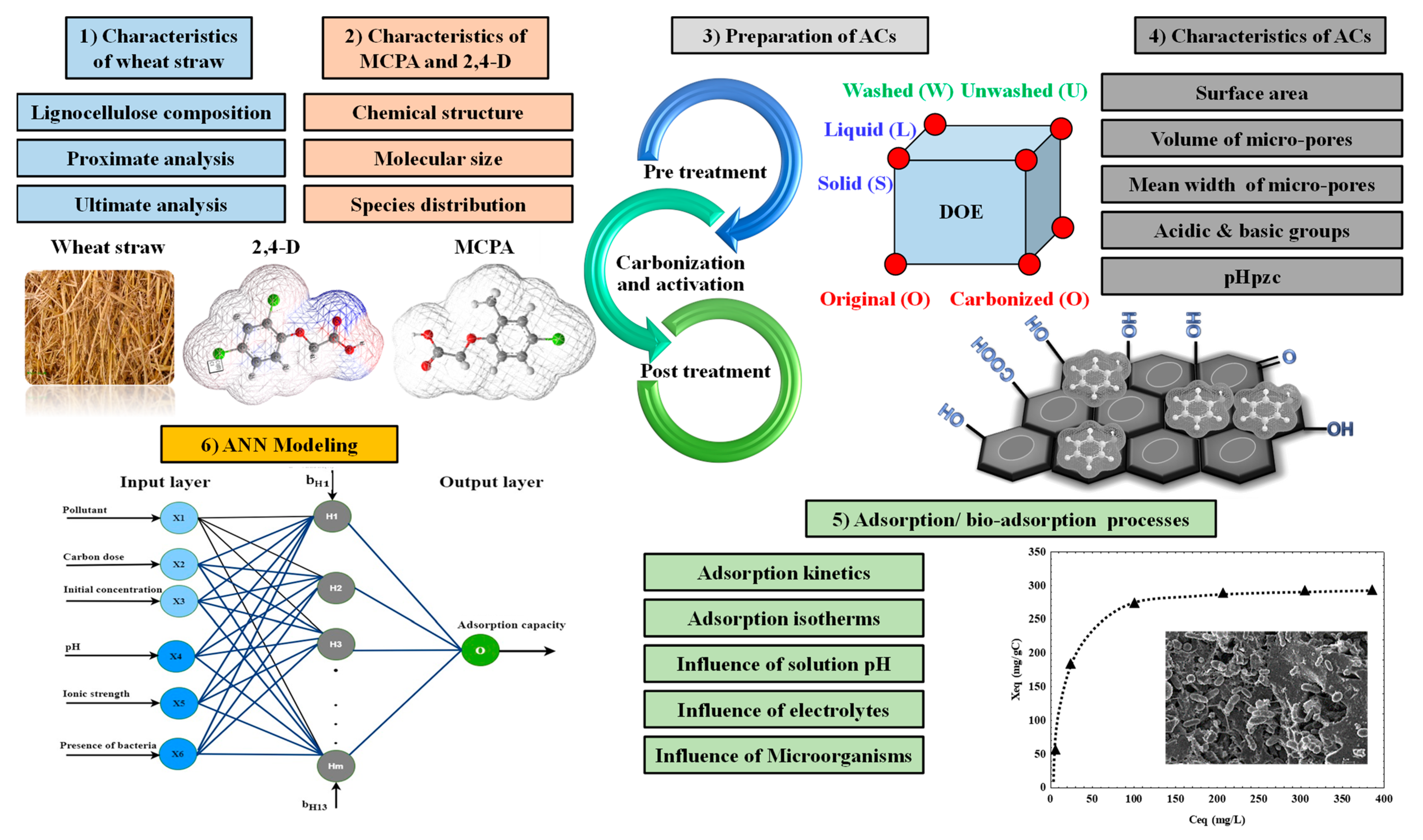
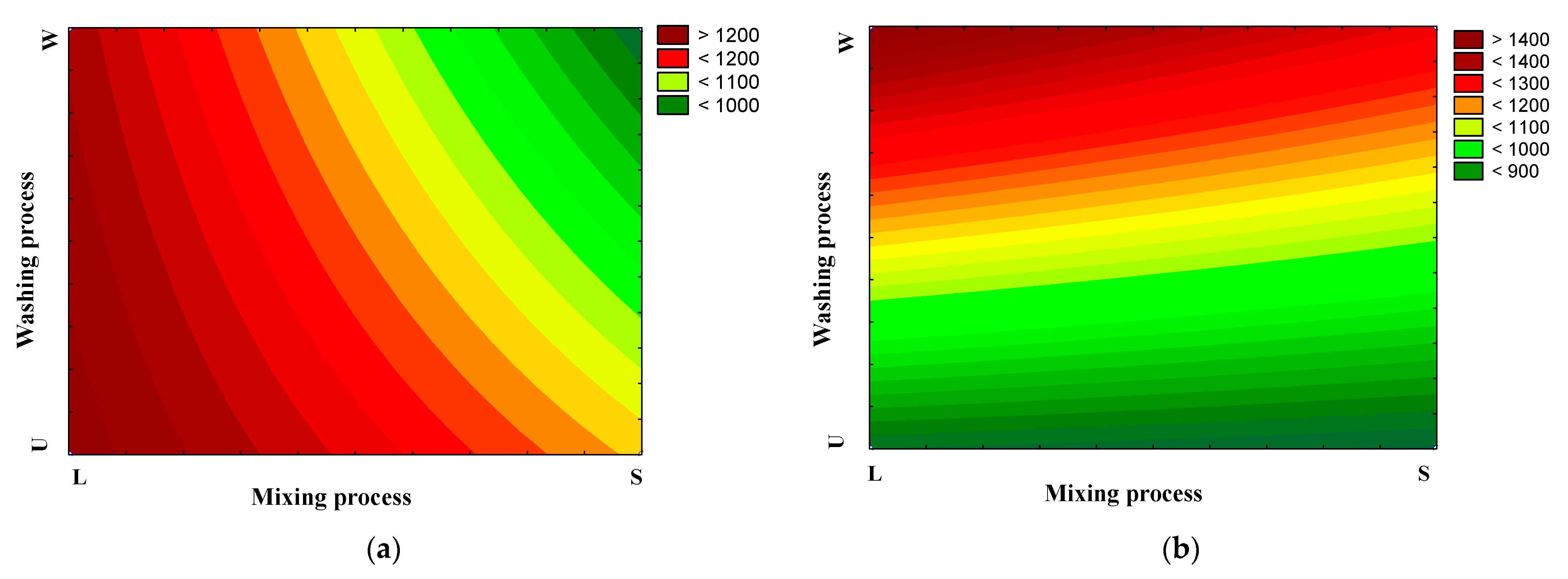
2.2. Preparation of AC
2.3. Adsorption Processes
2.3.1. Adsorption Kinetics
2.3.2. Adsorption Isotherm
2.3.3. Operational Parameters
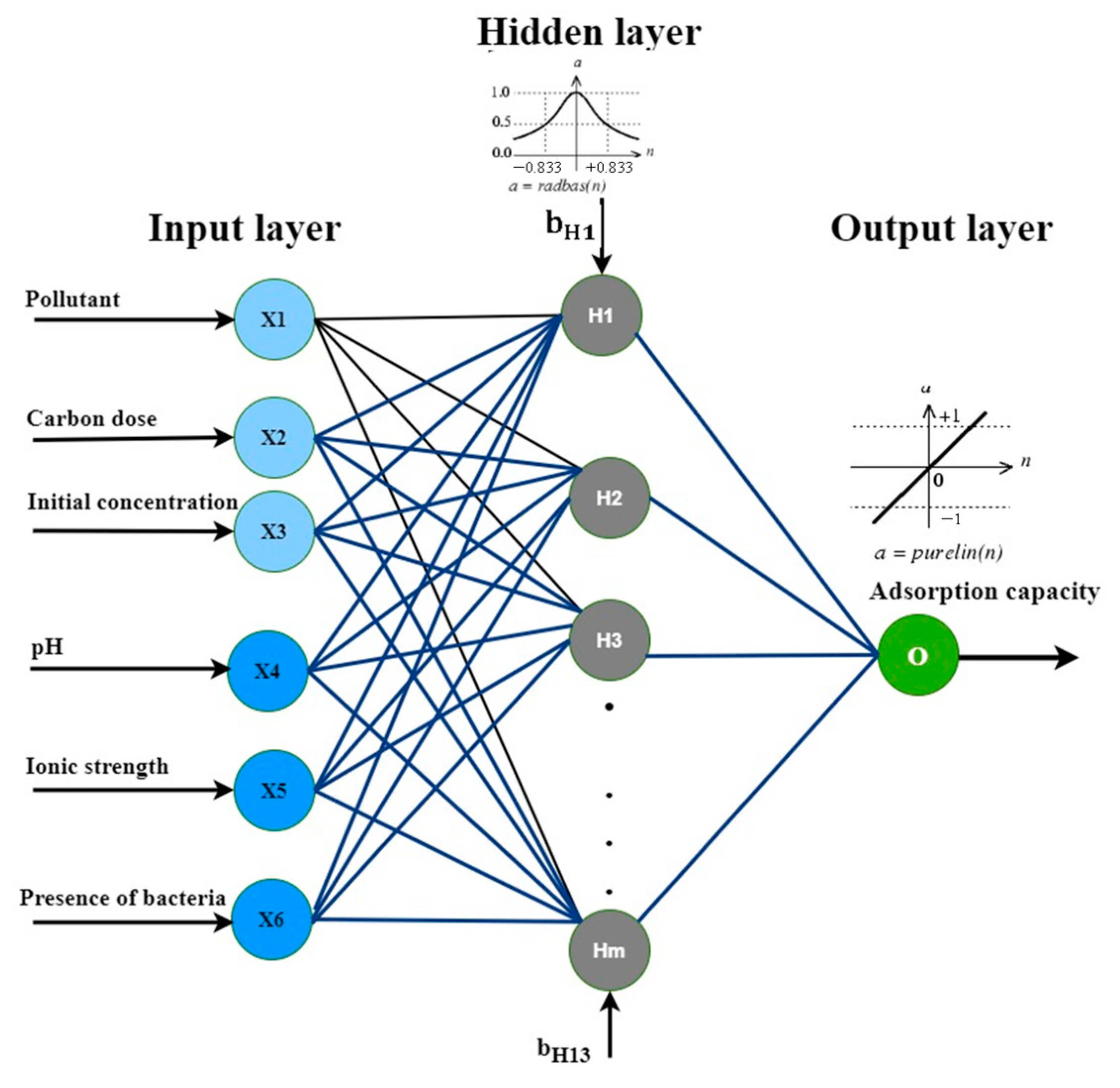
2.4. Artificial Neural Networks
3. Results and Discussion
3.1. Characteristics of Activated Carbon
3.2. Adsorption Kinetics
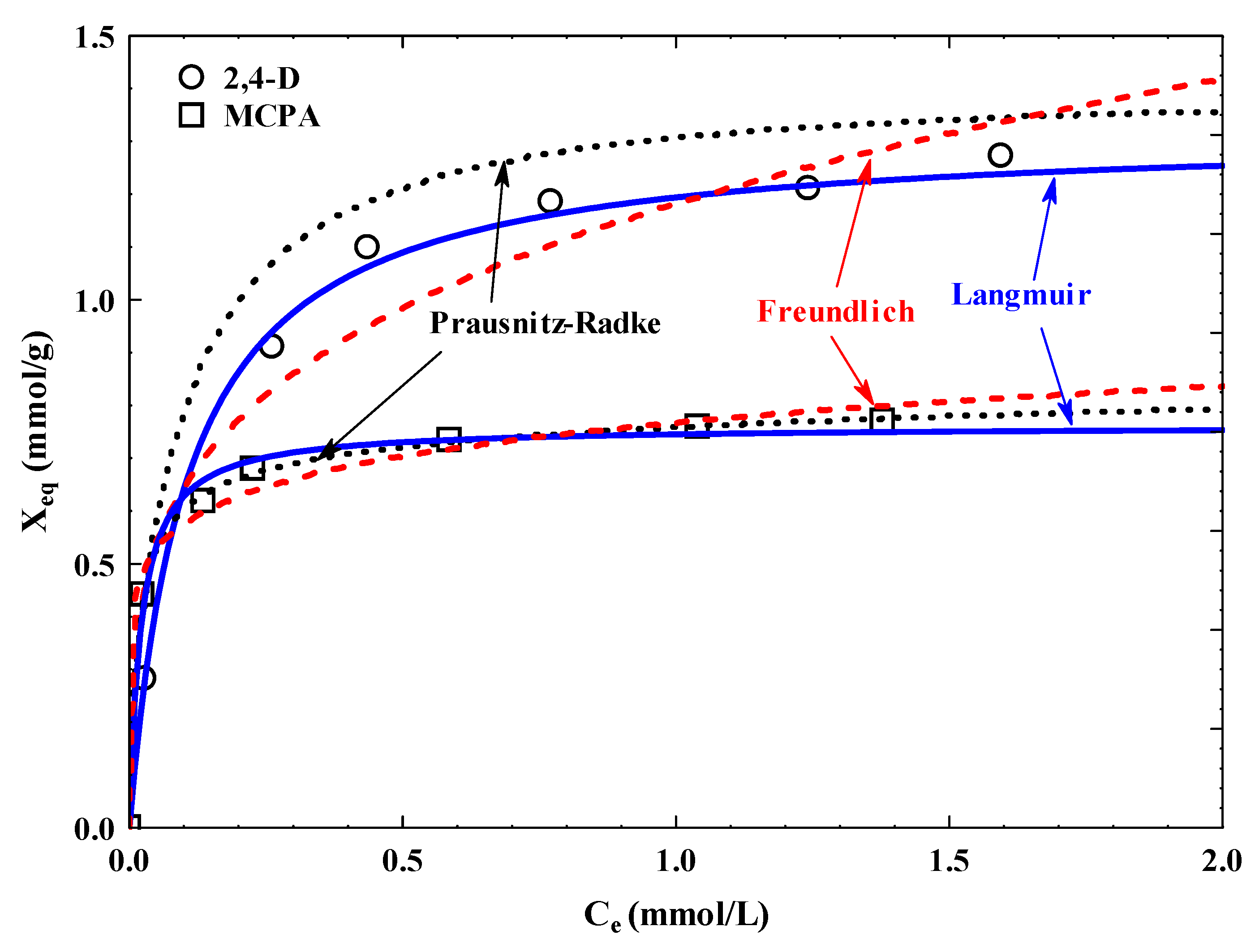
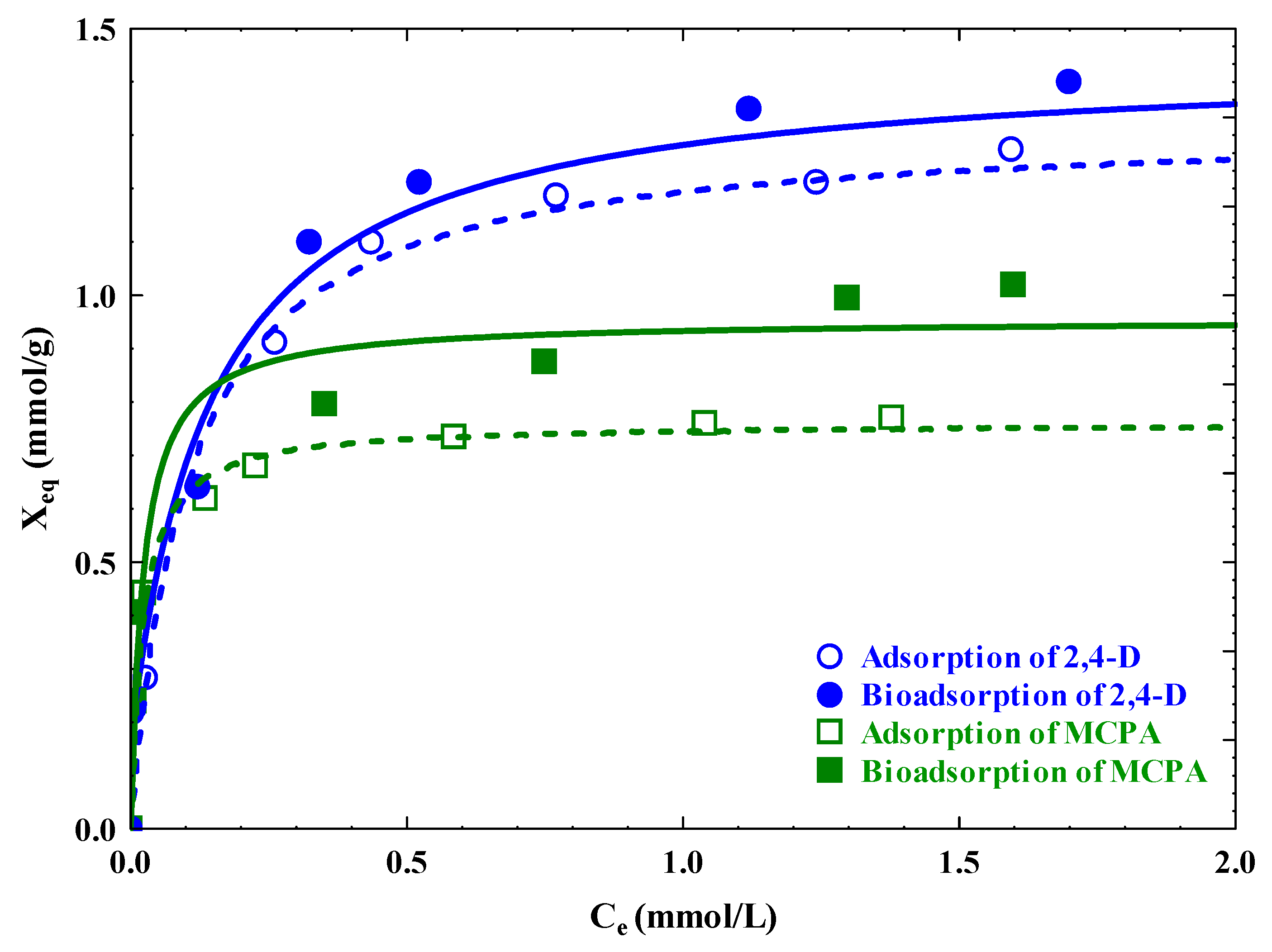
3.3. Adsorption Isotherms
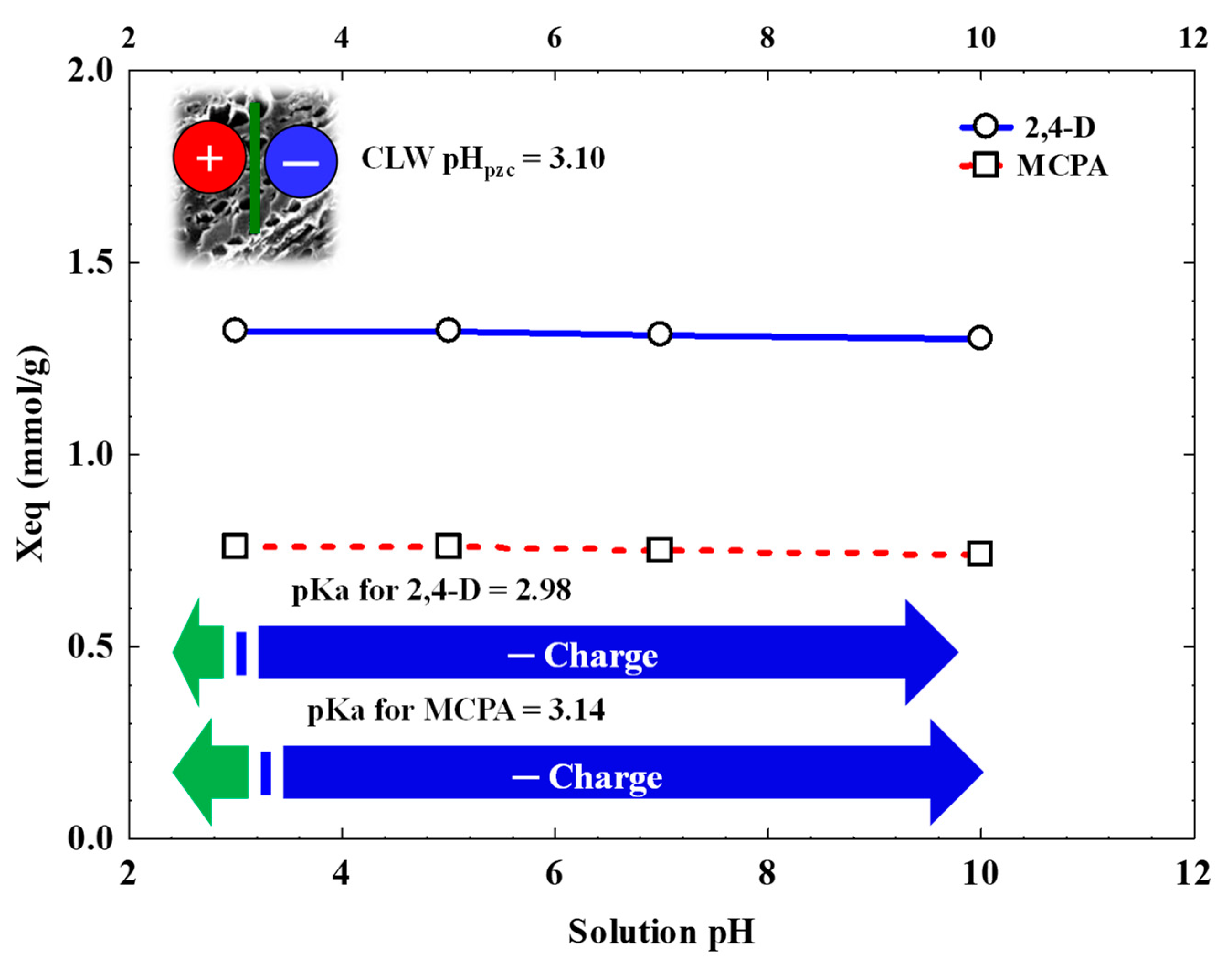
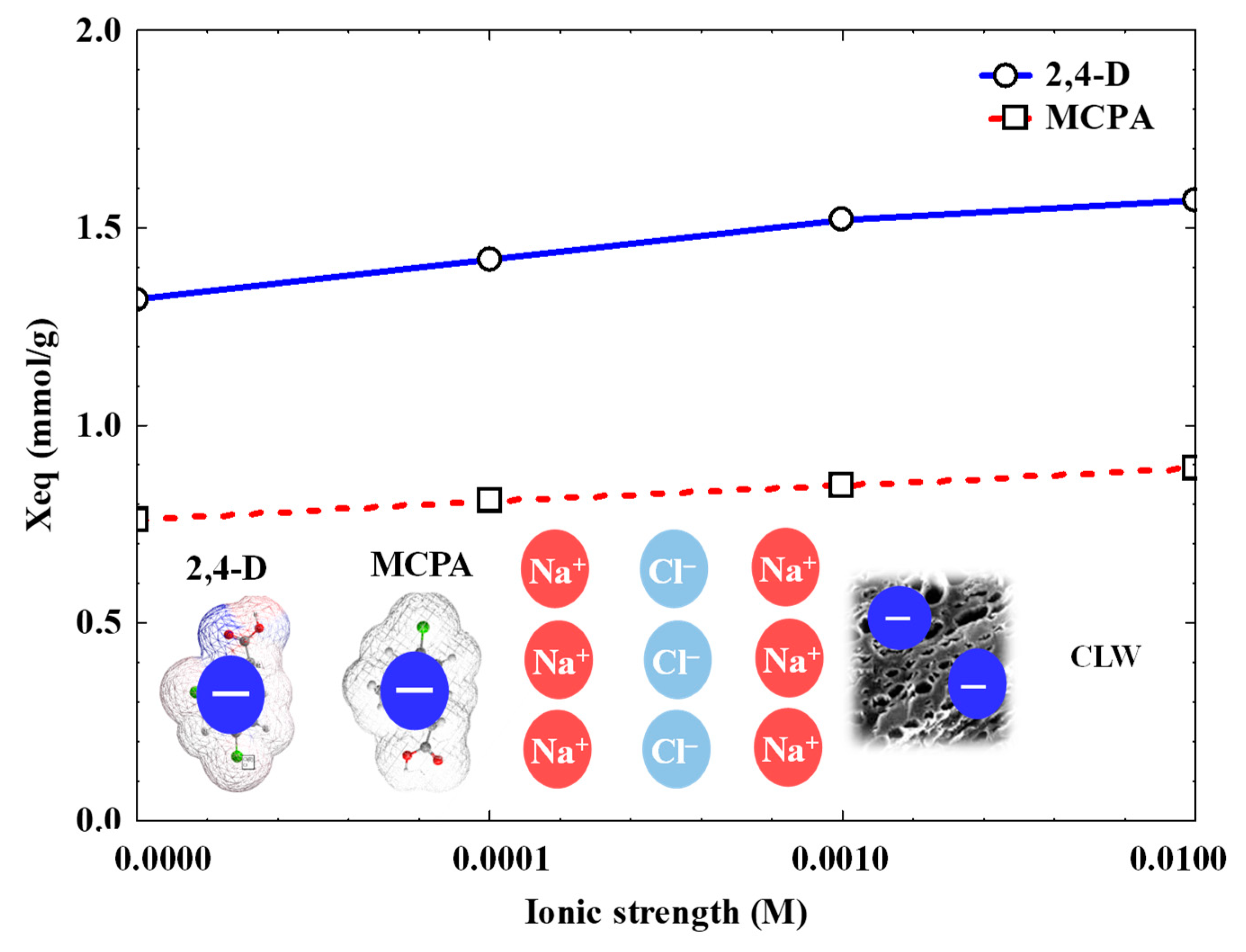
3.4. Influence of Operational Variables
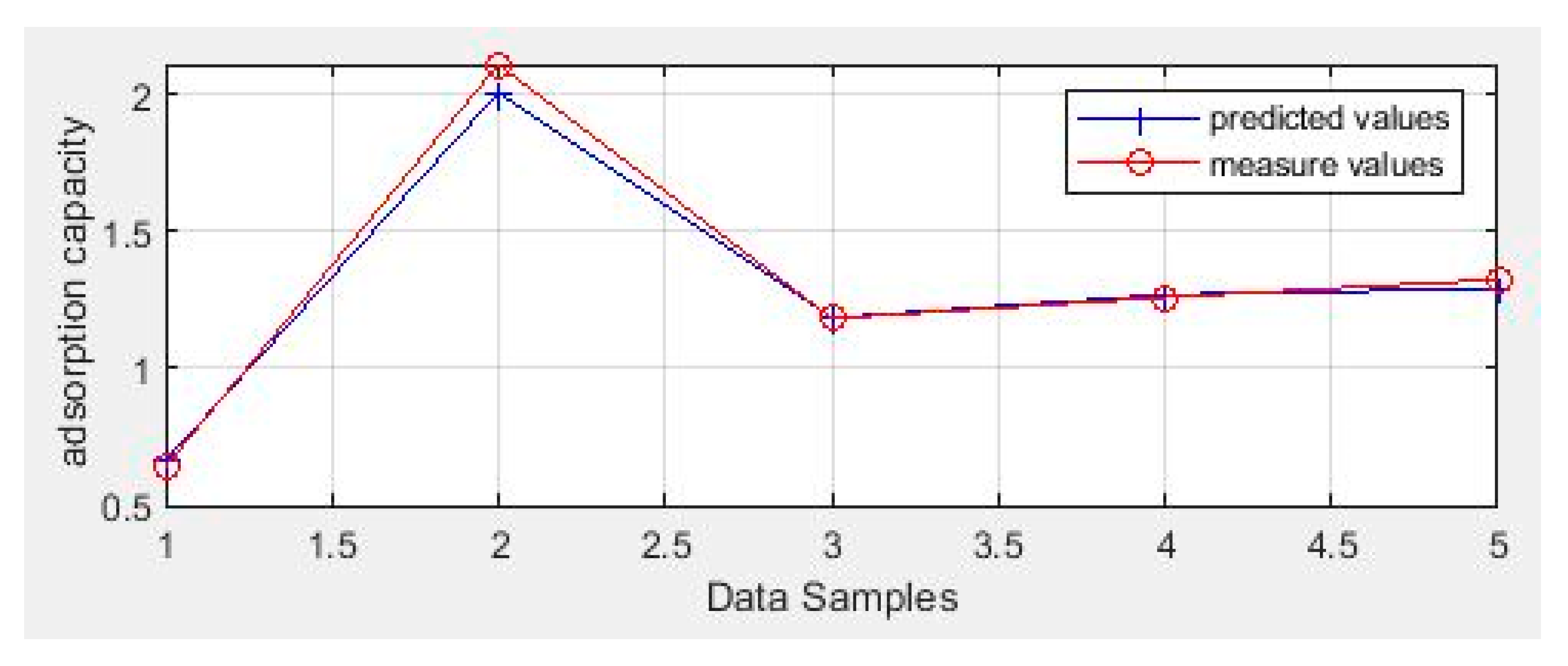
3.5. Modeling via Neural Networks
4. Conclusions
5. Relevant Topics for Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGinley, J.; Healy, M.G.; Ryan, P.C.; Mellander, P.-E.; Morrison, L.; O’Driscoll, J.H.; Siggins, A. Batch adsorption of herbicides from aqueous solution onto diverse reusable materials and granulated activated carbon. J. Environ. Manag. 2022, 323, 116102. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Naeem, M.A.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; la Cecilia, D.; Tang, F.H.M.; McBratney, A. The global environmental hazard of glyphosate use. Sci. Total Environ. 2020, 717, 137167. [Google Scholar] [CrossRef] [PubMed]
- Samadi-Maybodi, A.; Ghezel-Sofla, H.; BiParva, P. Simultaneous removal of phenoxy herbicides, 2-methyl-4-chlorophenoxyacetic acid and 2.4-dichlorophenoxyacetic acid from aqueous media by magnetized MgAl-LDH@ Fe3O4 composite: Application of partial least squares and Doehlert experimental design. Design, 2022; preprint. [Google Scholar] [CrossRef]
- Pouchieu, C.; Piel, C.; Carles, C.; Gruber, A.; Helmer, C.; Tual, S.; Marcotullio, E.; Lebailly, P.; Baldi, I. Pesticide use in agriculture and Parkinson’s disease in the AGRICAN cohort study. Int. J. Epidemiol. 2018, 47, 299–310. [Google Scholar] [CrossRef]
- Sahin, C.; Karpuzcu, M.E. Mitigation of organophosphate pesticide pollution in agricultural watersheds. Sci. Total Environ. 2020, 710, 136261. [Google Scholar] [CrossRef]
- FAO. Pesticides Use, Pesticides Trade and Pesticides Indicators. Global, Regional and Country Trends, 1990–2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Deyris, P.-A.; Pelissier, F.; Grison, C.M.; Hesemann, P.; Petit, E.; Grison, C. Efficient removal of persistent and emerging organic pollutants by biosorption using abundant biomass wastes. Chemosphere 2023, 313, 137307. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P. Pesticides in agriculture and environment: Impacts on human health. Contam. Agric. Environ. Health Risks Remediat. 2019, 1, 76–95. [Google Scholar]
