Purification of Pesticide-Contaminated Water Using Activated Carbon from Prickly Pear Seeds for Environmentally Friendly Reuse in a Circular Economy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorbate
2.2. Synthesis of Activated Carbon (AC)
2.3. Characterization of Activated Carbon
2.3.1. Microporosity Approximated According to the Iodine Number and the Methylene Blue Number
- : Initial concentration of iodine (molL−1) or methylene blue (mg L−1).
- : Final concentration of iodine (mol L−1) or methylene blue (mg L−1).
- : Volume of solution of iodine or methylene blue (mL).
- : Mass of activated carbon (g).
2.3.2. Acidic and Basic Surface: Boehm Method
2.3.3. pH of Zero Charge of Surface (pHZC)
2.3.4. Brunauer–Emmett–Teller BET Analysis
2.3.5. Scanning Electron Microscopy (SEM) Analysis
2.4. Adsorption Experiments
2.4.1. Determination of Pesticide Elimination Rate R (%)
- : Initial concentration of deltamethrin (mg L−1).
- : Equilibrium concentration of deltamethrin (mg L−1).
2.4.2. Kinetics Adsorption
2.4.3. Adsorption Equilibrium Models
2.4.4. Thermodynamic Study
2.5. Density Functional Theory (DFT)
3. Results and Discussion
3.1. Characterization of Activated Carbon
3.1.1. Iodine and Methylene Blue Indices (, QMB)
3.1.2. Boehm Titration
3.1.3. pH of Zero Charge Value
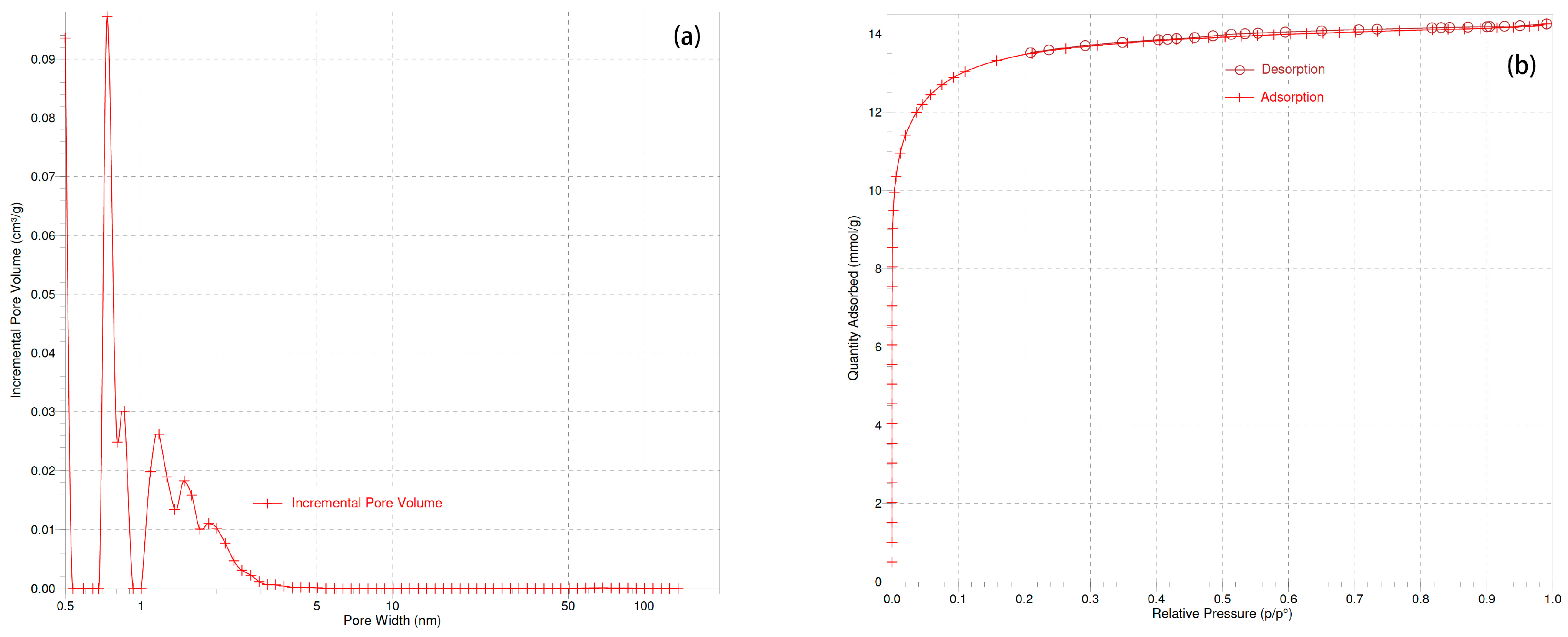
3.1.4. Specific Surface Area: BET Method
3.1.5. Morphological Study: SEM-EDX Analysis
3.2. Adsorption Studies
3.2.1. Mass Effect
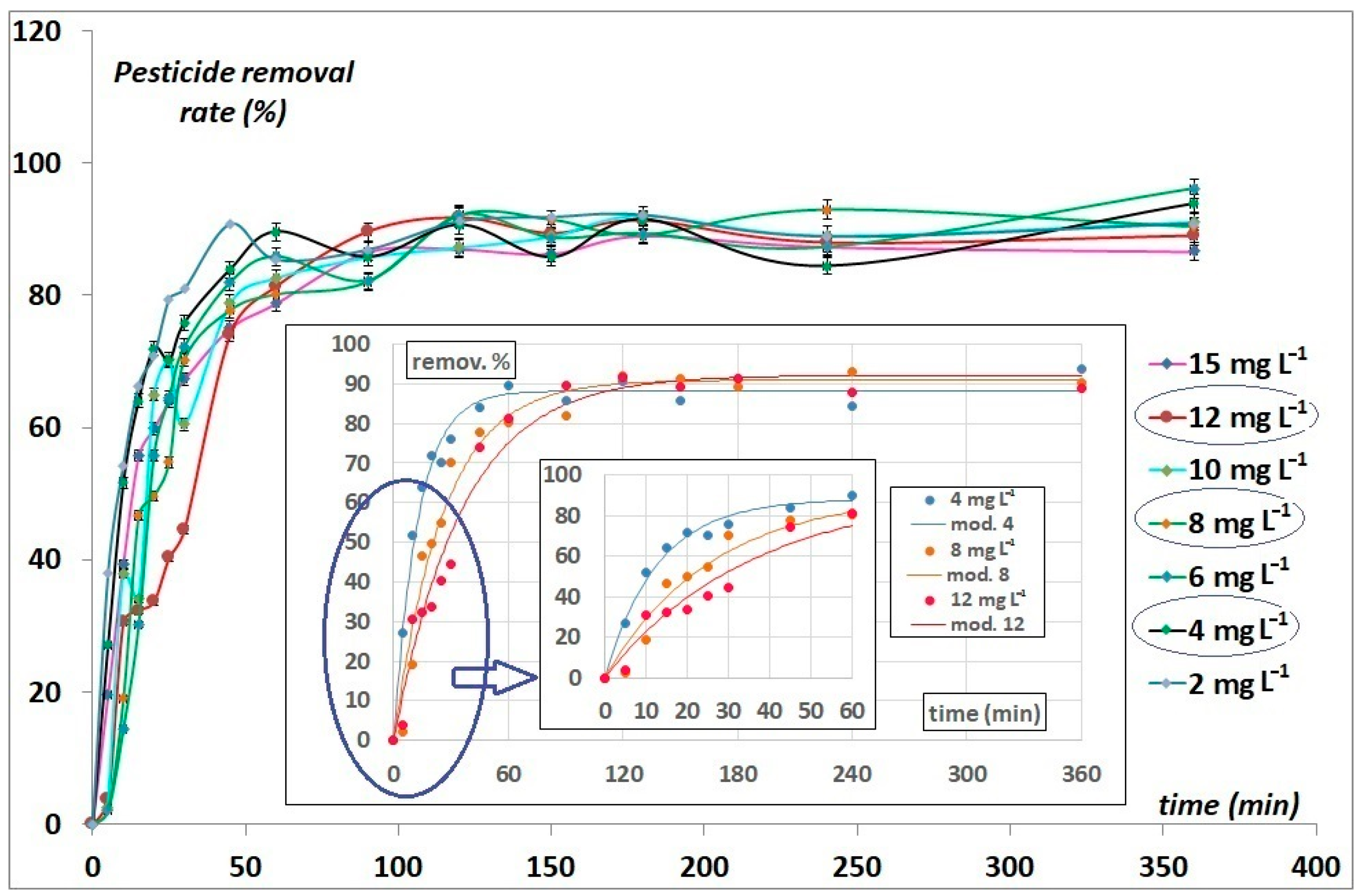
3.2.2. Contact Time Effect
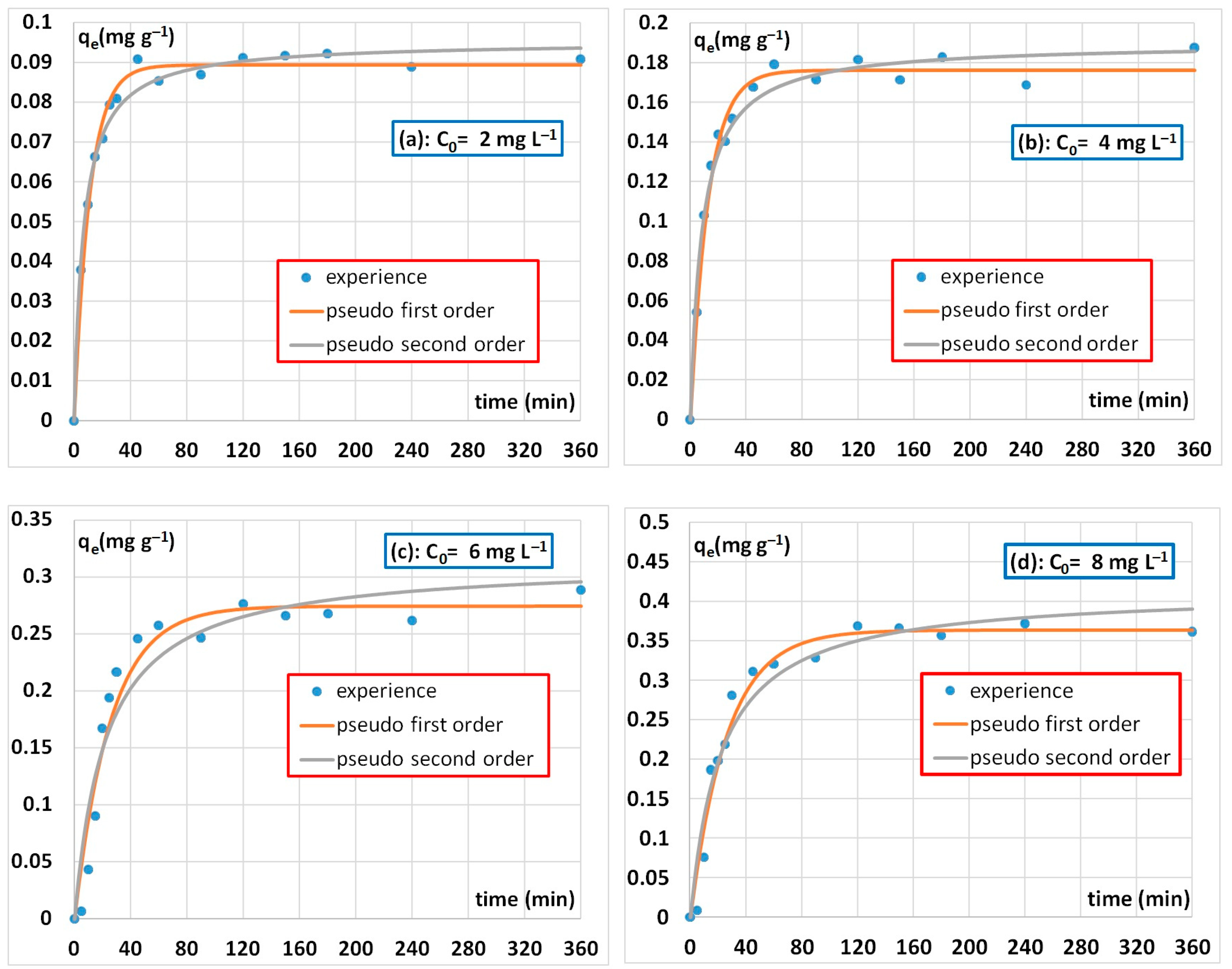
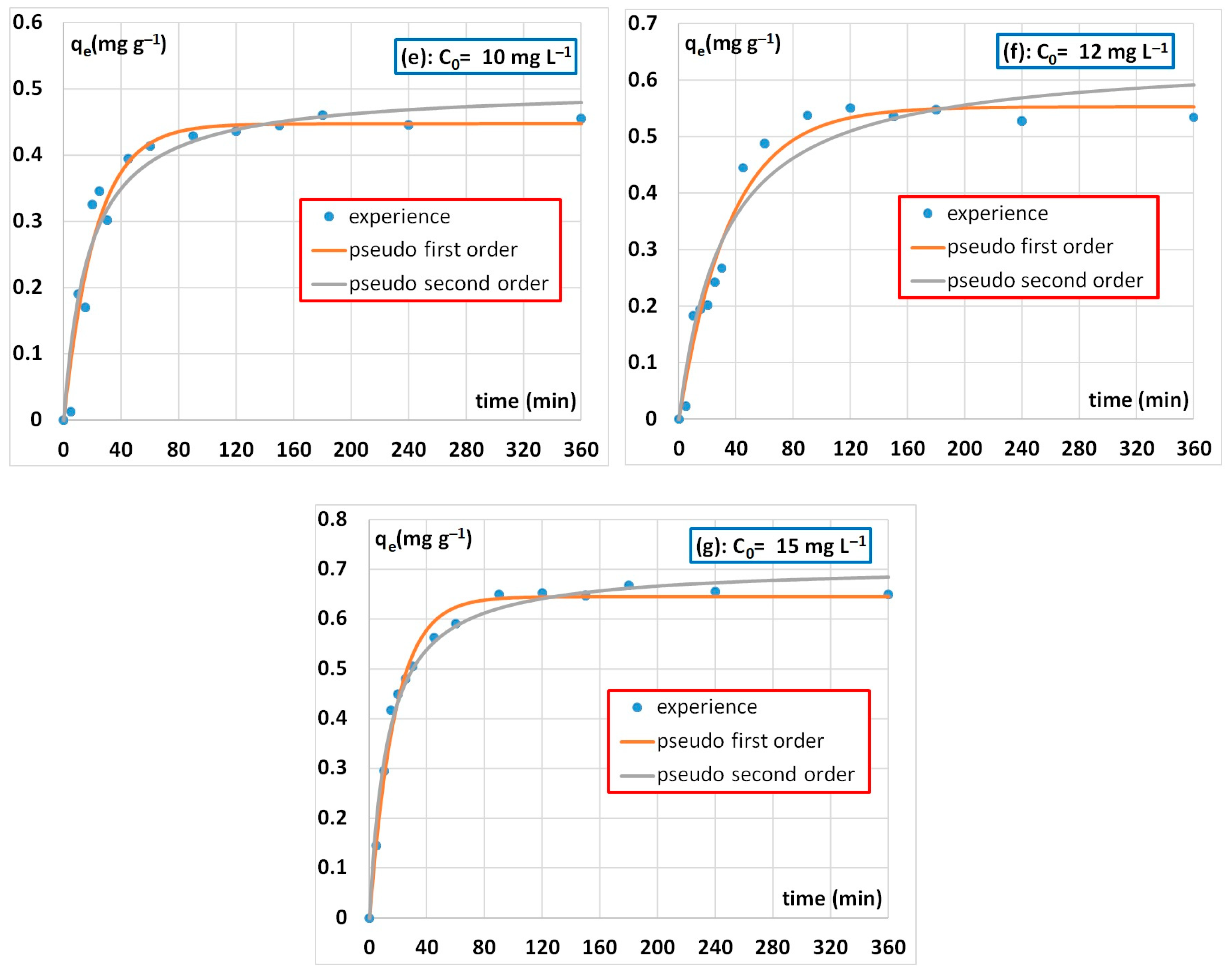
3.2.3. Adsorption Kinetics Results
3.2.4. Adsorption Isotherms and Modeling
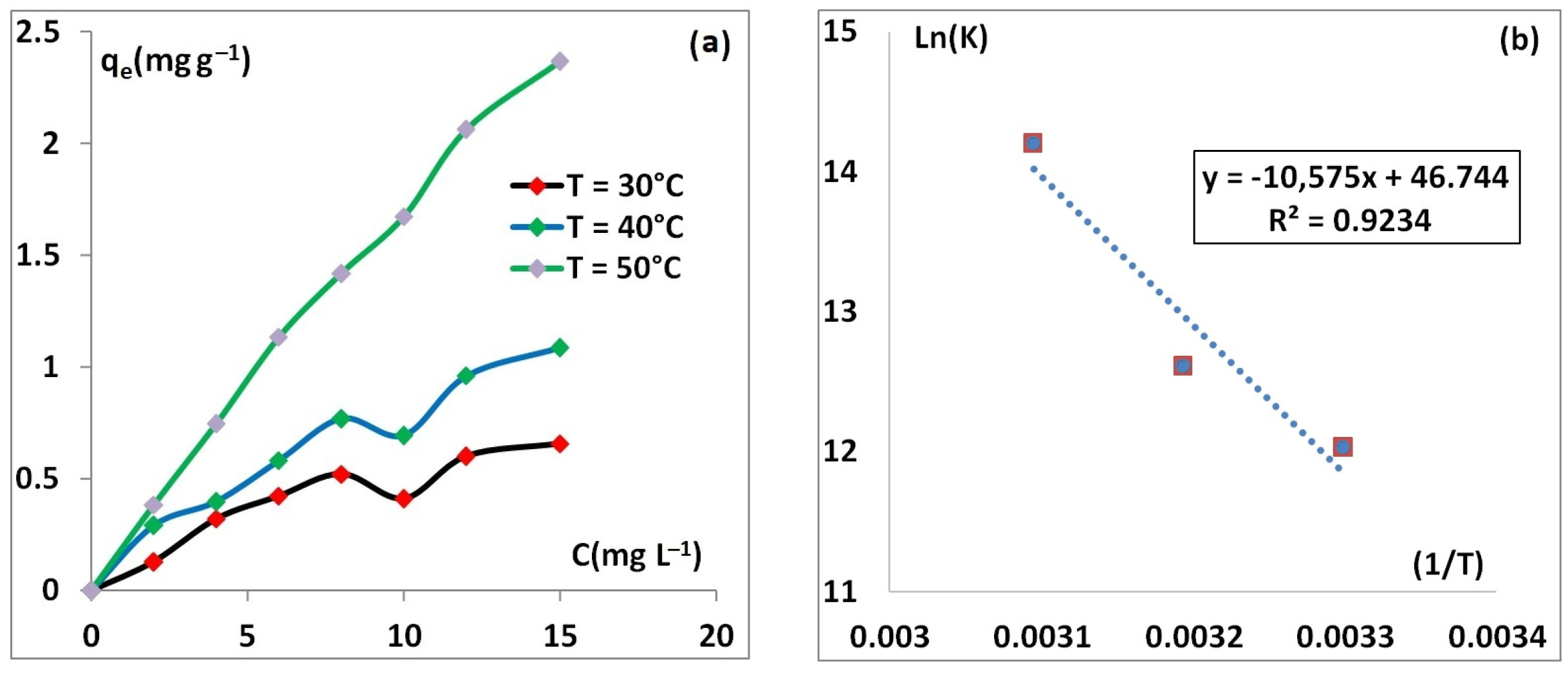
3.2.5. Thermodynamics Study
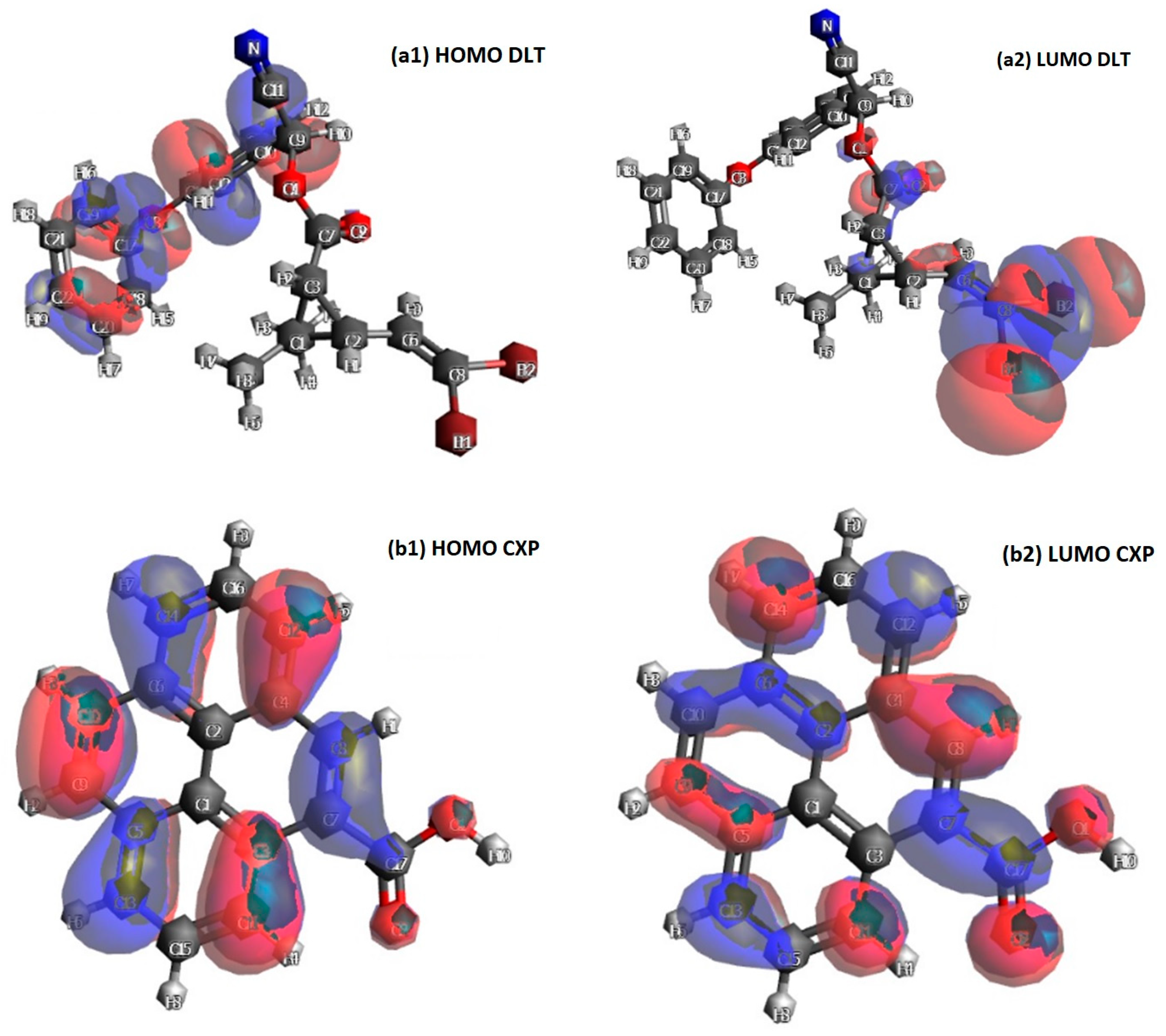
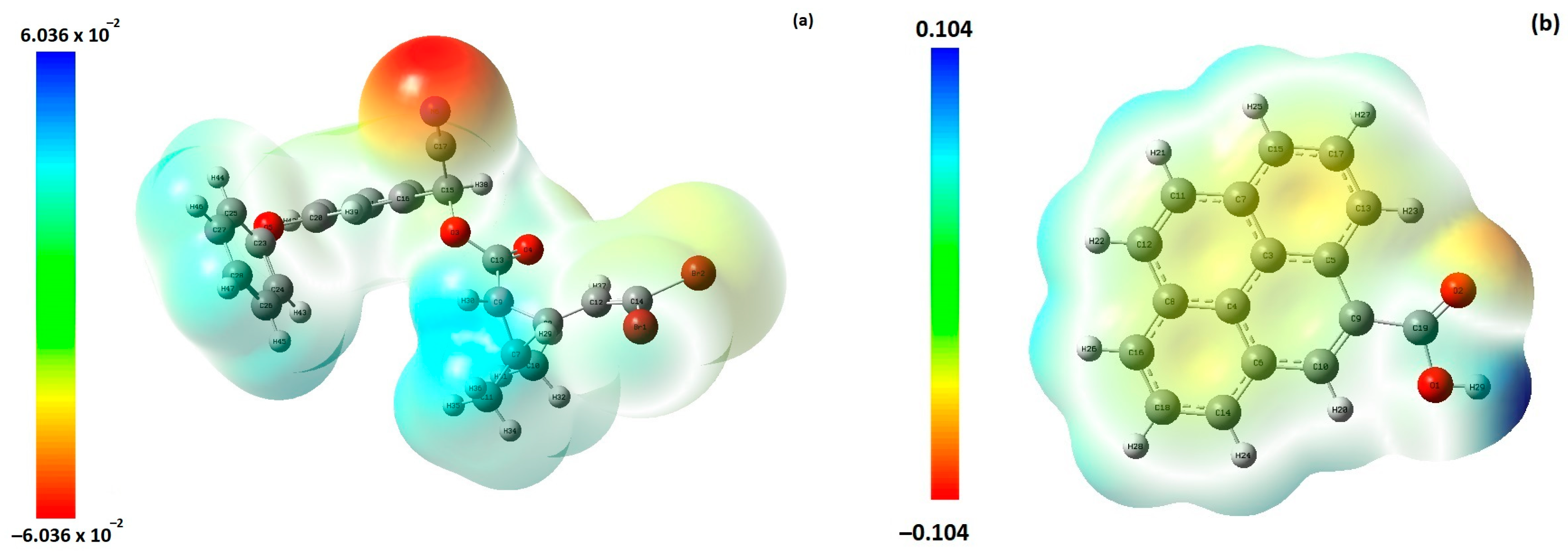
3.3. DFT and Retention Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Kumar, V.; Anil, A.G.; Kapoor, D.; Khasnabis, S.; Shekar, S.; Pavithra, N.; Samuel, J.; Subramanian, S.; Singh, J. Adsorption and detoxification of pharmaceutical compounds from wastewater using nanomaterials: A review on mechanism, kinetics, valorization and circular economy. J. Environ. Manag. 2021, 300, 113569. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-García, V.; Rosales, E.; Puga, A.; Pazos, M.; Sanromán, M. Synthesis and use of efficient adsorbents under the principles of circular economy: Waste valorisation and electroadvanced oxidation process regeneration. Sep. Purif. Technol. 2020, 242, 116796. [Google Scholar] [CrossRef]
- Kasprzyk, M.; Czerwionka, K.; Gajewska, M. Waste materials assessment for phosphorus adsorption toward sustainable application in circular economy. Resour. Conserv. Recycl. 2021, 168, 105335. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.; Swiatkowski, A.; Tarasiuk, B. Adsorption of selected herbicides from aqueous solutions on activated carbon. J. Therm. Anal. Calorim. 2010, 101, 785–794. [Google Scholar] [CrossRef]
