Growth Dynamics and Nutrient Removal from Biogas Slurry Using Water Hyacinth
Abstract
:1. Introduction
2. Phytoremediation Potentiality of WH: A Bibliometric Study
3. Collection and Characterization of Biogas Slurry and Plant Samples
4. Experimental Setup for the Study
4.1. Experimental Setup 1: Removal of Nutrients by WH
4.2. Experimental Setup 2: Measurement of the Biomass Quality
5. Materials and Methods
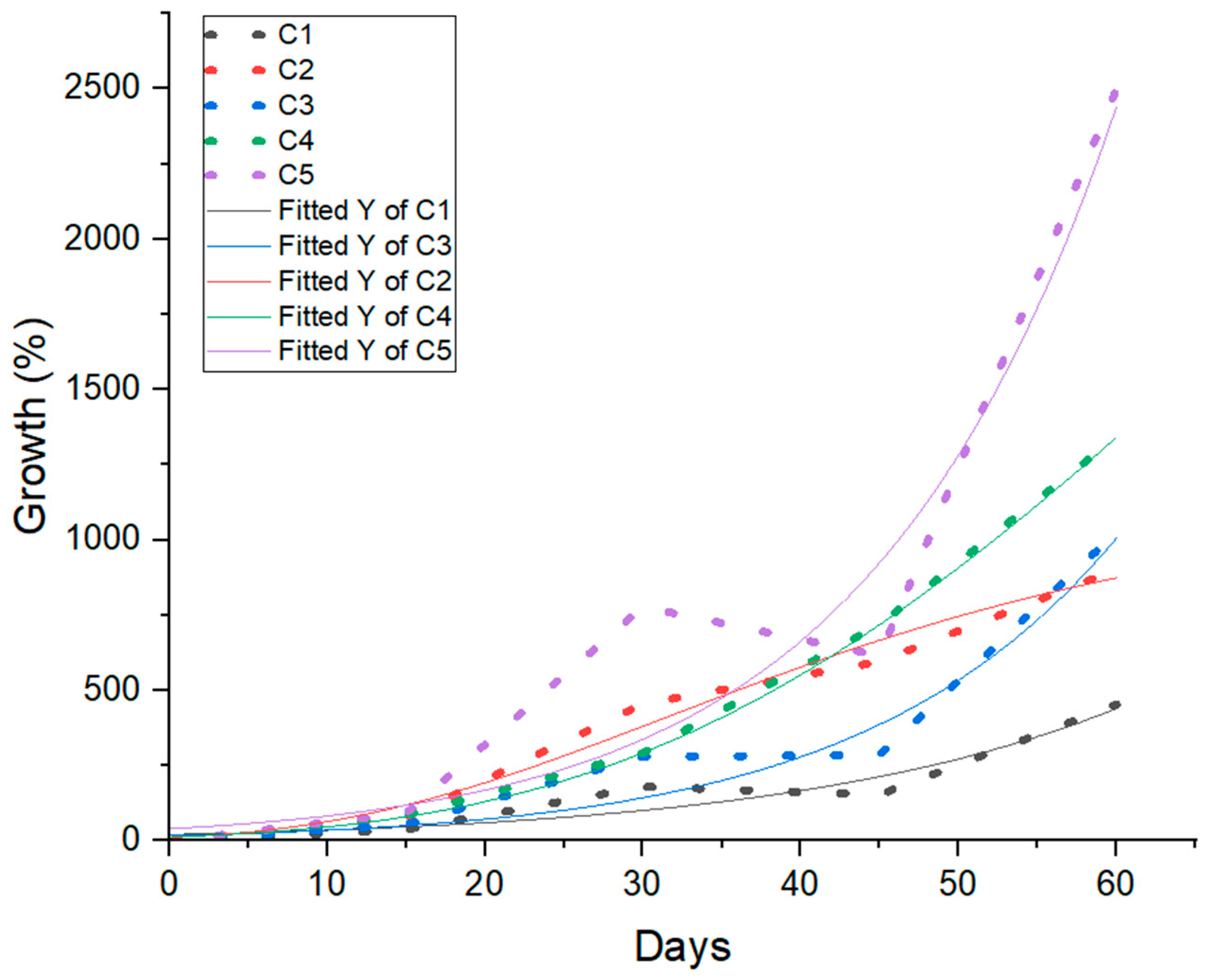
5.1. Relative Growth Rate of the Biomass
5.2. S-Gompertz Model
- Y: This represents the growth of WH at a given time (X). It is the dependent variable we are trying to model or predict.