- Blachnio, M.; Kusmierek, K.; Swiatkowski, A.; Derylo-Marczewska, A. Adsorption of Phenoxyacetic Herbicides from Water on Carbonaceous and Non-Carbonaceous Adsorbents. Molecules 2023, 28, 5404. [Google Scholar] [CrossRef]
- Pandiarajan, A.; Kamaraj, R.; Vasudevan, S.; Vasudevan, S. OPAC (orange peel activated carbon) derived from waste orange peel for the adsorption of chlorophenoxyacetic acid herbicides from water: Adsorption isotherm, kinetic modelling and thermodynamic studies. Bioresour. Technol. 2018, 261, 329–341. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Sankowska, M.; Świątkowski, A. Kinetic and equilibrium studies of simultaneous adsorption of monochlorophenols and chlorophenoxy herbicides on activated carbon. Desalination Water Treat. 2014, 52, 178–183. [Google Scholar] [CrossRef]
- Loomis, D.; Guyton, K.; Grosse, Y.; El Ghissasi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015, 16, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Spaltro, A.; Pila, M.; Simonetti, S.; Álvarez-Torrellas, S.; Rodríguez, J.G.; Ruiz, D.; Compañy, A.D.; Juan, A.; Allegretti, P. Adsorption and removal of phenoxy acetic herbicides from water by using commercial activated carbons: Experimental and computational studies. J. Contam. Hydrol. 2018, 218, 84–93. [Google Scholar] [CrossRef] [PubMed]
- El-Shatoury, S.; El-Baz, A.; Abdel Daiem, M.; El-Monayeri, D. Enhancing wastewater treatment by commercial and native microbial Inocula with factorial design. Life Sci. J. 2014, 11, 736–742. [Google Scholar]
- Alrowais, R.; Said, N.; Bashir, M.T.; Ghazy, A.; Alwushayh, B.; Abdel daiem, M.M. Adsorption of Diphenolic Acid from Contaminated Water onto Commercial and Prepared Activated Carbons from Wheat Straw. Water 2023, 15, 555. [Google Scholar] [CrossRef]
- Abdel daiem, M.M.; Sánchez-Polo, M.; Rashed, A.S.; Kamal, N.; Said, N. Adsorption mechanism and modelling of hydrocarbon contaminants onto rice straw activated carbons. Pol. J. Chem. Technol. 2019, 21, 1–12. [Google Scholar] [CrossRef]
- Blachnio, M.; Derylo-Marczewska, A.; Charmas, B.; Zienkiewicz-Strzalka, M.; Bogatyrov, V.; Galaburda, M. Activated carbon from agricultural wastes for adsorption of organic pollutants. Molecules 2020, 25, 5105. [Google Scholar] [CrossRef]
- Nyijime, T.A.; Ayuba, A.M.; Chahul, H.F. Experimental and computational studies on activated Bambara groundnut (Vigna subterranean) hulls for the adsorptive removal of herbicides from aqueous solution. Bull. Natl. Res. Cent. 2021, 45, 189. [Google Scholar] [CrossRef]