- Bartram, J. Water and Health in Europe: A Joint Report from the European Environment Agency and the WHO Regional Office for Europe; WHO Regional Office Europe: Copenhagen, Denmark, 2002. [Google Scholar]
- Akçay, G.; Akçay, M.; Yurdakoç, K. Removal of 2, 4-dichlorophenoxyacetic acid from aqueous solutions by partially characterized organophilic sepiolite: Thermodynamic and kinetic calculations. J. Colloid Interface Sci. 2005, 281, 27–32. [Google Scholar] [CrossRef]
- Rebich, R.; Coupe, R.; Thurman, E. Herbicide concentrations in the Mississippi River Basin—The importance of chloroacetanilide herbicide degradates. Sci. Total Environ. 2004, 321, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Reigart, J.R.; Roberts, J.R. Pesticides in children. Pediatr. Clin. N. Am. 2001, 48, 1185–1198. [Google Scholar] [CrossRef]
- Salman, J.M.; Njoku, V.; Hameed, B.H. Adsorption of pesticides from aqueous solution onto banana stalk activated carbon. Chem. Eng. J. 2011, 174, 41–48. [Google Scholar] [CrossRef]
- Corsini, E.; Liesivuori, J.; Vergieva, T.; Van Loveren, H.; Colosio, C. Effects of pesticide exposure on the human immune system. Hum. Exp. Toxicol. 2008, 27, 671–680. [Google Scholar] [CrossRef]
- Dich, J.; Zahm, S.H.; Hanberg, A.; Adami, H.-O. Pesticides and cancer. Cancer Causes Control 1997, 8, 420–443. [Google Scholar] [CrossRef]
- Vallack, H.W.; Bakker, D.J.; Brandt, I.; Broström-Lundén, E.; Brouwer, A.; Bull, K.R.; Gough, C.; Guardans, R.; Holoubek, I.; Jansson, B. Controlling persistent organic pollutants–what next? Environ. Toxicol. Pharmacol. 1998, 6, 143–175. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.; Hameed, B. Detoxification of pesticide waste via activated carbon adsorption process. J. Hazard. Mater. 2010, 175, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Trostanetsky, A.; Quinn, E.; Rapaport, A.; Harush, A.; Gottlieb, D. Efficacy of deltamethrin emulsifiable concentrate against stored-product insects. J. Stored Prod. Res. 2023, 101, 102072. [Google Scholar] [CrossRef]
- Arthur, F.H.; Athanassiou, C.G.; Morrison, W.R., III. Mobility of stored product beetles after exposure to a combination insecticide containing deltamethrin, methoprene, and a piperonyl butoxide synergist depends on species, concentration, and exposure time. Insects 2020, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Ao, S.; Ji, P.; Zhou, Y.; Tang, H.; Zhou, L.; Zhang, X. Assessment of deltamethrin toxicity in Macrobrachium nipponense based on histopathology, oxidative stress and immunity damage. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 246, 109040. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, Y.; Ares, I.; Anadón, A.; Martínez, M.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Wang, X.; Martínez, M.-A. Deltamethrin toxicity: A review of oxidative stress and metabolism. Environ. Res. 2019, 170, 260–281. [Google Scholar] [CrossRef]
