Abstract
Water pollution has become a major environmental menace due to municipal and industrial effluents discharged into water bodies. Several processes have been devised for the treatment and disposal of wastewater and sludge. Yet, most of the conventional technologies do not meet the requirements of sustainability as they impose a higher load on the environment in terms of resource depletion and toxic waste generation. Recently, sustainable innovative technologies, like hydrodynamic cavitation (HC), have emerged as energy-efficient methods, which can enhance the conventional wastewater treatment processes. HC is a very effective technique for the intensification of processes, like aeration, activated sludge treatment, and anaerobic digestion processes in conventional wastewater treatment plants, particularly for the enhanced degradation of persistent pollutants. On the other hand, advanced oxidation is a proven enhancement method for wastewater treatment. This review provides a comprehensive overview of recently published literature on the application of HC for the treatment of persistent organic pollutants. The potential synergistic impact of HC coupled with advanced oxidation and alternative pre-treatment methods was also reviewed in this study. Moreover, an overview of the present state of model-based research work for HC reactors and a feasibility analysis of various advanced oxidation process is also covered. Options for the pilot-to-large scale implementation of HC and advanced oxidation technologies to ensure the better sustainability of wastewater treatment plants are recommended.
1. Introduction
The biological treatment of water involves the utilization of either aerobic or anaerobic processes to remove significant quantities of digested or activated sludge [1]. The wastewater treatment has the potential to generate substantial quantities of waste, typically ranging from 5 to 35% of the total amount of wastewater that has been treated [2]. The composition of bio-sludge primarily comprises extracellular polymeric substances, water, and biomass. Through the use of microorganisms in biomass, biological treatment techniques can oxidize or reduce organic contaminants [3]. The secondary treatment process is typically carried out by simulating suitable conditions, such as the pH and oxygen concentration, within a bioreactor. The running costs associated with biological processes are typically low, while the capital expenses associated with the agitators and aeration pumps that are employed can be quite substantial [4,5]. A significant limitation of biological processes pertains to their exclusive capacity to metabolize solely biodegradable substances. The presence of persistent compounds, noxious and recalcitrant, in wastewater may hinder the biological treatment procedure and the metabolic activity of microorganisms [6]. The capacity of the biomass to metabolize complex molecules that result in a significant chemical oxygen demand (COD) is limited. When the organic load is high, it may result in longer retention times for biological processes, which can lead to increased costs associated with aeration [7,8].
The integration of biological processes with advanced oxidation techniques can be a viable approach for the degradation of persistent and recalcitrant pollutants in wastewater. This can lead to a reduction in processing costs by facilitating biological treatment [9]. Recent decades have seen a rise in interest in cavitation, a novel enhanced oxidation process. Cavitation refers to the rapid formation, expansion, and collapse of vapor-filled cavities within a very short time frame, resulting in the production of powerful shockwaves. Furthermore, the disintegration of voids results in the production of extremely reactive hydroxyl radicals, which contribute to chemical phenomena, and the generation of high turbulence and shear mixing, which contribute to physical phenomena [10,11].
The generation of cavitation can be achieved through two distinct methods, which involve the utilization of sudden constriction and high pressure flow, while acoustic cavitation involves the application of ultrasound processes [12]. Numerous studies have reported the occurrence of cavitation as a means of degrading organic pollutants in wastewater. However, it is noteworthy that the majority of these studies have focused on the degradation of a single contaminant [7,13,14].
Cavitation has been the subject of recent research as a potential method for treating actual industrial effluents and as a viable pre-treatment option in lieu of advanced oxidation processes [7,12]. The combination of biological and cavitation processes presents significant potential in terms of economic and environmental benefits. Frequently, the occurrence of enduring transitional stages during cavitation results in a comparatively lesser decline in COD by as much as 40% from the initial reduction. However, the hydroxyl radicals have the potential to decompose substantial biorefractory compounds into smaller biodegradable molecules, thereby facilitating a more effective biological oxidation process [7,15].
The utilization of cavitation as a pre-treatment method has been observed to effectively disrupt hemicellulose structures and enhance the digestibility of sludge, thereby increasing its solubility [16]. The utilization of cavitation as a pre-treatment method is highly advantageous owing to its cost-effectiveness, absence of supplementary chemical usage, and absence of additional waste generation, as reported in previous studies [8]. Cavitation has demonstrated the effective degradation of various compounds, including phenol, dimethylformamide, magenta dye, 2-chlorophenol, methylene blue, triclosan, rhodamine B, octanol, cyclohexanol, and naproxen [17,18]. HC is deemed to possess superior energy efficiency and is frequently deemed appropriate for commercial-scale implementation and expansion.
This study aims to provide a comprehensive review of HC as a mean of treating wastewater that contains hazardous and complex structured pollutants. The paper objectives are to conduct a comprehensive analysis of all operational parameters that may impact HC and evaluate the effectiveness of HC in combination with hydrogen peroxide for the purpose of mitigating the overall organic burden and persistent organic pollutants in wastewater treatment. Additionally, this paper provides an overview of current modelling approaches for HC reactors and compares the costs of various advanced oxidation processes, as reported in the existing literature. Finally, the pilot and full-scale implementation of HC and advanced oxidation for water purification are discussed.
2. Overview of Cavitation
Cavitation is commonly classified as an advanced oxidation process (AOP), which falls within the category of emerging technologies that have demonstrated the efficient degradation of nonbiodegradable pollutants [4]. Various methods, such as hydrodynamic or acoustic cavitation, have been utilized and documented for their effectiveness, either individually or in conjunction with hydrogen peroxide, catalysts, ultraviolet (UV) irradiation, persulfates, the Fenton process, and ozonation [9].
It is imperative to acknowledge that the phenomenon of acoustic cavitation presents significant challenges in terms of scaling up, primarily due to the substantial increase in energy demands and associated treatment expenses. In contrast, the utilization of HC reactors is deemed a viable alternative due to their superior energy efficiencies and cost-effectiveness in operation. The level of mineralization in wastewater treatment, particularly for complex compounds utilizing hydroxyl radicals alone, is limited due to the inadequate production of oxidizing radicals [6,8,19]. The efficacy of the treatment utilizing hydroxyl radicals can be enhanced through the integration of AOPs. This combination results in a greater production of oxidizing radicals, leading to an augmented degradation rate of the pollutant.
In their study, Gagol et al. [20] investigated the degradation of various organic compounds, including different derivatives of Benzene Tetracarboxylic Acid (BTEX), phenol, and organosulfur compounds. They employed both HC and acoustic cavitation techniques, in conjunction with ozone, hydrogen peroxide, and peroxone, to carry out the degradation processes. These experiments were conducted under basic pH conditions. The utilization of a combination of HC and AOPs in the complete oxidation of organic compounds has demonstrated the superior efficacy of HC-based combinations when compared to individual operations [20]. The degradation of volatile organic compounds was investigated by Mohod et al. [11] through the utilization of a combined method that incorporates HC and AOPs. According to the report, the application of HC in conjunction with ozonation has resulted in a maximum reduction of 40% in COD and 50% in the biochemical oxygen demand. The findings of the study provide confirmation that the use of heterogeneous catalysts in conjunction with AOPs is an effective method for the degradation of volatile organic compounds [11].
Nevertheless, the utilization of heterogeneous catalysts and AOPs results in the significant consumption of chemical substances in order to achieve complete pollutant removal. Consequently, this leads to an overall escalation of the overall price of treatment. Hence, the utilization of HC-based processes as a pre-treatment method for biological oxidation presents an intriguing approach. Several literature sources documented the utilization of AOPs as a means of pre-treatment for biological oxidation processes. This approach aims to facilitate the degradation of harmful pollutants found in wastewater [21,22].
The combined approach of Fenton and biological oxidation was investigated by [7] for the removal of bio recalcitrant pollutants from industrial effluent. Three distinct types of effluents, originating from landfill leachate, an industrial hazardous waste landfill, and a plywood manufacturing factory, were subjected to a treatment process involving Fenton as a pre-treatment method, followed by conventional biological treatment. The reported reduction in both the COD and biochemical oxygen demand exceeded 90% for all effluents. Gostisa, along with their colleagues, conducted a study on the elimination of pesticide compounds from aqueous solutions through the implementation of AOPs in conjunction with a biological treatment approach. According to the report, a significant COD-removal efficiency exceeding 95% was achieved through the utilization of a combined O3/UV system in conjunction with biological oxidation [23]. In a similar vein, Esmaeeli and colleagues, conducted a study on the elimination of ethylbenzene and pnitrophenol from artificially prepared wastewater. They employed a hybrid approach that combined ultrasound-assisted Fenton methodology with the conventional aerobic process. The study documented a notable increase in the biodegradability index from 0.15 to 0.36, which was successfully accomplished in a span of 40 min through the utilization of the ultrasound-assisted Fenton process [16]. Table 1 provides a comprehensive summary of the existing research that examines the efficacy of different hydraulic conductivity geometries and hybrid methodologies in the degradation of diverse water pollutants.

Table 1.
Synopsis of treating wastewater with HC in conjunction with AOPs.
3. Hydrodynamic Cavitation Mechanism
Cavitation bubbles, also known as cavities, usually occur when the pressure in a specific area drops below the vapor pressure of the liquid [18]. Bernoulli’s principle provides a framework for attaining pressure reduction in a flow system, where changes in the liquid velocity and pressure distribution are given by Equation (1). Constricting fluid passageways is a widely used technique to increase fluid velocity, resulting in a corresponding drop in pressure. Instances of such limitations encompass venturi tubes and spray nozzles [31]. The equation involves the fluid density, denoted by q, and the pressures at two places in the flow system, commonly referred to as p1 and p2 (upstream and downstream, respectively). Additionally, v1 and v2 indicate the fluid velocities at these respective sites. The fluid velocity within the tube escalates, resulting in a decrease in pressure. The narrowest part of the system, where the liquid reaches its highest speed (v2), corresponds to the location of the lowest pressure (p2).
Vapor bubble production is hypothesized to happen when the pressure in a specific area drops below the vapor pressure of the liquid at the current temperature. If the pressure of p2 falls below the vapor pressure, it is possible for vapor bubbles to form. After the narrowing, there is a simultaneous occurrence of rapid pressure restoration and bubble implosion, resulting in the release of a substantial amount of energy. The degree of cavitation and the strength of energy release are directly linked to the pressure at the throat, highlighting the need for accurately forecasting the onset of cavitation. This prediction not only enhances the comprehension of cavitation physics but also facilitates the analysis of flow patterns in hydrodynamic cavitation processes and the development of cavitation devices [20].
Initiation of Hydrodynamic Cavitation
The initiation of cavitation, which signifies the beginning of cavitation phenomena, is a critical factor in both preventing the formation of cavitation and utilizing its advantages in the dynamics of liquid flow. This parameter is crucial in determining the hydrodynamics of a liquid system. The complexity of cavitation inception stems from its reliance on multiple parameters, such as the presence of seeding nuclei, fluid velocity, physical characteristics, and system pressure. Despite extensive study efforts, a thorough comprehension of the initiation of cavitation remains difficult. Thoma’s Cavitation index (α) is used for the purpose of characterizing cavitation. In this index, ps represents the suction pressure of the pump, pv is the vapor pressure of the liquid at its temperature, and ∆p is the pressure increase from suction to discharge at the pump’s best efficiency point. While originally suggested for use in pump applications, this parameter showed a disadvantage as a result of variability in the parameters among different pumps [6].
Plesset [1] proposed a cavitation parameter, ξ, to solve the constraint in studying liquid flow over a submerged object. This value allows for a qualitative correlation of flow patterns.
