Andiroba Oil (Carapa guianensis Aubletet) as a Functionalizing Agent for Titica Vine (Heteropsis flexuosa) Nanofibril Films: Biodegradable Products from Species Native to the Amazon Region
Abstract
1. Introduction
2. Methods
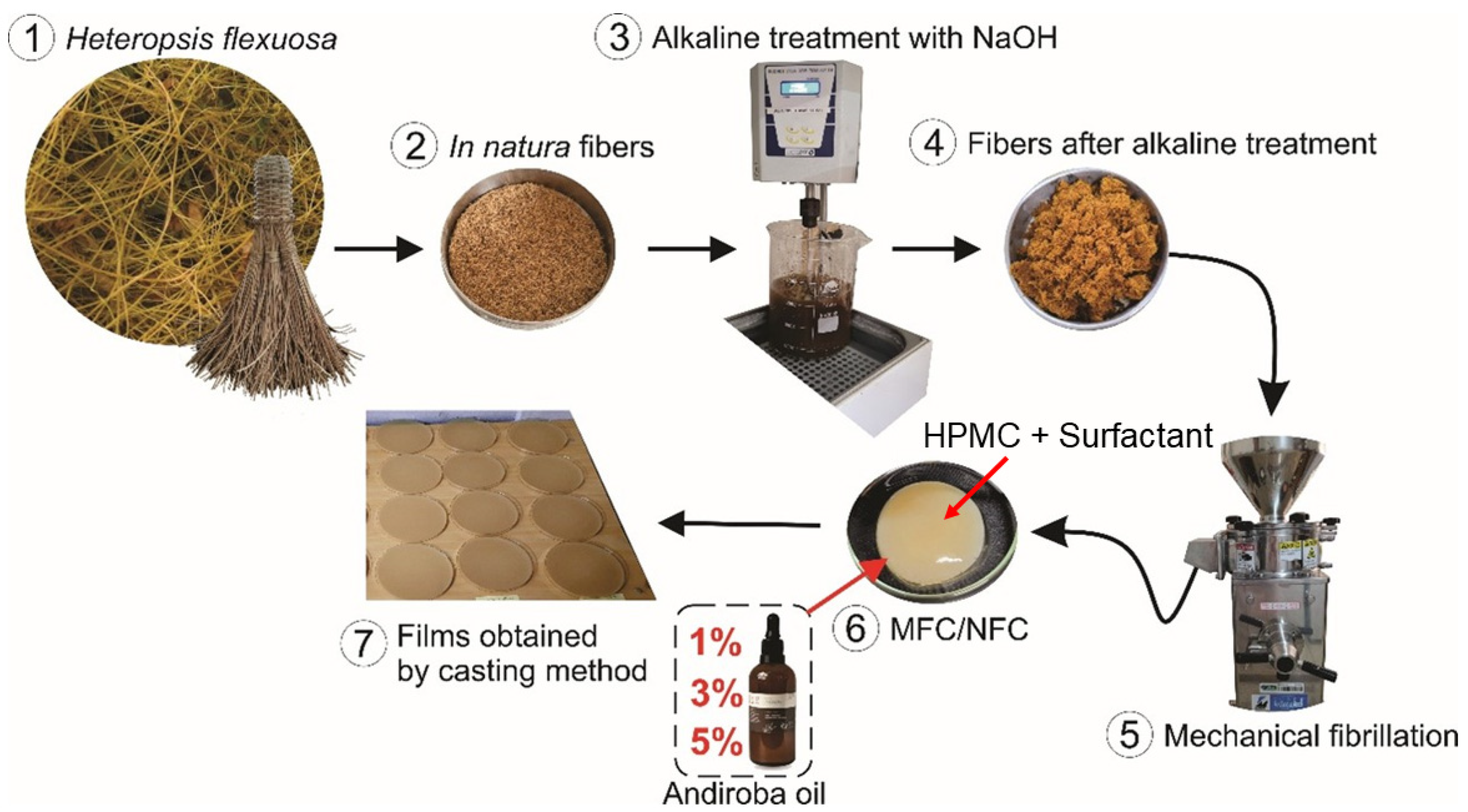
2.1. Obtainment of Materials
2.2. Alkaline Treatment of Fibers
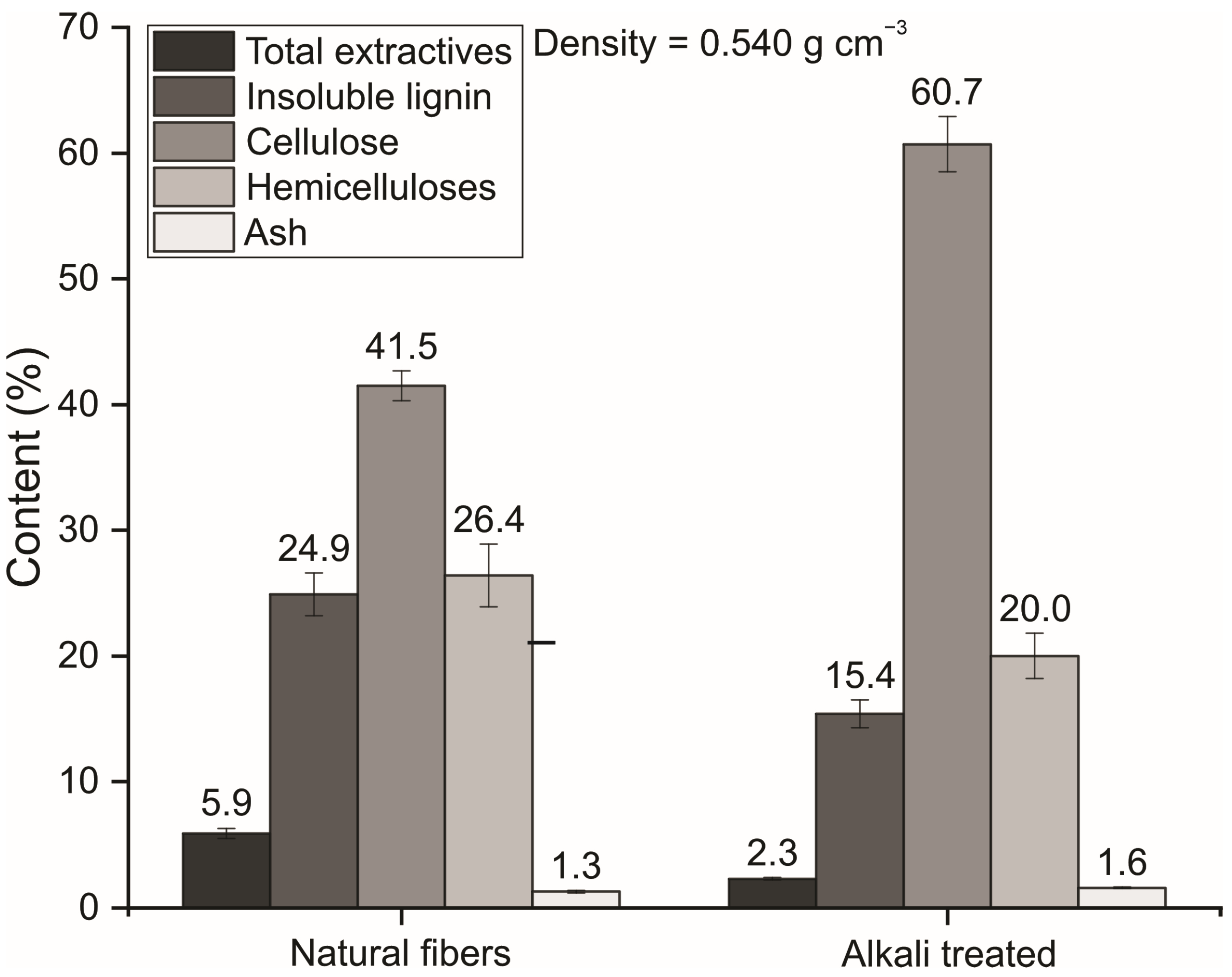
2.3. Density Determination and Chemical Composition of the Fibers
2.4. Mechanical Fibrillation of H. flexuosa
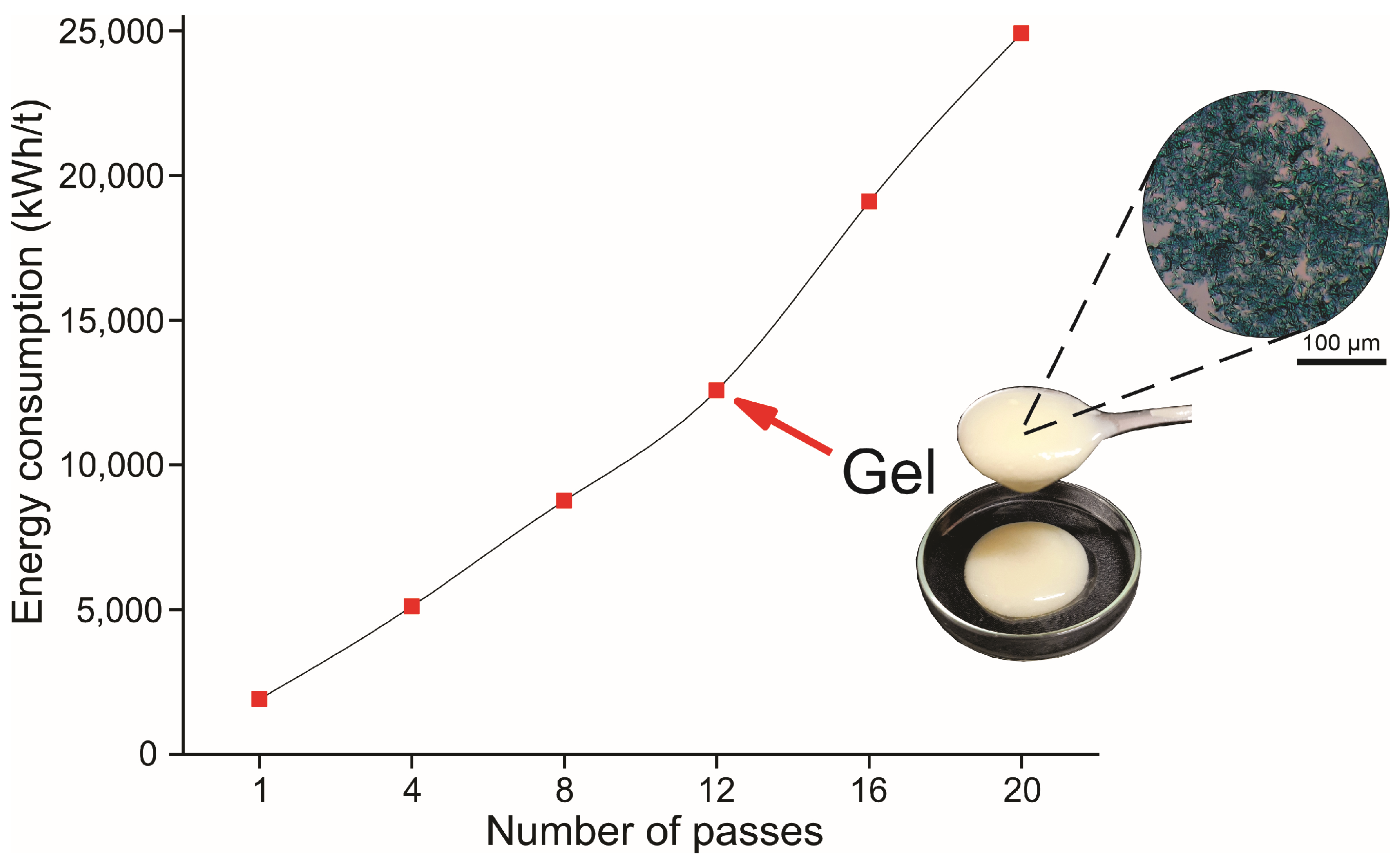
2.5. Energy Consumption of MFC/NFC Production
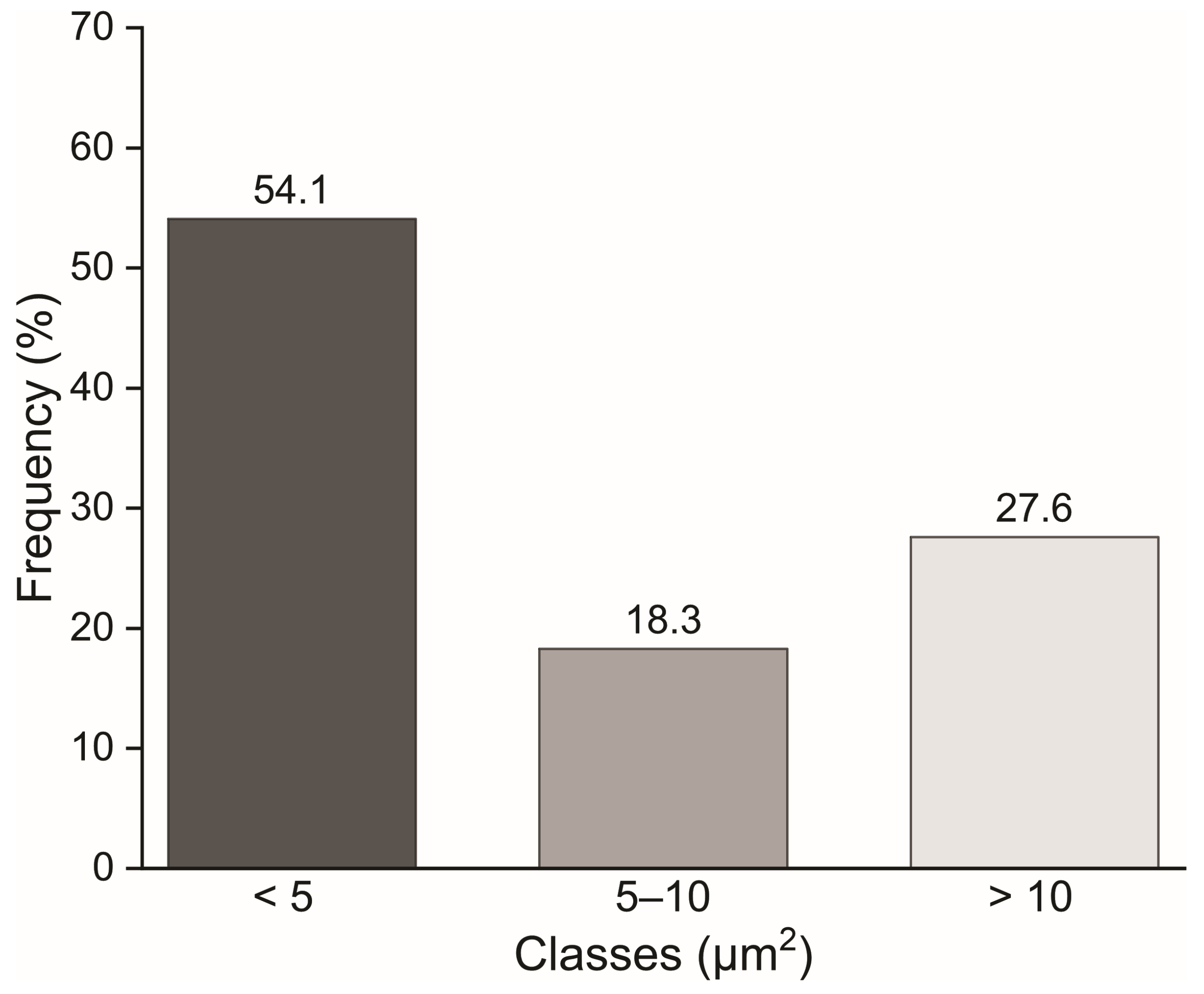
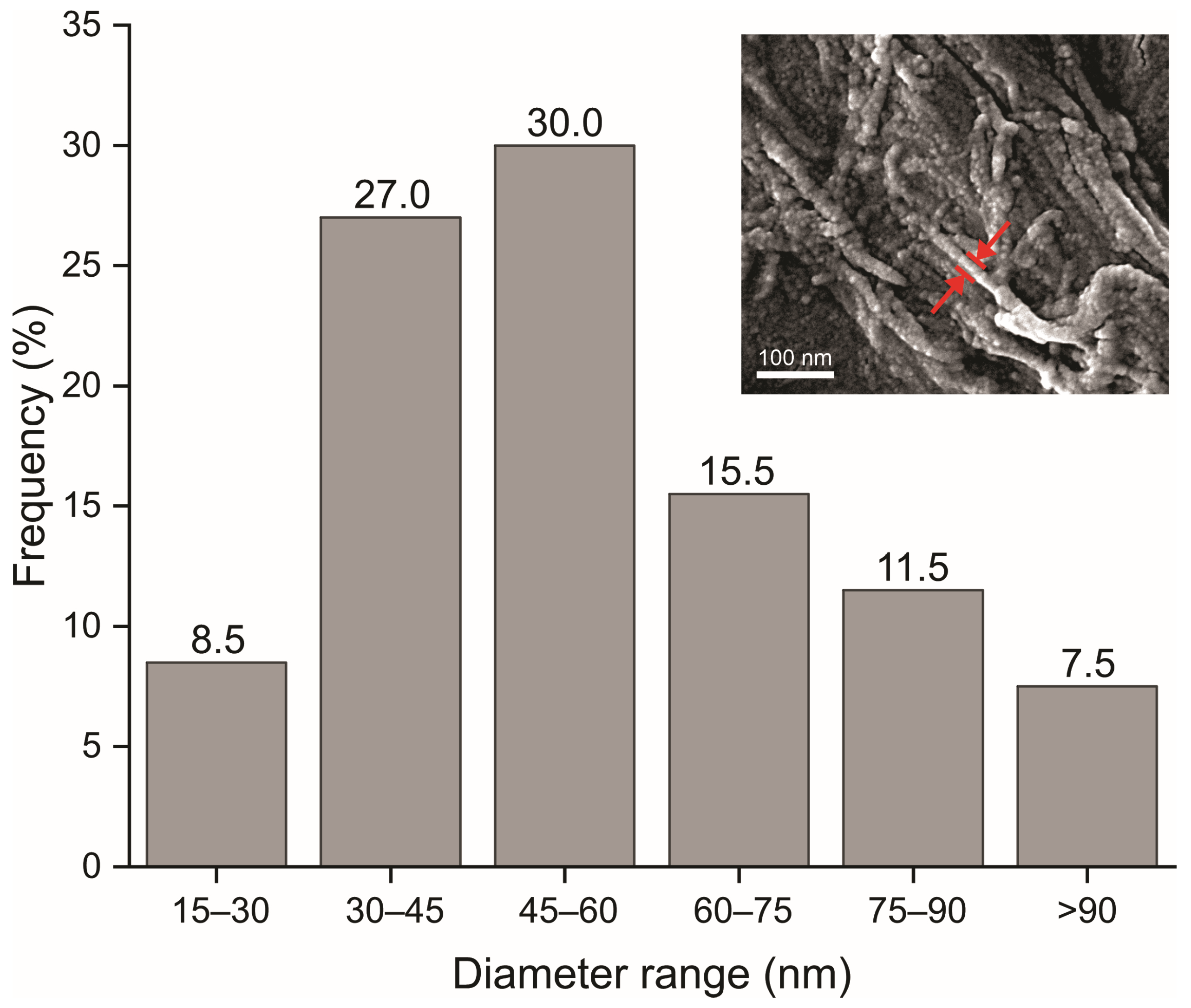
2.6. Structural and Dimensional Analysis of the MFC/NFC
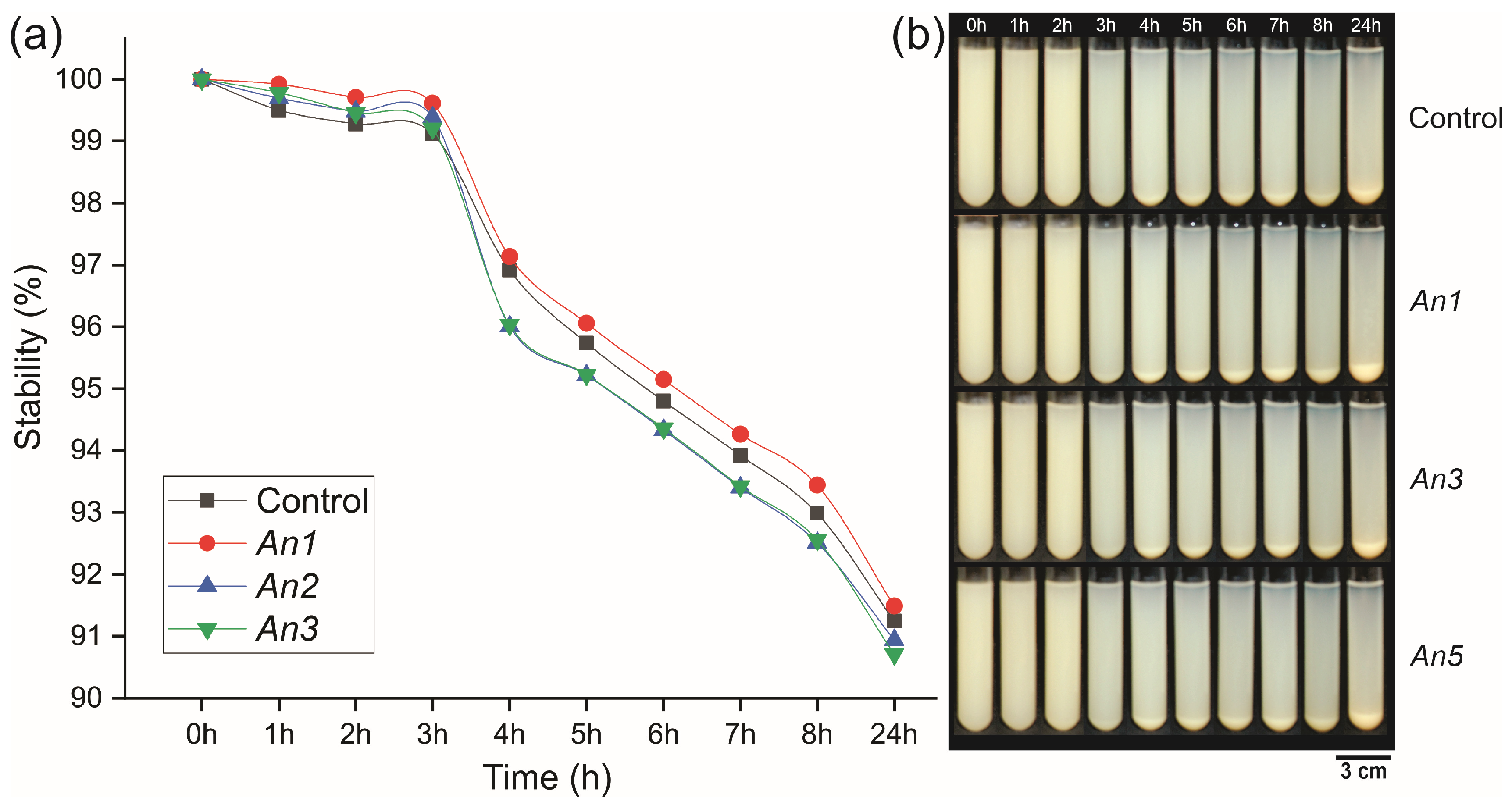
2.7. Stability of the MFC/NFC Suspension
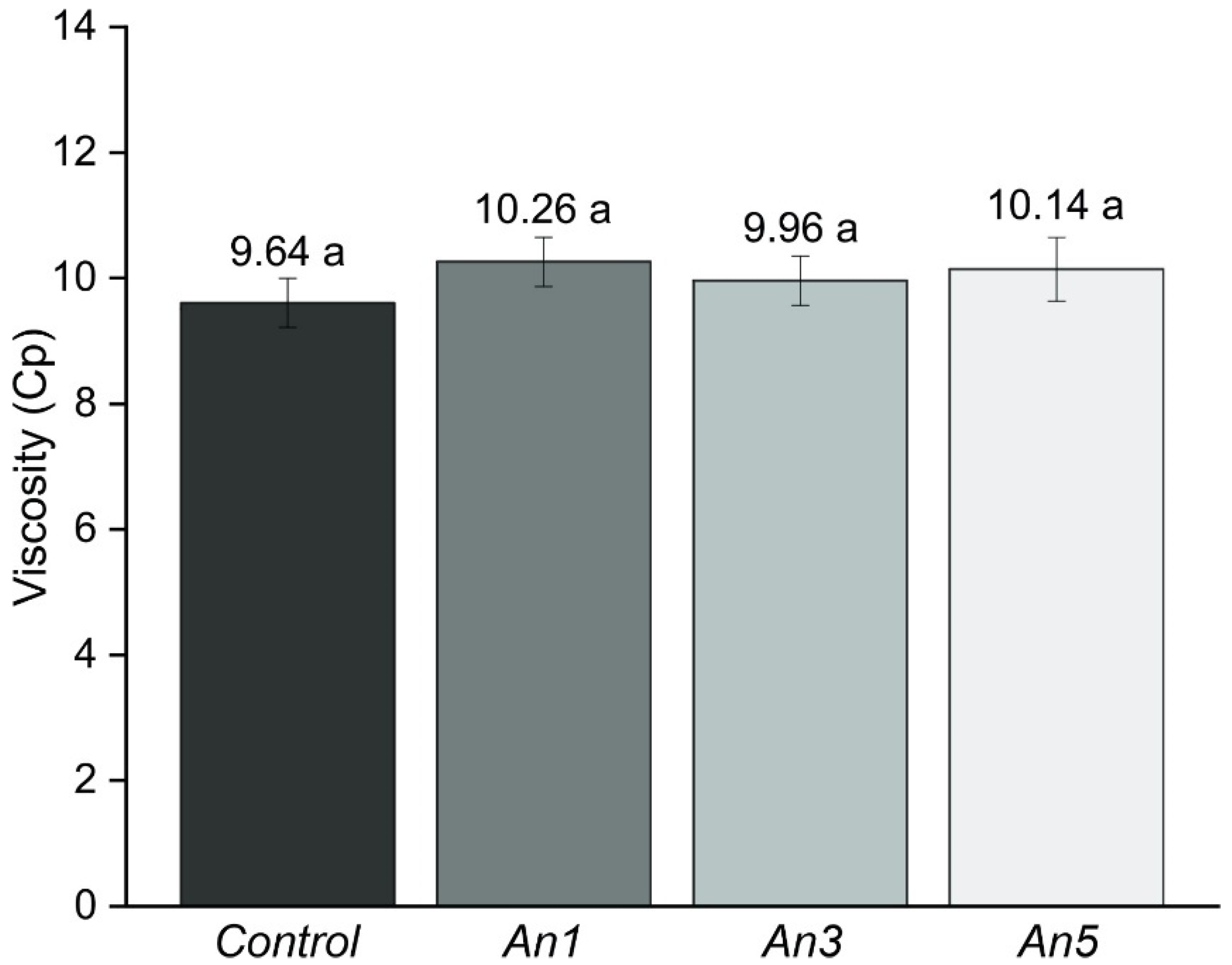
2.8. Viscosity of the Suspensions
2.9. Production of the MFC/NFC Films
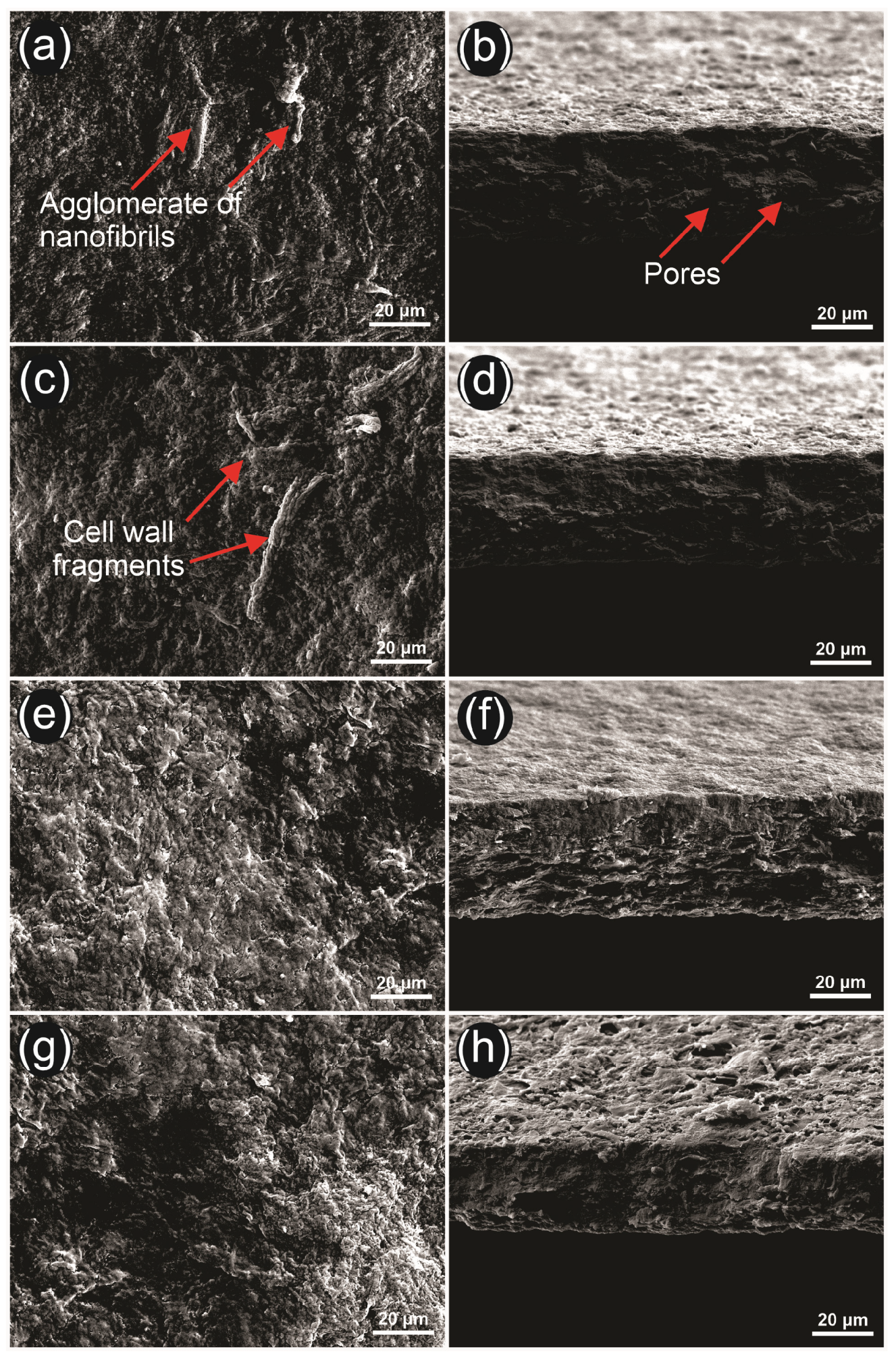
2.10. Physical and Morphological Properties of the Films
2.11. Fourier Transform Infrared Spectroscopy (FTIR)
2.12. Light Transmission and Transparency of the Films
2.13. Coloration and Opacity of the Films
2.14. Thermal Degradation of the Films
2.15. Degradation in Water of the Films
2.16. Barrier to Water Vapor and Grease
2.17. Surface Properties of the Films
2.18. Mechanical Properties
2.19. Antioxidant Activity
2.20. Biodegradation of Films
2.21. Data Analysis
3. Results and Discussion
3.1. Chemical and Physical Characterization of the Fibers
3.2. Energy Consumption