- Abdel-Daim, M.; El-Bialy, B.E.; Rahman, H.G.A.; Radi, A.M.; Hefny, H.A.; Hassan, A.M. Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: Biochemical and histopathological studies. Biomed. Pharmacother. 2016, 77, 79–85. [Google Scholar] [CrossRef]
- Yildirim, M.Z.; Benlı, A.Ç.K.; Selvı, M.; Özkul, A.; Erkoc, F.; Kocak, O. Acute toxicity, behavioral changes, and histopathological effects of deltamethrin on tissues (gills, liver, brain, spleen, kidney, muscle, skin) of Nile tilapia (Oreochromis niloticus L.) fingerlings. Environ. Toxicol. Int. J. 2006, 21, 614–620. [Google Scholar] [CrossRef]
- Martínez-Escudero, C.; Garrido, I.; Ros, C.; Flores, P.; Hellín, P.; Contreras, F.; Fenoll, J. Remediation of pesticides in commercial farm soils by solarization and ozonation techniques. J. Environ. Manag. 2023, 329, 117062. [Google Scholar] [CrossRef]
- de Almeida Lopes, T.S.; Hessler, R.; Bohner, C.; Junior, G.B.A.; de Sena, R.F. Pesticides removal from industrial wastewater by a membrane bioreactor and post-treatment with either activated carbon, reverse osmosis or ozonation. J. Environ. Chem. Eng. 2020, 8, 104538. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Semiconductor based photocatalytic degradation of pesticides: An overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Zhenlu, L.; Dengrong, S.; Changshui, W.; Bo, Y.; Bei, L.; Jingquan, H.; Shaohua, J.; Chunmei, Z.; Shuijian, H. Zeolitic imidazolate framework-67 and its derivatives for photocatalytic applications. Coord. Chem. Rev. 2024, 502, 215612. [Google Scholar]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Martinez, S.S. Advanced oxidation processes for the removal of organophosphorus pesticides in aqueous matrices: A systematic review and meta-analysis. Process Saf. Environ. Prot. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Lee, D.-Y.; Oh, K.-Y.; Jeong, D.-K.; Lee, D.-Y.; Kim, J.-H. Photochemical advanced oxidative process treatment effect on the pesticide residues reduction and quality changes in dried red peppers. Sci. Rep. 2023, 13, 4444. [Google Scholar] [CrossRef]
- Moradi, M.; Elahinia, A.; Vasseghian, Y.; Dragoi, E.-N.; Omidi, F.; Khaneghah, A.M. A review on pollutants removal by Sono-photo-Fenton processes. J. Environ. Chem. Eng. 2020, 8, 104330. [Google Scholar] [CrossRef]
- Brillas, E. Fenton, photo-Fenton, electro-Fenton, and their combined treatments for the removal of insecticides from waters and soils. A review. Sep. Purif. Technol. 2022, 284, 120290. [Google Scholar] [CrossRef]
