Assessing the Use of Supercritical Carbon Dioxide as a Carrier for Alkoxysilanes to Consolidate Degraded PUR Ester Foams: An Alternative to Traditional Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ester-Based PUR Foams and Artificial Ageing
2.2. Consolidation Materials
2.3. Consolidation Methodology
2.3.1. Spray Consolidation (Traditional Method)
2.3.2. Supercritical CO2-Assisted Consolidation
2.4. Characterisation
3. Results and Discussion
3.1. Ester-Based PUR Foam, and APDEMS and OTMS Mixture References: ATR-FTIR Characterisation
3.2. Supercritical CO2-Assisted Consolidation of Ester-Based PUR Foams with APDEMS and OTMS
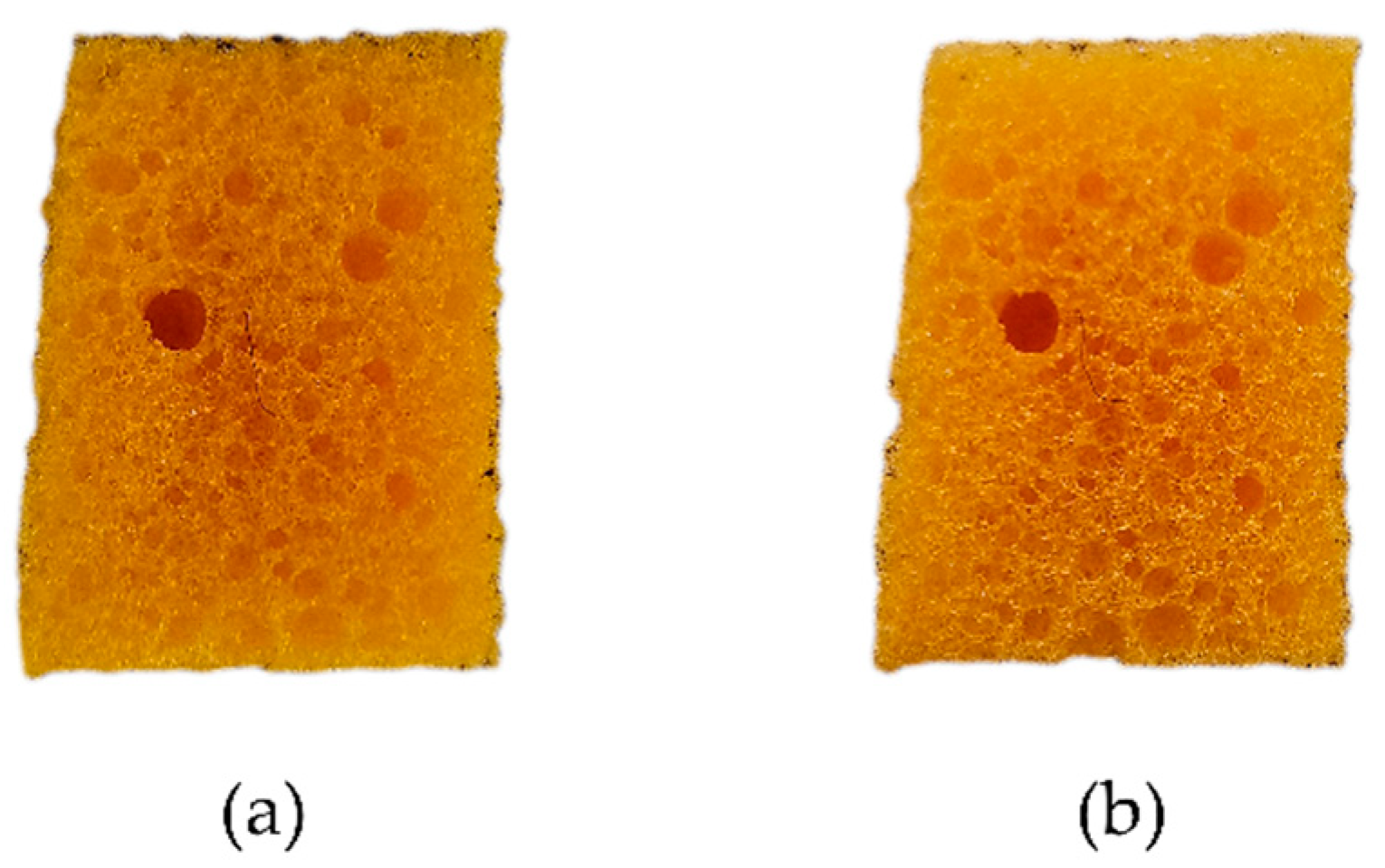
3.2.1. Visual, Tactile Properties, and Weight Changes
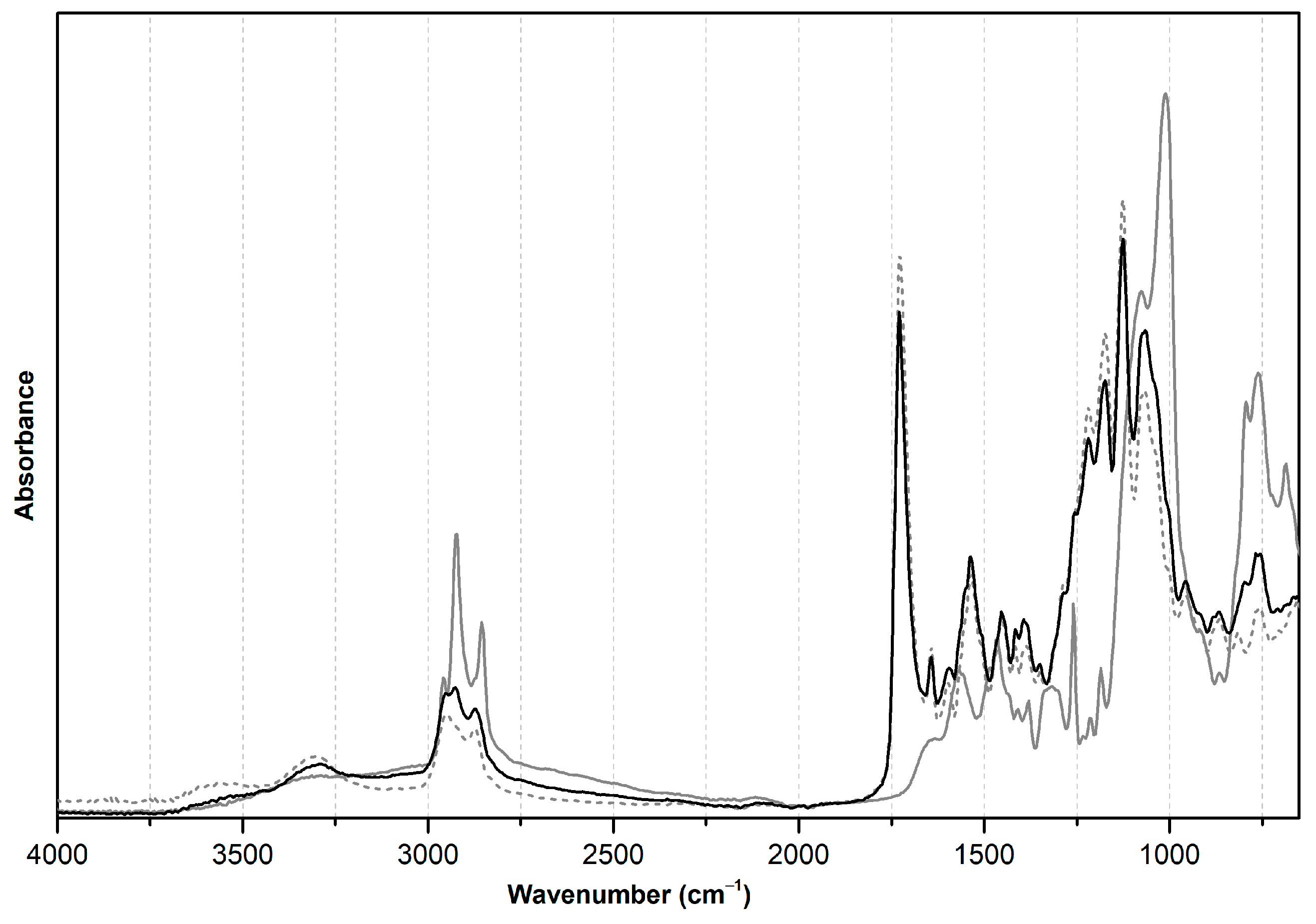
3.2.2. ATR-FTIR Analysis
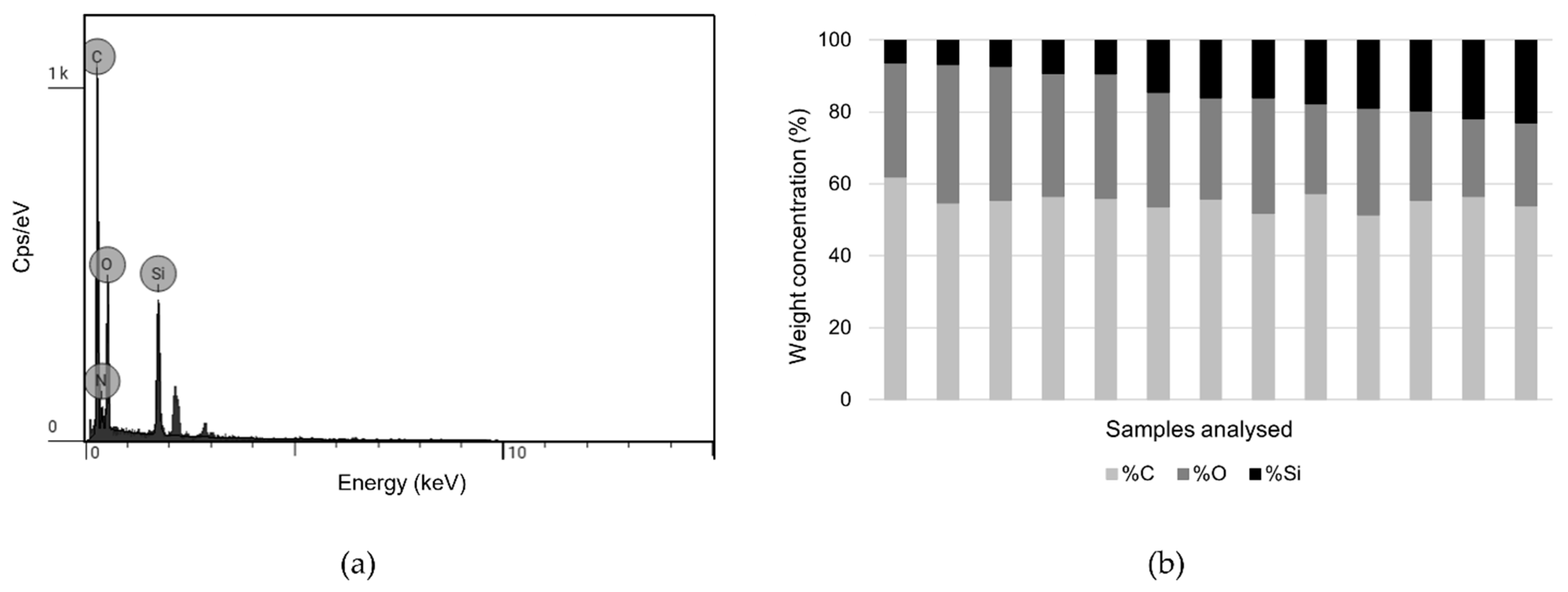
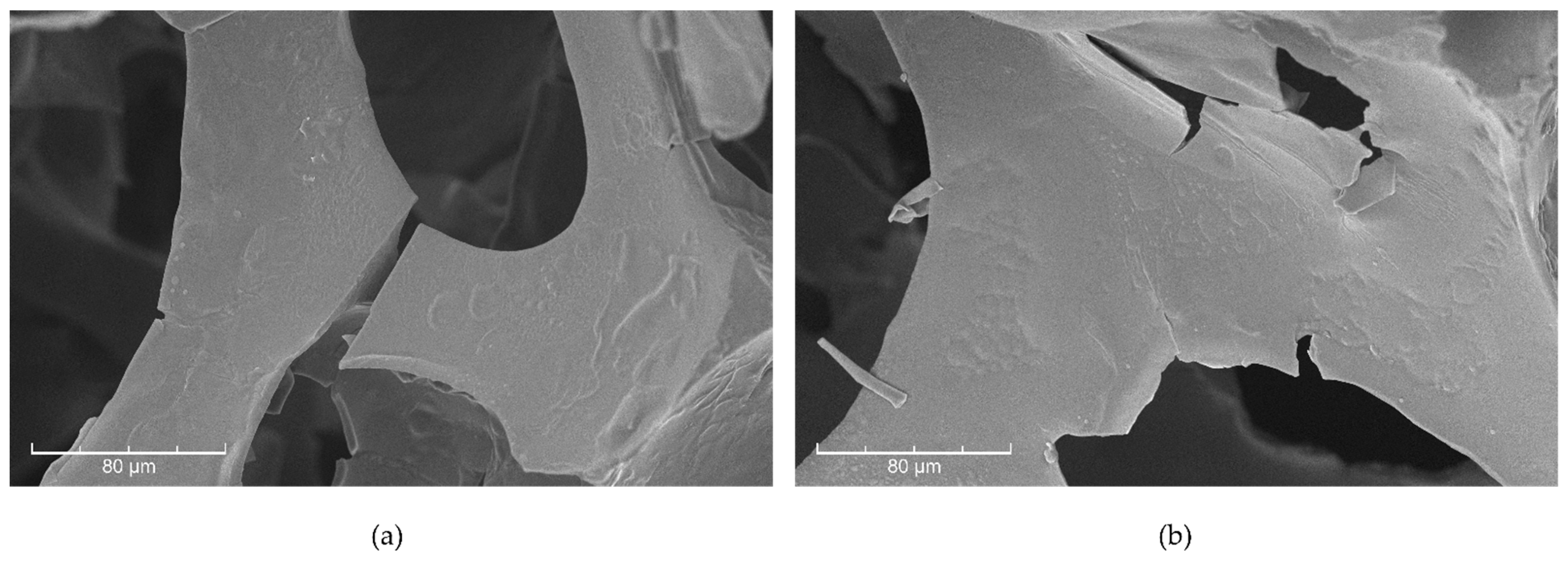
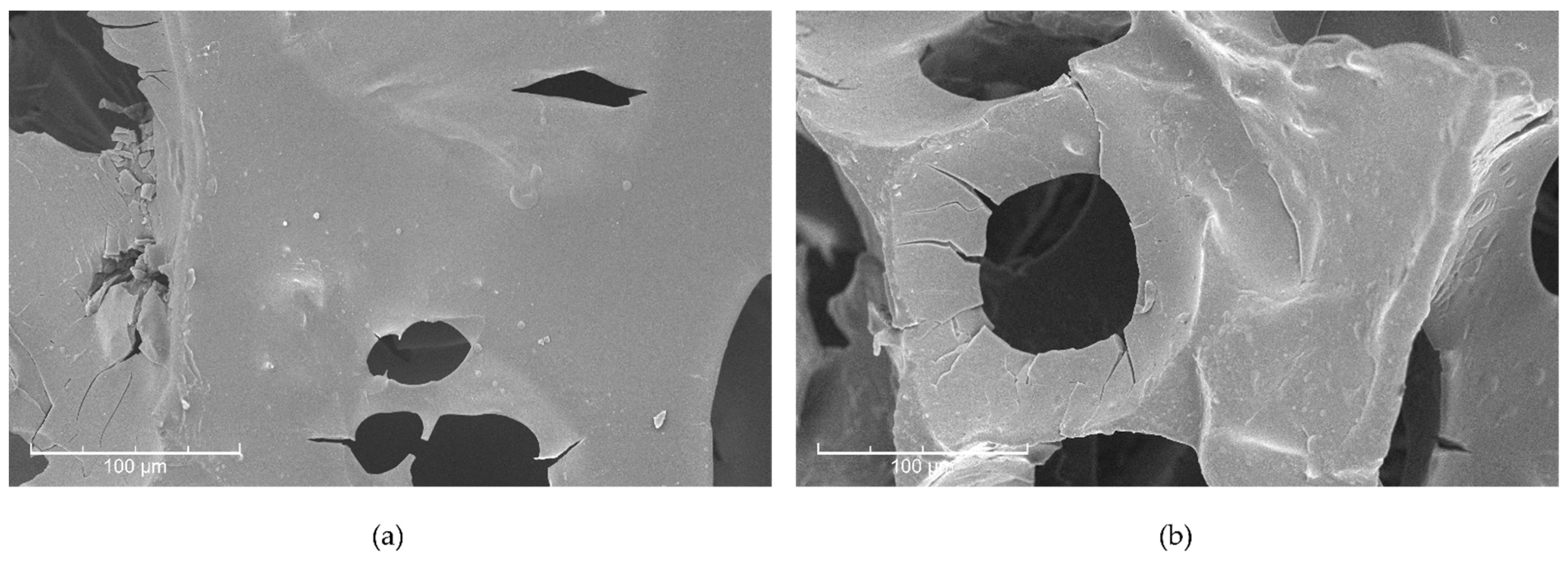
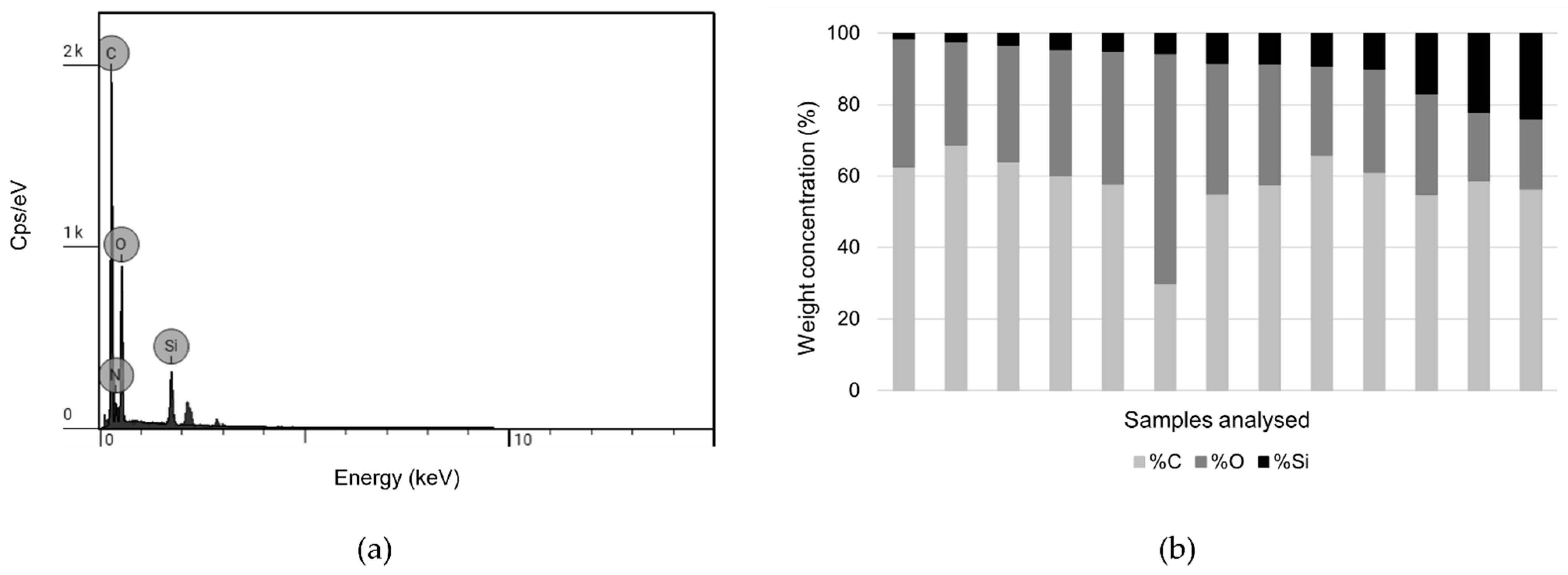
3.2.3. SEM Imaging and SEM-EDS Analysis
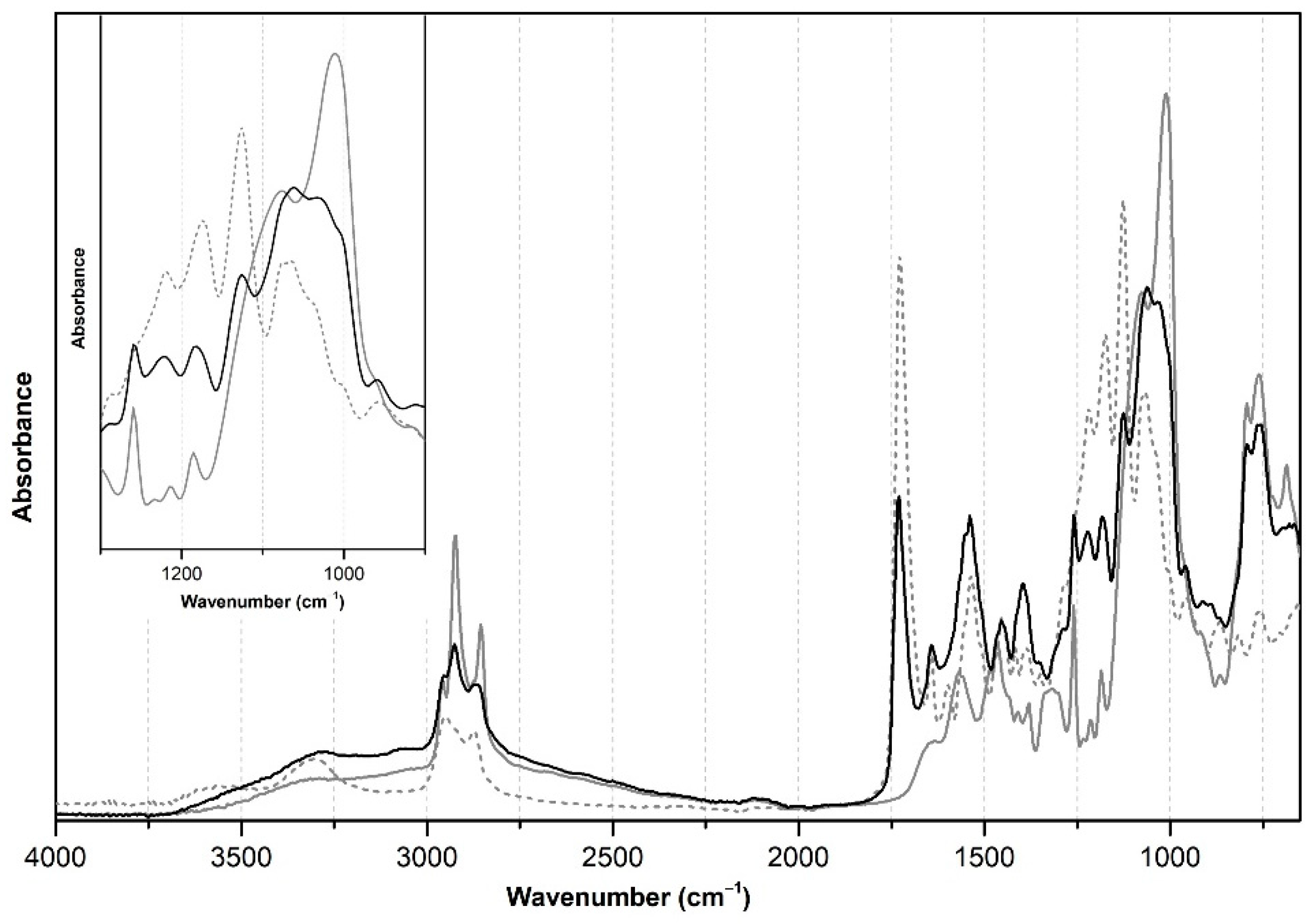
3.3. Supercritical CO2-Assisted Consolidation vs. Spray Consolidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Oosten, T. PUR Facts: Conservation of Polyurethane Foam in Art and Design; RCE Publications; Amsterdam University Press: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Pellizzi, E. Étude Du Vieillissement Des Mousses de Polyuréthane Ester et Consolidation Par Les Aminoalkylalkoxysilanes. Ph.D. Thesis, Université D’Évry val D’Essonne, Évry-Courcouronnes, Paris, France, 2012. [Google Scholar]
- Lattuati-Derieux, A.; Thao-Heu, S.; Lavédrine, B. Assessment of the Degradation of Polyurethane Foams after Artificial and Natural Ageing by Using Pyrolysis-Gas Chromatography/Mass Spectrometry and Headspace-Solid Phase Microextraction-Gas Chromatography/Mass Spectrometry. J. Chromatogr. A 2011, 1218, 4498–4508. [Google Scholar] [CrossRef] [PubMed]