3.3. Microstructure of the Suspensions
3.4. Stability of the Suspensions
3.5. Viscosity of the Suspensions
3.6. Physical and Morphological Properties of the Films
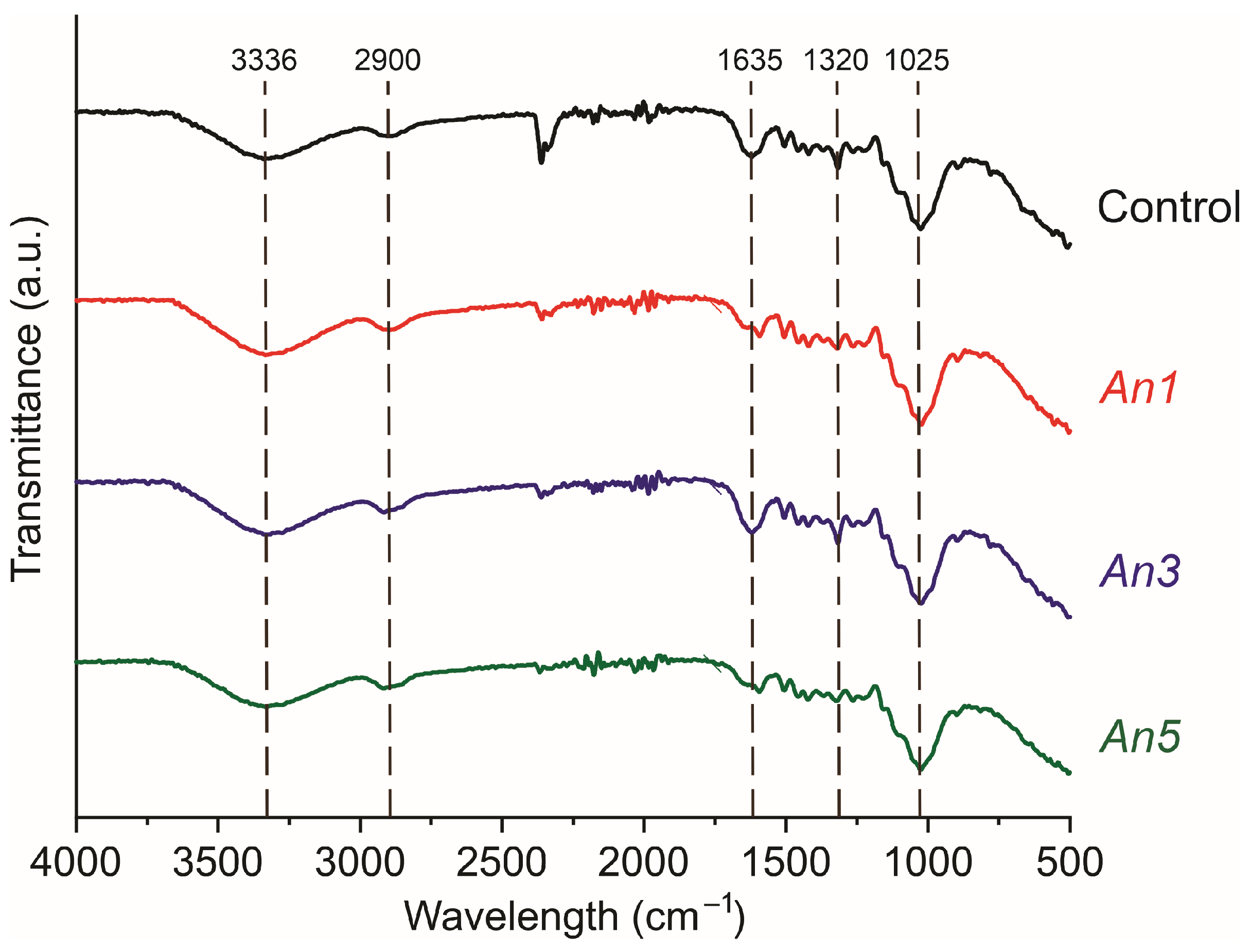
3.7. Fourier-Transformed Infrared Spectrometry (FTIR)
3.8. Transmittance and Transparency of the Films
3.9. Opacity and Color of the Films
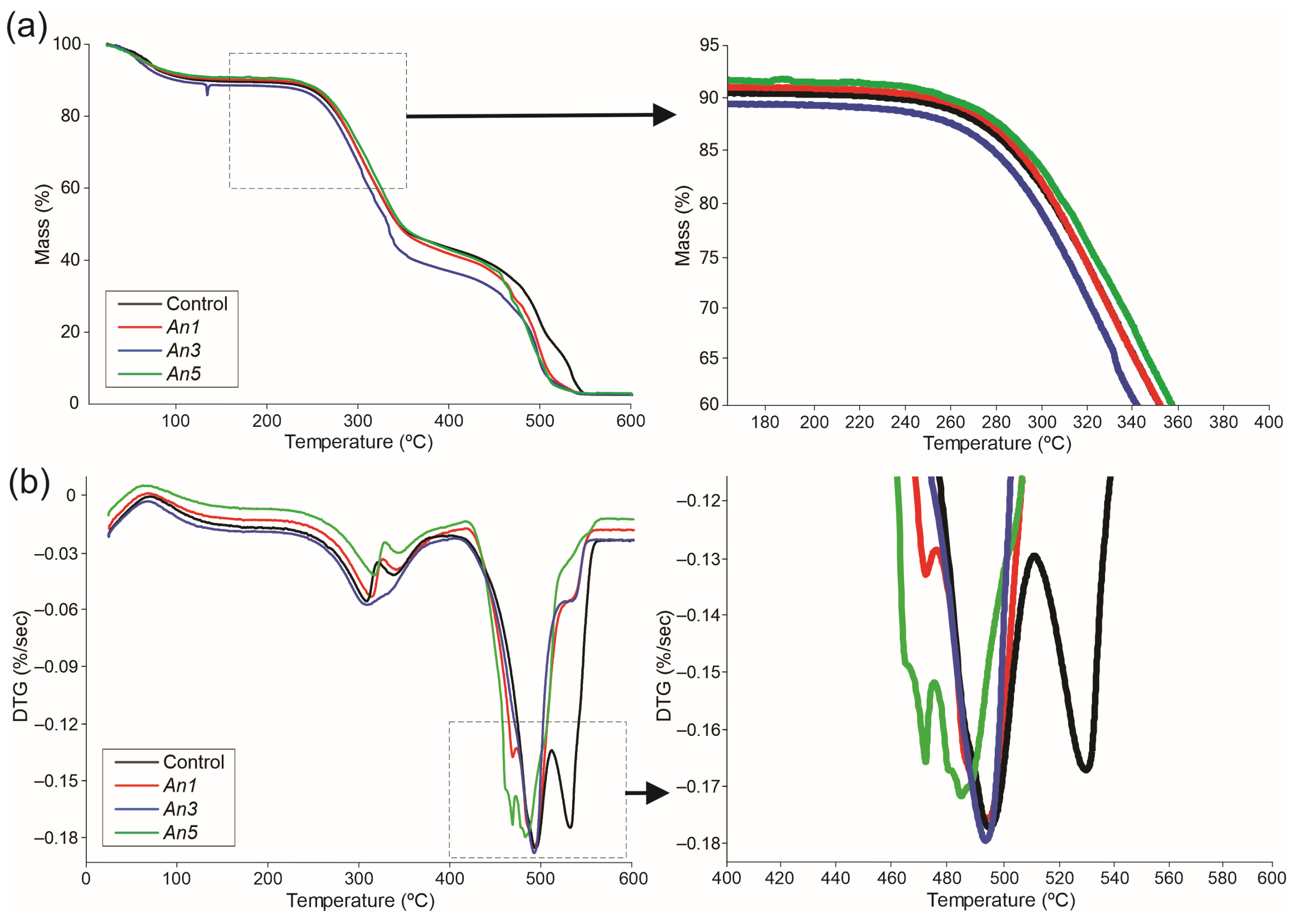
3.10. Thermal Degradation of Films
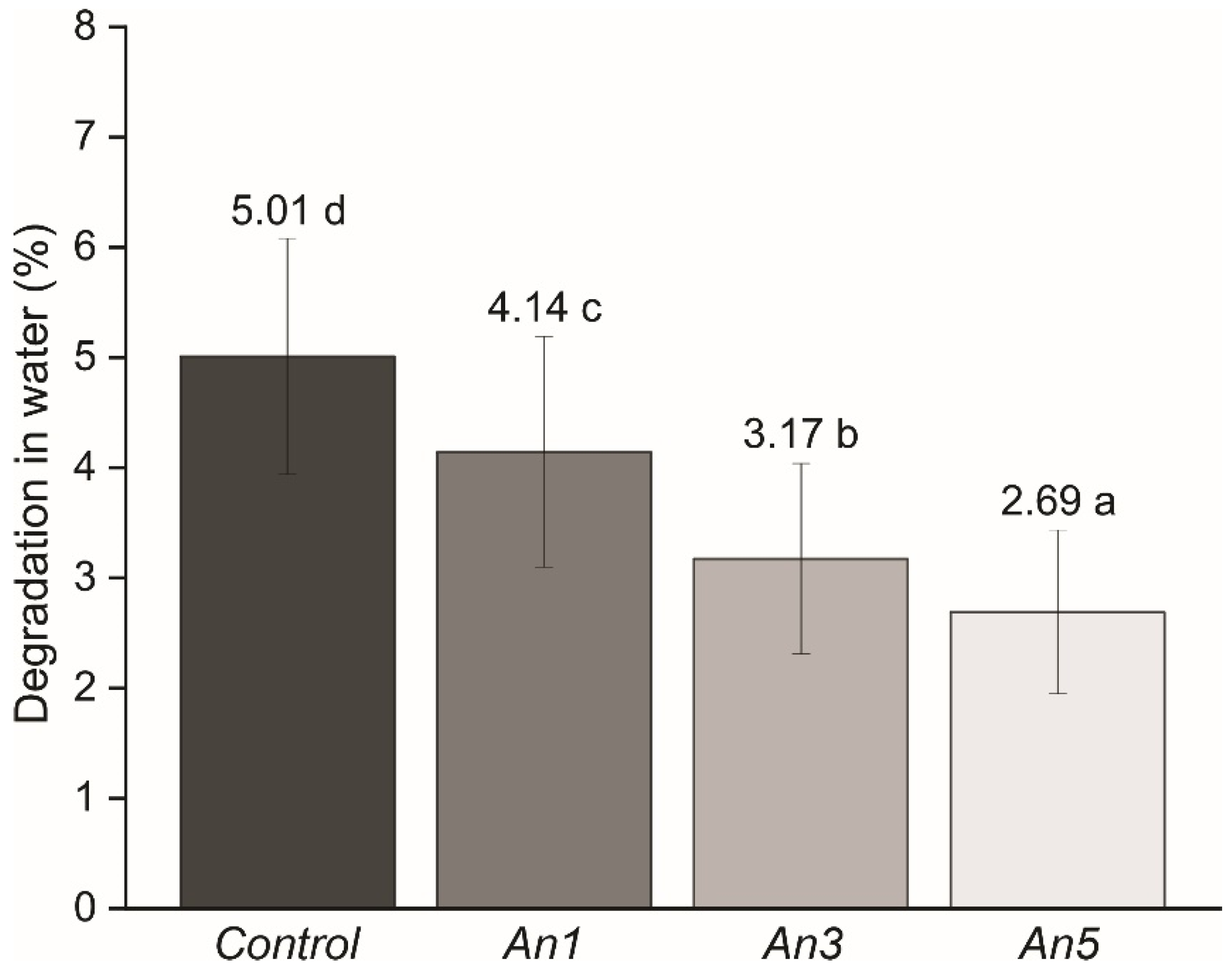
3.11. Degradation in Water of the Films
3.12. Barrier Properties and Grease Resistance
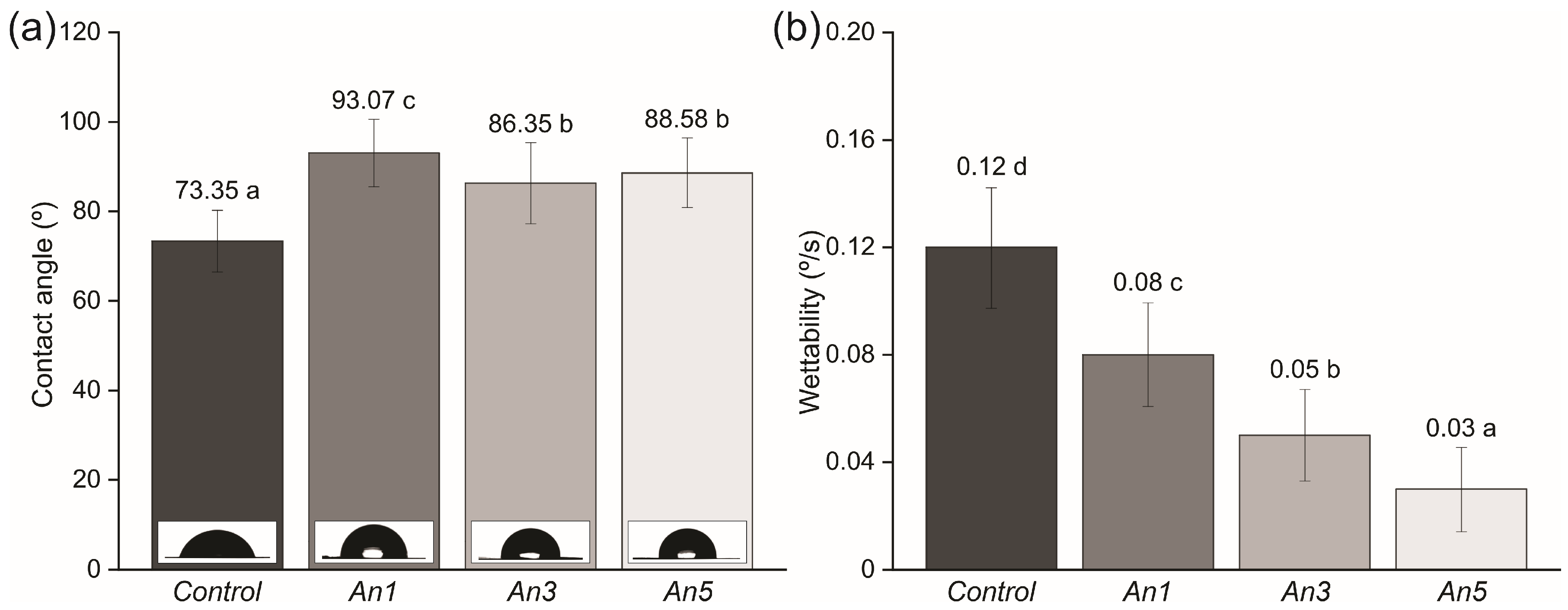
3.13. Surface Properties of the Films
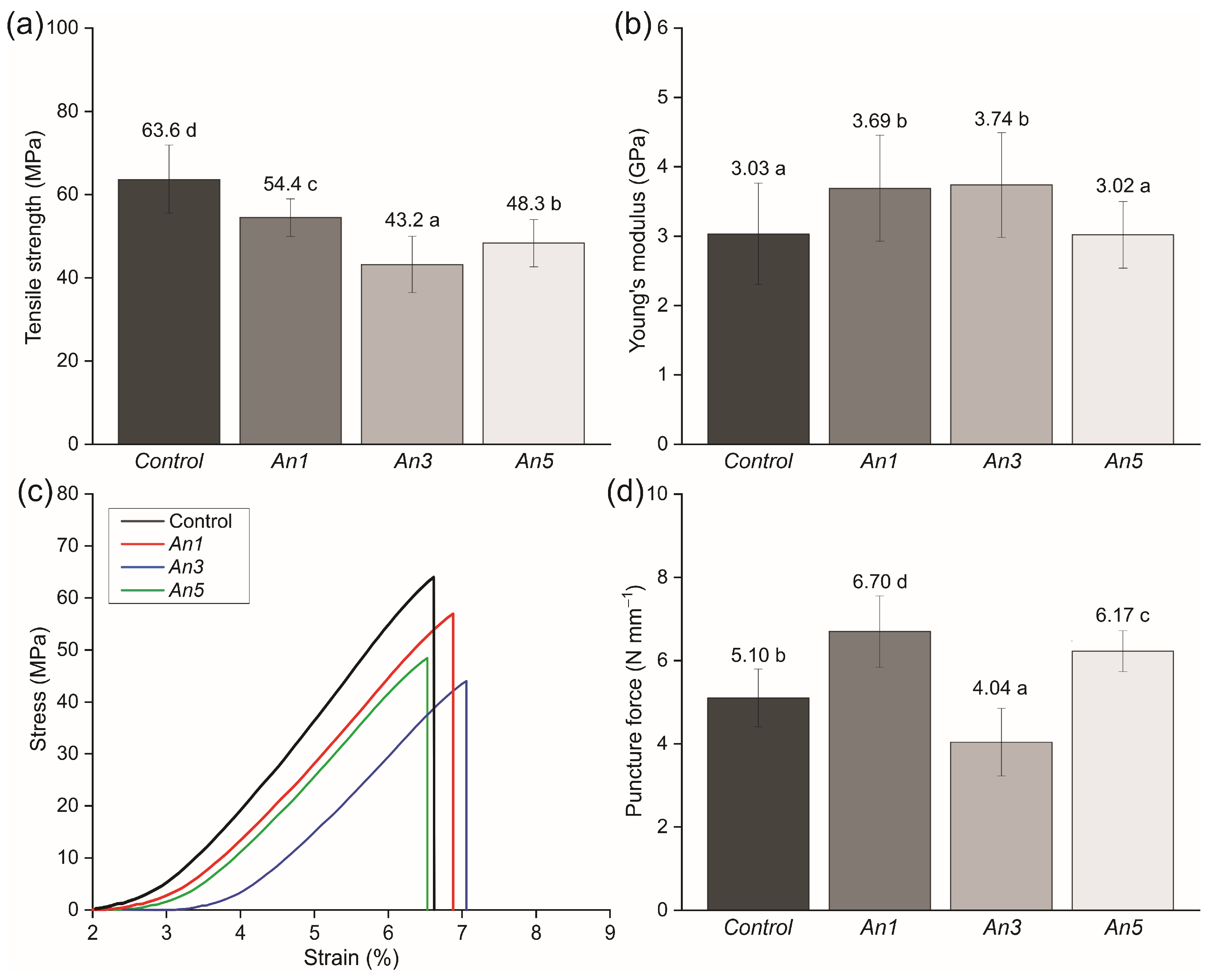
3.14. Mechanical Properties of the Films
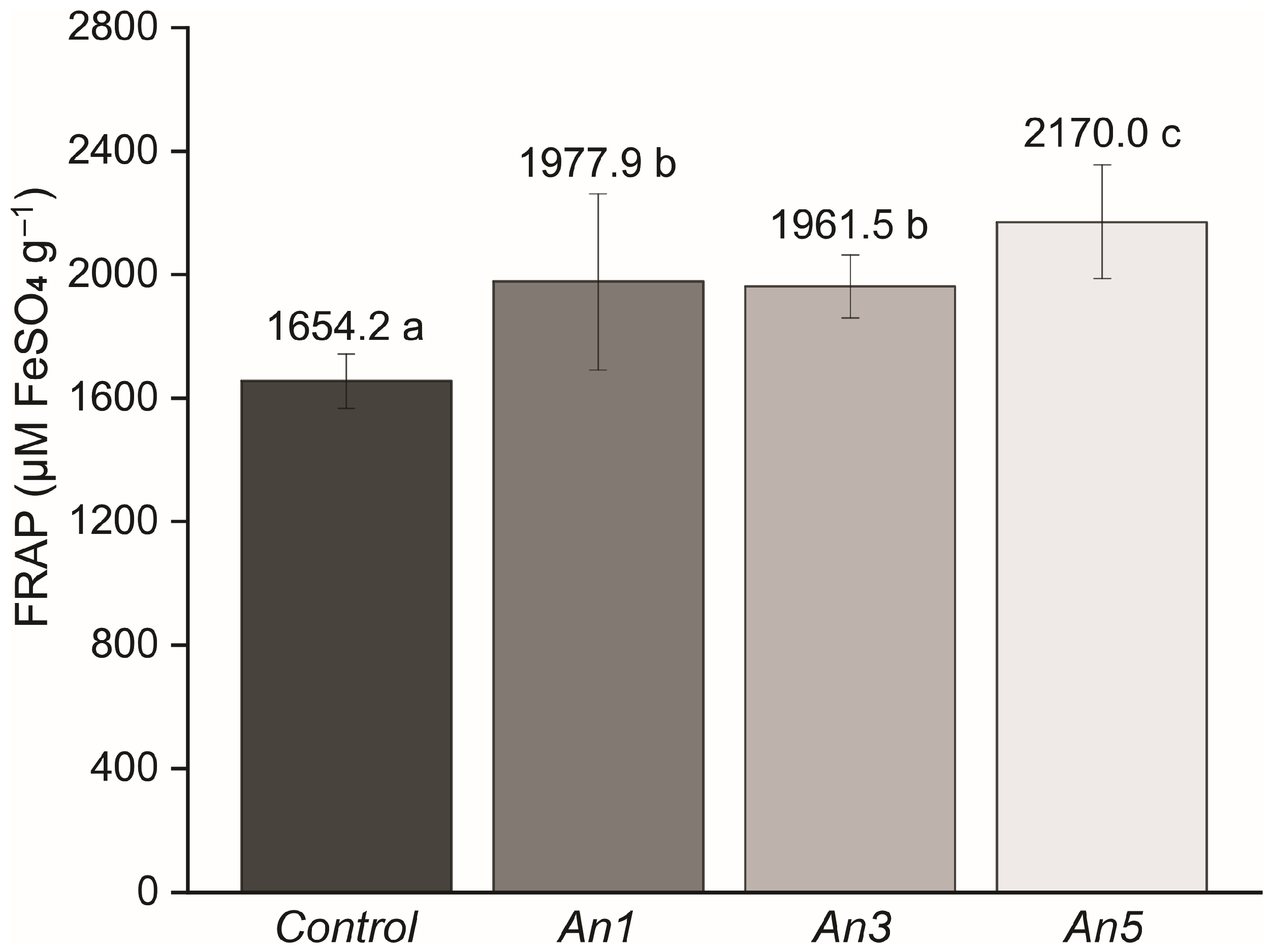
3.15. Antioxidant Activity of Films
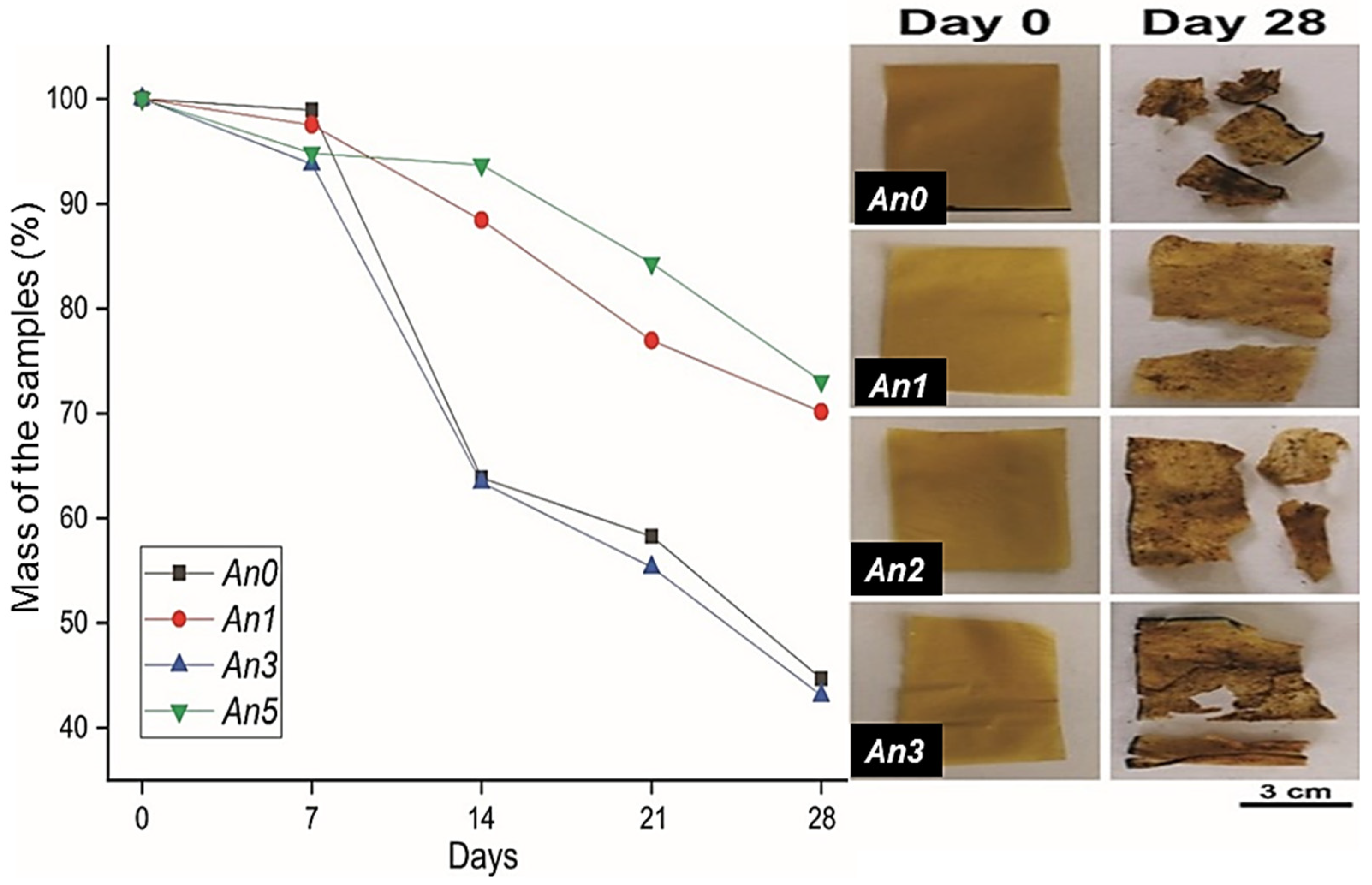
3.16. Biodegradability of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunetta, E.; Cacciotti, I. An overview of the packaging industry: State of the art, opportunities, challenges, criticisms, and solutions. Nanostruct. Mater. Food Packag. Appl. 2024, 2024, 1–30. [Google Scholar] [CrossRef]
- Silva, D.W.; Batista, F.G.; Scatolino, M.V.; Mascarenhas, A.R.P.; Medeiros, D.T.; Tonoli, G.H.D.; Lazo, T.A.A.; Caselli, F.T.R.; Souza, T.M.; Alves Junior, F.T. Developing a Biodegradable Film for Packaging with Lignocellulosic Materials from the Amazonian Biodiversity. Polymers 2023, 15, 3646. [Google Scholar] [CrossRef] [PubMed]