The cavitation parameter, ξ, often known as the “cavitation number” in current research, is an important parameter for describing the features of cavitation flow. In open water systems, the cavitation number of each flow is inversely proportional to the fluid velocity. The onset of cavitation is characterized by the formation of a cloud of cavitation. According to Equation (3), the cavitation number is influenced by both the static pressure and the velocity of the liquid. This dimensionless quantity is used in closed systems, such as venturi and orifices, where it is important to specify pressures and liquid velocities. A lower cavitation number increases the chances of cavitation happening or makes it more intense, as emphasized by Bagal and Gogate [32], who stated that cavitation usually starts when the cavitation number reaches around 1, with the best cavitation performance observed within a cavitation number range of 0.1 to 1.0. Cavitation inception, a complex occurrence, is linked to several characteristics, with cavitation nuclei being of utmost significance [21,22].
The distinctive environment enables the formation of cavitation by decreasing the required tensile strength of the liquid, resulting in a series of dynamic processes. The analysis of these processes entails a comprehensive investigation, considering factors, such as intermolecular forces at the interface, the influence of viscosity, and the presence of non-condensable gases. Although the cavitation number is commonly used to evaluate the probability of cavitation genesis, its shortcomings become evident when considering the complex dynamics involved. The cavitation number alone is insufficient in capturing the numerous intricacies and is inadequate for accurately determining the specific conditions for cavitation inception, as it heavily relies on several physical features.
Šarc et al. [33] underlined the several parameters that contribute to the beginning of cavitation, with particular attention to the shape of the constriction, the temperature of the medium, and the size and density of the cavitation nuclei. The study conducted by Yan and Thorpe [34] provides more understanding of the complex relationship between the cavitation number and shape. Their studies unveiled a strong correlation, indicating that the cavitation inception number exhibited considerable variation, ranging from 1.7 to 2.4, for orifice-to-pipe diameter ratios ranging from 0.4 to 0.8. Expanding on these discoveries, Cioncolini et al. [35] suggested that micro-orifices could potentially have significantly lower cavitation numbers at the beginning of cavitation, introducing an additional level of intricacy to the forecasting of the cavitation origin. Table 2 provides a complete compilation of cavitation inception numbers found in the literature, aiming to consolidate the extensive knowledge on this topic. This table demonstrates the significant range of values for the cavitation number that indicate the start of cavitation, ranging from less than 1 to greater than 3. The variability depends on a wide range of operational settings, geometric parameters, and the distinct properties of cavitation nuclei. The task of precisely forecasting the onset of cavitation is a persistent difficulty, as our current comprehension mainly depends on experimental findings that provide limited insight into the complex realm of cavitation dynamics. As researchers explore the intricacies of these processes, the pursuit of a more accurate and thorough comprehension of cavitation initiation remains ongoing [36].

Table 2.
Cavitation inception numbers reported in the literature.
In a previous study by Agarkoti et al. [12], a comparative analysis was performed to evaluate the dimensions of bubbles produced at the outlet of both the HC and bubbling reactors. The research findings indicated a notable discrepancy in the sizes of bubbles observed in the HC reactor (0.62 mm) compared to the bubbling reactor (23.19 mm). As illustrated in Figure 1, the high-velocity solution is introduced into the HC reactor, leading to the creation of reduced suction pressure. According to the findings of Gujar’s research, it has been noted that the pressure experienced during cavitation tends to be lower than the saturated vapor pressure at the corresponding temperature [4]. When pressure is low, the liquid film in the cavitation evaporates inward, equalizing the pressure difference between the interior and exterior. As the decline in pressure persists, the bubble experiences a swift expansion. The cavity undergoes a perpetual influx of gaseous molecules that have transitioned from the liquid film through evaporation. Gas-filled bubbles are formed in an environment of low pressure and are subsequently compressed by the restoring pressure of the HC reactor. This compression causes the bubbles to decrease in volume, reaching a range of 0.50–1.50 mm [12,38]. Furthermore, the prevention of the collapse of gas-filled bubbles is attributed to their gradual compression. However, the limited space within these areas increases the occurrence of collisions between •OH molecules, thus facilitating chemical reactions in the gas phase. Moreover, the compression of the gas-filled bubbles causes an increase in temperature. As a result, the gas liquid mass transfer increases as the velocity and frequency with which NO molecules strike the surface of the gas-filled bubbles [13].
Figure 1.
Hydrodynamic cavitation process in a venture cavitator.
The generation of radicals inside the collapsing bubble and their dispersion in the bulk solutions are two essential phases that determine the oxidation potential of cavitation-induced radicals for the bubbles undergoing stable oscillation. The distribution in the second step affects the likelihood of the interaction with the pollutants, whereas the radicals produced in the first step define the types and concentrations of the oxidants [39].
4. Effect of Operating Parameters
4.1. Geometry of Hydrodynamic Cavitation
The typical configurations for processing systems utilizing HC reactors often involve a recirculation-type setup. The setup consists of multiple components, including control valves, a positive displacement pump, gauges, and a holding tank [40]. The suction side of the pump is connected to the lower section of the holding tank. The discharge from the pump is divided into two lines, specifically the primary conduit and a secondary conduit. The main channel is provided with a cavitation chamber, such as a slit venturi or orifice plates, while the secondary channel is fitted with a valve to control the flow within the main channel. Moreover, the arrangement encompasses pressure gauges. In order to mitigate the ingress of air into the liquid, it is ensured that both lines are terminated at a position below the liquid level within the holding tank [16].
Orifice plates have the capability to be fitted with either a solitary or multiple openings. In cases where multiple apertures are utilized, a range of various combinations involving hole diameters and quantities can be employed. The utilization of an orifice plate with multiple holes allows for the acquisition of different levels of cavitation intensity in this variation [10]. According to reference [41], the relationship between intensity and the ratio of the hole perimeter to cross-sectional area is directly proportional. Hence, the utilization of an orifice plate in the HC reactor offers the benefit of adjusting the intensity to fulfil the specific demands of a given application, while also enabling control over the inlet flow rate, inlet pressure, and temperature [42]. The impact of multiple-hole-orifice-plate geometry on the rhodamine B solution degradation, a cationic dye, was investigated by [43]. They have come to the conclusion that the cavitational yield in hydrodynamic cavitation might be increased by changing the flow geometry and, therefore, the turbulent pressure fluctuation frequency. By adjusting the cavitation device’s geometry and flow conditions, the ideal frequency of turbulence can be reached. They have found that, in order to obtain a bigger area occupied by the shear layer due to a higher perimeter value, it is desirable to utilize a plate with a smaller hole size opening, hence increasing the number of holes, for the plates having the same cross-sectional flow area. The venturi device demonstrates a greater velocity at the constriction point in comparison to an orifice when exposed to an equivalent pressure differential. The smooth convergence and divergence of the venturi sections are responsible for this phenomenon. The venturi configuration is frequently utilized in processes characterized by lower intensity, typically requiring pressures ranging from 15 to 20 bar [44,45], and involving physical transformations. In contrast, the utilization of the orifice flow configuration is commonly observed in the context of more intense chemical reactions [17,46]. However, a research investigation in the field of disinfection against pathogenic microorganisms has demonstrated that venturi-type reactors demonstrate enhanced efficacy in comparison to orifice plates [47].
According to previous studies, venturi tubes have been theoretically shown to produce denser cavitation clouds and provide extended durations for bubble growth and collapse [41]. Typically, these circumstances result in increased turbulence and heightened collapse intensity beyond the constriction. The application of device geometry to practical scenarios necessitates a meticulous examination and analysis. Imperfect conditions often yield poor COD reductions even when optimum pressures may have been applied; they include collapse conditions, the turbulence intensity, and velocity gradients. More holes in an orifice could theoretically result in more jet streams, which should increase the cavitational effects. In practice, though, the pressure and geometry play more significant roles [48].
The study conducted by [31] has established two parameters, α and β0, which have been specifically devised to analyze the performance of orifices in relation to their geometric characteristics. In another study [49] t was observed that the α parameter, which represents the ratio of the total perimeter to total area, exhibited the greatest degradation in the constriction with the highest velocity, as indicated in Table 3. Likewise, a decreased β0 value, which represents the ratio of the total area to the cross-section of the pipe, is associated with increased cavitational intensity. Gogate and Pandit [13] emphasized the necessity of optimizing the diameter of orifices in accordance with the specific application in each study. In the context of orifice plates, the velocities experienced by the fluid passing through the orifice are observed to increase as a consequence of the abrupt reduction in the available area for flow. This increase in velocities subsequently leads to a decrease in the pressure. When the velocities reach a level where their increase is adequate to cause the local pressure to drop below the vapor pressure of the medium under operating conditions, the formation of cavities occurs. Cavities of this nature are observed at various locations within the reactor, with their occurrence being closely tied to the quantity of holes present in the orifice plates. At the location downstream of the orifice, there is an expansion in the cross-sectional area, resulting in a reduction in velocities, which leads to an increase in pressures and the occurrence of pressure fluctuations. These pressure variations play a crucial role in governing the various phases of cavitation, including formation, growth, and collapse [26,50,51].

Table 3.
Hydraulic characteristics of three distinct orifice plate geometries.
4.2. Hydrodynamic Cavitation Pressure
The induction of HC is significantly influenced by the inlet pressure and cavitation number. The formation and severity of cavities are dependent upon the pressure exerted on the liquid medium during its passage through a constriction [26,27]. The efficiency of wastewater treatment can be improved by promoting contaminant degradation through the use of optimal cavity numbers. The parameter known as the cavitation number exhibits a direct correlation with the pressure and serves as a means of assessing cavitational apparatuses, as well as facilitating cross-process comparisons of outcomes [21].
The formation of cavities through cavitation is postulated to occur when the cavitation number descends below the limit of unity. If the pressure is increased beyond the optimal level, it can lead to a significant reduction in the cavitation number. This, in turn, can result in the formation of an excessive number of coalescence, cavities, and the creation of a cloud of cavities. At elevated pressures and cavitation numbers, multiple concerns emerge.
Research findings suggest that an ideal range of inlet pressure between 4 to 10 bars results in enhanced biodegradability [17]. The impact of pressure variation on the treatment of distillery wastewater was investigated. Results indicated that the increase in pressure from bar 8 to 13 bar did not have a significant effect on COD reduction, with both pressures resulting in 32% and 34% reduction, respectively. However, the final biodegradability was enhanced at the higher pressure, with a value of 0.32 compared to 0.13 at the lower pressure. This was reported in a previous study [26]. The utilization of high pressures (>11 bars) in the process has several drawbacks, such as increased treatment expenses, impeller and pump damage caused by overheating, the creation of cavity clouds, and insufficient cavitation effects. The majority of research endeavors have reported an ideal pressure range of no more than 8 bars, accompanied by a cavitation number exceeding 0.07. The utilization of elevated pressures in cavitation presents benefits provided that it circumvents adverse consequences, such as corrosion, and remains within operational thresholds. Equation (4) defines the cavitation number, which is a useful parameter for characterizing cavitation conditions. The equation includes downstream pressure (P2), the vapor pressure of the liquid (Pv), and liquid velocity at the throat of the orifice (Vo).
4.3. Effect of Temperature
The impact of temperature on the evaporation pressure of liquids makes it a critical factor in cavitation-based processes. Extensive research has been conducted on the correlation between elevated temperatures and the augmentation of cavitation bubble quantities [19]. In cases in which the temperature is excessively elevated, the increased vapor pressure of the liquid causes a significant increase in the vapor content within the cavities. Consequently, this results in the cushioning of the cavity implosion [1]. In the majority of studies conducted, it has been consistently observed that there is an optimal temperature at which HC exhibits its highest level of activity. The applicability of an optimal solution may vary across different applications, and the specific value of the optimum will be contingent upon the particular application. Consequently, it is crucial to conduct laboratory-scale studies tailored to the specific application in order to meet this requirement [52].