- Shawaqfeh, A.T. Removal of pesticides from water using anaerobic-aerobic biological treatment. Chin. J. Chem. Eng. 2010, 18, 672–680. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Seczkowska, M.; Deryło-Marczewska, A.; Blachnio, M. Adsorption equilibrium and kinetics of selected phenoxyacid pesticides on activated carbon: Effect of temperature. Adsorption 2016, 22, 777–790. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.-F.; Wang, Z.; Song, L.; Yao, J. Flexible cellulose foams with a high loading of attapulgite nanorods for Cu2+ ions removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126038. [Google Scholar] [CrossRef]
- Lu, T.; Cao, W.; Liang, H.; Deng, Y.; Zhang, Y.; Zhu, M.; Ma, W.; Xiong, R.; Huang, C. Blow-Spun nanofibrous membrane for simultaneous treatment of emulsified oil/water mixtures, dyes, and bacteria. Langmuir 2022, 38, 15729–15739. [Google Scholar] [CrossRef]
- Wang, S.-L.; Tzou, Y.-M.; Lu, Y.-H.; Sheng, G. Removal of 3-chlorophenol from water using rice-straw-based carbon. J. Hazard. Mater. 2007, 147, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Baccar, R.; Blánquez, P.; Bouzid, J.; Feki, M.; Sarrà, M. Equilibrium, thermodynamic and kinetic studies on adsorption of commercial dye by activated carbon derived from olive-waste cakes. Chem. Eng. J. 2010, 165, 457–464. [Google Scholar] [CrossRef]
- Janković, B.; Manić, N.; Dodevski, V.; Radović, I.; Pijović, M.; Katnić, Đ.; Tasić, G. Physico-chemical characterization of carbonized apricot kernel shell as precursor for activated carbon preparation in clean technology utilization. J. Clean. Prod. 2019, 236, 117614. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Modarressi, A.; Rogalski, M. Adsorption of dyes on activated carbon prepared from apricot stones and commercial activated carbon. J. Taiwan Inst. Chem. Eng. 2015, 53, 112–121. [Google Scholar] [CrossRef]
- de Yuso, A.M.; Rubio, B.; Izquierdo, M.T. Influence of activation atmosphere used in the chemical activation of almond shell on the characteristics and adsorption performance of activated carbons. Fuel Process. Technol. 2014, 119, 74–80. [Google Scholar] [CrossRef]
- Dolas, H.; Sahin, O.; Saka, C.; Demir, H. A new method on producing high surface area activated carbon: The effect of salt on the surface area and the pore size distribution of activated carbon prepared from pistachio shell. Chem. Eng. J. 2011, 166, 191–197. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, K. Preparation of activated carbons from walnut shells via vacuum chemical activation and their application for methylene blue removal. Chem. Eng. J. 2010, 165, 209–217. [Google Scholar] [CrossRef]