- Ahari, H.; Golestan, L.; Anvar, S.A.A.; Cacciotti, I.; Garavand, F.; Rezaei, A.; Sani, M.A.; Jafari, S.M. Bio-nanocomposites as food packaging materials; the main production techniques and analytical parameters. Adv. Colloid Interface Sci. 2022, 310, 102806. [Google Scholar] [CrossRef]
- Scatolino, M.V.; Bufalino, L.; Dias, M.C.; Mendes, L.M.; Silva, M.S.; Tonoli, G.H.D. Copaiba oil and vegetal tannin as functionalizing agents for açai nanofibril films: Valorization of forest wastes from Amazonia. Environ. Sci. Pollut. Res. 2022, 29, 66422–66437. [Google Scholar] [CrossRef]
- Dias, M.C.; Zidanes, U.L.; Mascarenhas, A.R.P.; Setter, C.; Scatolino, M.V.; Martins, M.A.; Mori, F.A.; Belgacem, M.N.; Tonoli, G.H.D.; Ferreira, S.R. Mandacaru cactus as a source of nanofibrillated cellulose for nanopaper production. Int. J. Biol. Macromol. 2023, 235, 123850. [Google Scholar] [CrossRef]
- Cunha, J.D.S.C.; Nascimento, L.F.C.; Luz, F.S.; Monteiro, S.N.; Lemos, M.F.; Silva, C.G.; Simonassi, N.T. Physical and Mechanical Characterization of Titica Vine (Heteropsis flexuosa) Incorporated Epoxy Matrix Composites. Polymer 2021, 13, 4079. [Google Scholar] [CrossRef]
- Lira, G.B.; Lopes, A.; Nascimento, F.; Conceição, G.; Brasil, D. Extraction processes and industrial uses of andiroba and açaí oils: A review. Res. Soc. Dev. 2021, 10, 12. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Evon, P. Twin-screw extrusion technology for vegetable oil extraction: A review. J. Food Eng. 2017, 212, 190–200. [Google Scholar] [CrossRef]