The results of the discoloration experiments conducted on Orange acid-II [27] and Orange 7 [10] indicate that lower temperatures have a positive effect on enhancing the discoloration process. However, the experimental investigation on the degradation of 2,4-dinitrophenol revealed that the degree of degradation exhibited an upward trend as the temperature increased, reaching its peak at temperatures above 35 °C, after which it experienced a slight decline [53]. The degradation of alachlor also exhibited a similar trend, as reported in a previous study [27]. The degradation rate exhibited an upward trend as the temperature increased within the range of 30–40 °C but experienced a decline once the temperature surpassed 40 °C. It is imperative to acknowledge that maintaining an appropriate temperature is crucial in order to prevent adverse consequences, such as thermal degradation of the fluid or harm to the equipment employed during the cavitation process. Moreover, elevated temperatures have the potential to result in an increase in energy usage and subsequent financial burdens [54].
4.4. Effect of pH
The consideration of hydrogen ion activity and pH is crucial in the treatment of wastewater. The selection of optimum pH conditions, based on comprehensive experimental studies, can significantly enhance the degradation efficiency by over 50%. The determination of optimum pH values can be broadly applied to specific categories of compounds, such as aromatic amines and certain azo dyes. However, when dealing with actual wastewaters, it becomes necessary to conduct experiments that specifically investigate the effects of pH variations. Although several studies have neglected to investigate the specific parameter of cavitation utilization as a pre-treatment for biological oxidation [10,13,55], several reviews have underscored the significance of examining pH in processes based on cavitation [49,56].
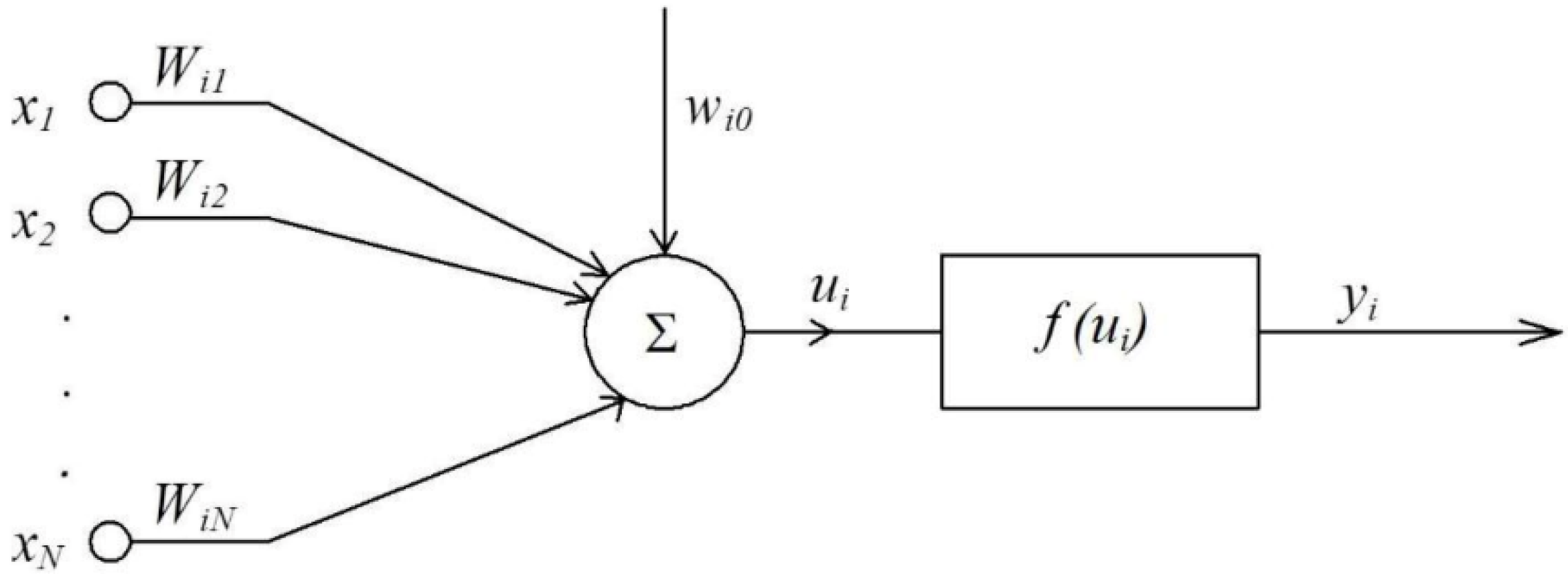
The model of the neural network emerged with McCulloch’s and Pitts’ seminal work, which was the first to describe a neuron in mathematical terms and associate it with data processing. One of the key elements of the model is summing up the input signals with an appropriate weight, and subjecting the result to the nonlinear activation function as shown in Figure 2 [57].
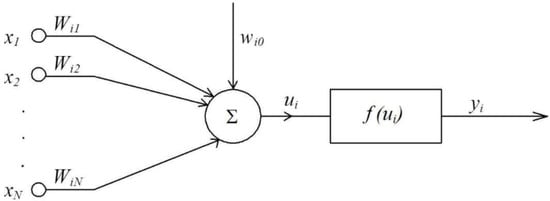
Figure 2.
McCulloch–Pitts’ single neuron model [57].
The degradation of organic compounds is generally more pronounced under acidic conditions as a result of the enhanced dissociation of peroxide in water and the diminished recombination of radicals [19,45,58]. The pH levels that are deemed optimal for tannery wastewaters and fish processing wastewaters generally lie within the range of 4 to 7. The outcomes of the research conducted on pH adjustments for these wastewaters did not demonstrate any significant improvements [9,41]. The study conducted on vegetable oil refinery wastewater found that the greatest enhancement in BI was observed at a pH level of 4 [59]. Furthermore, it is imperative to perform the pH adjustment prior to engaging in biological oxidation processes. In instances where a low pH level results in the most favorable enhancement of BI, it becomes necessary to incorporate a pH adjustment tank both prior to and following the cavitating unit. This adjustment introduces a shift in attention towards increased capital and operational expenses associated with the treatment process.
The optimal pH range for biological processes can vary from 6.0 to 8.0. However, the utilization of wastewater with a low pH of 2–3 can lead to the degradation of microfauna, significantly diminishing the effectiveness of wastewater treatment [9]. The presence of a significantly high alkaline pH in wastewater has the potential to induce corrosion or the precipitation of metals. It is advisable to utilize a pH level appropriate for biological oxidation during cavitation pre-treatment, unless there is a substantial impact of pH observed in the cavitation treatment that justifies the additional expenses associated with the pH adjustment [18].
4.5. Effect of Addition of Oxidants
Hydrogen peroxide is the prevailing oxidant typically employed in conjunction with cavitation. The inclusion of hydrogen peroxide has consistently demonstrated enhanced COD reduction and increased biodegradability across various wastewater compositions. Increasing the loading of hydrogen peroxide (H2O2) up to an optimal level results in a greater reduction in COD due to the increased presence of hydroxyl radicals [60]. It is worth emphasizing once more that elevated peroxide loadings result in the presence of residual peroxide in the wastewater, which hinders and impedes subsequent biological processes. Therefore, determining the optimal peroxide loading is crucial. However, the amalgamation appears to yield noteworthy enhancement effects.
As an illustration, in the case of wastewater from vegetable oil refineries, the augmentation of the hydrogen peroxide (H2O2) concentration from 20 to 30 gL−1 resulted in an elevation of COD removal from 68 to 72% and an increase in the BI from 0.60 to 0.72 [16]. The findings indicated comparable outcomes in the case of fish processing wastewater, with an optimal loading rate of 15 gL−1 resulting in a significantly elevated BI of 0.93. A high BI has the potential to result in a highly efficient process of biological oxidation [18].
Other AOPs have also been documented to exhibit similar effective combinations. An example of a process reported by Gogate involved the combination of HC and Fenton’s reagent. This process exhibited a 75% reduction in the energy requirement and cost, as well as improved degradation, compared to the individual HC process [61]. The utilization of a combination of UV irradiation and hydrogen peroxide has also been documented as a means to augment degradation. The utilization of titanium dioxide (TiO2) in conjunction with cavitation has been observed to result in a significant enhancement in the value of the BI parameter, reaching a maximum of 0.98 [52]. One limitation associated with TiO2 processes is the extended duration required for degradation. When comparing hydrogen peroxide and ozone, it has been observed that ozone results in a lower reduction in COD and a lower improvement in BI when compared to hydrogen peroxide and Fenton [38].
4.6. Effect of Physicochemical Properties of a Liquid
Based on the sources cited [31,52,53,62], it has been established that the promotion of cavitation effects is facilitated by specific favorable conditions, namely low viscosity, high surface tension, and low liquid vapor pressure. An elevation in the vapor pressure will augment the concentration of vapor within the cavity. Consequently, the intensity of bubble collapse is reduced as a result of the cushioning effect created by the ongoing condensation of vapor. Consequently, liquids exhibiting lower vapor pressure will yield greater levels of cavitation intensity. Elevating the viscosity of a fluid will result in an augmented threshold pressure for the occurrence of cavitation, as it necessitates exceeding a more robust inherent cohesive force. Therefore, fluids with lower viscosity exhibit a more pronounced cavitation effect. The Equation (5) represents the expression for the pressure resulting from surface tension, as symbolized by [31]. This equation is given below where s represents the surface tension coefficient. The contraction process of the bubble will be accelerated by the pressure resulting from surface tension. Therefore, it can be observed that the violent collapse of bubbles is directly proportional to the increase in surface tension. Consequently, the final collapse temperature and pressure also experience a slight elevation [26].
4.7. Effect of Initial Pollutant Concentration
The degradation reaction induced by cavitation can be characterized as a pseudo-first-order process, as described by Equations (6) and (7).
In this equation, C represents the concentration of the pollutant at a given time t, C0 represents the initial concentration, and k represents the rate constant for degradation. The degradation rate is inversely proportional to the initial pollutant concentration, as indicated by Equation (6) [27]. If the conditions of cavitation remain unchanged, the quantity of HO. radicals within the system would remain constant. However, it is observed that the overall quantity of pollutants in the solution exhibits an upward trend as the initial concentration of pollutants increases [18]. Consequently, the rate of degradation is diminished. However, it should be noted that an increase in the initial concentration of pollutants will result in the greater capture of pollutants by HO., consequently leading to a higher overall decomposition quantity [16]. The experimental investigation on the degradation of imidacloprid utilizing the slit venturi apparatus demonstrated that the rate constant for the pseudo-first-order reaction exhibited an increase from 0.79 × 10−3 to 1.27 × 10−3 min−1 as the initial concentration decreased from 60 to 20 ppm [7].
5. The Present State of Modeling HC Processes
To create a simulation model for the overall performance of the HC device/process, it is crucial to design a model that measures the performance of the overall products (hydroxyl radicals/shear) generated in the cavitation reactor for each pass. Sarvothaman et al. [63] have proposed a model to estimate the performance of pollutant degradation per pass. The technique is highly versatile and can be expanded to accommodate other desired transformation processes.
The discussions primarily revolved around the degradation of organic effluents through HC, with the exception of the modeling of biomass pre-treatment data using artificial neural networks (ANNs). HC is always undergoing advancements in its applications. It is utilized in various applications, such as emulsion production, the enhancement of liquid–liquid reactions and extractions, the control of particle size distribution in crystallization, and improvements in bioactive extraction from algae.
It is crucial to apply the modeling methodologies to a wide range of applications. Hydrodynamic cavitation is employed to either decrease a specific property (such as the concentration of pollutants or the size of droplets) or to increase a specific property (such as the concentration of the extractant or the pace of biogas generation). Cavitation induces extreme conditions that give rise to various physicochemical transformations, spanning a broad spectrum of spatiotemporal scales. These scales range from molecular dimensions and timeframes to the micron-scale cavities, and extend further to encompass reactor and cavitation device-scale processes [56]. At present, there is a shortage of predictive models based on first-principles for the multiple scales encompassing a wide range of magnitudes and the intricate physics involved in the phase change and high temperature chemical reactions. In the absence of these predictive models, process engineers have employed a wide range of approaches and models to design processes based on HC [21]. The methodologies employed thus far can be categorized into three main groups: semiempirical models, data-driven models, and physics-based models. The following sections, namely Section 5.1, Section 5.2 and Section 5.3, provide a concise discussion of the advantages and disadvantages of models falling within these three categories. Additionally, recommendations for enhancing these models are also presented.