- Boontham, W.; Habaki, H.; Egashira, R. Removal of phenol from oil mill effluent using activated carbon prepared from Kernel Shell in Thailand’s Palm Industry. J. Chem. Eng. Jpn. 2020, 53, 682–688. [Google Scholar] [CrossRef]
- Ibeh, P.O.; García-Mateos, F.J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Activated carbon monoliths from lignocellulosic biomass waste for electrochemical applications. J. Taiwan Inst. Chem. Eng. 2019, 97, 480–488. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2021, 283, 124671. [Google Scholar] [CrossRef]
- Feng, P.; Li, J.; Wang, H.; Xu, Z. Biomass-based activated carbon and activators: Preparation of activated carbon from corncob by chemical activation with biomass pyrolysis liquids. ACS Omega 2020, 5, 24064–24072. [Google Scholar] [CrossRef] [PubMed]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of activated carbon production from coconut shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef]
- Mao, H.; Zhou, D.; Hashisho, Z.; Wang, S.; Chen, H.; Wang, H.H.; Lashaki, M.J. Microporous activated carbon from pinewood and wheat straw by microwave-assisted KOH treatment for the adsorption of toluene and acetone vapors. RSC Adv. 2015, 5, 36051–36058. [Google Scholar] [CrossRef]
- Ma, Y. Comparison of activated carbons prepared from wheat straw via ZnCl2 and KOH activation. Waste Biomass Valorization 2017, 8, 549–559. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Emran, M.; El-Sadek, M.H.; El-Shanshory, A.A.; Soliman, H.M.A.; Akl, M.A.; Rashad, M. Enhanced removal of cationic dye by eco-friendly activated biochar derived from rice straw. Appl. Water Sci. 2020, 10, 45. [Google Scholar] [CrossRef]
- Li, M.; Lu, X.; Xia, H.; Zhang, C.; Wang, X.; Chen, Z.; Qu, K.; Huang, B.; Moore, S.; Mao, Y. In-depth characterization of the pituitary transcriptome in Simmental and Chinese native cattle. Domest. Anim. Endocrinol. 2019, 66, 35–42. [Google Scholar] [CrossRef]
- Wan Ibrahim, W.M.H.; Mohamad Amini, M.H.; Sulaiman, N.S.; Kadir, W.R.A. Powdered activated carbon prepared from Leucaena leucocephala biomass for cadmium removal in water purification process. Arab. J. Basic Appl. Sci. 2019, 26, 30–40. [Google Scholar] [CrossRef]
- Cansado, I.P.P.; Mourão, P.A.M.; Gomes, J.; Almodôvar, V. Adsorption of MCPA, 2,4-D and diuron onto activated carbons from wood composites. Ciência Tecnol. Dos Mater. 2017, 29, e224–e228. [Google Scholar] [CrossRef]
- Ramamoorthy, M.; Ragupathy, S.; Sakthi, D.; Arun, V.; Kannadasan, N. Synthesis of SnO2 loaded on corn cob activated carbon for enhancing the photodegradation of methylene blue under sunlight irradiation. J. Environ. Chem. Eng. 2020, 8, 104331. [Google Scholar] [CrossRef]