- Brito, A.D.; Coelho, R.F.R.; Rosal, L.F. Saberes e práticas tradicionais da extração do óleo de Carapa guianenses Abul. (Andiroba) em área de várzea do município de igarapé-miri, PA. Rev. Bras. Agroecol. 2020, 15, 13. [Google Scholar] [CrossRef]
- Costa-Silva, J.H.; Lima, C.R.; Silva, E.J.R.; Araújo, A.V.; Fraga, M.C.C.A.; Ribeiro, A.R.; Arruda, A.C.; Lafayette, S.S.L.; Wanderley, A.G. Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J. Ethnopharmacol. 2008, 116, 495–500. [Google Scholar] [CrossRef]
- Garavand, F.; Nooshkam, M.; Khodaei, D.; Yousefi, S.; Cacciotti, I.; Ghasemlou, M. Recent advances in qualitative and quantitative characterization of nanocellulose-reinforced nanocomposites: A review. Adv. Colloid Interface Sci. 2023, 318, 102961. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, F.; Chen, R.; Deng, G.; Chao, Y.; Yang, Z.; Wu, H.; Bai, M.; Zhang, P.; Hu, Y. Effect of cellulose nanocrystal-stabilized cinnamon essential oil Pickering emulsions on structure and properties of chitosan composite films. Carbohydr. Polym. 2022, 275, 118704. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, M.; Wang, C.; Yan, S.; Lu, P.; Wang, S. Developed chitosan/oregano essential oil biocomposite packaging film enhanced by cellulose nanofibril. Polymer 2020, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.A.; Oliveira, A.C.S.; Santos, T.A.; Dias, M.V.; Yoshida, M.I.; Borges, S.V. WPI and cellulose nanofibres bio-nanocomposites: Effect of thyme essential oil on the morphological, mechanical, barrier and optical properties. J. Polym. Environ. 2020, 28, 231–241. [Google Scholar] [CrossRef]