- Alothaid, H. Evaluation of cytotoxicity, oxidative stress and organ-specific effects of activated carbon from Al-Baha date palm kernels. Saudi J. Biol. Sci. 2022, 29, 103387. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Optimization of rambutan peel based activated carbon preparation conditions for Remazol Brilliant Blue R removal. Chem. Eng. J. 2011, 168, 280–285. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Optimization of preparation conditions for mangosteen peel-based activated carbons for the removal of Remazol Brilliant Blue R using response surface methodology. Chem. Eng. J. 2010, 165, 883–890. [Google Scholar] [CrossRef]
- Sun, Y.; Webley, P.A. Preparation of activated carbons from corncob with large specific surface area by a variety of chemical activators and their application in gas storage. Chem. Eng. J. 2010, 162, 883–892. [Google Scholar] [CrossRef]
- Bourahla, S.; Nemchi, F.; Belayachi, H.; Belayachi, A.; Harrats, C.; Belhakem, M. Removal of the AO7 dye by adsorption on activated carbon based on grape marc: Equilibrium, regeneration, and FTIR spectroscopy. J. Iran. Chem. Soc. 2023, 20, 669–681. [Google Scholar] [CrossRef]
- Wongvitvichot, W.; Thitiprasert, S.; Thongchul, N.; Chaisuwan, T. Metal ion removal using a low-cost coconut shell activated carbon bioadsorbent in the recovery of lactic acid from the fermentation broth. Bioresour. Bioprocess. 2023, 10, 58. [Google Scholar] [CrossRef]
- Garg, D.; Kumar, S.; Sharma, K.; Majumder, C. Application of waste peanut shells to form activated carbon and its utilization for the removal of Acid Yellow 36 from wastewater. Groundw. Sustain. Dev. 2019, 8, 512–519. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Wang, Z.; Lv, M.; Xu, Q.; Chen, Z.; Wen, Q.; Li, A. La-doped activated carbon as high-efficiency phosphorus adsorbent: DFT exploration of the adsorption mechanism. Sep. Purif. Technol. 2022, 298, 121585. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Zhou, J.-L.; Guo, R.; Liu, Y.-j.; Yu, Y.-f.; Zhang, B.; Wu, Y.-W.; Zhao, L.; Lu, Q. Effect of flue gas components on the removal of cadmium pollutants by transition metal-modified activated carbon: Density functional theory and thermodynamic study. Fuel 2023, 343, 128011. [Google Scholar] [CrossRef]
- Rong, S.; Zhang, L.; Yue, Z.; Song, Z.; Tang, W.; Zhu, X.; Wang, T.; Zhou, X.; Cheng, S.; Xia, X. Directional introduction of pyridine nitrogen functional groups in activated carbon catalysts for the catalytic production of hydrogen: An experimental and DFT calculation. Chem. Eng. J. 2023, 453, 139744. [Google Scholar] [CrossRef]
- Naboulsi, A.; El Himri, M.; El Haddad, M. Adsorption mechanism of a pesticide/herbicide mixture on a new eco-friendly activated carbon of Ritama-Monosperma (L.) Boiss used DFT/B3LYP. Biomass Convers. Biorefinery 2023, 13, 13715–13728. [Google Scholar] [CrossRef]