- Pellizzi, E.; Lattuati-Derieux, A.; Lavédrine, B.; Cheradame, H. Degradation of Polyurethane Ester Foam Artifacts: Chemical Properties, Mechanical Properties and Comparison between Accelerated and Natural Degradation. Polym. Degrad. Stab. 2014, 107, 255–261. [Google Scholar] [CrossRef]
- Horie, C.V. Materials for Conservation: Organic Consolidants, Adhesives and Coatings, 2nd ed.; Butterworth-Heinemann: Amterdam, The Netherlands, 2010. [Google Scholar]
- Micheluz, A.; Angelin, E.M.; Sawitzki, J.; Pamplona, M. Plastics in Robots: A Degradation Study of a Humanoid Skin Mask Made of Soft Urethane Elastomer. Herit. Sci. 2022, 10, 4. [Google Scholar] [CrossRef]
- França de Sá, S. What Does the Future Hold for Polyurethane Fashion and Design? Conservation Studies Regarding the 1960s and 1970s Objects from the MUDE Collection. Ph.D. Thesis, Universidade NOVA de Lisboa, Lisboa, Portugal, 2017. [Google Scholar]
- Chaumat, G.; Tran, K.; Dekkers, J.M.; Pellizzi, E.; Lattuati-Derieux, A. On-Going Studies in Consolidation of Polyurethane (PUR) Foams. In POPART: Preservation of Plastic Artefacts in Museum Collections; Lavédrine, B., Fournier, A., Martin, G., Eds.; Comité Des Travaux Historiques Et Scientifiques: Paris, France, 2012; pp. 271–293. [Google Scholar]
- Waentig, F. Plastics in Art : A Study from the Conservation Point of View; Imhof: Petersberg, Germany, 2008. [Google Scholar]
- Royan, L.; Daher, C.; Balcar, N.; Barabant, G.; Lattuati-Derieux, A. Conservation Des Mousses Polyuréthane Ester: Consolidation Par Application de Mélanges de Polysiloxanes. Techne 2018, 46, 119–125. [Google Scholar] [CrossRef]
- Damjanović, R.B.; Jović, M.L.; Jančić-Heinemann, R.M.; Živković, I.D. Conservation and Restoration of Works of Art and Museum Artifacts Made from Polymer Materials—Field of Close Connection of Science and Art: Overview of Current Practice. In Art and Science Applied: Experience and Vision; Prosen, M., Dimković, D., Eds.; University of Belgrade: Beograd, Serbia, 2022; pp. 170–194. ISBN 978-86-80245-45-4. [Google Scholar]
