Electrokinetic Remediation in Marine Sediment: A Review and a Bibliometric Analysis
Abstract
1. Introduction
1.1. Electrokinetic Remediation: Principles
1.2. Electrokinetic Base Methodology Currently Applied in Different Environments
- Electrokinetic biobarriers, also known as electrokinetic biofences, are a static in situ approach used to enclose, contain and purify contaminated groundwater. This technology involves positioning a row of electrodes at the border of a contaminated area, perpendicularly to the predominant groundwater flow direction, and placing them at the lowest depth where contaminants are observed. Different layouts can be used depending on the type of contamination, whether it is inorganic or organic.
- Electrolytic reactive barriers, also known as e-barriers, consist of a panel of permeable electrodes closely laid in the channel of polluted groundwater. For more information on this methodology, refer to Sale, Petersen and Gilbert [37].
- Electrokinetic-permeable reactive barriers (PRBs) are an in situ method used to enclose a reactive zone in groundwater for the elimination of halogenated organic compounds and inorganic salts. If the barrier is loaded with microorganisms, it works as a biological reactor to break down the target contaminants. On the other hand, if the barrier is loaded with limestone, hydroxyapatite, activated carbon, or zeolite, it works as a chemical precipitator or adsorbent.
- Electrokinetic–chemical oxidation/reduction is used for wastewater treatment. It is based on the concepts of chemical oxidation and reduction reactions, which reduce organic contaminants by adding oxidants such as hydrogen peroxide, permanganate, persulfate or reductants in the form of nanoscale iron particles.