- Lafi, R.; Montasser, I.; Hafiane, A. Adsorption of congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt. Sci. Technol. 2019, 37, 160–181. [Google Scholar] [CrossRef]
- Borah, L.; Goswami, M.; Phukan, P. Adsorption of methylene blue and eosin yellow using porous carbon prepared from tea waste: Adsorption equilibrium, kinetics and thermodynamics study. J. Environ. Chem. Eng. 2015, 3, 1018–1028. [Google Scholar] [CrossRef]
- Özsin, G.; Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Chemically activated carbon production from agricultural waste of chickpea and its application for heavy metal adsorption: Equilibrium, kinetic, and thermodynamic studies. Appl. Water Sci. 2019, 9, 56. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S.; Zamani, S.; Akbari, S.; Salehi, M. Optimization and modeling of aqueous Cr (VI) adsorption onto activated carbon prepared from sugar beet bagasse agricultural waste by application of response surface methodology. Surf. Interfaces 2020, 18, 100444. [Google Scholar] [CrossRef]
- Alrowais, R.; Abdel daiem, M.M.; Li, R.; Maklad, M.A.; Helmi, A.M.; Nasef, B.M.; Said, N. Groundwater Quality Assessment for Drinking and Irrigation Purposes at Al-Jouf Area in KSA Using Artificial Neural Network, GIS, and Multivariate Statistical Techniques. Water 2023, 15, 2982. [Google Scholar] [CrossRef]
- Alrowais, R.; Yousef, R.S.; Ahmed, O.K.; Mahmoud-Aly, M.; Abdel daiem, M.M.; Said, N. Enhanced detoxification methods for the safe reuse of treated olive mill wastewater in irrigation. Environ. Sci. Eur. 2023, 35, 95. [Google Scholar] [CrossRef]
- Naz, M.; Uyanik, S.; Yesilnacar, M.I.; Sahinkaya, E. Side-by-side comparison of horizontal subsurface flow and free water surface flow constructed wetlands and artificial neural network (ANN) modelling approach. Ecol. Eng. 2009, 35, 1255–1263. [Google Scholar] [CrossRef]
- Ghaedi, A.M.; Vafaei, A. Applications of artificial neural networks for adsorption removal of dyes from aqueous solution: A review. Adv. Colloid Interface Sci. 2017, 245, 20–39. [Google Scholar] [CrossRef]
- Reynel-Ávila, H.E.; Aguayo-Villarreal, I.A.; Diaz-Muñoz, L.L.; Moreno-Pérez, J.; Sánchez-Ruiz, F.J.; Rojas-Mayorga, C.K.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. A review of the modeling of adsorption of organic and inorganic pollutants from water using artificial neural networks. Adsorpt. Sci. Technol. 2022, 2022, 9384871. [Google Scholar] [CrossRef]