- Albuquerque, R.M.; Meira, H.M.; Silva, I.D.; Silva, C.J.G.; Almeida, F.C.G.; Amorim, J.D.; Vinhas, G.M.; Costa, A.F.S.; Sarubbo, L.A. Production of a bacterial cellulose/poly (3-hydroxybutyrate) blend activated with clove essential oil for food packaging. Polym. Polym. Compos. 2021, 29, 259–270. [Google Scholar] [CrossRef]
- Scatolino, M.V.; Bufalino, L.; Mendes, L.M.; Guimaraes Junior, M.; Tonoli, G.H.D. Impact of nanofibrillation degree of eucalyptus and Amazonian hardwood sawdust on physical properties of cellulose nanofibril films. Wood Sci. Technol. 2017, 51, 1095–1115. [Google Scholar] [CrossRef]
- ABNT NBR 11941; Wood: Determination of Basic Density. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2003; 6p.
- TAPPI T 204 om-97; Solvent Extractives of Wood and Pulp. Technical Association of the Pulp and Paper Industry Press: Norcross, Georgia, 2007.
- TAPPI T 222 om-02; Acid-Insoluble Lignin in Wood Pulp. Technical Association of the Pulp and Paper Industry Press: Norcross, Georgia, 2002.
- Browning, B.L. The Chemistry of Wood; Interscience: Warrenvile, IL, USA, 1963; 689p. [Google Scholar]
- Kennedy, F.; Phillips, G.O.; Willians, P.A. Wood and Cellulosics, Industrial Utilization, Biotechnology, Structure and Properties; Ellis Horwood: Chichester, UK, 1987. [Google Scholar]
- TAPPI T 211 om-02; Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C. Technical Association of the Pulp and Paper Industry Press: Norcross, Georgia, 2002.