- Electrokinetic bioremediation, also known as electro-bioremediation or electro-bioreclamation, is a group of cleanup techniques that use microbes for transformation and electrokinetics for the transport of subsurface pollutants, as well as any substances present or produced during the process. This technique is a combination of electrokinetics and bioremediation.
- Electrokinetic phytoremediation is a plant-based technology that involves the use of plants to remove elemental contaminants or reduce their bioavailability in contaminated areas. This is due to plants’ ability to absorb ionic materials in the soil through their root system, even in small amounts.
- Electrokinetic stabilization is mainly used to remove heavy metals. It acts by precipitating contaminants through the injection of alkaline solutions or reducing agents based on the specific contaminant conditions.
- Electrokinetic-thermal treatment is a process that involves the application of electrical energy to a contaminated site, resulting in resistive heating. This heating accelerates many chemical and biological reactions, modifying physical properties, and can be used to remove volatile organic contaminants.
1.3. Electrokinetic Bioremediation in Marine Environments
1.4. The Impact of Electrokinetic on Microbial Communities
1.5. Chemical Changes Generated by the Electric Field in Sediments
2. Materials and Methods
3. Results
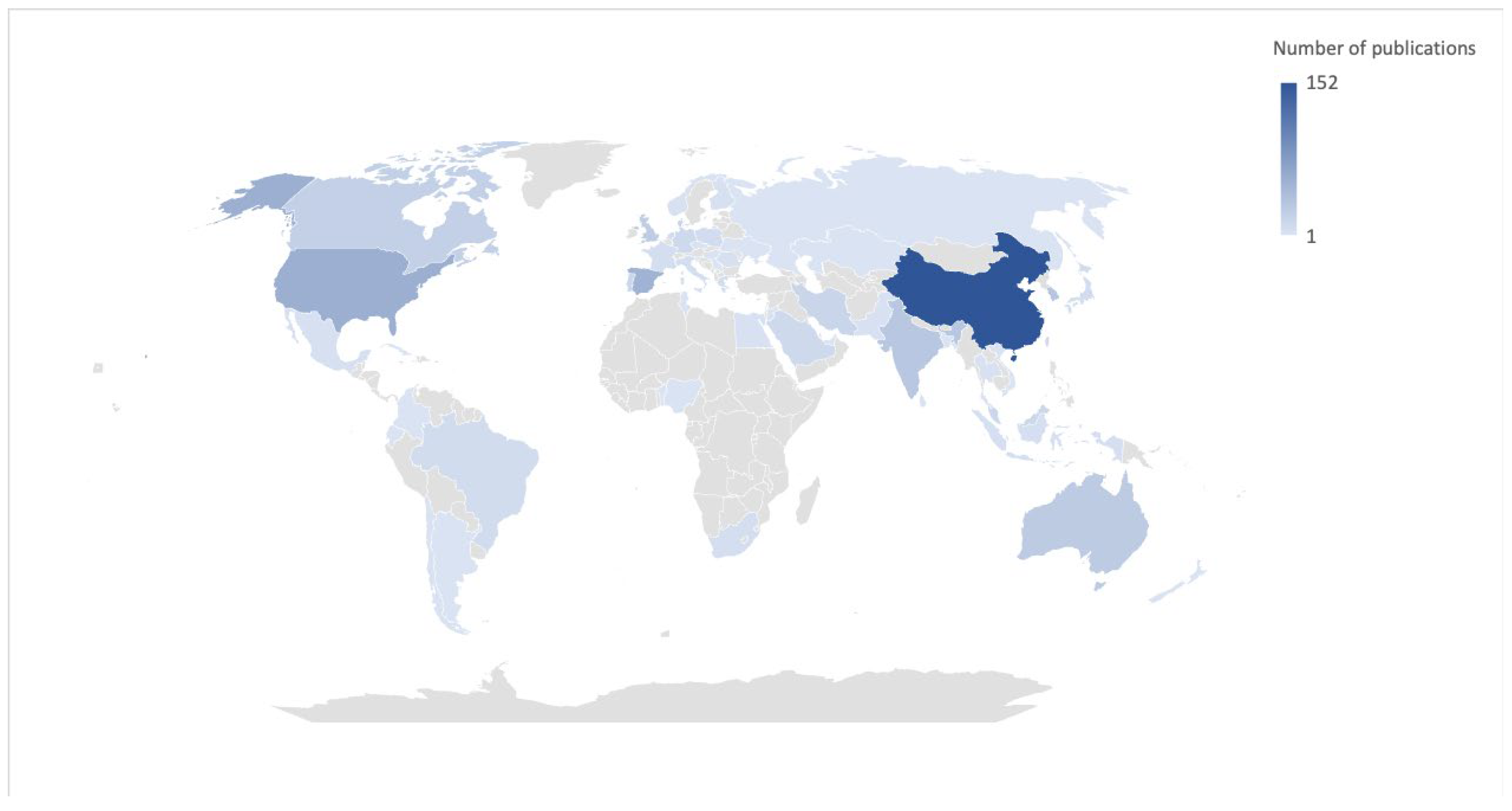
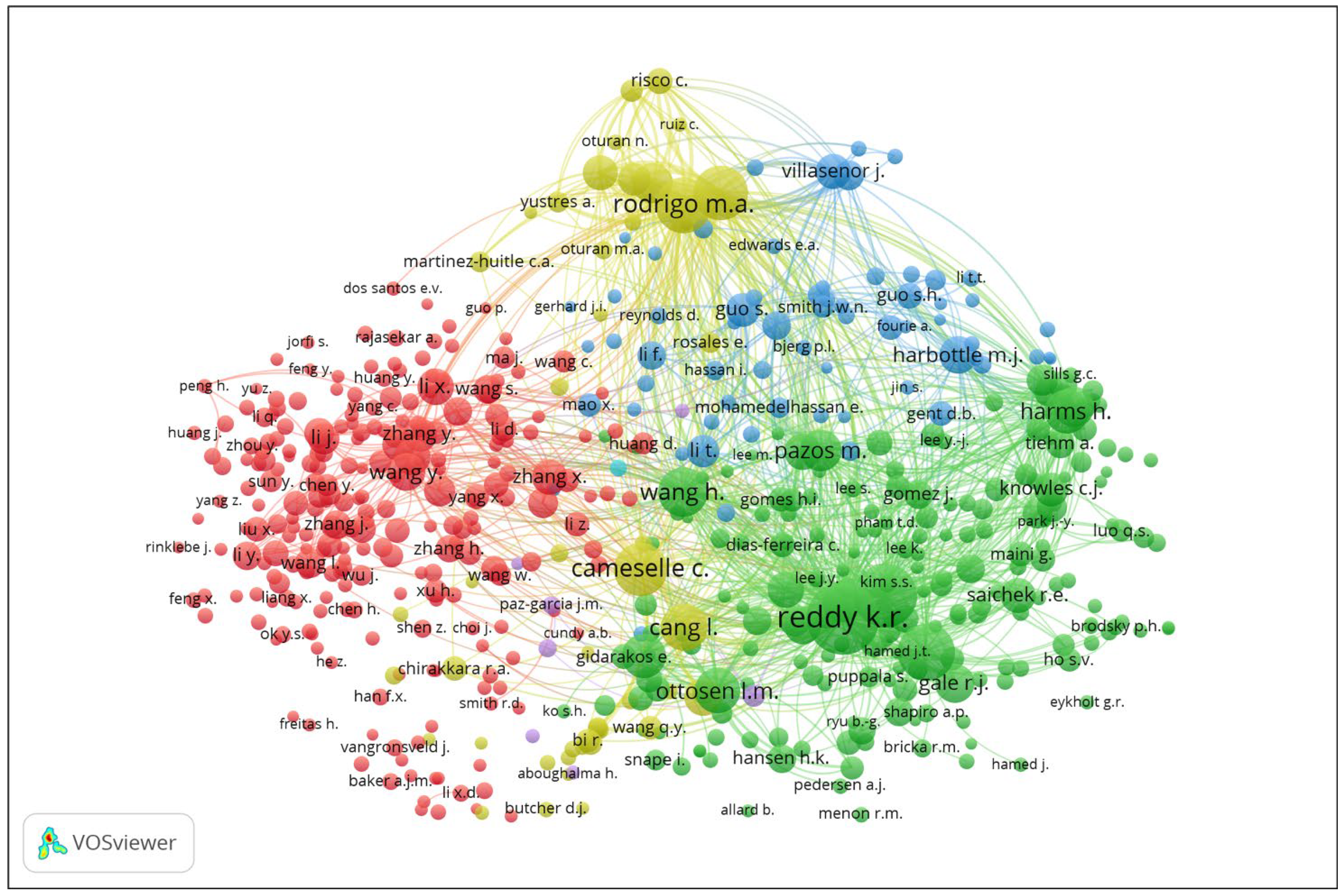
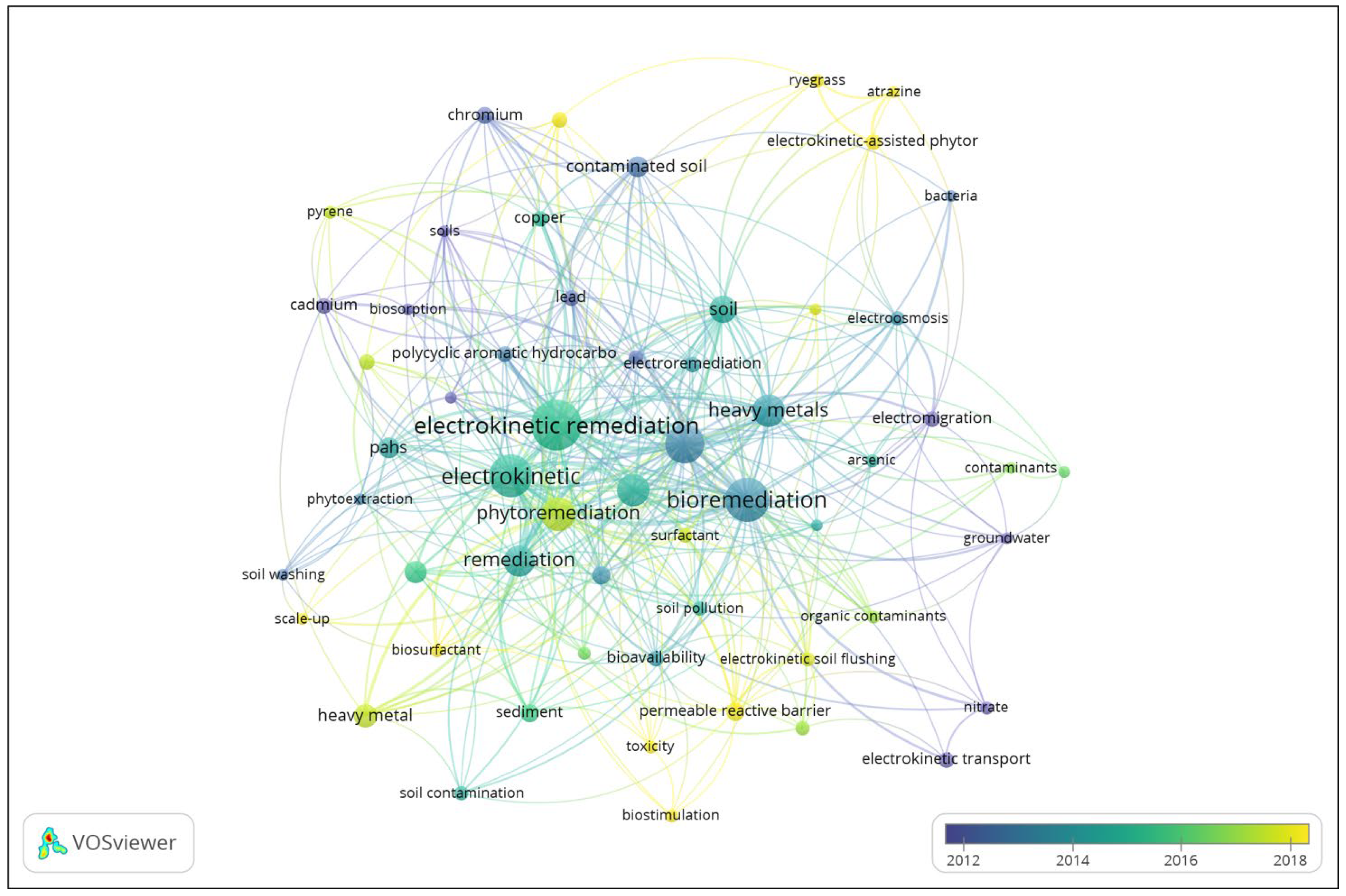
3.1. Bibliometric Analysis
3.2. Publication Trends
3.3. Country of Authorship and Affiliation
3.4. Authorship
3.5. Keywords Co-Occurrence Analysis
3.6. Publications with the Highest Citation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Soil Pollution: A Hidden Realty; FAO: Rome, Italy, 2018. [Google Scholar]