Table 4 shows various types of models from the literature along with their limitations.

Table 4.
This illustration displays various types of models from the literature along with their limitations.
5.1. Semiempirical Models
The present methodology involves the utilization of a lumped parameter model to represent the intricate physicochemical conversions taking place within an HC reactor. Primarily, two methodologies are employed for this objective. The initial methodology employs a first-order reaction to depict all physicochemical transformations, with the observed data being described using simply pseudo-first-order kinetics. While it is theoretically feasible to employ pseudo-nth order kinetics, the majority of studies documenting empirical observations regarding the degradation of pollutants through HC have predominantly relied on pseudo-first order kinetics [53].
Numerous research investigations have been conducted employing this particular methodology. Vicharen et al. [66] have demonstrated that the utilization of a pseudo-first-order rate constant to characterize the cavitation caused by the hydrodynamic phenomenon is unsuitable. The authors have effectively demonstrated that this methodology yields distinct values for the effective rate constant in HC reactors operating under identical conditions, but with different volumes in the holding tank (specifically, kapp = 1.06 × 10−3 min−1 for a holding tank volume of 0.005 m3, and kapp = 2.41 × 10−4 min−1 for a holding tank volume of 0.024 m3) [66]. The establishment of a causal relationship between the effective rate constant and the volume of the holding tank is not physically feasible. Therefore, it is more beneficial to utilize a per-pass performance factor to characterize the HC reactor. This factor will solely depend on the reactor’s design and operating conditions. In their study, Teng et al. [56] employed the per-pass performance factor, which incorporates an empirical parameter represented by the symbol Φ, to quantitatively assess the per-pass performance of the HC reactor, as illustrated in Equation (8). Within this particular context, the variable V is utilized to symbolize the volume of liquid that is encompassed within the holding tank. The variable q is used to represent the overall net flow rate across the holding tank, whereas Q is employed to denote the flow rate that is specifically associated with the flow through the HC reactor. Finally, the variable C represents a specific attribute of significance, such as the level of concentration in the context of water treatment or extraction processes, or the size of particles or drops in the scenario of size reduction applications. It is imperative to acknowledge that each of these variables must be accompanied by their corresponding units of measurement. The value of Φ is determined through empirical observations and subsequently utilized for analysis, conceptualization, and improvement [21,56].
In the field of HC research, it is common to utilize semiempirical models that employ either the pseudo rate constant or per-pass performance factor approach. This is well-documented in various published studies. The preference for employing the per-pass performance factor approach over the pseudo rate constant approach stems from its capability to accurately evaluate the per-pass performance of the cavitation device, while mitigating any possible confounding variables, such as the existence of a holding tank. However, it is evident that relying solely on the per-pass performance factor approach is inadequate for acquiring significant insights or extrapolating outcomes. The per-pass performance factor is influenced by several factors, including the pressure drop across the cavitation device, the design of the cavitation device, the operating temperature, the downstream pressure, the pH level, and various other process parameters [13,38,67].
The study conducted by Qin et al. [68] provides evidence of the degradation of chlorocarbons through the utilization of HC. The research highlights the importance of considering the solubility and hydrophobicity of organic compounds when assessing the effectiveness of the degradation process. The study’s results revealed that species possessing greater solubility and hydrophobicity tend to concentrate at the cavity’s surface, making them more susceptible to degradation. The per-pass performance factor exhibits an optimal response with respect to different operational parameters, including the temperature, flow rate, and pressure drop. An augmentation of the flow rate (or pressure drop) and temperature results in a surge in the number density of cavities that are formed. However, the higher ratio of the gas phase leads to an elevated compressibility of the gas–liquid mixture. As a result, this phenomenon leads to a reduction in the intensity of collapse, characterized by a decrease in the temperature at which collapse occurs and the diminished generation of hydroxyl radicals [25]. Consequently, the overall degradation performance is reduced. Goodarzi et al. [42] have observed a decrease in the per-pass performance factor of cavitation devices when the size of the device increases, assuming that the devices have identical geometries. Therefore, it is crucial to improve the simplified semiempirical models by integrating data-driven and physics-based models.
5.2. Data-Driven Models
Efforts have been undertaken to employ solely data-driven methodologies in order to characterize the performance of HC reactors, given the intricate nature of the diverse physicochemical processes taking place within them. Various data-driven modeling formalisms are currently accessible, but the artificial neural network (ANN) seems to be well-suited for characterizing the performance of HC-based processes. The ANNs possess the ability to effectively model intricate relationships [69].
In practice, ANNs are commonly employed in functions that involve extensive datasets. Nevertheless, experiments utilizing HC exhibit a high level of complexity, necessitating substantial quantities of materials and consuming significant amounts of time, consequently resulting in elevated costs [60]. The available data obtained from experiments utilizing HC is inherently limited in nature. Overfitting may occur in the context of ANNs, wherein the model captures spurious relationships that do not actually exist. This phenomenon often results in unphysical interpolation and extrapolation outcomes.
In order to mitigate the issue of overfitting, it is recommended to commence with a neural network architecture that is as simplistic as feasible. In general, ANN models that incorporate a solitary hidden layer are often regarded as a favorable initial choice [44,70,71]. In a recent study, Salmi et al. demonstrated the development of an ANN model to describe the outcomes of HC in two distinct applications: pre-treatment of waste biomass to improve biogas production and degradation of organic pollutants in water. ANN architectures employing a solitary hidden layer were employed [21].
ANN models that were developed demonstrated a strong ability to accurately represent the experimental data. Although the experimental data were accurately captured within its specified range, the extrapolated trends of bio methane generation over time exhibited unphysical characteristics. The ANN model, on the other hand, exhibited exceptional performance and demonstrated the capability to interpolate effectively within the experimental data range. Although these models can be employed for efficient interpolation, they are limited in their ability to offer insights beyond the existing experimental data and are not particularly valuable for extrapolation purposes [69,72].
There exists considerable potential for the development of enhanced models for the various components depicted in this approach. In addition to formulating model equations based on fundamental principles, it is necessary to conduct further research to quantify the impact of numerical factors, including the degree of the convergence time step, grid spacing, and other related considerations. Additionally, simulated results are required to accurately simulate real-world applications of HC with a high level of fidelity.
The existing knowledge regarding various physicochemical transformations that take place through the initiation, expansion, and subsequent collapse of voids (and their interaction with the surrounding environment) is insufficient [73]. The majority of cavity dynamics models in the current literature continue to rely on the classical Rayleigh−Plesset (RP) cavity dynamics model [1]. This model assumes a cavity situated within an infinite medium, thereby assuming symmetric expansion and collapse. In actuality, the majority of cavities tend to experience asymmetrical collapse due to the presence of adjacent cavities and other disruptive factors. Hence, the development of quantitative models to simulate asymmetric cavity collapse and subsequent physicochemical transformations is of utmost importance.
In a recent study conducted by Berstad et al. [19], the researchers employed a volume-of-fluid method to simulate the asymmetric collapse of a cavity in close proximity to a liquid droplet. The simulations conducted were characterized by a two-dimensional nature and axis-symmetry. The development of comprehensive, three-dimensional transient flow models is crucial in order to make accurate quantitative predictions regarding the physicochemical transformations resulting from asymmetric cavity collapse.
The Pseudophase Lattice Boltzmann (PPLB) approach emerges as a viable option for addressing this matter. The PPLB approach, as demonstrated by Wang et al. [58], effectively incorporates phase separation and the formation or the collapse of interfaces, eliminating the need for front tracking methods. Consequently, this approach holds promise for advancing our comprehension of the initiation, expansion, and disintegration of cavities. The PPLB approach has been employed by Nogueira et al. [15] and Fedorov et al. [14] to conduct three-dimensional simulations of collapsing cavities. In a recent study conducted by Sarvothaman et al. [37], comprehensive three-dimensional computational fluid dynamics simulations were employed, utilizing the volume-of-fluid approach. The primary objective of this investigation was to gain insights into the impact of adjacent surfaces, whether rigid or flexible, on the phenomenon of cavity collapse [9].
5.3. Physics-Based Models
The processes that rely on HC primarily utilize shear forces, localized areas of high temperature, and hydroxyl radicals to facilitate a range of physicochemical transformations [36]. Hence, it is imperative to cultivate the skill of making “a priori” predictions regarding these variables based on device design and operating parameters. Despite numerous efforts spanning over a century, beginning with Rayleigh in 1934, the achievement of quantitative simulations for the overall performance of HC devices and processes remains challenging, as it necessitates the incorporation of adjustable parameters, including the flow rate, suction, and discharge pressure.
The primary factor contributing to this phenomenon is the simultaneous presence of significant temporal and spatial scales that encompass a wide range of magnitudes, encompassing entities, such as radicals and molecules at the smallest scale, to micron-sized cavities, and even cavitation devices spanning tens of centimeters in size. The effectiveness of an HC reactor or process is contingent upon several subprocesses, including the positioning, generation rate, and paths of cavities. These factors are influenced by the design of the flow rate, cavitation reactor, dissolved gases, pressure, temperature, and other relevant parameters [17]. The collapse of cavities and the resulting physicochemical effects occur when cavities are formed and transported by a flowing fluid [30,74]. During this process, the cavities undergo pressure fluctuations and, under specific conditions, undergo a violent collapse. The phenomenon of collapse results in the formation of concentrated regions with significantly elevated pressures and temperatures, accompanied by the occurrence of vigorous shear forces, high-velocity jets, and shock waves.
The determination of reactor performance will heavily rely on the crucial factors of the location, quantity, and magnitude of collapsing cavities. The effectiveness of the contact between collapsing cavities and the application process of interest can be influenced by various factors, including the pH, hydrophobicity, and the duration of contact [49,75]. These additional factors should be considered in conjunction with the factors mentioned earlier. The existing comprehension of various physicochemical transformations that take place through the initiation, expansion, and dissolution of voids (and their interaction with the surrounding environment) is insufficient. The majority of cavity dynamics models in the current literature continue to rely on the classical Rayleigh Plesset (RP) cavity dynamics model [56,76]. This model assumes a cavity within an infinite medium, thereby assuming symmetric expansion and collapse. In actuality, the majority of cavities exhibit asymmetrical collapse due to the presence of surrounding cavities and other disruptive factors. Hence, it is imperative to construct quantitative models that can simulate the phenomenon of asymmetric cavity collapse and the ensuing physicochemical transformations [55].
6. Methods for Assessing Cavitation Efficiency
6.1. Wastewater Pollutant Degradation
In recent years, numerous methods have been proposed to examine the effectiveness of various HC mechanisms at wastewater treatment plants (WWTPs). These methods primarily focus on evaluating both the efficiency of pollutant removal and the total energy supplied to the system. The selection of these methods is contingent upon the specific characteristics of the treated matter.
The degradation of pollutants in wastewaters has been a subject of recent interest. The HC technique has emerged as a potential method for addressing this issue, either as a standalone approach or in combination with additives. This technique aims to break down the toxic and carcinogenic compounds present in polluted water bodies. Various studies have assessed the extent of degradation (ED) of pollutants, including methylene blue dye [10], Rhodamine 6G (Rh6G) and Rhodamine B [10,30,77,78,79,80], orange acid II [80], orange 4 dye, reactive brilliant red K-2BP [23], brilliant green [80], pharmaceuticals [18], and pharmaceutical micro-pollutants [81]. The ED is determined as the extent of degradation. The calculation of ED can be performed using the formula presented in Equation (9).