- A: This parameter represents the upper asymptote of the growth curve. In the context of WH growth, it signifies the maximum growth level that can be reached. As time (X) approaches infinity, Y will asymptotically approach A.
- exp: This is the exponential function with the base e (approximately 2.71828). It is used to model exponential growth or decay.
- k: This is the growth rate coefficient. It controls the steepness of the S-shaped growth curve. A higher value of k implies a steeper curve, indicating faster growth.
- XC: This represents the time at which the growth curve undergoes an inflection point. The inflection point is the point where the growth rate transitions from accelerating to decelerating. In other words, it is the time when the rate of growth is the highest.
5.3. Physiochemical Analysis of Biogas Slurry
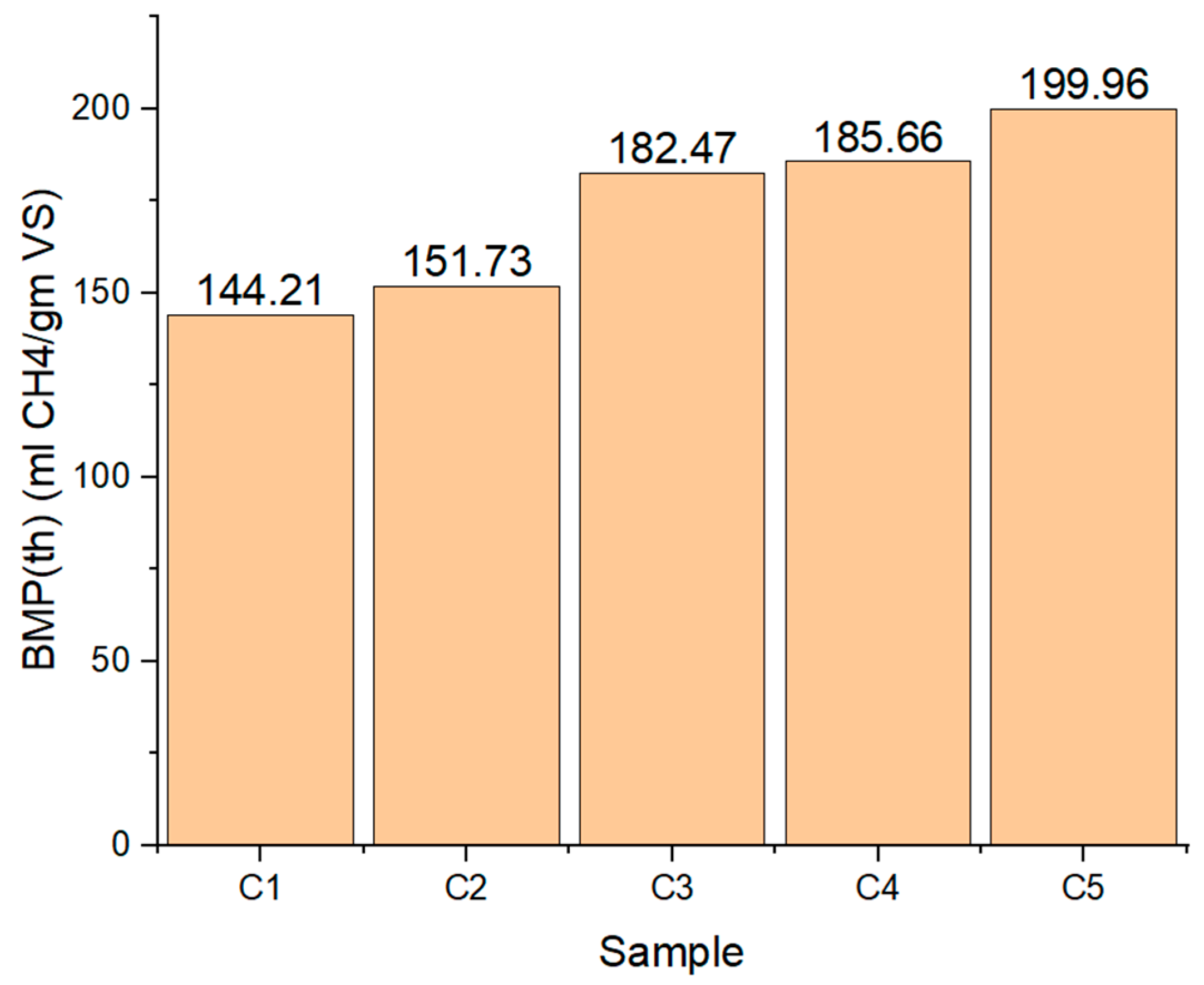
5.4. Theoretical BMP (BMPth) Analysis
5.5. Statistical Analysis
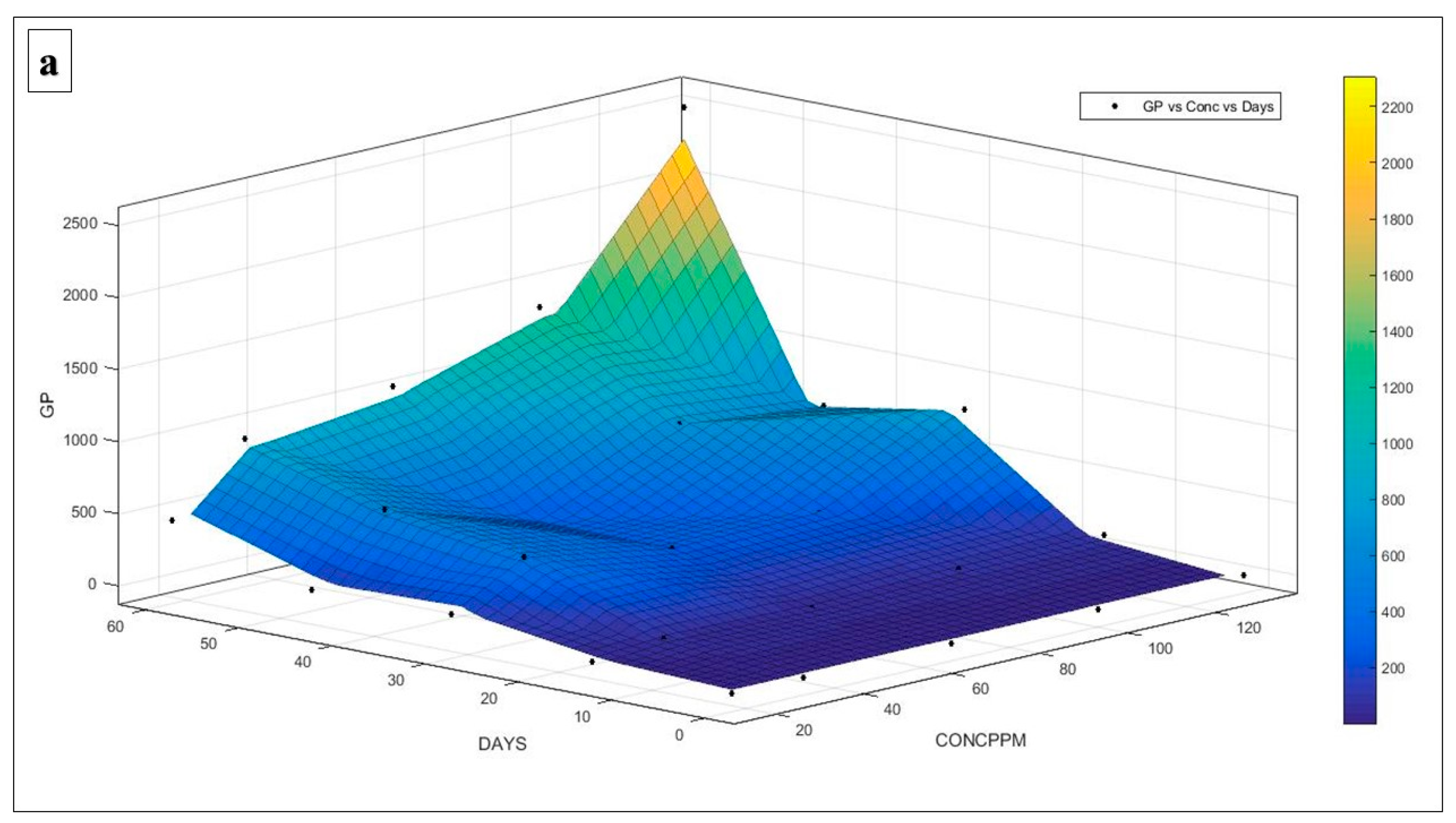
5.6. Mathematical Modelling
6. Results
6.1. Relative Growth Rate (RGR)
6.2. Changes in Physiochemical Parameters
6.3. Pearson Correlation and Principal Component Analysis (PCA)
6.4. Establishment of an Appropriate Model
7. Theoretical Methane Production Potentiality
8. Future Prospects
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koley, A.; Bray, D.; Banerjee, S.; Sarhar, S.; Thahur, R.G.; Hazra, A.K.; Mandal, N.C.; Chaudhury, S.; Ross, A.B.; Camargo-Valero, M.A.; et al. Water hyacinth (Eichhornia crassipes) a sustainable strategy for heavy metals removal from contaminated waterbodies. In Bioremediation of Toxic Metals; CRC Press: Boca Raton, FL, USA, 2022; pp. 95–114. [Google Scholar]
- Ullah, A.; Mushtaq, H.; Ali, H.; Munis, M.F.H.; Javed, M.T.; Chaudhary, H.J. Diazotrophs-assisted phytoremediation of heavy metals: A novel approach. Environ. Sci. Pollut. Res. 2015, 22, 2505–2514. [Google Scholar] [CrossRef]
- Jeong, S.; Moon, H.S.; Nam, K. Increased ecological risk due to the hyperaccumulation of As in Pteris cretica during the phytoremediation of an As-contaminated site. Chemosphere 2015, 122, 1–7. [Google Scholar] [CrossRef]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Mazumdar, K.; Das, S. Phytoremediation of Pb, Zn, Fe, and Mg with 25 wetland plant species from a paper mill contaminated site in North East India. Environ. Sci. Pollut. Res. 2015, 22, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Rai, S.; Gautam, K.; Sharma, S. Phytoremediation strategies of plants: Challenges and opportunities. In Plants and Their Interaction to Environmental Pollution; Elsevier: Amsterdam, The Netherlands, 2023; pp. 211–229. [Google Scholar]