- Zhou, Q.; Wang, S.; Liu, J.; Hu, X.; Liu, Y.; He, Y.; He, X.; Wu, X. Geological Evolution of Offshore Pollution and Its Long-Term Potential Impacts on Marine Ecosystems. Geosci. Front. 2022, 13, 101427. [Google Scholar] [CrossRef]
- Costa, E.; Piazza, V.; Gambardella, C.; Moresco, R.; Prato, E.; Biandolino, F.; Cassin, D.; Botter, M.; Maurizio, D.; D’Adamo, R.; et al. Ecotoxicological Effects of Sediments from Mar Piccolo, South Italy: Toxicity Testing with Organisms from Different Trophic Levels. Environ. Sci. Pollut. Res. 2016, 23, 12755–12769. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Visciano, P.; Giammarino, A.; Manera, M.; Di Nardo, W.; Amorena, M. Polycyclic Aromatic Hydrocarbons in Marine Organisms from the Adriatic Sea, Italy. Chemosphere 2007, 66, 1904–1910. [Google Scholar] [CrossRef] [PubMed]
- Borja, A.; Belzunce, M.; Garmendia, J.; Rodriguez, J.G.; Solaun, O.; Zorita, I. Impact of Pollutants on Coastal and Benthic Marine Communities; Bentham Science Publishers: Sharjah, United Arab Emirates, 2011; ISBN 978-1-60805-121-2. [Google Scholar]
- Negrin, V.; Girones, L.; Serra, A. Eco-Friendly Strategies of Remediation in the Marine System. In Coastal and Deep Ocean Pollution; CRC Press: Boca Raton, FL, USA, 2020; pp. 184–214. ISBN 978-0-203-70427-1. [Google Scholar]
- Ferrucci, A.; Vocciante, M.; Bagatin, R.; Ferro, S. Electrokinetic Remediation of Soils Contaminated by Potentially Toxic Metals: Dedicated Analytical Tools for Assessing the Contamination Baseline in a Complex Scenario. J. Environ. Manag. 2017, 203, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Rosestolato, D.; Bagatin, R.; Ferro, S. Electrokinetic Remediation of Soils Polluted by Heavy Metals (Mercury in Particular). Chem. Eng. J. 2015, 264, 16–23. [Google Scholar] [CrossRef]