The variable C0 [mg L−1] represents the initial concentration of the pollutant, while the variable C [mg L−1] represents the concentration of the pollutant at a given moment in time. All studies consistently demonstrate that greater effectiveness in terms of pollutant degradation have been noticed for higher ED and higher hydraulic conductivity HC conditions. Another parameter that has been considered is the cavitation yield, denoted as C.Y. [9,38,72,77,82].
It is determined according to Equation (10) and defined as the ratio of the observed cavitation effect, measured in terms of the quantity of the degraded pollutant, typically expressed in [mg L−1] using HC, to the total energy input into the system. The degraded matter refers to the quantity of pollutant (expressed in milligrams per liter) that is eliminated during the HC treatment. On the other hand, the power density (measured in joules per liter) can be calculated using Equation (11) [49]. In the given equation, V represents the volume of the treated matter in liters, Pabs denotes the absorbed power of the pump in the HC system in watts, and it signifies the duration of the treatment in seconds. Higher C.Y. values are indicative of greater efficiencies in the context of both degradation and the overall energy provided to the HC system. However, the use of C.Y. is limited to comparing multiple HC systems only when the pollutant being analyzed is consistent across all systems. In contrast, due to the varying properties and molecular structures of pollutants, it is not feasible to directly compare the treatment efficiencies of different contaminants using different HC systems. This is primarily because the distinct characteristics of pollutants can result in varying levels of resistance to the HC treatment [83]
6.2. Biological Wastewater Treatments
In the area of biological treatments, the efficacy of HC can be assessed through the quantification of enhancements in the solubilization of activated sludge, specifically in terms of the increase in soluble COD (SCOD). Additionally, the evaluation can be conducted by examining the ratio between the alteration in SCOD subsequent to cavitation and the particulate chemical oxygen demand as shown in Equation (12). The variable SCODcav represents the concentration of SCOD in the treated sludge at time t, measured in milligrams per liter (mg L−1), while SCOD0 represents the concentration of soluble COD in the untreated sludge, also measured in mg L−1 [74].
7. HC for Degradation of Various Organics in Wastewater
Industrial wastewater is defined as the collective term encompassing sewage and liquid waste generated during the course of industrial manufacturing processes [11,77]. There exists a wide variety of wastewater types, with industrial wastewater being particularly intricate in terms of its constituent components. An instance to consider is the presence of phenols in the wastewater generated by the petroleum-refining sector. The effluent generated by the pesticide-manufacturing sector comprises a diverse array of pesticides. The effluent generated by the heavy-metal-smelting industry comprises cadmium, plumbum, and various other metallic elements. According to Kausley et al. [76], the wastewater generated by the electroplating industry is characterized by the presence of cyanide, chromium, and various other heavy metals. The composition of industrial wastewater is intricate and frequently comprises a multitude of deleterious substances, necessitating compliance with discharge criteria prior to release. The efficacy of HC as a standalone method for industrial wastewater treatment is limited due to the presence of a significant quantity of organic compounds and macromolecules in the wastewater [7]. Consequently, the integration of HC with other techniques has emerged as the predominant focus of scholarly investigation in the field of industrial wastewater treatment. Furthermore, as a result of the intricate composition of industrial wastewater, researchers often employ universally recognized variables for sewage assessment in quantitative experimental studies.
These variables include the VSS (Volatile Suspended Solids), COD, SVI (Sludge Volume Index), TOC (Total Organic Carbon), kinetic rate coefficient, and others [20]. In their study, Thanekar and Gogate [84] integrated HC with an oxidation process utilizing hydrogen peroxide (H2O2), ozone (O3), and persulfate. In an effort to mitigate COD, researchers conducted a comparative analysis of the efficacy of HC as a standalone treatment method for industrial wastewater, as well as its potential synergistic effects when combined with other oxidation processes. HC exhibits superior energy efficiency and presents a notably reduced treatment cost in comparison to ultrasonic cavitation [54,85]. In their study, Dixit et al. [86] investigated the treatment of refining wastewater through the utilization of an orifice plate and venturi tube. They analyzed the degradation rate and energy consumption ratio, assessed the treatment efficiency of HC treatment for refining wastewater, and subsequently optimized the design of the cavitation reactor [86]. Table 5 provides an overview of the industrial wastewater treatment via hydrodynamic cavitation.

Table 5.
Overview on the treatment of industrial wastewater via hydrodynamic cavitation.
8. Studies on Pilot-Scale and Full-Scale Implementation
The efficacy of a technology is evaluated based on its capacity for widespread and comprehensive implementation, taking into account its practical utility. Ozone is widely recognized as a highly reactive and selective oxidant for organic pollutants, particularly polyphenols, within the range of available Advanced Oxidation/Reduction Processes (AO/RPs). For instance, the application of ozonation as a standalone method or in combination with UV-C light (referred to as O3/UV-C) or peroxidation has demonstrated significant efficacy in the treatment of polyphenol-contaminated wastewater. Polyphenol, a commonly identified water pollutant, primarily infiltrates the aquatic ecosystem through the cork manufacturing industry [83].
In a recent study conducted by Amr et al. [26], it was demonstrated that the combined processes of O3/UV and O3/UV/H2O2, operating at the natural pH level of 4, exhibit significantly higher efficacy compared to individual UV and ozone systems in treating distillery wastewater. This enhanced performance can be attributed to the generation of highly reactive radicals within the system [18]. In addition, the O3/UV and O3/UV/H2O2 processes exhibited the significant removal of COD and the total oxygen content. As a result, there is extensive documentation on the use of ozonation and ozone-related AOPs for wastewater treatment, both at small- and full-scale levels [10]. In their study, Nogueira et al. [15] conducted the purification and complete mineralization of distillery wastewater through the implementation of an ozone, UV-A, and TiO2 system, with a specific focus on COD removal. Based on the findings, the researchers reached the conclusion that the total mineralization, as determined by the removal of COD, exhibited an increase when employing a combined system of UV-A/visible light/ozone/TiO2, as compared to ozone treatment alone.
Prado et al. [40] investigated the efficacy of three different treatment methods for wastewater. These methods included the photocatalytic process, the Fenton reaction, and a UV-peroxidation system. The experiments were conducted using 100 mL glass vessels containing iron-rich clays. The researchers posited that the addition of a heterogeneous photocatalyst, such as TiO2, could potentially decrease the quantity of H2O2 needed to achieve a greater percentage of COD removal. In their study, Hilares et al. [67] examined the elimination of trichloroethylene, perchloroethylene, cis-1,2 dichloroethylene (cis-1,2 DCE), and methyl tert-butyl ether through a pilot-scale investigation. This investigation involved the implementation of an in-line application of ozone (O3) and hydrogen peroxide (H2O2). The researchers reached the conclusion that employing an ozone dosage of 8 mg L−1, with a contact time ranging from 3 to 6 min, and an H2O2/O3 ratio of 0.5 is a more appropriate approach for effectively eliminating trichloroethylene, perchloroethylene, cis-1,2 DCE, and MTBE compared to the use of ozone alone [16].
In a study conducted by Liang et al. [60], the authors investigated the efficacy of ozone (O3) as a standalone treatment and in combination with hydrogen peroxide (H2O2) for the removal of MTBE from surface wastewater. The findings from the experiments conducted with a total flow capacity of 12 gallons per minute (gpm) indicate that the combination of H2O2/O3 showed greater potential than O3 alone in terms of MTBE removal [55]. The findings of the study revealed that an optimal combination of 4 mg/L of O3 and 1.3 mg/L of H2O2 resulted in a removal rate of approximately 78% for MTBE. However, in a separate study conducted by [87], it was observed that ozonation leads to the generation of six nitrosamines predominantly, both in pilot- and full-scale operations. The researchers primarily directed their attention towards eight treatment plants that employed ozonation for the treatment of secondary or tertiary wastewater effluents. Additionally Mohod et al. [11] included two other treatment plants for a comparative analysis, which utilized chlorination or UV disinfection for the treatment of tertiary wastewater effluent. Furthermore, the researchers conducted an analysis of alternative comprehensive treatment methods, including advanced oxidation and reverse osmosis.
It was revealed that N-nitrosomorpholine and N-nitrosodimethylamine were identified as the predominant nitrosamines in both untreated wastewater, with concentrations reaching up to 89 and 67 mg/L, respectively, and treated wastewater. Moreover, the concentrations of N-nitrosodiethylamine exhibited variability within the provided range [88]. In their study, Mukherjee et al. [52] put forth a simplified model for the production of drinking water. The model elucidates the interplay among organic carbon removal, ozone decomposition, disinfection, and bromate formation in the context of ozonation [11]. The data for calibration and simulation were obtained from a full-scale ozonation system that was installed at the waterworks of the Flemish Water Supply Company in Kluizen, Belgium. The results of the study showed the effectiveness of the developed model in accurately predicting various outcomes, including excess ozone concentration, bacteria inactivation, optical density removal, and BrO3–formation [15].
Nevertheless, it is crucial to undertake a thorough and rigorous examination in order to further substantiate the suggested framework [18]. During the intervening period, the output of the model was notably affected by parameters, such as the optical density, as evidenced by the findings of the sensitivity analysis. A further practical analysis may be required to test this model, specifically in relation to the impact of the ozone dosage on reactor performance [75]. It is anticipated that this model will have a substantial role in investigations pertaining to drinking water modeling.
The comprehensive evaluation of UV/H2O2 processes has also been investigated using an established UV/H2O2 model that incorporates a widely accepted radical mechanism [53]. This model was adjusted and verified using real influent samples under various operational parameters [11]. The findings of the study indicate that the UV/H2O2 process is significantly influenced by a subset of the operational and chemical parameters.
The pilot-scale studies conducted by Bhat et al. [81] focused on the elimination of micropollutants from a WWTP through the implementation of ozonation and adsorption onto powdered activated carbon [10]. The findings of their investigation revealed that the elimination of sulfamethoxazole was more effective through the process of ozonation, whereas the reduction of benzotriazole and iomeprol was more efficient when employing activated carbon.
A comprehensive investigation was undertaken by [5] a to examine the efficacy of micropollutant removal in selected WWTPs in Switzerland. The authors proposed that the WWTPs should be enhanced by implementing more effective and cost-efficient alternatives, such as powdered activated carbon or ozonation. The findings of their research indicated that an ozone dosage of 0.55 g of ozone per gram of dissolved organic carbon is the most appropriate for achieving a removal efficiency of over 79% on average for nearly 550 micropollutants [5]. The authors also proposed the implementation of a supplementary biological post-treatment following ozonation in order to mitigate or reduce the adverse ecotoxicological impact resulting from the presence of biodegradable ozonation transformation products and oxidation by-products.
9. Economic Feasibility
Advanced oxidation processes are costly in comparison to conventional biological oxidation processes. Despite its economic viability, biological oxidation is constrained by the biodegradability potential of the effluent. Numerous effluents contain a substantial quantity of recalcitrant and persistent pollutants that are resistant to biological oxidation and may also impede bacterial activity [89]. Given the requirement of pre-treatment to enhance biodegradability in these effluents, cavitation shows great promise owing to its cost-effectiveness [16]. Various chemical and advanced oxidation processes have been extensively documented in the literature for the purpose of enhancing BI and treating sludge [15,75]. However, it is important to note that these processes may have certain limitations.
For instance, Fenton’s process may lead to the generation of additional sludge, while also incurring higher costs compared to cavitation. Furthermore, the presence of reactive radicals in these processes can potentially hinder the growth and activity of biomass. Several studies have conducted comprehensive cost analyses and comparisons, which indicate that cavitation can serve as a viable alternative to other oxidation processes [20]. When considering the comparison between cavitation and biological oxidation, it is important to acknowledge the existence of a tradeoff between the cost of the process and the efficiency of the treatment, as indicated in Table 6 [17]. For instance, the financial expenditure associated with biological treatment is significantly lower than that of cavitation [52]. However, it is frequently observed that biological treatment is accompanied by relatively low degradation efficiencies [90].