- Guimarães, B.M.R.; Scatolino, M.V.; Martins, M.A.; Ferreira, S.R.; Mendes, L.M.; Lima, J.T.; Guimarães Junior, M.; Tonoli, G.H.D. Bio-based films/nanopapers from lignocellulosic wastes for production of added-value micro-/nanomaterials. Environ. Sci. Pollut. Res. 2021, 29, 8665–8683. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef]
- TAPPI T 230 om-19; Viscosity of Pulp (Capillary Viscometer Method). Technical Association of the Pulp and Paper Industry Press: Norcross, Georgia, 2019.
- TAPPI T 411 om-15; Thickness (Caliper) of Paper, Paperboard and Combined Board, Phys. Prop. Comm. Process Prod. Qual. Div. Technical Association of the Pulp and Paper Industry: Norcross, Georgia, 2015; pp. 3–6.
- TAPPI Revision of T 410 om-08; Grammage of Paper and Paperboard (Weight per Unit Area). Technical Association of the Pulp and Paper Industry: Norcross, Georgia, 2013.
- ASTM D1746-15; Standard Test Method for Transparency of Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 2015.
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef]
- Lago, R.C.; Oliveira, A.L.M.; Dias, M.C.; Carvalho, E.E.N.; Tonoli, G.H.D.; Vilas Boas, E.V.B. Obtaining cellulosic nanofibrils from oat straw for biocomposite reinforcement: Mechanical and barrier properties. Ind. Crops Prod. 2020, 148, 112264. [Google Scholar] [CrossRef]
- Silva, D.W.; Batista, F.G.; Scatolino, M.V.; Mascarenhas, A.R.P.; Medeiros, D.T.; Denzin Tonoli, G.H.; Mendes, L.M.; Souza, T.M.; Alves Junior, F.T. Nanofibrillated pulps from Amazonian species as a potential raw material for ecological packaging. Nord. Pulp Pap. Res. J. 2024. [CrossRef]