- Koley, A.; Ghosh, A.; Banerjee, S.; Gupta, N.; Thakur, R.G.; Show, B.K.; Chaudhury, S.; Hazra, A.K.; Ross, A.B.; Nahar, G.; et al. Phytoremediation of Wastewater Discharged from Paper and Pulp, Textile and Dairy Industries using Water Hyacinth (Eichhornia crassipes). In Bioremediation for Sustainable Environmental Cleanup; CRC Press: Boca Raton, FL, USA, 2024; pp. 238–261. [Google Scholar]
- Getahun, S.; Kefale, H. Problem of Water Hyacinth (Eichhornia crassipes (Mart.)) in Lake Tana (Ethiopia): Ecological, Economic, and Social Implications and Management Options. Int. J. Ecol. 2023, 2023, 4618069. [Google Scholar] [CrossRef]
- Bajpai, S.; Nemade, P.R. An integrated biorefinery approach for the valorization of water hyacinth towards circular bioeconomy: A review. Environ. Sci. Pollut. Res. 2023, 30, 39494–39536. [Google Scholar] [CrossRef]
- Kushwaha, A.; Hans, N.; Upadhyay, N.; Goswami, S.; Pal, P.; Benettayeb, A.; Choi, Y.; Goswami, L.; Kim, B.S. Valorization of water hyacinth: A sustainable route for bioenergy generation and other value-added products. In Bio-Based Materials and Waste for Energy Generation and Resource Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–30. [Google Scholar]
- Madikizela, L.M. Removal of organic pollutants in water using water hyacinth (Eichhornia crassipes). J. Environ. Manag. 2021, 295, 113153. [Google Scholar] [CrossRef]
- Reddy, K.R.; DeBusk, T.A. State-of-the-art utilization of aquatic plants in water pollution control. Water Sci. Technol. 1987, 19, 61–79. [Google Scholar] [CrossRef]
- Erdoğan, E.M.; Özkan, M. Chromium and Lead Phytoremediation with Water Hyacinth and Use of Contaminated Biomass as Feedstock for Biofuel Production. Biomass Convers. Biorefin. 2023, 1–14. [Google Scholar] [CrossRef]
- Das, E.J.; Bhuiyan, M.A.R.; Hasan, M.M. Implementation of water hyacinth (Eichhornia crassipes) and water lettuce (Pistia stratiotes) in the re-treatment of conventionally treated pharmaceutical wastewater: A case study of Radiant Pharmaceuticals Limited, Dhaka, Bangladesh. Environ. Monit. Assess. 2023, 195, 1210. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, M. Aquatic plant remediation to control pollution. In Biological Approaches to Controlling Pollutants; Woodhead Publishing: Sawston, UK, 2022; pp. 365–397. [Google Scholar]