Table 6.
Analysis of the relative costs and degradation rates of HC and biological oxidation.
Pre-treatment is a necessary step in these instances due to the prioritization of degradation as a precursor to cost reduction in the treatment process. Furthermore, it is advisable to combine HC with other established advanced oxidation processes, such as ozonation [26]. HC in conjunction with AOPs have been found to exhibit a greater enhancement in biodegradability and reduced expenses [25]. Regarding the pre-treatment of anaerobic digestion, it was observed that the digestion of HC-pre-treated sludge not only achieved cost neutrality in terms of the expenses associated with HC pumping but also yielded an energy output that was 3.5 to 9 times greater than the energy consumed during the pre-treatment process [91].
Furthermore, the quantity of sludge intended for disposal exhibited a reduction as a result of the implementation of pre-treatment measures. The implementation of a combination of pre-treatments for biological oxidation and anaerobic digestion within a single treatment plant has the potential to yield several benefits. These include cost recovery for pre-treatment, increased levels of COD reduction, energy generation, and reduced challenges associated with sludge disposal [68]. Previous studies have provided evidence that cavitation plays a role in enhancing the production of short-chain fatty acids (SCFAs) during anaerobic digestion, thereby facilitating the recovery of nutrients in subsequent stages of the digestion process [16].
The aforementioned integrated process is also applicable to the concept of ‘nutrient removal-enhanced recovery’ in wastewater treatment plants. If the wastewater treatment plant achieves an energy-positive status, the implementation of a well-designed continuous-flow cavitation process holds great potential as a viable technique. Based on the findings presented in the study conducted by [58], it has been demonstrated that employing concepts similar to the one described can result in potential annual savings of up to $3.5 million for a treatment plant with a daily capacity of 105 m3, when utilizing ultrasound-based cavitation [75].
Furthermore, it is suggested that the utilization of HC, which has been demonstrated to be significantly more cost-effective compared to ultrasound, has the potential to yield greater cost savings and promote sustainability. Cavitation and advanced oxidation processes have garnered significant attention for their efficacy in eliminating a wide range of nonbiodegradable water contaminants [15]. The majority of existing studies examining the cost estimation of advanced oxidation/reduction processes (AO/RPs) have primarily focused on operational costs, particularly the energy consumption associated with treatment (specifically, electrical energy per order calculations).
However, there is a need for more comprehensive economic analyses pertaining to different AO/RPs, particularly those utilizing ozone and hydrogen peroxide, which are extensively employed in large-scale applications. The cost of water treatment involving the removal of organic contaminants from the aquatic environment via advanced oxidation/reduction processes (AO/RPs) is influenced by several factors. These factors include the absorbed dose, wastewater volume that needs to be treated, size of the treatment facility, utilization time of the facility, and the approach taken for capital recovery. According to a prediction made in reference [68], the projected cost for establishing a permanent full-scale 1.5MeV treatment facility in Miami was approximately $2.150 million. It is pertinent to acknowledge that the aforementioned cost encompasses the construction of the necessary infrastructure for the treatment system [18]. The operational and maintenance costs are incorporated at a rate of $41 per hour. Nevertheless, it is important to note that certain indirect costs, such as supervision and overhead expenses, have not been taken into account.
Gopalakrishnan et al. [5] conducted a separate study, which provided evidence of the significant efficacy of ionizing radiation technology in the deactivation of microorganisms commonly found in treated wastewater. Based on the findings derived from their investigation conducted in 2007, an approximate total cost of 3.0 million US$ was projected for the construction of a gamma irradiation WWTP, with operational expenses estimated to be around 1.0 million US$ annually.
In their study, [10] conducted a cost comparison between the UV/H2O2 method and the adsorption method employing activated carbon for the treatment of water contaminated with tetrachloroethylene. The analysis was performed with a flow rate of 50 cubic meters per day, an annual plant operation of 360 days per year, and a tetrachloroethylene-removal efficiency of 99% from an inlet concentration of 200 milligrams per liter. The cost analysis encompassed both the initial investment, which comprised the purchase price of the primary equipment, control systems, and building, as well as the ongoing operational expenditures, such as maintenance, energy consumption, chemical usage, utility expenses, labor costs, and analytical services. The findings indicated that the UV/H2O2 process and adsorption method exhibited comparable costs. Specifically, the cost for treating tetrachloroethylene-contaminated water using the UV/H2O2 process was approximately $2 per cubic meter, while the cost for employing carbon adsorption was approximately $1.70 per cubic meter [52].
In their study, Lakshmi et al. [92] also conducted a cost estimation analysis of acoustic cavitation as a means of wastewater treatment. The findings indicated that the utilization of acoustic cavitation in the process incurs greater costs compared to alternative AOPs, such as ozonation, O3/H2O2, and UV/H2O2. However, the integration of ultrasonic cavitation with AOPs presents a more economically appealing option [68].
10. Prospects for Future Research
Published reports exhibit a deficiency in empirical data to substantiate the efficacy of biodegradability and sludge disintegration. The industrial application can be significantly enhanced by implementing optimization techniques that account for all operational process parameters. Moreover, it is imperative to prioritize the consideration of temperature and pH that have been overlooked in numerous reports.
The cost of biological oxidation processes is significantly lower compared to advanced oxidation processes, such as cavitation. The necessity of pre-treatment for specific wastewater demands a thorough investigation. In certain instances, the enhancement of efficiency may be achieved through the modification or alteration of biomass selection. It is imperative to include alternative biological processes, such as biofilm processes or Annamox processes, in pre-treatment studies. In order to conduct a more comprehensive analysis of the bio refractory compounds requiring degradation, studies need to include a detailed report on the degradation mechanism. The utilization of such data has the potential to enhance the optimization of research efforts aimed at selectively degrading large compounds. The establishment of effective synergistic interactions among cavitation, ozone, Fenton, and photocatalysis mechanisms holds the potential to enhance the comprehension of these processes at a more profound level. Further research should be directed towards investigating the application of HC in conjunction with oxidants for the purpose of sludge treatment.
The available literature on HC is relatively limited compared to the extensive body of research on ultrasound, indicating a scarcity of data in the former field. Currently, there is a lack of available data regarding the impact of the ultrasound duty-cycle and signal type on the process of sludge disintegration. These studies are recommended due to their potential to facilitate cost-effective operations. The implementation of a continuous-flow system is necessary for the industrial-scale application of processes. A substantial volume of wastewater and sludge can be effectively treated using either a semi-batch or continuous processing approach. However, there is a lack of available literature documenting specific details and data pertaining to these processes. The majority of previous studies examining the impact of HC degradation have relied on the assumption of a solitary spherical bubble, which does not accurately represent the actual scenario.
There is an urgent need for the development of realistic models that take into account the bubble–bubble/bubble–liquid interface and the collapse of nonspherical bubbles. In the context of new models, it is imperative to adopt pressure distribution that is characterized by enhanced accuracy. Extensive research has been conducted on the chemical impacts of HC, specifically in relation to the generation of hydroxyl radicals. However, there remains a notable absence of direct simulation of the degradation process based on chemical reaction kinetics. The current studies lack adequate direct and quantitative descriptions of the reaction between free radicals and contaminants. Moreover, it is necessary to conduct further investigations into the lifetimes of various radicals, as well as the predominant reaction sites.
11. Conclusions
The utilization of cavitation in conjunction with oxidants presents a prospective, environmentally friendly, and cost-effective approach for pre-treatment. The reduction of COD is of utmost importance in conjunction with the improvement of the biodegradability index, as it effectively alleviates the impact on biological oxidation processes. A minimum biodegradable index of 0.4 is necessary, although numerous studies have documented an elevation exceeding 0.5 and, in certain instances, reaching as high as 0.8 to 1. The meticulous choice of operating conditions and additives is needed for improving biodegradability while simultaneously minimizing the cost of pre-treatment, thereby ensuring overall economic efficiency. The combination of cavitation with Fenton or hydrogen peroxide has been documented to enhance the reduction of COD by as much as 50–75%. It is recommended that a minimum reduction of 50% in COD can be achieved in order to ensure the commercial viability of the pre-treatment process. The existing agreement regarding the cavitation number typically falls within the range of 0.07 to 0.2. In most instances, the optimal cavitation number has been observed to result in increased efficiency. Furthermore, the geometric characteristics of orifice and venturi devices have a significant impact on their performance. HC has the capability to induce elevated temperature and pressure conditions, resulting in the dissociation of water molecules and subsequent liberation of active radicals. A significant number of chemical reactions occur under these specified conditions. The magnitudes of pressure and temperature, as well as the quantity of free radicals, are influenced by the geometrical and operating parameters. It is highly advisable to conduct a theoretical analysis and laboratory-scale studies in order to optimize these parameters prior to implementing the new technology. The viability of HC in wastewater treatment is contingent upon the development of energy-efficient and cost-effective reactors, thus necessitating its integration with AOPs. It is important to acknowledge that the operational expenses associated with a cavitation reactor, particularly an acoustic cavitation reactor, are comparatively higher in comparison to a conventional reactor. However, the strategic choice of operational parameters and the implementation of intensification methods can significantly enhance the overall benefits achieved. In conclusion, it can be asserted that HC is a widely recognized technology at the laboratory level, necessitating further endeavors to effectively advocate for its implementation in industrial settings.