- Messikh, N.; Bougdah, N.; Bousba, S.; Djazi, F. Modeling the adsorption of chlorobenzene on modified bentonite using an artificial neural network. Curr. Res. Green Sustain. Chem. 2020, 3, 100026. [Google Scholar] [CrossRef]
- Azqhandi, M.H.A.; Ghaedi, M.; Yousefi, F.; Jamshidi, M. Application of random forest, radial basis function neural networks and central composite design for modeling and/or optimization of the ultrasonic assisted adsorption of brilliant green on ZnS-NP-AC. J. Colloid Interface Sci. 2017, 505, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, A.; Ghaedi, M.; Azqhandi, M.H.A.; Goudarzi, A.; Hajati, S. Ultrasound-assisted binary adsorption of dyes onto Mn@ CuS/ZnS-NC-AC as a novel adsorbent: Application of chemometrics for optimization and modeling. J. Ind. Eng. Chem. 2017, 54, 377–388. [Google Scholar] [CrossRef]
- Silva, T.S.; de Freitas Souza, M.; da Silva Teófilo, T.M.; Dos Santos, M.S.; Porto, M.A.F.; Souza, C.M.M.; Dos Santos, J.B.; Silva, D.V. Use of neural networks to estimate the sorption and desorption coefficients of herbicides: A case study of diuron, hexazinone, and sulfometuron-methyl in Brazil. Chemosphere 2019, 236, 124333. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, H.; Bhat, R.; Selvaraj, R. Removal of an agricultural herbicide (2,4-Dichlorophenoxyacetic acid) using magnetic nanocomposite: A combined experimental and modeling studies. Environ. Res. 2023, 238, 117124. [Google Scholar]
- Dahlan, I.; Azhar, E.E.M.; Hassan, S.R.; Aziz, H.A.; Hung, Y.-T. Statistical Modeling and Optimization of Process Parameters for 2, 4-Dichlorophenoxyacetic Acid Removal by Using AC/PDMAEMA Hydrogel Adsorbent: Comparison of Different RSM Designs and ANN Training Methods. Water 2022, 14, 3061. [Google Scholar] [CrossRef]
- Isiyaka, H.A.; Jumbri, K.; Sambudi, N.S.; Zango, Z.U.; Abdullah, N.A.F.; Saad, B.; Mustapha, A. Adsorption of dicamba and MCPA onto MIL-53 (Al) metal–organic framework: Response surface methodology and artificial neural network model studies. RSC Adv. 2020, 10, 43213–43224. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Mohamed, M. Integrated Technologies Based on the Use of Activated Carbon and Radiation to Remove Contaminants Present in Landfill Leachates. Ph.D. Thesis, Granada University, Granada, Spain, 2013. [Google Scholar]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Mokti, N.; Borhan, A.; Zaine, S.N.A.; Zaid, H.F.M. Synthesis and Characterisation of Pyridinium-Based Ionic Liquid as Activating Agent in Rubber Seed Shell Activated Carbon Production for CO2 Capture. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 82, 85–95. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Benavente, V.; Weidemann, E.; Gentili, F.G.; Jansson, S. Influence of hydrothermal carbonization conditions on the porosity, functionality, and sorption properties of microalgae hydrochars. Sci. Rep. 2023, 13, 8562. [Google Scholar] [CrossRef]