- Van Oosten, T.; Learner, T. Identification and Characterisation of Plastic Artefacts. In Preservation of Plastic Artefacts in Museum Collections.; Lavédrine, B., Fournier, A., Martin, G., Eds.; CTHS: Sydney, Australia, 2012; pp. 29–36. [Google Scholar]
- Pellizzi, E.; Lattuati-Derieux, A.; d’Espinose de Lacaillerie, J.-B.; Lavédrine, B.; Cheradame, H. Reinforcement Properties of 3-Aminopropylmethyldiethoxysilane and N-(2-Aminoethyl)-3-Aminopropylmethyldimethoxysilane on Polyurethane Ester Foam. Polym. Degrad. Stab. 2012, 97, 2340–2346. [Google Scholar] [CrossRef]
- Daher, C.; Fabre-Francke, I.; Balcar, N.; Barabant, G.; Cantin, S.; Fichet, O.; Chéradame, H.; Lavédrine, B.; Lattuati Derieux, A. Consolidation of Degraded Polyurethane Foams by Means of Polysiloxane Mixtures: Polycondensation Study and Application Treatment. Polym. Degrad. Stab. 2018, 158, 92–101. [Google Scholar] [CrossRef]
- Pellizzi, E. Flexible Polyurethane Ester Foam Consolidation, Preliminary Study of Aminopropylmethyldiethoxysilane Reinforcement Treatment. In Proceedings of the CCI Symposium 2011—Adhesives and Consolidants for Conservation: Research and Applications, Ottawa, Canada, 17–21 October 2011; Volume 18. [Google Scholar]
- Pellizzi, E.; Lattuati-Derieux, A.; d’Espinose de Lacaillerie, J.-B.; Lavédrine, B.; Cheradame, H. Consolidation of Artificially Degraded Polyurethane Ester Foam with Aminoalkylalkoxysilanes. Polym. Degrad. Stab. 2016, 129, 106–113. [Google Scholar] [CrossRef]
- ECHA. Decision on Substance Evaluation Pursuant to Article 46(1) of Regulation (EC) NO 1907/2006 for Hexamethyldisiloxane, CAS No 107-46-0 (EC No 203-492-7); ECHA: Helsinky, Finland, 2015. [Google Scholar]