- Kan, Y.; Zhang, R.; Xu, X.; Wei, B.; Shang, Y. Comparative study of raw and HNO3-modified porous carbon from waste printed circuit boards for sulfadiazine adsorption: Experiment and DFT study. Chin. Chem. Lett. 2023, 34, 108272. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, Z.; He, M.; Yang, X.; Lai, S.; An, K.; Han, S.; Qu, Z.; Zhou, W.; Wang, Z. Effect of oxygen functional groups on competitive adsorption of benzene and water on carbon materials: Density functional theory study. Sci. Total Environ. 2023, 863, 160772. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Zhang, C.; Bao, N.; Cheng, C. Activated carbons with well-developed microporosity and high surface acidity prepared from lotus stalks by organophosphorus compounds activations. Carbon 2013, 60, 289–291. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys. 2016, 6, 651–658. [Google Scholar] [CrossRef]
- Deng, W.-N.; Li, Y.-H.; Xu, D.-F.; Zhou, W.; Xiang, K.-X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, Y.; Lou, X.; Chen, H.; Jiang, S.; Zhou, W. Vanadium oxide/carbonized chestnut needle composites as cathode materials for advanced aqueous zinc-ion batteries. J. Energy Storage 2024, 77, 109859. [Google Scholar] [CrossRef]
- Raut, E.R.; Bedmohata, M.A.; Chaudhari, A.R. Study of synthesis and characterization of raw bagasse, its char and activated carbon prepared using chemical additive. Water Sci. Technol. 2023, 87, 2233–2249. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Liu, B.; Hu, J.; Li, H. Determination of iodine number of activated carbon by the method of ultraviolet–visible spectroscopy. Mater. Lett. 2021, 285, 129137. [Google Scholar] [CrossRef]
- Shrestha, R.M. Effect of preparation parameters on methylene blue number of activated carbons prepared from a locally available material. J. Inst. Eng. 2016, 12, 169–174. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Noh, J.S.; Schwarz, J.A. Effect of HNO3 treatment on the surface acidity of activated carbons. Carbon 1990, 28, 675–682. [Google Scholar] [CrossRef]
- Yağmur, H.K.; Kaya, İ. Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. J. Mol. Struct. 2021, 1232, 130071. [Google Scholar] [CrossRef]
- de Vargas Brião, G.; da Silva, M.G.C.; Vieira, M.G.A.; Chu, K.H. Correlation of type II adsorption isotherms of water contaminants using modified BET equations. Colloid Interface Sci. Commun. 2022, 46, 100557. [Google Scholar] [CrossRef]
- Dehmani, Y.; Lamhasni, T.; Mohsine, A.; Tahri, Y.; Lee, H.-s.; Lgaz, H.; Alrashdi, A.A.; Abouarnadasse, S. Adsorption removal of phenol by oak wood charcoal activated carbon. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Cortés, J.-C.; Navarro-Quiles, A.; Santonja, F.-J.; Sferle, S.-M. Statistical analysis of randomized pseudo-first/second order kinetic models. Application to study the adsorption on cadmium ions onto tree fern. Chemom. Intell. Lab. Syst. 2023, 240, 104910. [Google Scholar] [CrossRef]