- Ibrahim, H.S.; Ammar, N.S.; Soylak, M.; Ibrahim, M. Removal of Cd (II) and Pb (II) from aqueous solution using dried water hyacinth as a biosorbent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Fedler, C.B.; Duan, R. Biomass production for bioenergy using recycled wastewater in a natural waste treatment system. Resour. Conserv. Recycl. 2011, 55, 793–800. [Google Scholar] [CrossRef]
- Ansari, A.A.; Naeem, M.; Gill, S.S.; AlZuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Pang, Y.L.; Quek, Y.Y.; Lim, S.; Shuit, S.H. Review on phytoremediation potential of floating aquatic plants for heavy metals: A promising approach. Sustainability 2023, 15, 1290. [Google Scholar] [CrossRef]
- Aoyama, I.; Nishizaki, H. Uptake of nitrogen and phosphate, and water purification by water hyacinth Eichhornia crassipes (Mart.) Solms. Water Sci. Technol. 1993, 28, 47–53. [Google Scholar] [CrossRef]
- Malik, A. Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ. Int. 2007, 33, 122–138. [Google Scholar] [CrossRef]
- Chaudhuri, S.R.; Salodkar, S.; Sudarshan, M.; Mukherjee, I.; Thakur, A.R. Role of water hyacinth-mediated phytoremediation in wastewater purification at East Calcutta wetland. Environ. Sci. 2008, 5, 53–62. [Google Scholar] [CrossRef]
- Zheng, J.; Chan, Z.; Chen, L.; Zhu, P.; Shen, J. Feasibility studies on N and P removal using water hyacinth in Taihu Lake region. Jiangsu Agric. Sci. 2008, 3, 247–250. [Google Scholar]
- Fox, L.J.; Struik, P.C.; Appleton, B.L.; Rule, J.H. Nitrogen phytoremediation by water hyacinth (Eichhornia crassipes (Mart.) Solms). Water Air Soil Pollut. 2008, 194, 199–207. [Google Scholar] [CrossRef]
- Liao, S.W.; Chang, W.L. Heavy metal phytoremediation by water hyacinth at constructed wetlands in Taiwan. J. Aquat. Plant Manag. 2004, 42, 60–68. [Google Scholar]
- Fazal, S.; Zhang, B.; Mehmood, Q. Biological treatment of combined industrial wastewater. Ecol. Eng. 2015, 84, 551–558. [Google Scholar] [CrossRef]
- Buhari, J.; Hasan, H.A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R. Future and challenges of co-biofilm treatment on ammonia and Bisphenol A removal from wastewater. J. Water Process Eng. 2023, 54, 103969. [Google Scholar] [CrossRef]