- UN DESA. The Sustainable Development Goals Report 2023: Special Edition—July 2023; UN DESA: New York, NY, USA, 2023. [Google Scholar]
- Kirby, C.F.; McHugh, M.A. No Titlehase Behavior of Polymers in Supercritical Fluid Solvents. Chem. Rev. 1999, 99, 565–602. [Google Scholar] [CrossRef] [PubMed]
- Kampasakali, E.; Fardi, T.; Pavlidou, E.; Christofilos, D. Towards Sustainable Museum Conservation Practices: A Study on the Surface Cleaning of Contemporary Art and Design Objects with the Use of Biodegradable Agents. Heritage 2021, 4, 2023–2043. [Google Scholar] [CrossRef]
- Fahmy, S.M. Solubility of Fluorinated Polymers in Supercritical Carbon Dioxide. Ph.D. Thesis, Fakultät für Mathematik, Informatik und Naturwissenschaften der Rheinisch-Westfälischen Technischen Hochschule Aachen, Aachen, Germany, 2005. [Google Scholar]
- Kemmere, M.F.; Meyer, T. (Eds.) Supercritical Carbon Dioxide for Sustainable Polymer Processes. In Supercritical Carbon Dioxide in Polymer Reaction Engineering; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2005; pp. 1–19. [Google Scholar]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical Carbon Dioxide as a Green Solvent for Processing Polymer Melts: Processing Aspects and Applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Tomás Ferreira, J. Supercritical CO2 Based Green Technologies for the Consolidation of Foams in Cultural Heritage: The Case Study of Robert Enke’s Pair of Gloves. Master’s Thesis, NOVA School of Science and Technology, Lisbon, Portugal, 2020. [Google Scholar]
- Aguiar-Ricardo, A.; Casimiro, T.; Sousa, M.; Melo, M.J.; Tomaz, P.M. Supercritical Carbon Dioxide: The Art of Technology in Art Conservation. Cons. Património 2007, 6, 3–9. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, H.; Xu, L.; Li, Y.; Dong, M. Effects of Cosolvent on Dissolution Behaviors of PVAc in Supercritical CO2: A Molecular Dynamics Study. Chem. Eng. Sci. 2019, 206, 22–30. [Google Scholar] [CrossRef]