- Boakye, P.; Tran, H.N.; Lee, D.S.; Woo, S.H. Effect of water washing pretreatment on property and adsorption capacity of macroalgae-derived biochar. J. Environ. Manag. 2019, 233, 165–174. [Google Scholar] [CrossRef]


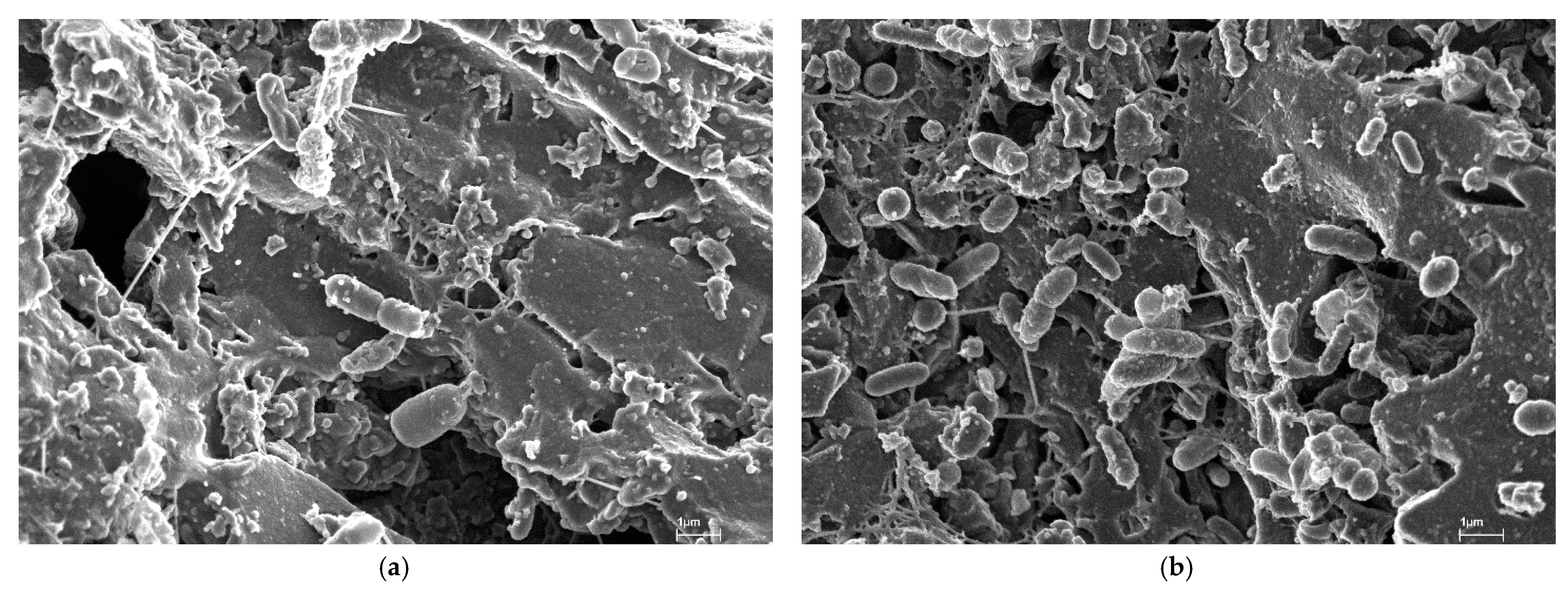
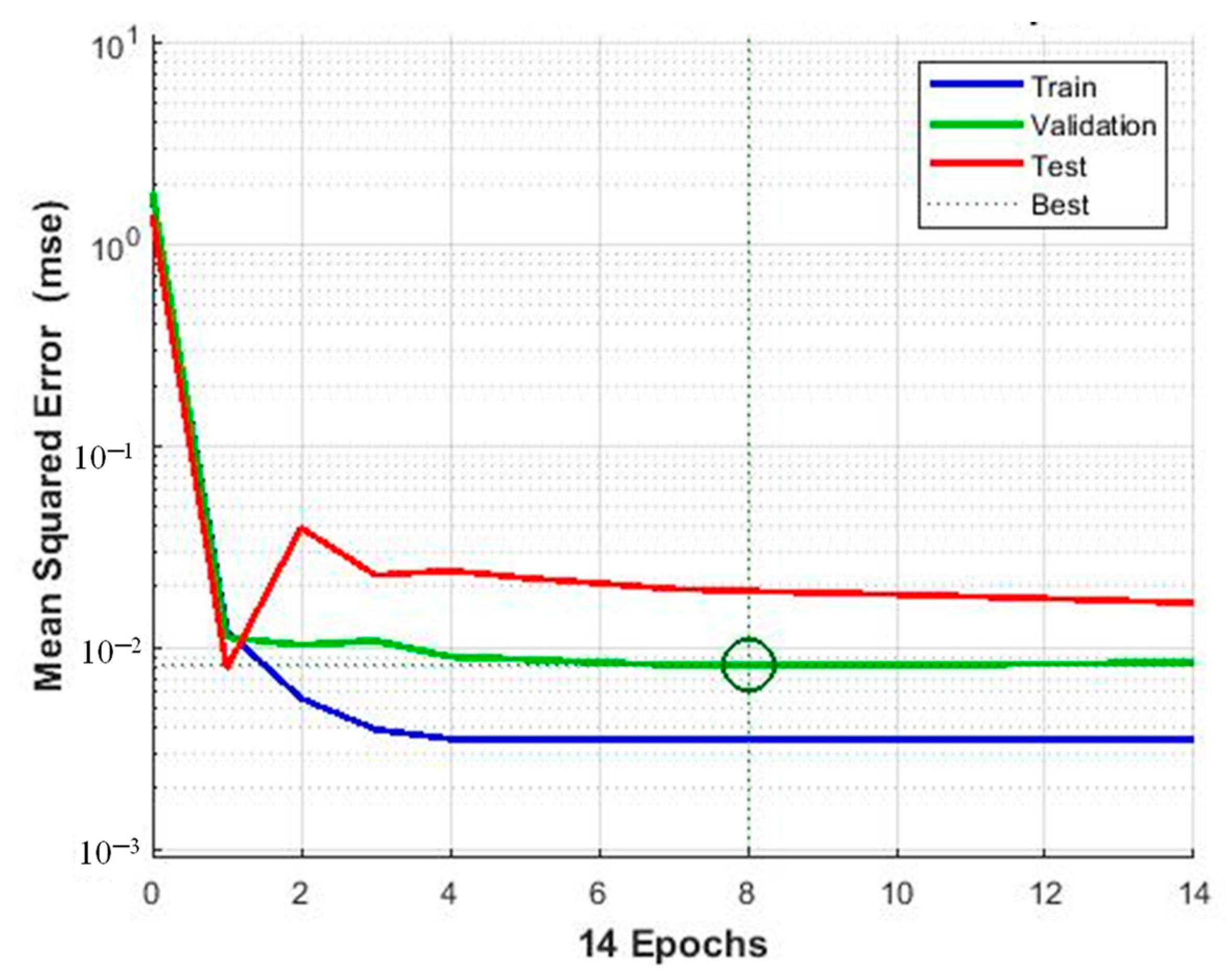
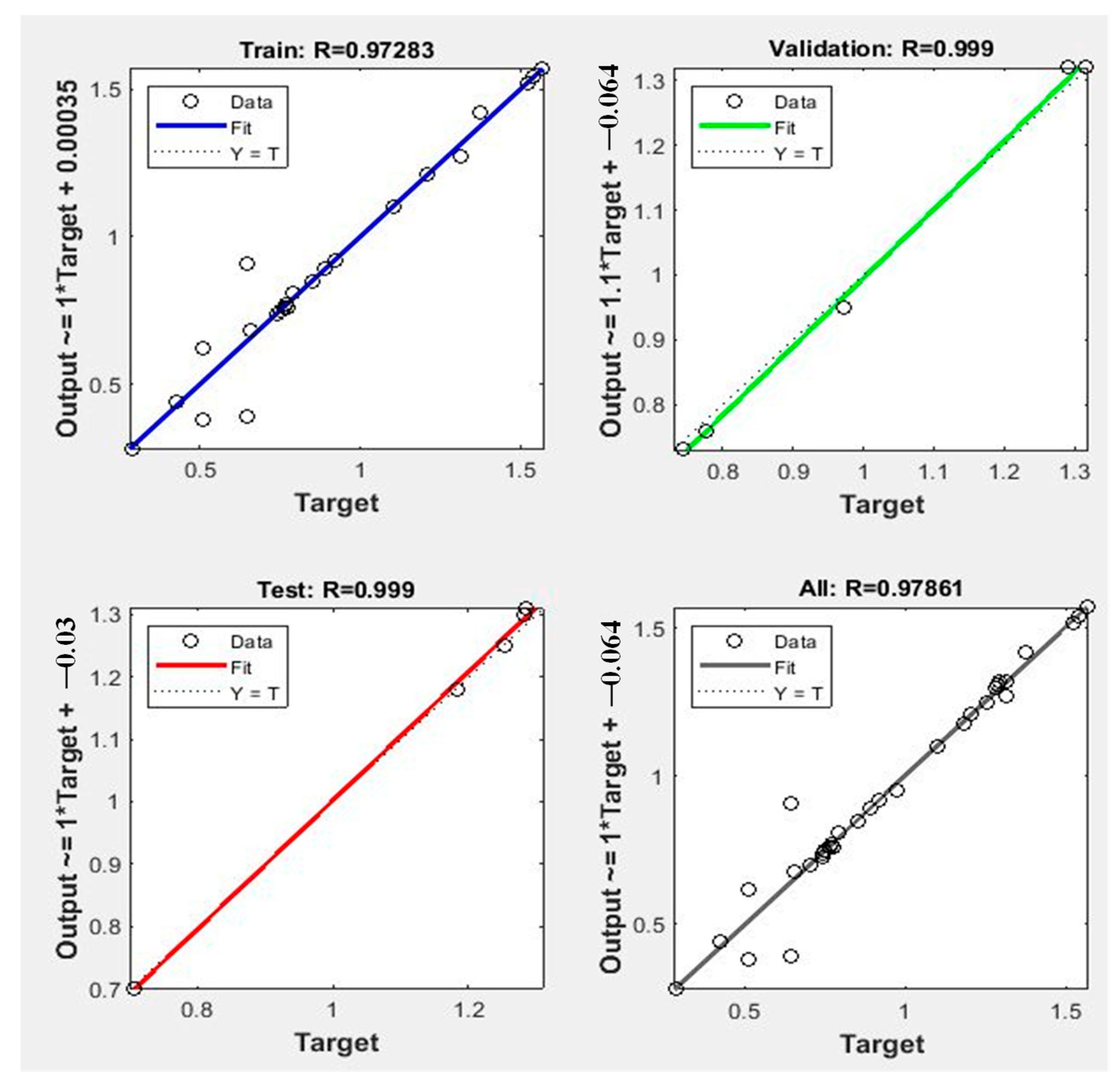
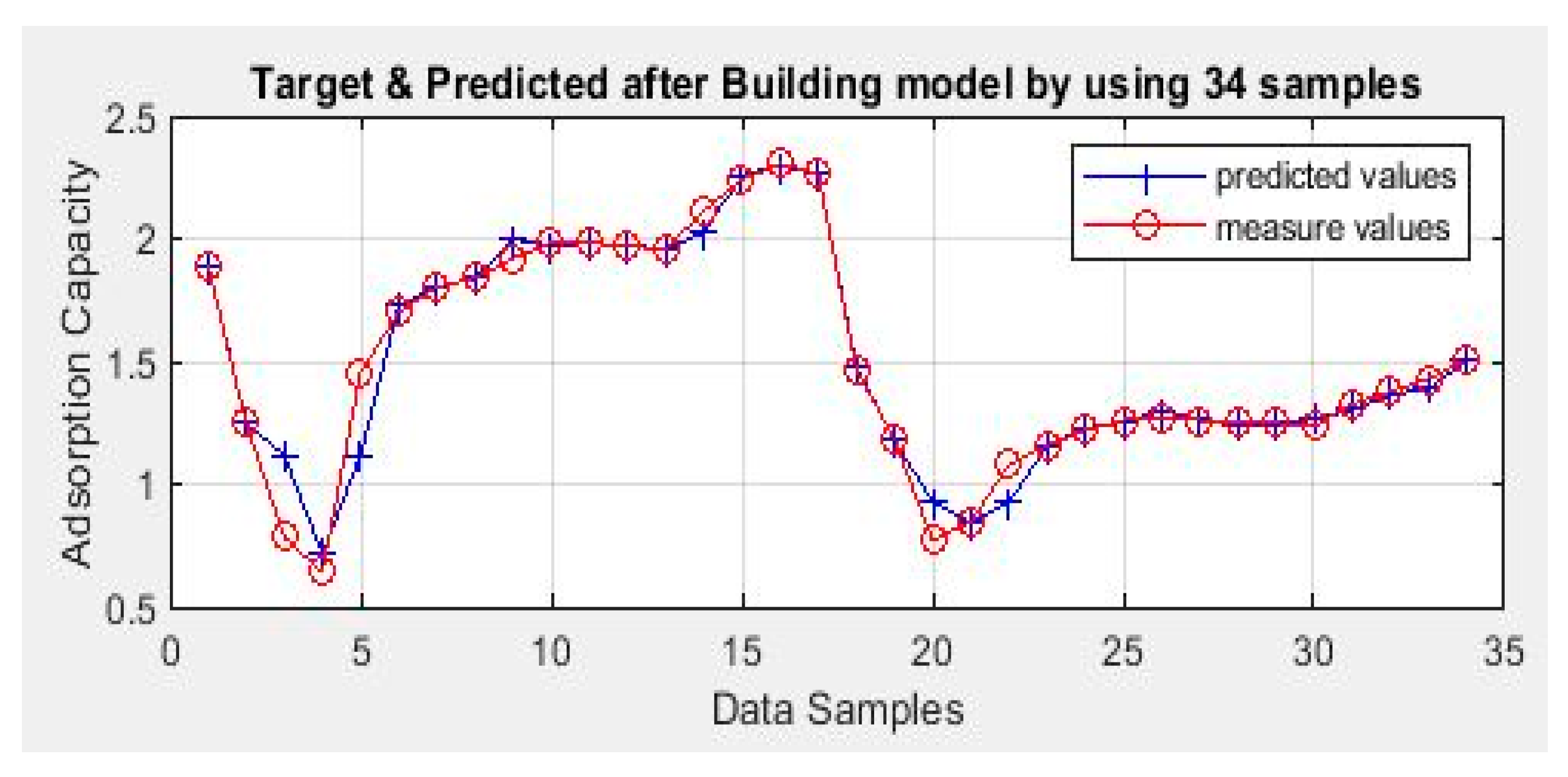
| Raw Material | Activation Agent | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|
| Wheat straw | KOH | 1164 | 0.51 | 265 | [16] |
| Wheat straw | KOH | 1250 | 0.60 | 200 | [25] |
| Wheat straw | KOH | 552 | -- | 147 | [26] |
| Wheat straw | ZnCl2 | 907 | -- | 266 | [26] |
| Rice straw | H3PO4 | 613 | 0.25 | 176 to 329 | [17] |
| Rice straw | ZnCl2 | 771 | 0.23 | 155 to 329 | [17] |
| Rice straw | Wet attrition | 223 | -- | 46 to 91 | [27] |
| Rice husk | CO2 | 1097 | 0.83 | 163 | [28] |
| Leucaena leucocephala biomass | NaOH | 185 to 776 | -- | 70 | [29] |
| Wood | CO2 | 805 to 1211 | 0.33 to 0.58 | 226 to 539 | [30] |
| Wood | KOH | 2044 | 0.93 | 200 | [25] |
| Corn cob | H3PO4 | 293 | 0.35 | -- | [31] |
| Coffee waste | CH3CO2K | 220 | 0.13 | 91 | [32] |
| Tea precursors | H3PO4 | 2054 | 1.75 | 400 to 402 | [33] |
| Chickpea husk | KOH and K2CO3 | 2082 | 1.07 | 56 to 136 | [34] |
| Sugar beet bagasse | H3PO4 | 748 | 0.36 | 10 | [35] |
| ANN Model | Experiment | Data Used (Training- Testing-Validation) | Input Variables | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|
| RDB feed forward, Levenberg–Marquardt back-propagation | Kinetics and isotherm | -- | 4 | 0.92 mmol/g | [40] |
| RDB | Equilibrium RSM (CCD) | 36-0-18 | 3 | 98.89% | [41] |
| RDB neural network with Kernel stone algorithm | Binary, RSM (CCD) | 22-10-0 | 5 | 126.42 to 115.08 mg/g | [42] |
| FFNN | Adsorption in soil | 12-9-1 | 12 | -- | [44] |
| FFNN | RSM (CCD) | 4-9-1 | 4 | 7.29 mg/g | [45] |
| FFNN | RSM | 3-9-2 | 3 | 94.52 mg/g | [46] |
| FFNN | RSM (CCD), ANOVA | 5-7-1 | 5 | 272.2 to 232.5 mg/g | [47] |
| Molecular Size (x, y, z) (nm) | Molecular Weight (g/mol) | VA (a) (cm3/mol) | S (b) (g/L) | log Kow (c) | pKa1 (d) | |
|---|---|---|---|---|---|---|
| 2,4-D | 1.29 × 0.73 × 0.42 | 221.00 | 182 | 2.16 | 2.69 | 2.98 |
| MCPA | 1.24 × 0.84× 0.42 | 200.62 | 185 | 1.38 | 2.22 | 3.14 |