- Kumar, S.; Malav, L.C.; Malav, M.K.; Khan, S.A. Biogas slurry: Source of nutrients for eco-friendly agriculture. Int. J. Extens. Res. 2015, 2, 42–46. [Google Scholar]
- Musse, Z.A.; Yoseph Samago, T.; Beshir, H.M. Effect of liquid bio-slurry and nitrogen rates on soil physico-chemical properties and quality of green bean (Phaseolus vulgaris L.) at Hawassa Southern Ethiopia. J. Plant Interact. 2020, 15, 207–212. [Google Scholar] [CrossRef]
- Tang, J.; Yin, J.; Davy, A.J.; Pan, F.; Han, X.; Huang, S.; Wu, D. Biogas slurry as an alternative to chemical fertilizer: Changes in soil properties and microbial communities of fluvo-aquic soil in the North China Plain. Sustainability 2022, 14, 15099. [Google Scholar] [CrossRef]
- Malhotra, M.; Aboudi, K.; Pisharody, L.; Singh, A.; Banu, J.R.; Bhatia, S.K.; Varjani, S.; Kumar, S.; González-Fernández, C.; Singh, R. Biorefinery of anaerobic digestate in a circular bioeconomy: Opportunities, challenges and perspectives. Renew. Sustain. Energy Rev. 2022, 166, 112642. [Google Scholar] [CrossRef]
- Feng, L.; Guldberg, L.B.; Hansen, M.J.; Ma, C.; Ohrt, R.V.; Møller, H.B. Impact of slurry removal frequency on CH4 emission and subsequent biogas production; a one-year case study. Waste Manag. 2022, 149, 199–206. [Google Scholar] [CrossRef]
- Ke, L.; Liu, X.; Du, B.; Wang, Y.; Zheng, Y.; Li, Q. Component analysis and risk assessment of biogas slurry from biogas plants. Chin. J. Chem. Eng. 2022, 44, 182–191. [Google Scholar] [CrossRef]
- Palmer-Felgate, E.J.; Mortimer, R.J.; Krom, M.D.; Jarvie, H.P. Impact of point-source pollution on phosphorus and nitrogen cycling in stream-bed sediments. Environ. Sci. Technol. 2010, 44, 908–914. [Google Scholar] [CrossRef]
- Selvaraj, P.S.; Periasamy, K.; Suganya, K.; Ramadass, K.; Muthusamy, S.; Ramesh, P.; Palanisami, T. Novel resources recovery from anaerobic digestates: Current trends and future perspectives. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1915–1999. [Google Scholar] [CrossRef]
- Koley, A.; Mukhopadhyay, P.; Gupta, N.; Singh, A.; Ghosh, A.; Show, B.K.; GhoshThakur, R.; Chaudhury, S.; Hazra, A.K.; Balachandran, S. Biogas production potential of aquatic weeds as the next-generation feedstock for bioenergy production: A review. Environ. Sci. Pollut. Res. 2023, 30, 111802–111832. [Google Scholar] [CrossRef]