- Teixeira, F.S.; dos Reis, T.A.; Sgubin, L.; Thomé, L.E.; Bei, I.W.; Clemencio, R.E.; Corrêa, B.; Salvadori, M.C. Disinfection of Ancient Paper Contaminated with Fungi Using Supercritical Carbon Dioxide. J. Cult. Herit. 2018, 30, 110–116. [Google Scholar] [CrossRef]
- Frade, C.S.C.; Cruz, P.; Lopes, E.; Sousa, M.M.; Hallett, J.; Santos, R.; Aguiar-Ricardo, A.; Casimiro, T. Cleaning Classical Persian Carpets with Silk and Precious Metal Thread: Conservation and Ethical Considerations. In Proceedings of the ICOM Committee for Conservation 16th Triennial Meeting, Lisbon, Portugal, 19–23 September 2011; Critério Artes Gráficas, Lda. ICOM Committee for Conservation: Almada, Portugal, 2011. [Google Scholar]
- Yanjuan, W.; Yanxiong, F.; Wei, T.; Chunying, L. Preservation of Aged Paper Using Borax in Alcohols and the Supercritical Carbon Dioxide System. J. Cult. Herit. 2013, 14, 16–22. [Google Scholar] [CrossRef]
- Tan, W.; Cheng, L.F.; Fang, Y.X. Deacidification of Paper Using Supercritical Carbon Dioxide Containing Calcium Propionate or Magnesium Bicarbonate. AMR 2013, 781, 2637–2640. [Google Scholar] [CrossRef]
- Kang, S.M.; Unger, A.; Morrell, J.J. The Effect of Supercritical Carbon Dioxide Extraction on Color Retention and Pesticide Reduction of Wooden Artifacts. J. Am. Inst. Conserv. 2004, 43, 151–160. [Google Scholar] [CrossRef]
- Hammond, G. Using Supercritical Carbon Dioxide as a Tool for Preserving Culturally Significant Items. Ph.D. Thesis, The University of Birmingham, Birmingham, UK, 2017. [Google Scholar]
- Sousa, M.; Melo, M.J.; Casimiro, T.; Aguiar-Ricardo, A. The Art of CO2 for Art Conservation: A Green Approach to Antique Textile Cleaning. Green Chem. 2007, 9, 943–947. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuneable Textile Cleaning and Disinfection Process Based on Supercritical CO2 and Pickering Emulsions. J. Supercrit. Fluids 2016, 118, 128–139. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. A Novel Approach for Textile Cleaning Based on Supercritical CO2 and Pickering Emulsions. J. Supercrit. Fluids 2013, 76, 83–93. [Google Scholar] [CrossRef]