Author Contributions
K.A.B. and A.M.Y. carried out the design of the article and document write-up; T.J. and A.S.A.M. carried out manuscript review and editing. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the support of the Ministry of Higher Education, Research and Innovation of Oman through BFP/RGP/EBR/22/079 block funding.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gogate, P.R.; Pandit, A.B. Engineering Design Methods for Cavitation Reactors II: Hydrodynamic Cavitation. AIChE J. 2000, 46, 1641–1649. [Google Scholar] [CrossRef]
- Patil, P.B.; Bhandari, V.M.; Ranade, V.V. Wastewater Treatment and Process Intensification for Degradation of Solvents Using Hydrodynamic Cavitation. Chem. Eng. Process.-Process Intensif. 2021, 166, 108485. [Google Scholar] [CrossRef]
- Sivagami, K.; Sakthivel, K.P.; Nambi, I.M. Advanced Oxidation Processes for the Treatment of Tannery Wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Gujar, S.K.; Gogate, P.R.; Kanthale, P.; Pandey, R.; Thakre, S.; Agrawal, M. Combined Oxidation Processes Based on Ultrasound, Hydrodynamic Cavitation and Chemical Oxidants for Treatment of Real Industrial Wastewater from Cellulosic Fiber Manufacturing Sector. Sep. Purif. Technol. 2021, 257, 117888. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Iyer, G.M.; Liu, L.; Zhang, C. Hydrocarbon Separations by Glassy Polymer Membranes. J. Polym. Sci. 2020, 58, 2482–2517. [Google Scholar] [CrossRef]
- Popat, A.; Nidheesh, P.V.; Anantha Singh, T.S.; Suresh Kumar, M. Mixed Industrial Wastewater Treatment by Combined Electrochemical Advanced Oxidation and Biological Processes. Chemosphere 2019, 237, 124419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Koros, W.J. Tailoring the Transport Properties of Zeolitic Imidazolate Frameworks by Post-Synthetic Thermal Modification. ACS Appl. Mater. Interfaces 2015, 7, 23407–23411. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Recent Advances and Prospects of Catalytic Advanced Oxidation Process in Treating Textile Effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karimi, B.; Rajaei, M.S. The Effect of Aeration on Advanced Coagulation, Flotation and Advanced Oxidation Processes for Color Removal from Wastewater. J. Mol. Liq. 2016, 223, 75–80. [Google Scholar] [CrossRef]
- Mohod, A.V.; Teixeira, A.C.S.C.; Bagal, M.V.; Gogate, P.R.; Giudici, R. Degradation of Organic Pollutants from Wastewater Using Hydrodynamic Cavitation: A Review. J. Environ. Chem. Eng. 2023, 11, 109773. [Google Scholar] [CrossRef]
- Agarkoti, C.; Thanekar, P.D.; Gogate, P.R. Cavitation Based Treatment of Industrial Wastewater: A Critical Review Focusing on Mechanisms, Design Aspects, Operating Conditions and Application to Real Effluents. J. Environ. Manag. 2021, 300, 113786. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Pandit, A.B. A Review of Imperative Technologies for Wastewater Treatment I: Oxidation Technologies at Ambient Conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Fedorov, K.; Rayaroth, M.P.; Shah, N.S.; Boczkaj, G. Activated Sodium Percarbonate-Ozone (SPC/O3) Hybrid Hydrodynamic Cavitation System for Advanced Oxidation Processes (AOPs) of 1,4-Dioxane in Water. Chem. Eng. J. 2023, 456, 141027. [Google Scholar] [CrossRef]
- Nogueira, A.A.; Bassin, J.P.; Cerqueira, A.C.; Dezotti, M. Integration of Biofiltration and Advanced Oxidation Processes for Tertiary Treatment of an Oil Refinery Wastewater Aiming at Water Reuse. Environ. Sci. Pollut. Res. 2016, 23, 9730–9741. [Google Scholar] [CrossRef] [PubMed]
- Esmaeeli, A.; Sarrafzadeh, M.H.; Zeighami, S.; Kalantar, M.; Bariki, S.G.; Fallahi, A.; Asgharnejad, H.; Ghaffari, S.B. A Comprehensive Review on Pulp and Paper Industries Wastewater Treatment Advances. Ind. Eng. Chem. Res. 2023, 62, 8119–8145. [Google Scholar] [CrossRef]
- Patil, P.B.; Thanekar, P.; Bhandari, V.M. A Strategy for Complete Degradation of Metformin Using Vortex-Based Hydrodynamic Cavitation. Ind. Eng. Chem. Res. 2022, 62, 19262–19273. [Google Scholar] [CrossRef]
- Thapa, B.S.; Pandit, S.; Patwardhan, S.B.; Tripathi, S.; Mathuriya, A.S.; Gupta, P.K.; Lal, R.B.; Tusher, T.R. Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives. Sustainability 2022, 14, 8379. [Google Scholar] [CrossRef]
- Berstad, D.; Anantharaman, R.; Wilhelmsen, Ø.; Skjervold, V.; Nekså, P. Large-Scale Hydrogen Production and Liquefaction for Regional and Global Export. In Proceedings of the International Hydrogen and Fuel Cells Conference, Trondheim, Norway, 14–15 May 2018. [Google Scholar]
- Gągol, M.; Cako, E.; Fedorov, K.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Hydrodynamic Cavitation Based Advanced Oxidation Processes: Studies on Specific Effects of Inorganic Acids on the Degradation Effectiveness of Organic Pollutants. J. Mol. Liq. 2020, 307, 113002. [Google Scholar] [CrossRef]
- Al Salmi, H.; Khan, F.R. A Comparative Case Study on Accountability of Corporate Social Responsibility (CSR) Practices in Oman Lng and Omifco at Sur City in Oman. Humanit. Soc. Sci. Rev. 2019, 7, 490–502. [Google Scholar] [CrossRef]
- Thanekar, P.; Lakshmi, N.J.; Shah, M.; Gogate, P.R.; Znak, Z.; Sukhatskiy, Y.; Mnykh, R. Degradation of Dimethoate Using Combined Approaches Based on Hydrodynamic Cavitation and Advanced Oxidation Processes. Process Saf. Environ. Prot. 2020, 143, 222–230. [Google Scholar] [CrossRef]
- Gostiša, J.; Širok, B.; Repinc, S.K.; Levstek, M.; Stražar, M.; Bizjan, B.; Zupanc, M. Performance Evaluation of a Novel Pilot-Scale Pinned Disc Rotating Generator of Hydrodynamic Cavitation. Ultrason. Sonochem. 2021, 72, 105431. [Google Scholar] [CrossRef] [PubMed]
- Rokesh, K.; Mohan, S.C.; Karuppuchamy, S.; Jothivenkatachalam, K. Photo-Assisted Advanced Oxidation Processes for Rhodamine B Degradation Using ZnO-Ag Nanocomposite Materials. J. Environ. Chem. Eng. 2018, 6, 3610–3620. [Google Scholar] [CrossRef]
- Babu, S.G.; Ashokkumar, M.; Neppolian, B. The Role of Ultrasound on Advanced Oxidation Processes. Top. Curr. Chem. 2016, 374, 75. [Google Scholar] [CrossRef] [PubMed]
- Abu Amr, S.S.; Aziz, H.A.; Bashir, M.J.K.; Aziz, S.Q.; Alsaibi, T.M. Comparison and Optimization of Ozone–Based Advanced Oxidation Processes in the Treatment of Stabilized Landfill Leachate. J. Eng. Res. Technol. 2015, 2, 122–130. [Google Scholar]
- Min, B.; Korde, A.; Yang, S.; Kim, Y.; Jones, C.W.; Nair, S. Separation of C2–C4 Hydrocarbons from Methane by Zeolite MFI Hollow Fiber Membranes Fabricated from 2D Nanosheets. AIChE J. 2021, 67, e17048. [Google Scholar] [CrossRef]
- Chatel, G.; Colmenares, J.C. Sonochemistry: From Basic Principles to Innovative Applications. Top. Curr. Chem. 2017, 375, 8. [Google Scholar] [CrossRef] [PubMed]
- Chakinala, A.G.; Gogate, P.R.; Burgess, A.E.; Bremner, D.H. Industrial Wastewater Treatment Using Hydrodynamic Cavitation and Heterogeneous Advanced Fenton Processing. Chem. Eng. J. 2009, 152, 498–502. [Google Scholar] [CrossRef]
- Saharan, V.K.; Rizwani, M.A.; Malani, A.A.; Pandit, A.B. Effect of Geometry of Hydrodynamically Cavitating Device on Degradation of Orange-G. Ultrason. Sonochem. 2013, 20, 345–353. [Google Scholar] [CrossRef]
- Khatri, J.; Nidheesh, P.V.; Anantha Singh, T.S.; Suresh Kumar, M. Advanced Oxidation Processes Based on Zero-Valent Aluminium for Treating Textile Wastewater. Chem. Eng. J. 2018, 348, 67–73. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater Treatment Using Hybrid Treatment Schemes Based on Cavitation and Fenton Chemistry: A Review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Šarc, A.; Stepišnik-Perdih, T.; Petkovšek, M.; Dular, M. The Issue of Cavitation Number Value in Studies of Water Treatment by Hydrodynamic Cavitation. Ultrason. Sonochem. 2017, 34, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Thorpe, R.B. Flow Regime Transitions Due to Cavitation in the Flow through an Orifice. Int. J. Multiph. Flow 1990, 16, 1023–1045. [Google Scholar] [CrossRef]
- Cioncolini, A.; Scenini, F.; Duff, J.; Szolcek, M.; Curioni, M. Choked Cavitation in Micro-Orifices: An Experimental Study. Exp. Therm. Fluid Sci. 2016, 74, 49–57. [Google Scholar] [CrossRef]
- Gostiša, J.; Zupanc, M.; Dular, M.; Širok, B.; Levstek, M.; Bizjan, B. Investigation into Cavitational Intensity and COD Reduction Performance of the Pinned Disc Reactor with Various Rotor-Stator Arrangements. Ultrason. Sonochem. 2021, 77, 105669. [Google Scholar] [CrossRef] [PubMed]
- Sarvothaman, V.P.; Velisoju, V.K.; Subburaj, J.; Panithasan, M.S.; Kulkarni, S.R.; Castaño, P.; Turner, J.; Guida, P.; Roberts, W.L.; Nagarajan, S. Is Cavitation a Truly Sensible Choice for Intensifying Photocatalytic Oxidation Processes?–Implications on Phenol Degradation Using ZnO Photocatalysts. Ultrason. Sonochem. 2023, 99, 106548. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A Detailed Review on Advanced Oxidation Process in Treatment of Wastewater: Mechanism, Challenges and Future Outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Peng, K.; Qin, F.G.F.; Jiang, R.; Qu, W.; Wang, Q. Production and Dispersion of Free Radicals from Transient Cavitation Bubbles: An Integrated Numerical Scheme and Applications. Ultrason. Sonochem. 2022, 88, 106067. [Google Scholar] [CrossRef]
- Prado, C.A.; Antunes, F.A.F.; Rocha, T.M.; Sánchez-Muñoz, S.; Barbosa, F.G.; Terán-Hilares, R.; Cruz-Santos, M.M.; Arruda, G.L.; da Silva, S.S.; Santos, J.C. A Review on Recent Developments in Hydrodynamic Cavitation and Advanced Oxidative Processes for Pretreatment of Lignocellulosic Materials. Bioresour. Technol. 2022, 345, 126458. [Google Scholar] [CrossRef]
- Ge, M.; Sun, C.; Zhang, G.; Coutier-Delgosha, O.; Fan, D. Combined Suppression Effects on Hydrodynamic Cavitation Performance in Venturi-Type Reactor for Process Intensification. Ultrason. Sonochem. 2022, 86, 106035. [Google Scholar] [CrossRef]
- Goodarzi, S.; Jahanshahi Javaran, E.; Rahnama, M.; Ahmadi, M. Techno-Economic Evaluation of a Multi Effect Distillation System Driven by Low-Temperature Waste Heat from Exhaust Flue Gases. Desalination 2019, 460, 64–80. [Google Scholar] [CrossRef]
- Sivakumar, M.; Pandit, A.B. Wastewater Treatment: A Novel Energy Efficient Hydrodynamic Cavitational Technique. Ultrason. Sonochem. 2002, 9, 123–131. [Google Scholar] [CrossRef]
- Moholkar, V.S.; Pandit, A.B. Modeling of Hydrodynamic Cavitation Reactors: A Unified Approach. Chem. Eng. Sci. 2001, 56, 6295–6302. [Google Scholar] [CrossRef]
- Ozonek, J.; Lenik, K. Effect of Different Design Features of the Reactor on Hydrodynamic Cavitation Process. Arch. Mater. Sci. Eng. 2011, 52, 112–117. [Google Scholar]
- Chu, Y. Transition Metal-Containing Carbon Molecular Sieve Membranes for Advanced Olefin/Paraffin Separations. Ph.D. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2017. [Google Scholar]