- ASTM E96; Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016.
- TAPPI T 559 cm-12; Grease Resistance Test for Paper and Paperboard. Technical Association of the Pulp and Paper Industry Press: Norcross, Georgia, 2012.
- TAPPI T 458 cm-14; Surface Wettability of Paper (Angle of Contact Method). Technical Association of the Pulp and Paper Industry TAPPI Press: Norcross, Georgia, 2014.
- Mascarenhas, A.R.P.; Scatolino, M.V.; Santos, A.A.; Norcino, L.B.; Duarte, P.J.; Melo, R.R.; Dias, M.C.; Faria, C.E.T.; Mendonça, M.C.; Tonoli, G.H.D. Hydroxypropyl methylcellulose films reinforced with cellulose micro/nanofibrils: Study of physical, optical, surface, barrier and mechanical properties. N. Nord. Pulp Pap. Res. J. 2022, 37, 366–384. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 2018.
- Norcino, L.B.; Mendes, J.F.; Natarelli, C.V.L.; Manrich, A.; Oliveira, J.E.; Mattoso, L.H.C. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Braz. J. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Kenny, J.K.; Medlin, J.W.; Beckham, G.T. Quantification of Phenolic Hydroxyl Groups in Lignin via 19F NMR Spectroscopy. ACS Sustain. Chem. Eng. 2023, 11, 5644–5655. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- LPF. Forest Products Laboratory, National Forest Information System. Brazilian Forest Service. Database of Brazilian Woods. Brasília, 2023. Available online: https://snif.florestal.gov.br/ (accessed on 14 May 2023).