- Memet, J. Conservation of Underwater Cultural Heritage: Characteristics and New Technologies. Mus. Int. 2008, 60, 42–49. [Google Scholar] [CrossRef]
- Gregory, D.; Jensen, P.; Strætkvern, K. Conservation and in Situ Preservation of Wooden Shipwrecks from Marine Environments. J. Cult. Herit. 2012, 13, S139–S148. [Google Scholar] [CrossRef]
- Cretté, S.A.; Näsänen, L.M.; González-Pereyra, N.G.; Rennison, B. Conservation of Waterlogged Archaeological Corks Using Supercritical CO2 and Treatment Monitoring Using Structured-Light 3D Scanning. J. Supercrit. Fluids 2013, 79, 299–313. [Google Scholar] [CrossRef]
- D’Andrea, A.; Mariani, S.; Aliboni, A.; Tagliacozzo, A.; Cerilli, E. Supercritical Drying Process in Conservation of Waterlogged Osteological Remains. In Proceedings of the International Society for the Advancement of Supercritical Fluids (I.S.A.S.F.) 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003. [Google Scholar]
- Devièse, T.; Ribechini, E.; Querci, D.; Higham, T. Assessing the Efficiency of Supercritical Fluid Extraction for the Decontamination of Archaeological Bones Prior to Radiocarbon Dating. Analyst 2019, 144, 6128–6135. [Google Scholar] [CrossRef]
- Devièse, T.; Ham-Meert, A.V.; Hare, V.J.; Lundy, J.; Hommel, P.; Bazaliiskii, V.I.; Orton, J. Supercritical Fluids for Higher Extraction Yields of Lipids from Archeological Ceramics. Anal. Chem. 2018, 90, 2420–2424. [Google Scholar] [CrossRef]
- Bartoletti, A.; Soares, I.; Ramos, A.M.; Shashoua, Y.; Quye, A.; Casimiro, T.; Ferreira, J.L. Assessing the Impact and Suitability of Dense Carbon Dioxide as a Green Solvent for the Treatment of PMMA of Historical Value. Polymers 2023, 15, 566. [Google Scholar] [CrossRef] [PubMed]