- Vocciante, M.; Dovì, V.; Ferro, S. Sustainability in ElectroKinetic Remediation Processes: A Critical Analysis. Sustainability 2021, 13, 770. [Google Scholar] [CrossRef]
- Yeung, A.T.; Gu, Y.-Y. A Review on Techniques to Enhance Electrochemical Remediation of Contaminated Soils. J. Hazard. Mater. 2011, 195, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, X.; Li, Q.; Wei, J.; Li, Z. Research and Application Progress of Electrochemical Water Treatment Technology. Water Treat. Technol. 2019, 45, 11–16. [Google Scholar]
- Zheng, T.; Wang, J.; Wang, Q.; Meng, H.; Wang, L. Research Trends in Electrochemical Technology for Water and Wastewater Treatment. Appl. Water Sci. 2017, 7, 13–30. [Google Scholar] [CrossRef]
- Vocciante, M.; Bagatin, R.; Ferro, S. Enhancements in ElectroKinetic Remediation Technology: Focus on Water Management and Wastewater Recovery. Chem. Eng. J. 2017, 309, 708–716. [Google Scholar] [CrossRef]
- Irfan, S.; Ranjha, M.M.A.N.; Shafique, B.; Ullah, M.I.; Siddiqui, A.R.; Wang, L. Bioremediation of Soil: An Overview. In Advances in Bioremediation and Phytoremediation for Sustainable Soil Management: Principles, Monitoring and Remediation; Malik, J.A., Ed.; Springer: Cham, Switzerland, 2022; pp. 1–16. ISBN 978-3-030-89984-4. [Google Scholar]
- Hussain, S.; Siddique, T.; Arshad, M.; Saleem, M. Bioremediation and Phytoremediation of Pesticides: Recent Advances. Crit. Rev. Environ. Sci. Technol. 2009, 39, 843–907. [Google Scholar] [CrossRef]
- Murillo-Tovar, M.A.; Saldarriaga-Noreña, H.; Saeid, A. (Eds.) Indu Sharma Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment; IntechOpen: Rijeka, Croatia, 2020; Chapter 12; ISBN 978-1-83880-332-2. [Google Scholar]