- Lahtinen, P.; Liukkonen, S.; Pere, J.; Sneck, A.; Kangas, H. A comparative study of fibrillated fibers from different mechanical and chemical pulps. BioResources 2014, 9, 2115–2127. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Balea, A.; Fuente, E.; Blanco, A.; Negro, C. Nanocelluloses: Natural-based materials for fiber-reinforced cement composites. A critical review. Polymers 2019, 11, 518. [Google Scholar] [CrossRef]
- Jaramillo, C.M.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Marwanto, M.; Maulana, M.I.; Febrianto, F.; Wistara, N.J.; Nikmatin, S.; Masruchin, N.; Zaini, L.H.; Lee, S.H.; Kim, N.H. Characteristics of nanocellulose crystals from balsa and kapok fibers at different ammonium persulfate concentrations. Wood Sci. Technol. 2021, 55, 1319–1335. [Google Scholar] [CrossRef]
- Salem, K.S.; Starkey, R.H.; Pal, L.; Lucia, L.; Jameel, H. The topochemistry of cellulose nanofibrils as a function of mechanical generation energy. ACS Sustain. Chem. Eng. 2019, 8, 1471–1478. [Google Scholar] [CrossRef]
- Qin, D.; Ma, X.; Zhang, B.; Luo, Q.; Na, H.; Chen, J.; Zhu, J. Ultra-high gas barrier and enhanced mechanical properties of corn cellulose nanocomposite films filled with graphene oxide nanosheets. Carbohydr. Polym. Technol. Appl. 2021, 2, 100066. [Google Scholar] [CrossRef]
- Silva, D.F.; Lima, K.T.; Bastos, G.N.; Oliveira, J.A.R.; Nascimento, L.A.S.; Costa, C.E.F.; Rocha Filho, G.N.; Concha, V.O.C.; Passos, M.F. PCL/andiroba oil (Carapa guianensis Aubl.) hybrid film for wound healing applications. Polymer 2021, 13, 1591. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Rosanti, S.D. Effect of heat treatment on thermal resistance, transparency and antimicrobial activity of sonicated ginger cellulose film. Carbohyd. Polym. 2020, 240, 116287. [Google Scholar] [CrossRef]
- Qing, Y.; Sabo, R.; Wu, Y.; Zhu, J.Y.; Cai, Z. Self-assembled optically transparent cellulose nanofibril films: Effect of nanofibril morphology and drying procedure. Cellulose 2015, 22, 1091–1102. [Google Scholar] [CrossRef]
- Okahisa, Y.; Furukawa, Y.; Ishimoto, K.; Narita, C.; Intharapichai, K.; Ohara, H. Comparison of cellulose nanofiber properties produced from different parts of the oil palm tree. Carbohydr. Polym. 2018, 198, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible films for food packaging: A review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Martins, M.P.; Dagostin, J.L.A.; Franco, T.S.; Muñiz, G.I.B.; Masson, M.L. Application of cellulose nanofibrils isolated from an agroindustrial residue of peach palm in cassava starch films. Food Biophys. 2020, 15, 323–334. [Google Scholar] [CrossRef]
- Costa, M.N.F.S.; Muniz, M.A.P.; Negrão, C.A.B.; Costa, C.E.F.; Lamarão, M.L.N.; Morais, L.; Silva Junior, J.O.C.; Costa, R.M.R. Characterization of Pentaclethra macroloba oil: Thermal stability, gas chromatography and Rancimat. J. Therm. Anal. Calorim. 2014, 115, 2269–2275. [Google Scholar] [CrossRef]
- Wang, W.; Gu, F.; Deng, Z.; Zhu, Y.; Zhu, J.; Guo, T.; Song, J.; Xiao, H. Multilayer surface construction for enhancing barrier properties of cellulose-based packaging. Carbohydr. Polym. 2021, 255, 117431. [Google Scholar] [CrossRef] [PubMed]
- Sahraee, S.; Milani, J.M.; Ghanbarzade, B.; Hamishekar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Abbasi, H.; Fahim, H.; Mahboubi, M. Fabrication and characterization of composite film based on gelatina and electrospun cellulose acetate fibers incorporating essential oil. J. Food Meas. Charact. 2021, 15, 2108–2118. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Effect of post-treatments and concentration of cotton linter cellulose nanocrystals on the properties of agar-based nanocomposite films. Carbohydr. Polym. 2015, 134, 20–29. [Google Scholar] [CrossRef]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Chitosan coated liposome dispersions loaded with cacao hull waste extract: Effect of spray drying on physico-chemical stability and in vitro bioaccessibility. J. Food Eng. 2018, 223, 91–98. [Google Scholar] [CrossRef]
- Bardi, M.A.G.; Rosa, D.S. Evaluation of biodegradation in simulated soil of poli (ε-caprolactone), cellulose acetate and its blends. Rev. Bras. Apl. Vácuo 2007, 26, 43–47. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Malekzadeh, E.; Tatari, A.; Firouzabadi, M.D. Preparation, characteristics, and soil-biodegradable analysis of corn starch/nanofibrillated cellulose (CS/NFC) and corn starch/nanofibrillated lignocellulose (CS/NFLC) films. Carbohydr. Polym. 2023, 309, 120699. [Google Scholar] [CrossRef]

| Tratament | Andiroba Oil (%) |
|---|---|
| Control | 0 |
| An1 | 1 |
| An3 | 3 |
| An5 | 5 |
| Treatments | Thickness (µm) | Grammage (g m−2) | Bulk Density (g cm−3) | Porosity (%) |
|---|---|---|---|---|
| Control | 43.0 ± 0.9 * a | 33.7 ± 1.9 a | 0.82 ± 0.01 a | 46.7 ± 2.0 b |
| An1 | 48.8 ± 1.3 c | 41.8 ± 1.4 b | 0.85 ± 0.02 b | 44.6 ± 2.3 b |
| An3 | 46.0 ± 1.9 b | 42.3 ± 1.3 b | 0.92 ± 0.05 c | 40.1 ± 3.4 a |
| An5 | 49.6 ± 0.7 d | 45.5 ± 1.2 c | 0.93 ± 0.02 c | 39.3 ± 1.4 a |
| Treatments | Opacity (%m−1) | L* | C* | hue |
|---|---|---|---|---|
| Control | 0.987 ± 0.03 * b | 40.78 ± 2.0 a | 11.23 ± 2.64 a | 91.34 ± 0.72 c |
| An1 | 1.220 ± 0.02 d | 44.92 ± 1.1 c | 15.11 ± 0.79 b | 89.53 ± 1.50 b |
| An3 | 0.875 ± 0.01 a | 40.46 ± 1.1 a | 16.07 ± 0.98 c | 86.49 ± 1.80 a |
| An5 | 1.013 ± 0.08 c | 42.59 ± 0.7 b | 16.69 ± 1.20 c | 87.68 ± 1.53 a |
| Treatments | WVTR | WVP | Grease Resistance Score |
|---|---|---|---|
| Control | 710.3 ± 7.7 * b | 2.84 ± 0.03 a | 12 |
| An1 | 686.9 ± 11.7 a | 3.35 ± 0.07 c | 12 |
| An3 | 729.4 ± 20.9 b | 3.17 ± 0.09 b | 12 |
| An5 | 754.5 ± 11.3 c | 4.60 ± 0.16 d | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, C.S.d.; Batista, F.G.; Silva, D.W.; Scatolino, M.V.; Medeiros, D.T.d.; Mascarenhas, A.R.P.; Lago, R.C.d.; Setter, C.; Borges, I.O.; Tonoli, G.H.D.; et al. Andiroba Oil (Carapa guianensis Aubletet) as a Functionalizing Agent for Titica Vine (Heteropsis flexuosa) Nanofibril Films: Biodegradable Products from Species Native to the Amazon Region. Sustainability 2024, 16, 4395. https://doi.org/10.3390/su16114395
Paiva CSd, Batista FG, Silva DW, Scatolino MV, Medeiros DTd, Mascarenhas ARP, Lago RCd, Setter C, Borges IO, Tonoli GHD, et al. Andiroba Oil (Carapa guianensis Aubletet) as a Functionalizing Agent for Titica Vine (Heteropsis flexuosa) Nanofibril Films: Biodegradable Products from Species Native to the Amazon Region. Sustainability. 2024; 16(11):4395. https://doi.org/10.3390/su16114395
Chicago/Turabian StylePaiva, Cleyson Santos de, Felipe Gomes Batista, Danillo Wisky Silva, Mário Vanoli Scatolino, Dayane Targino de Medeiros, Adriano Reis Prazeres Mascarenhas, Rafael Carvalho do Lago, Carine Setter, Ianca Oliveira Borges, Gustavo Henrique Denzin Tonoli, and et al. 2024. "Andiroba Oil (Carapa guianensis Aubletet) as a Functionalizing Agent for Titica Vine (Heteropsis flexuosa) Nanofibril Films: Biodegradable Products from Species Native to the Amazon Region" Sustainability 16, no. 11: 4395. https://doi.org/10.3390/su16114395
APA StylePaiva, C. S. d., Batista, F. G., Silva, D. W., Scatolino, M. V., Medeiros, D. T. d., Mascarenhas, A. R. P., Lago, R. C. d., Setter, C., Borges, I. O., Tonoli, G. H. D., Souza, T. M. d., Mendes, L. M., Bufalino, L., Alves Junior, F. T., Felix, F. d. S., & Dias, M. V. (2024). Andiroba Oil (Carapa guianensis Aubletet) as a Functionalizing Agent for Titica Vine (Heteropsis flexuosa) Nanofibril Films: Biodegradable Products from Species Native to the Amazon Region. Sustainability, 16(11), 4395. https://doi.org/10.3390/su16114395