- Tomás Ferreira, J.; Bartoletti, A.; França de Sá, S.; Quye, A.; Shashoua, Y.; Casimiro, T.; Ferreira, J.L. Proof-of-Concept Study on the Feasibility of Supercritical Carbon Dioxide-Assisted Consolidation Treatment for a Pair of Goalkeeper Gloves on Synthetic Latex-Based Foam Mock-Ups. Sustainability 2024, 16, 1562. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Xu, W.Z.; Li, X. A Novel Approach to the Synthesis of SiO2-PVAc Nanocomposites Using a One-Pot Synthesis in Supercritical CO2. Green Chem. 2007, 9, 768–776. [Google Scholar] [CrossRef]
- Mitchell, G.; France, F.; Nordon, A.; Tang, P.L.; Gibson, L.T. Assessment of Historical Polymers Using Attenuated Total Reflectance-Fourier Transform Infra-Red Spectroscopy with Principal Component Analysis. Herit. Sci. 2013, 1, 28. [Google Scholar] [CrossRef]
- Wilhelm, C.; Gardette, J. Infrared Analysis of the Photochemical Behaviour of Segmented Polyurethanes: 1. Aliphatic Poly(Ester-Urethane). Polymer 1997, 38, 4019–4031. [Google Scholar] [CrossRef]
- Srichatrapimuk, V.; Cooper, S. Infrared Thermal Analysis of Polyurethane Block Polymers. J. Macromol. Sci. Part B 1978, 15, 267–311. [Google Scholar] [CrossRef]
- Hummel, D.O. Atlas of Plastics Additives, Analysis by Spectrometric Methods; Springer: Berlin, Germany, 2002. [Google Scholar]
- Huber, M.P.; Kelch, S.; Berke, H. FTIR Investigations on Hydrolysis and Condensation Reactions of Alkoxysilane Terminated Polymers for Use in Adhesives and Sealants. Int. J. Adhes. Adhes. 2016, 64, 153–162. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
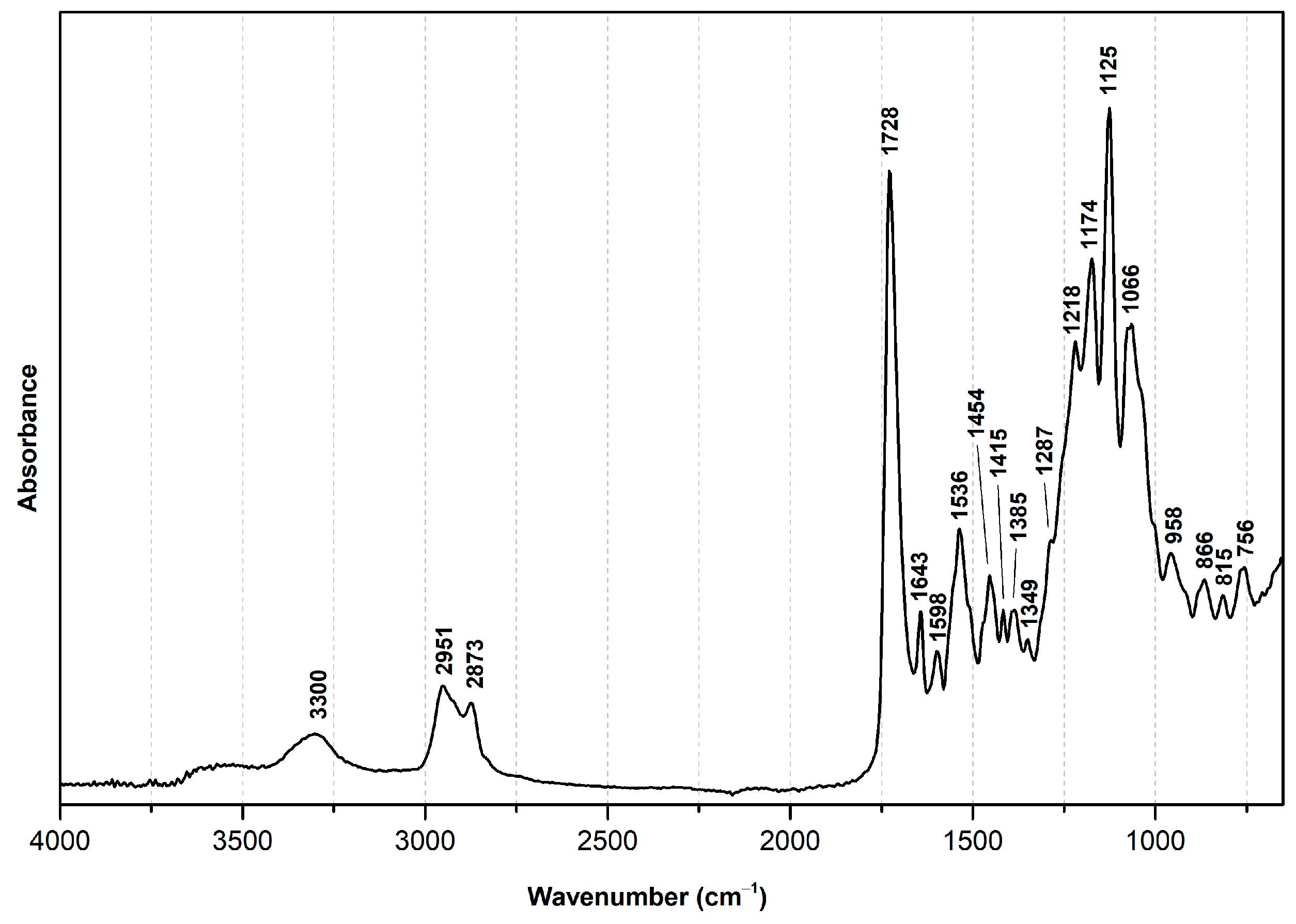
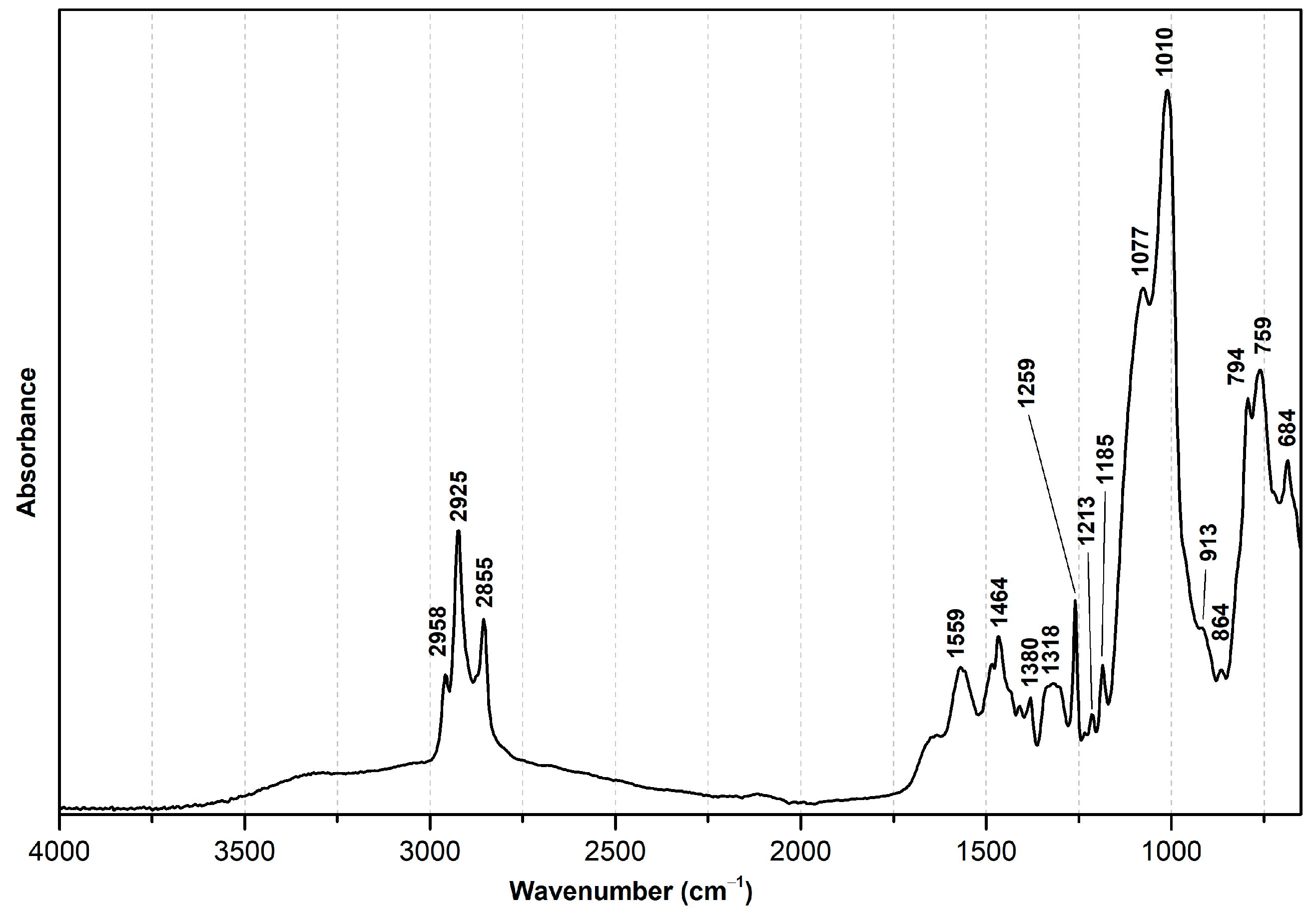

| Wavenumber (cm−1) | Assignment [4,7,45,46,47] 1 |
|---|---|
| 3300 | ν(N-H), H- |
| 2951 | νas(C-H2) |
| 2873 | νs (C-H2) |
| 1728 | ν(C=O), ester |