- Sharma, R.; Saini, H.; Paul, D.R.; Chaudhary, S.; Nehra, S.P. Removal of organic dyes from wastewater using Eichhornia crassipes: A potential phytoremediation option. Environ. Sci. Pollut. Res. 2021, 28, 7116–7122. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, X.; Srishti, A.; Song, S.; Tan, H.T.W.; Sweeney, D.J.; Ghosh, S.; Wang, C.H. Water hyacinth for energy and environmental applications: A review. Bioresour. Technol. 2021, 327, 124809. [Google Scholar] [CrossRef]
- Basu, A.; Hazra, A.K.; Chaudhury, S.; Ross, A.B.; Balachandran, S. State of the art research on sustainable use of water hyacinth: A bibliometric and text mining analysis. Informatics 2021, 8, 38. [Google Scholar] [CrossRef]
- Gupta, N.; Banerjee, S.; Koley, A.; Basu, A.; Gogoi, N.; Hoque, R.R.; Mandal, N.C.; Balachandran, S. Fungal Strategies for the Remediation of Polycyclic Aromatic Hydrocarbons. In Bioremediation for Sustainable Environmental Cleanup; CRC Press: Boca Raton, FL, USA, 2024; pp. 86–108. [Google Scholar]
- Banerjee, S.; Gupta, N.; Pramanik, K.; Gope, M.; GhoshThakur, R.; Karmakar, A.; Gogoi, N.; Hoque, R.R.; Mandal, N.C.; Balachandran, S. Microbes and microbial strategies in carcinogenic polycyclic aromatic hydrocarbons remediation: A systematic review. Environ. Sci. Pollut. Res. 2024, 31, 1811–1840. [Google Scholar] [CrossRef] [PubMed]
- Bagdi, T.; Ghosh, S.; Sarkar, A.; Hazra, A.K.; Balachandran, S.; Chaudhury, S. Evaluation of research progress and trends on gender and renewable energy: A bibliometric analysis. J. Clean. Prod. 2023, 423, 138654. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, S.M.E.; Rahman, M.M.; Oh, D.H.; Ra, C.S. The effects of biogas slurry on the production and quality of maize fodder. Turk. J. Agric. For. 2010, 34, 91–99. [Google Scholar] [CrossRef]
- Yu, F.B.; Luo, X.P.; Song, C.F.; Zhang, M.X.; Shan, S.D. Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agric. Scand. B Soil Plant Sci. 2010, 60, 262–268. [Google Scholar]
- Liang, F.; Xu, L.; Ji, L.; He, Q.; Wu, L.; Yan, S. A new approach for biogas slurry disposal by adopting CO2-rich biogas slurry as the flower fertilizer of Spathiphyllum: Feasibility, cost and environmental pollution potential. Sci. Total Environ. 2021, 770, 145333. [Google Scholar] [CrossRef] [PubMed]
- ASTM, E. 647; Annual Book of ASTM Standards. ASTM: Philadelphia, PA, USA, 1983; pp. 710–730.