| Exp. No. | P | M | W | Designed Sample | |||
|---|---|---|---|---|---|---|---|
| 1 | O | −1 | L | −1 | U | −1 | OLU |
| 2 | O | −1 | S | +1 | U | −1 | OSU |
| 3 | O | −1 | L | −1 | W | +1 | OLW |
| 4 | O | −1 | S | +1 | W | +1 | OSW |
| 5 | C | +1 | L | −1 | U | −1 | CLU |
| 6 | C | +1 | S | +1 | U | −1 | CSU |
| 7 | C | +1 | L | −1 | W | +1 | CLW |
| 8 | C | +1 | S | +1 | W | +1 | CSW |
| AC | SN2 (a) (m2/g) | Wo (N2) (b) (cm3/g) | Wo (CO2) (c) (cm3/g) | Lo (N2) (d) (nm) | Lo (CO2) (e) (nm) | AGC (f) (meq/g) | BGC (g) (meq/g) | pHpzc (h) |
|---|---|---|---|---|---|---|---|---|
| OLU | 1263 ± 4.58 | 0.50 ± 0.03 | 0.34 ± 0.03 | 1.21 ± 0.02 | 0.68 ± 0.03 | 8.90 ± 0.26 | 10.12 ± 0.01 | 7.80 ± 0.02 |
| OSU | 1164 ± 2.65 | 0.51 ± 0.02 | 0.48 ± 0.02 | 1.27 ± 0.03 | 0.92 ± 0.02 | 8.71 ± 0.10 | 3.63 ± 0.06 | 5.99 ± 0.03 |
| OLW | 1285 ± 2.00 | 0.51 ± 0.03 | 0.53 ± 0.03 | 1.25 ± 0.03 | 0.72 ± 0.04 | 8.88 ± 0.19 | 3.95 ± 0.04 | 5.10 ± 0.05 |
| OSW | 934 ± 3.61 | 0.37 ± 0.03 | 0.33 ± 0.04 | 1.02 ± 0.03 | 0.67 ± 0.03 | 7.65 ± 0.13 | 0.41 ± 0.04 | 3.20 ± 0.02 |
| CLU | 870 ± 3.00 | 0.35 ± 0.03 | 0.42 ± 0.03 | 1.20 ± 0.02 | 0.68 ± 0.04 | 8.90 ± 0.17 | 3.24 ± 0.13 | 5.20 ± 0.05 |
| CSU | 785 ± 3.46 | 0.31 ± 0.03 | 0.38 ± 0.03 | 1.10 ± 0.09 | 0.65 ± 0.03 | 7.80 ± 0.26 | 3.01 ± 0.04 | 4.10 ± 0.04 |
| CLW | 1437 ± 2.52 | 0.60 ± 0.06 | 0.43 ± 0.02 | 1.20 ± 0.02 | 0.70 ± 0.02 | 7.02 ± 0.08 | 0.02 ± 0.02 | 3.10 ± 0.05 |
| CSW | 1342 ± 3.79 | 0.55 ± 0.04 | 0.40 ± 0.02 | 0.99 ± 0.05 | 0.65 ± 0.02 | 6.52 ± 0.04 | 0.03 ± 0.02 | 3.00 ± 0.02 |
| SN2 (m2/g) (a) | Wo (N2) (cm3/g) (b) | Lo (N2) (nm) (c) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| βi | SD | p-Value | βi | SD | p-Value | βi | SD | p-Value | |
| Intercept | +1135.00 | ±30.25 | 0.02 | +0.46 | ±0.02 | 0.07 | +1.16 | ±0.02 | 0.01 |
| P | −53.00 | ±60.50 | 0.54 | −0.02 | ±0.04 | 0.63 | −0.065 | ±0.05 | 0.39 |
| W | +229.00 | ±60.50 | 0.16 | +0.09 | ±0.04 | 0.57 | −0.08 | ±0.05 | 0.33 |
| M | −157.50 | ±60.50 | 0.23 | −0.06 | ±0.04 | 0.72 | −0.12 | ±0.05 | 0.23 |
| P × W | +333.00 | ±60.50 | 0.11 | +0.16 | ±0.04 | 0.69 | +0.03 | ±0.05 | 0.68 |
| P × M | +67.50 | ±60.50 | 0.47 | +0.01 | ±0.04 | 0.39 | −0.04 | ±0.05 | 0.58 |
| M × W | −65.50 | ±60.50 | 0.47 | −0.04 | ±0.04 | 0.43 | −0.10 | ±0.05 | 0.27 |
| R2 | 0.98 | 0.97 | 0.95 | ||||||
| Pollutant | Mass of Carbon (mg) | qe (exp.) (mmol/g) | 1st Order | 2nd Order | ||||
|---|---|---|---|---|---|---|---|---|
| qe (pred.) (mmol/g) | k1 (1/h) | %D | qe (pred.) (mmol/g) | k2 (g/mmol/h) | %D | |||
| 2,4-D | 25 | 1.273 | 1.216 | 0.308 | 11.03 | 1.254 | 0.410 | 6.65 |