| 1643 | ν(C=O), urethane (strong H-) |
| 1598 | ν(C=C), aromatic ring |
| 1536 | δ(N-H) and ν(C-N), urethane |
| 1454, 1415, 1349 | δ(C-H2) |
| 1385 | ω(C-H2) |
| 1287 | ν(C-N) |
| 1218 | ν(C-N) and δ(N-H) |
| 1174 | ν(C-O-C), ester |
| 1125 | ν(O-CH2) |
| 1066 | ν(C-O-C), urethane |
| 958 | δ(C-H2) |
| 866, 815, 756 | ω(C-H), aromatic ring |
| Wavenumber (cm−1) | Assignment [14,48,49,50] 1 |
|---|---|
| 2958, 2925 | ν(C-H) |
| 2855 | ρ(Si-O), CH3- |
| 1559 | δ(N-H) |
| 1464 | δ(C-H2) |
| 1380 | ω(C-H2) |
| 1318 | ν(C-N), masked |
| 1259 | ν(Si-CH3), APDEMS |
| 1213 | ν(Si-R), OTMS |
| 1185 | ν(Si-R), APDEMS; ρ(Si-O), CH3- |
| 1077 | ν(Si-O), CH3-CH2-; ν(Si-O), CH3- |
| 1010 | νas(Si-O-Si), polymer |
| 913 | ν(Si-OH) |
| 910–665 | ω(N-H2) |
| 864 | δ(Si-CH3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, I.; Viana, C.; Bartoletti, A.; França de Sá, S.; Quye, A.; Shashoua, Y.; Casimiro, T.; Ferreira, J.L. Assessing the Use of Supercritical Carbon Dioxide as a Carrier for Alkoxysilanes to Consolidate Degraded PUR Ester Foams: An Alternative to Traditional Methods. Sustainability 2024, 16, 4375. https://doi.org/10.3390/su16114375
Soares I, Viana C, Bartoletti A, França de Sá S, Quye A, Shashoua Y, Casimiro T, Ferreira JL. Assessing the Use of Supercritical Carbon Dioxide as a Carrier for Alkoxysilanes to Consolidate Degraded PUR Ester Foams: An Alternative to Traditional Methods. Sustainability. 2024; 16(11):4375. https://doi.org/10.3390/su16114375
Chicago/Turabian StyleSoares, Inês, Carolina Viana, Angelica Bartoletti, Susana França de Sá, Anita Quye, Yvonne Shashoua, Teresa Casimiro, and Joana Lia Ferreira. 2024. "Assessing the Use of Supercritical Carbon Dioxide as a Carrier for Alkoxysilanes to Consolidate Degraded PUR Ester Foams: An Alternative to Traditional Methods" Sustainability 16, no. 11: 4375. https://doi.org/10.3390/su16114375