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Waste Water, 19th ed.; American Public Health Association Inc.: New York, NY, USA, 1995; p. 1193. [Google Scholar]
- Buswell, A.M.; Mueller, H.F. Mechanism of methane fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Bray, D.G.; Ross, A.B. The Influence of Biochar Augmentation and Digestion Conditions on the Anaerobic Digestion of Water Hyacinth. Energies 2022, 15, 2524. [Google Scholar] [CrossRef]
- Bray, D.G.; Nahar, G.; Grasham, O.; Dalvi, V.; Rajput, S.; Dupont, V.; Camargo-Valero, M.A.; Ross, A.B. The Cultivation of Water Hyacinth in India as a Feedstock for Anaerobic Digestion: Development of a Predictive Model for Scaling Integrated Systems. Energies 2022, 15, 9599. [Google Scholar] [CrossRef]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of industrial mines wastewater using water hyacinth. Int. J. Phytoremediation 2017, 19, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Malik, S.A.; Hussain, S.T.; Aamir, S. Metallic phytoremediation and extraction of nanoparticles. Int. J. Phys. Sci. 2012, 7, 6105–6116. [Google Scholar]
- Panneerselvam, B.; Priya, K.S. Phytoremediation potential of water hyacinth in heavy metal removal in chromium and lead contaminated water. Int. J. Environ. Anal. Chem. 2023, 103, 3081–3096. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Chopra, A.K. Assessment of phytokinetic removal of pollutants of paper mill effluent using water hyacinth (Eichhornia crassipes [Mart.] Solms). Environ. Technol. 2018, 39, 2781–2791. [Google Scholar] [CrossRef]
- Rezania, S.; Fadhil, M.; Din, M.; Ponraj, M.; Sairan, F.M.; Fatimah, S.; Kamaruddin, B. Nutrient uptake and wastewater purification with Water Hyacinth and its effect on plant growth in batch system. J. Environ. Treat. Tech. 2013, 1, 81–85. [Google Scholar]
- Anipeddi, M.; Begum, S.; Anupoju, G.R. Integrated technologies for the treatment of and resource recovery from sewage and wastewater using water hyacinth. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 293–314. [Google Scholar]
- Jayaweera, M.W.; Kasturiarachchi, J.C. Removal of nitrogen and phosphorus from industrial wastewaters by phytoremediation using water hyacinth (Eichhornia crassipes (Mart.) Solms). Water Sci. Technol. 2004, 50, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, P.; Eid, E.M.; Taher, M.A.; El-Morsy, M.H.; Osman, H.E.; Al-Bakre, D.A.; Kumar, V. Phytoremediation of nitrogen and phosphorus pollutants from glass industry effluent by using water hyacinth (Eichhornia crassipes (Mart.) Solms): Application of RSM and ANN techniques for experimental optimization. Environ. Sci. Pollut. Res. 2023, 30, 20590–20600. [Google Scholar] [CrossRef] [PubMed]