- Karunarathne, H.; Amarasinghe, B. Fixed bed adsorption column studies for the removal of aqueous phenol from activated carbon prepared from sugarcane bagasse. Energy Procedia 2013, 34, 83–90. [Google Scholar] [CrossRef]
- Gholamiyan, S.; Hamzehloo, M.; Farrokhnia, A. RSM optimized adsorptive removal of erythromycin using magnetic activated carbon: Adsorption isotherm, kinetic modeling and thermodynamic studies. Sustain. Chem. Pharm. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Dong, W.; Zhang, L.; Kong, Q.; Wang, W. Efficient adsorption of sulfamethazine onto modified activated carbon: A plausible adsorption mechanism. Sci. Rep. 2017, 7, 12437. [Google Scholar] [CrossRef]
- Shaker, M.A. Dynamics and thermodynamics of toxic metals adsorption onto soil-extracted humic acid. Chemosphere 2014, 111, 587–595. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Socha, R.; Gurgul, J.; Wisniewski, M. XPS and NMR studies of phosphoric acid activated carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Martínez-Alonso, A.; Castro-Muñiz, A.; Suárez-García, F.; Tascón, J.M. Oxygen and phosphorus enriched carbons from lignocellulosic material. Carbon 2007, 45, 1941–1950. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M. Low-cost activated carbon preparation from Corn stigmata fibers chemically activated using H3PO4, ZnCl2 and KOH: Study of methylene blue adsorption, stochastic isotherm and fractal kinetic. Ind. Crops Prod. 2022, 178, 114546. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Silva, C.M. What’s wrong with Lagergreen pseudo first order model for adsorption kinetics? Chem. Eng. J. 2016, 306, 1138–1142. [Google Scholar] [CrossRef]
- Bujdák, J. Adsorption kinetics models in clay systems. The critical analysis of pseudo-second order mechanism. Appl. Clay Sci. 2020, 191, 105630. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, F.-C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant. J. Hazard. Mater. 2008, 155, 277–287. [Google Scholar] [CrossRef]
- Sellaoui, L.; Yazidi, A.; Taamalli, S.; Bonilla-Petriciolet, A.; Louis, F.; El Bakali, A.; Badawi, M.; Lima, E.C.; Lima, D.R.; Chen, Z. Adsorption of 3-aminophenol and resorcinol on avocado seed activated carbon: Mathematical modelling, thermodynamic study and description of adsorbent performance. J. Mol. Liq. 2021, 342, 116952. [Google Scholar] [CrossRef]
- Supong, A.; Bhomick, P.C.; Baruah, M.; Pongener, C.; Sinha, U.B.; Sinha, D. Adsorptive removal of Bisphenol A by biomass activated carbon and insights into the adsorption mechanism through density functional theory calculations. Sustain. Chem. Pharm. 2019, 13, 100159. [Google Scholar] [CrossRef]








| Adsorption Models | Used Models | Modeling Equation |