- Li, M.; Bussonnière, A.; Bronson, M.; Xu, Z.; Liu, Q. Study of Venturi Tube Geometry on the Hydrodynamic Cavitation for the Generation of Microbubbles. Miner. Eng. 2019, 132, 268–274. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, M.; Chen, B.; Sun, L.; Lu, H. Microbubble- and Nanobubble-Aeration for Upgrading Conventional Activated Sludge Process: A Review. Bioresour. Technol. 2022, 362, 127826. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Zhang, H.; Shi, W.; Xiong, M.; Gao, C.; Cui, M. Hydrodynamic Cavitation and Its Application in Water Treatment Combined with Ozonation: A Review. J. Ind. Eng. Chem. 2022, 114, 33–51. [Google Scholar] [CrossRef]
- Mittal, R.; Ranade, V. Bioactives from Microalgae: A Review on Process Intensification Using Hydrodynamic Cavitation. J. Appl. Phycol. 2023, 35, 1129–1161. [Google Scholar] [CrossRef]
- Moussavi, G.; Pourakbar, M.; Aghayani, E.; Mahdavianpour, M.; Shekoohyian, S. Comparing the Efficacy of VUV and UVC/S2 Advanced Oxidation Processes for Degradation and Mineralization of Cyanide in Wastewater. Chem. Eng. J. 2016, 294, 273–280. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mullick, A.; Teja, R.; Vadthya, P.; Roy, A.; Moulik, S. Performance and Energetic Analysis of Hydrodynamic Cavitation and Potential Integration with Existing Advanced Oxidation Processes: A Case Study for Real Life Greywater Treatment. Ultrason. Sonochem. 2020, 66, 105116. [Google Scholar] [CrossRef]
- Braeutigam, P.; Franke, M.; Wu, Z.L.; Ondruschka, B. Role of Different Parameters in the Optimization of Hydrodynamic Cavitation. Chem. Eng. Technol. 2010, 33, 932–940. [Google Scholar] [CrossRef]
- De-Nasri, S.J.; Sarvothaman, V.P.; Nagarajan, S.; Manesiotis, P.; Robertson, P.K.J.; Ranade, V.V. Quantifying OH Radical Generation in Hydrodynamic Cavitation via Coumarin Dosimetry: Influence of Operating Parameters and Cavitation Devices. Ultrason. Sonochem. 2022, 90, 106207. [Google Scholar] [CrossRef]
- Jankunaite, D.; Tichonovas, M.; Buivydiene, D.; Radziuniene, I.; Racys, V.; Krugly, E. Removal of Diclofenac, Ketoprofen, and Carbamazepine from Simulated Drinking Water by Advanced Oxidation in a Model Reactor. Water Air Soil Pollut. 2017, 228, 353. [Google Scholar] [CrossRef]
- Teng, S.Y.; Leong, W.D.; How, B.S.; Lam, H.L.; Máša, V.; Stehlík, P. Debottlenecking Cogeneration Systems under Process Variations: Multi-Dimensional Bottleneck Tree Analysis with Neural Network Ensemble. Energy 2021, 215, 119168. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Kamizela, T. Artificial Neural Networks in Modeling of Dewaterability of Sewage Sludge. Energies 2021, 14, 1552. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Yuan, R.; Wang, F.; Ma, F.; Zhou, B. Intensified Degradation of Textile Wastewater Using a Novel Treatment of Hydrodynamic Cavitation with the Combination of Ozone. J. Environ. Chem. Eng. 2020, 8, 103959. [Google Scholar] [CrossRef]
- Musmarra, D.; Prisciandaro, M.; Capocelli, M.; Karatza, D.; Iovino, P.; Canzano, S.; Lancia, A. Degradation of Ibuprofen by Hydrodynamic Cavitation: Reaction Pathways and Effect of Operational Parameters. Ultrason. Sonochem. 2016, 29, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Kang, L.; Liu, Y. The Flexible Design for Optimization and Debottlenecking of Multiperiod Hydrogen Networks. Ind. Eng. Chem. Res. 2016, 55, 2574–2583. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A Review and Assessment of Hydrodynamic Cavitation as a Technology for the Future. Ultrason. Sonochem. 2005, 12, 21–27. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Liang, L. Highly Efficient In-Situ Metal-Free Electrochemical Advanced Oxidation Process Using Graphite Felt Modified with N-Doped Graphene. Chem. Eng. J. 2018, 338, 700–708. [Google Scholar] [CrossRef]
- Sarvothaman, V.P.; Simpson, A.; Ranade, V.V. Comparison of Hydrodynamic Cavitation Devices Based on Linear and Swirling Flows: Degradation of Dichloroaniline in Water. Ind. Eng. Chem. Res. 2020, 59, 13841–13847. [Google Scholar] [CrossRef]
- Adama Maiga, M.; Coutier-Delgosha, O.; Buisine, D. Analysis of Sheet Cavitation with Bubble/Bubble Interaction Models. Phys. Fluids 2019, 31, 073302. [Google Scholar] [CrossRef]
- Zhao, M.; Wan, D.; Gao, Y. Comparative Study of Different Turbulence Models for Cavitational Flows around Naca0012 Hydrofoil. J. Mar. Sci. Eng. 2021, 9, 742. [Google Scholar] [CrossRef]
- Vichare, N.P.; Gogate, P.R.; Pandit, A.B. Optimization of Hydrodynamic Cavitation Using a Model Reaction. Chem. Eng. Technol. 2000, 23, 683–690. [Google Scholar] [CrossRef]
- Terán Hilares, R.; Dionízio, R.M.; Sánchez Muñoz, S.; Prado, C.A.; de Sousa Júnior, R.; da Silva, S.S.; Santos, J.C. Hydrodynamic Cavitation-Assisted Continuous Pre-Treatment of Sugarcane Bagasse for Ethanol Production: Effects of Geometric Parameters of the Cavitation Device. Ultrason. Sonochem. 2020, 63, 104931. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Song, Y.; Dai, Y.; Qiu, G.; Ren, M.; Zeng, P. Treatment of Berberine Hydrochloride Pharmaceutical Wastewater by O3/UV/H2O2 Advanced Oxidation Process. Environ. Earth Sci. 2015, 73, 4939–4946. [Google Scholar] [CrossRef]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Application of ANN to Hydrodynamic Cavitation: Preliminary Results on Process Efficiency Evaluation. Chem. Eng. Trans. 2014, 36, 199–204. [Google Scholar] [CrossRef]
- Ranade, V.V. Modeling of Hydrodynamic Cavitation Reactors: Reflections on Present Status and Path Forward. ACS Eng. Au 2022, 2, 461–476. [Google Scholar] [CrossRef]
- Song, L.; Wei, Y.; Deng, C.; Yang, J.; Sui, H.; Guo, F.; Meng, L.; Zhao, X.; Wei, S.; Sun, D.; et al. A Novel Method Based on Hydrodynamic Cavitation for Improving Nitric Oxide Removal Performance of NaClO2. Int. J. Environ. Res. Public Health 2023, 20, 3684. [Google Scholar] [CrossRef]
- Khan, S.; Raja, M.A.; Sayed, M.; Shah, L.A.; Sohail, M. Advanced Oxidation and Reduction Processes. In Advances in Water Purification Techniques: Meeting the Needs of Developed and Developing Countries; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–164. ISBN 9780128147900. [Google Scholar]
- Patil, Y.; Sonawane, S.H.; Shyam, P.; Sun, X.; Manickam, S. Hybrid Hydrodynamic Cavitation (HC) Technique for the Treatment and Disinfection of Lake Water. Ultrason. Sonochem. 2023, 97, 106454. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, J.; Huai, X.; Liu, B.; Guo, Z. Application of Hydrodynamic Cavitation to Wastewater Treatment. Chem. Eng. Technol. 2016, 39, 1363–1376. [Google Scholar] [CrossRef]
- Wang, B.; Su, H.; Zhang, B. Hydrodynamic Cavitation as a Promising Route for Wastewater Treatment–A Review. Chem. Eng. J. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Kausley, S.B.; Malhotra, C.P.; Pandit, A.B. Treatment and Reuse of Shale Gas Wastewater: Electrocoagulation System for Enhanced Removal of Organic Contamination and Scale Causing Divalent Cations. J. Water Process Eng. 2017, 16, 149–162. [Google Scholar] [CrossRef]
- Mancuso, G.; Langone, M.; Andreottola, G. A Critical Review of the Current Technologies in Wastewater Treatment Plants by Using Hydrodynamic Cavitation Process: Principles and Applications. J. Environ. Health Sci. Eng. 2020, 18, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.M.; Gogate, P.R. Intensification of Industrial Wastewater Treatment Using Hydrodynamic Cavitation Combined with Advanced Oxidation at Operating Capacity of 70 L. Ultrason. Sonochem. 2019, 52, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Badmus, K.O.; Tijani, J.O.; Massima, E.; Petrik, L. Treatment of Persistent Organic Pollutants in Wastewater Using Hydrodynamic Cavitation in Synergy with Advanced Oxidation Process. Environ. Sci. Pollut. Res. 2018, 25, 7299–7314. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Bhosale, G.S. Comparison of Effectiveness of Acoustic and Hydrodynamic Cavitation in Combined Treatment Schemes for Degradation of Dye Wastewaters. Chem. Eng. Process.-Process Intensif. 2013, 71, 59–69. [Google Scholar] [CrossRef]
- Bhat, A.P.; Gogate, P.R. Cavitation-Based Pre-Treatment of Wastewater and Waste Sludge for Improvement in the Performance of Biological Processes: A Review. J. Environ. Chem. Eng. 2021, 9, 104743. [Google Scholar] [CrossRef]
- Hilares, R.T.; Atoche-Garay, D.F.; Pagaza, D.A.P.; Ahmed, M.A.; Andrade, G.J.C.; Santos, J.C. Promising Physicochemical Technologies for Poultry Slaughterhouse Wastewater Treatment: A Critical Review. J. Environ. Chem. Eng. 2021, 9, 105174. [Google Scholar] [CrossRef]
- Mohammadi, K.; Saghafifar, M.; Ellingwood, K.; Powell, K. Hybrid Concentrated Solar Power (CSP)-Desalination Systems: A Review. Desalination 2019, 468, 114083. [Google Scholar] [CrossRef]
- Thanekar, P.; Gogate, P.R. Combined Hydrodynamic Cavitation Based Processes as an Efficient Treatment Option for Real Industrial Effluent. Ultrason. Sonochem. 2019, 53, 202–213. [Google Scholar] [CrossRef]
- Carpenter, J.; George, S.; Saharan, V.K. Low Pressure Hydrodynamic Cavitating Device for Producing Highly Stable Oil in Water Emulsion: Effect of Geometry and Cavitation Number. Chem. Eng. Process.-Process Intensif. 2017, 116, 97–104. [Google Scholar] [CrossRef]
- Dixit, D.; Thanekar, P.; Bhandari, V.M. Degradation of API Pollutants Using Hydrodynamic Cavitation and Process Intensification. Chem. Eng. Process.-Process Intensif. 2022, 172, 108799. [Google Scholar] [CrossRef]
- Roy, M.; Yadav, R.; Chiranjeevi, P.; Patil, S.A. Direct Utilization of Industrial Carbon Dioxide with Low Impurities for Acetate Production via Microbial Electrosynthesis. Bioresour. Technol. 2021, 320, 124289. [Google Scholar] [CrossRef] [PubMed]
- Gerrity, D.; Pisarenko, A.N.; Marti, E.; Trenholm, R.A.; Gerringer, F.; Reungoat, J.; Dickenson, E. Nitrosamines in Pilot-Scale and Full-Scale Wastewater Treatment Plants with Ozonation. Water Res. 2015, 72, 251–261. [Google Scholar] [CrossRef]
- Shokoohi, R.; Rahmani, A.; Asgari, G.; Ashrafi, M.; Ghahramani, E. Removal of Algae Using Hydrodynamic Cavitation, Ozonation and Oxygen Peroxide: Taguchi Optimization (Case Study: Raw Water of Sanandaj Water Treatment Plant). Process Saf. Environ. Prot. 2023, 169, 896–908. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient Degradation of 2,4-Dichlorophenoxyacetic Acid by Peroxymonosulfate/Magnetic Copper Ferrite Nanoparticles/Ozone: A Novel Combination of Advanced Oxidation Processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Lindsay, D.; Griffiths, M.; Sabitov, A.; Sioui, D.; Glover, B. How to Cost-Effectively Adapt to a Tight Oil World. In Hydrocarbon Processing; Gulf Publishing Co.: Houston, TX, USA, 2015. [Google Scholar]
- Lakshmi, N.J.; Gogate, P.R.; Pandit, A.B. Acoustic Cavitation for the Process Intensification of Biological Oxidation of CETP Effluent Containing Mainly Pharmaceutical Compounds: Understanding into Effect of Parameters and Toxicity Analysis. Ultrason. Sonochem. 2023, 98, 106524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).