- Mayo, A.W.; Hanai, E.E. Modeling phytoremediation of nitrogen-polluted water using water hyacinth (Eichhornia crassipes). Phys. Chem. Earth, Parts a/b/c 2017, 100, 170–180. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Yang, X.E.; Chang, H.Q.; Pu, P.M.; Ding, X.F.; Rengel, Z. Phytoremediation of nitrogen-polluted water using water hyacinth. J. Plant Nutr. 2007, 30, 1753–1765. [Google Scholar] [CrossRef]
- Barua, V.B.; Kalamdhad, A.S. Biochemical methane potential test of untreated and hot air oven pretreated water hyacinth: A comparative study. J. Clean. Prod. 2017, 166, 273–284. [Google Scholar] [CrossRef]
- Barua, V.B.; Kalamdhad, A.S. Anaerobic biodegradability test of water hyacinth after microbial pretreatment to optimise the ideal F/M ratio. Fuel 2018, 217, 91–97. [Google Scholar] [CrossRef]
- Barua, V.B.; Rathore, V.; Kalamdhad, A.S. Anaerobic co-digestion of water hyacinth and banana peels with and without thermal pretreatment. Renew Energy 2019, 134, 103–112. [Google Scholar] [CrossRef]
- Daniel, F.; Sekar, M.; Gavurová, B.; Govindasamy, C.; Boomadevi, P.; Praveenkumar, T.R. Recovering of biogas and nutrients via novel anaerobic co-digestion of pre-treated water hyacinth for the enhanced biogas production. Environ. Res. 2023, 231, 116216. [Google Scholar] [CrossRef]
- Show, B.K.; Shivakumaran, G.; Koley, A.; Ghosh, A.; Chaudhury, S.; Hazra, A.K.; Balachandran, S. Effect of thermal and NaOH pretreatment on water hyacinth to enhance the biogas production. Environ. Sci. Pollut. Res. 2023, 30, 120984–120993. [Google Scholar] [CrossRef]






| Current Study | Previous Studies | |||
|---|---|---|---|---|
| Characteristics | [46] | [47] | [48] | |
| Total Nitrogen (N) | 92 ± 6 mg/L | 5.88 mg/kg | 0.71 gm/L | 1255.05 ± 5.41 NH4+- N mg/L |
| Total Phosphorus | 15.23 ± 2 mg/L | 2.72 mg/kg | 15.5 gm/L | 64.01 ± 1.27 mg/L |
| Total Potassium (K) | 1600 mg/L | 1.33 mg/kg | 2.1 gm/L | 3006.81 ± 15.73 mg/L |
| pH | 7.53 | 7.8 | 7.80 ± 0.07 | |
| Electrical Conductivity | 18.6 dS/m | - | 30 dS/m | 5.67 ± 0.13 mS/cm |
| Chemical Oxygen Demand | 382.52 mg/L | - | 25,400 mg/L | - |
| TS (%) | 1.85 | |||
| VS (%) | 0.97 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koley, A.; GhoshThakur, R.; Das, K.; Gupta, N.; Banerjee, A.; Show, B.K.; Ghosh, A.; Chaudhury, S.; Hazra, A.K.; Nahar, G.; et al. Growth Dynamics and Nutrient Removal from Biogas Slurry Using Water Hyacinth. Sustainability 2024, 16, 4450. https://doi.org/10.3390/su16114450
Koley A, GhoshThakur R, Das K, Gupta N, Banerjee A, Show BK, Ghosh A, Chaudhury S, Hazra AK, Nahar G, et al. Growth Dynamics and Nutrient Removal from Biogas Slurry Using Water Hyacinth. Sustainability. 2024; 16(11):4450. https://doi.org/10.3390/su16114450
Chicago/Turabian StyleKoley, Apurba, Richik GhoshThakur, Kaushik Das, Nitu Gupta, Aishiki Banerjee, Binoy Kumar Show, Anudeb Ghosh, Shibani Chaudhury, Amit Kumar Hazra, Gaurav Nahar, and et al. 2024. "Growth Dynamics and Nutrient Removal from Biogas Slurry Using Water Hyacinth" Sustainability 16, no. 11: 4450. https://doi.org/10.3390/su16114450