|---|---|---|
| Kinetics | Pseudo-first-order | |
| Pseudo-second-order | ||
| Isotherms | Langmuir | |
| Freundlich |
| Adsorbent | Acidity Functions (meq g−1) | Basicity | |||
|---|---|---|---|---|---|
| Activated carbon | Carboxylic | Lactonic | Phenolic | Total | Total |
| 0.688 | 0.128 | 0.08 | 0.896 | 0.160 | |
| Compound | C | O | Mg | Al | Si | P | K | Ca | Na | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight of compound (%) | 80.77 | 15.77 | 0.04 | 0.05 | 0.15 | 3.54 | 0.03 | 0.23 | 0.02 | 0.06 |
| C (mgL−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | R2 | qe (mg g−1) | K2 (g mg−1 min−1) | R2 | |
| 2 | 0.089 | 0.091 | 0.99 | 0.095 | 1.58 | 0.99 |
| 4 | 0.17 | 0.078 | 0.97 | 0.19 | 0.63 | 0.97 |
| 6 | 0.27 | 0.039 | 0.95 | 0.31 | 0.14 | 0.91 |
| 8 | 0.36 | 0.038 | 0.97 | 0.40 | 0.11 | 0.95 |
| 10 | 0.44 | 0.045 | 0.95 | 0.5 | 0.11 | 0.93 |
| 12 | 0.55 | 0.028 | 0.97 | 0.64 | 0.05 | 0.94 |
| 15 | 0.64 | 0.057 | 0.99 | 0.70 | 0.11 | 0.99 |
| Models | Parameters of Isotherm | R2 | |
|---|---|---|---|
| Langmuir | qmax (mg g−1) | 1.13 | 0.942 |
| KL (L mg−1) | 0.676 | ||
| Freundlich | 1/n | 0.639 | 0.926 |
| KF (mg1−1/n L1/n g−1) | 0.437 | ||
| n | 1.56 | ||
| Temperature (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1 K−1) |
|---|---|---|---|
| 303 | −29.89 | 87.92 | 0.389 |
| 313 | −33.7 | ||
| 323 | −37.66 |
| Compound | HOMO (eV) | LUMO (eV) | GAP (eV) |
|---|---|---|---|
| Deltamethrin | −6.57 | −1.44 | 5.13 |
| Carboxypyrene | −5.79 | −2.30 | 3.50 |
| Deltamethrin–Carboxypyrene | −7.48 | 1.16 | 8.65 |
| Compound | µ (eV) | µ (eV) | µ (eV) |
|---|---|---|---|
| Deltamethrin | −4.01 | 2.57 | 3.13 |
| Carboxypyrene | −4.04 | 1.75 | 4.68 |
| Deltamethrin–Carboxypyrene | −3.16 | 4.32 | 1.16 |
| Compound | H (A.U.) | G (A.U.) |
|---|---|---|
| Deltamethrin | −6277.069 | −6277.154 |
| Carboxypyrene | −803.693 | −803.746 |
| Deltamethrin–Carboxypyrene | −7081.2048 | −7081.3127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zgolli, A.; Souissi, M.; Dhaouadi, H. Purification of Pesticide-Contaminated Water Using Activated Carbon from Prickly Pear Seeds for Environmentally Friendly Reuse in a Circular Economy. Sustainability 2024, 16, 406. https://doi.org/10.3390/su16010406
Zgolli A, Souissi M, Dhaouadi H. Purification of Pesticide-Contaminated Water Using Activated Carbon from Prickly Pear Seeds for Environmentally Friendly Reuse in a Circular Economy. Sustainability. 2024; 16(1):406. https://doi.org/10.3390/su16010406
Chicago/Turabian StyleZgolli, Amira, Marwa Souissi, and Hatem Dhaouadi. 2024. "Purification of Pesticide-Contaminated Water Using Activated Carbon from Prickly Pear Seeds for Environmentally Friendly Reuse in a Circular Economy" Sustainability 16, no. 1: 406. https://doi.org/10.3390/su16010406