- Padhye, L.P.; Srivastava, P.; Jasemizad, T.; Bolan, S.; Hou, D.; Shaheen, S.M.; Rinklebe, J.; O’Connor, D.; Lamb, D.; Wang, H.; et al. Contaminant Containment for Sustainable Remediation of Persistent Contaminants in Soil and Groundwater. J. Hazard. Mater. 2023, 455, 131575. [Google Scholar] [CrossRef]
- Benamar, A.; Ammami, M.T.; Song, Y.; Portet-Koltalo, F. Scale-up of Electrokinetic Process for Dredged Sediments Remediation. Electrochim. Acta 2020, 352, 136488. [Google Scholar] [CrossRef]
- Mena, E.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Effect of a Direct Electric Current on the Activity of a Hydrocarbon-Degrading Microorganism Culture Used as the Flushing Liquid in Soil Remediation Processes. Sep. Purif. Technol. 2014, 124, 217–223. [Google Scholar] [CrossRef]
- Carré, C.; Gunkel-Grillon, P.; Serres, A.; Jeannin, M.; Sabot, R.; Quiniou, T. Laboratory and In-Situ Investigations for Trapping Pb and Ni with an Unusual Electrochemical Device, the Calcareous Deposit in Seawater. Sci. Rep. 2019, 9, 3400. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic Remediation for the Removal of Heavy Metals in Soil: Limitations, Solutions and Prospection. Sci. Total Environ. 2023, 903, 165970. [Google Scholar] [CrossRef]
- Aulenta, F.; Canosa, A.; Reale, P.; Rossetti, S.; Panero, S.; Majone, M. Microbial Reductive Dechlorination of Trichloroethene to Ethene with Electrodes Serving as Electron Donors without the External Addition of Redox Mediators. Biotechnol. Bioeng. 2009, 103, 85–91. [Google Scholar] [CrossRef]
- Reddy, K.R.; Darko-Kagya, K.; Al-Hamdan, A.Z. Electrokinetic Remediation of Pentachlorophenol Contaminated Clay Soil. Water Air Soil Pollut. 2011, 221, 35–44. [Google Scholar] [CrossRef]