| 50 | 0.768 | 0.728 | 0.389 | 9.68 | 0.748 | 0.877 | 5.53 | |
| 100 | 0.410 | 0.381 | 0.326 | 12.09 | 0.391 | 1.453 | 8.17 | |
| MCPA | 25 | 0.937 | 0.918 | 0.064 | 19.29 | 1.017 | 0.083 | 27.84 |
| 50 | 0.670 | 0.644 | 0.066 | 15.81 | 0.703 | 0.141 | 7.43 | |
| 100 | 0.393 | 0.376 | 0.189 | 9.37 | 0.396 | 0.654 | 12.37 | |
| Pollutant | Langmuir | Freundlich | Prausnitz–Radke | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xm(a) (mmol/g) | B (b) (L/mmol) | BXm (c) (L/g) | X′m × 10−4 (mmol/m2/g) | %D | KF (d) (L/g) | 1/nF (e) | %D | a (f) (L/g) | b (g) (Lβ/mmolβ) | β (h) | %D | |
| 2,4-D | 1.32 | 9.44 | 12.46 | 9.19 | 2.07 | 1.18 | 0.26 | 40.96 | 12.05 | 9.09 | 1.01 | 2.37 |
| MCPA | 0.76 | 47.29 | 35.94 | 5.29 | 3.08 | 0.77 | 0.12 | 4.47 | 56.33 | 73.24 | 0.94 | 0.87 |
| Model | Error | Number of Neurons | |||
|---|---|---|---|---|---|
| 5 | 11 | 15 | 20 | ||
| RBFNN | RMSE | 0.0775 | 0.070 | 0.072 | 0.0706 |
| R2 | 0.9550 | 0.96 | 0.957 | 0.9560 | |
| Model | Activation Function of the Hidden Layers | Error | Training Algorithm | ||
|---|---|---|---|---|---|
| Trainlm | Trainscg | Trainbr | |||
| RBFNN | tansig | RMSE | 0.0560 | 0.0775 | 0.1862 |
| R2 | 0.9560 | 0.9400 | 0.8900 | ||
| radbas | RMSE | 0.0540 | 0.0723 | 0.1849 | |
| R2 | 0.9600 | 0.9560 | 0.9100 | ||
| tribas | RMSE | 0.0550 | 0.0851 | 0.4286 | |
| R2 | 0.9560 | 0.9400 | 0.7998 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrowais, R.; Abdel daiem, M.M.; Nasef, B.M.; Said, N. Activated Carbon Fabricated from Biomass for Adsorption/Bio-Adsorption of 2,4-D and MCPA: Kinetics, Isotherms, and Artificial Neural Network Modeling. Sustainability 2024, 16, 299. https://doi.org/10.3390/su16010299
Alrowais R, Abdel daiem MM, Nasef BM, Said N. Activated Carbon Fabricated from Biomass for Adsorption/Bio-Adsorption of 2,4-D and MCPA: Kinetics, Isotherms, and Artificial Neural Network Modeling. Sustainability. 2024; 16(1):299. https://doi.org/10.3390/su16010299
Chicago/Turabian StyleAlrowais, Raid, Mahmoud M. Abdel daiem, Basheer M. Nasef, and Noha Said. 2024. "Activated Carbon Fabricated from Biomass for Adsorption/Bio-Adsorption of 2,4-D and MCPA: Kinetics, Isotherms, and Artificial Neural Network Modeling" Sustainability 16, no. 1: 299. https://doi.org/10.3390/su16010299
APA StyleAlrowais, R., Abdel daiem, M. M., Nasef, B. M., & Said, N. (2024). Activated Carbon Fabricated from Biomass for Adsorption/Bio-Adsorption of 2,4-D and MCPA: Kinetics, Isotherms, and Artificial Neural Network Modeling. Sustainability, 16(1), 299. https://doi.org/10.3390/su16010299