- Reddy, K.; Cameselle, C. Electrochemical Remediation Technologies for Polluted Soils, Sediments and Groundwater; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Puri, A.N.; Anand, B. Reclamation of Alkali Soils by Electrodialysis. Soil Sci. 1936, 42, 23–28. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of Electrokinetic Remediation. Environ. Sci. Technol. 1993, 27, 2638–2647. [Google Scholar] [CrossRef]
- DeFlaun, M.F.; Condee, C.W. Electrokinetic Transport of Bacteria. J. Hazard. Mater. 1997, 55, 263–277. [Google Scholar] [CrossRef]
- Ugaz, A.; Puppala, S.; Gale, R.J.; Acar, Y.B. Electrokinetic Soil Processing Complicating Feature of Electrokinetic Remediation of Soils and Slurries: Saturation Effects and the Role of the Cathode Electrolysis. Chem. Eng. Commun. 1994, 129, 183–200. [Google Scholar] [CrossRef]
- Bennett, J.E. Electrodes for Generation of Hydrogen and Oxygen from Seawater. Int. J. Hydrog. Energy 1980, 5, 401–408. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Yu, J.; Zhou, W. Design Strategy of Corrosion-Resistant Electrodes for Seawater Electrolysis. Materials 2023, 16, 2709. [Google Scholar] [CrossRef] [PubMed]
- Consonni, V.; Trasatti, S.; Pollak, F.; O’Grady, W.E. Mechanism of Chlorine Evolution on Oxide Anodes Study of pH Effects. J. Electroanal. Chem. Interfacial Electrochem. 1987, 228, 393–406. [Google Scholar] [CrossRef]
- Karlsson, R.K.B.; Cornell, A. Selectivity between Oxygen and Chlorine Evolution in the Chlor-Alkali and Chlorate Processes. Chem. Rev. 2016, 116, 2982–3028. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Ibrahim, J.; Moussab, H. Recent Advances on Hydrogen Production through Seawater Electrolysis. Mater. Sci. Energy Technol. 2020, 3, 780–807. [Google Scholar] [CrossRef]
- Maril, M.; Delplancke, J.-L.; Cisternas, N.; Tobosque, P.; Maril, Y.; Carrasco, C. Critical Aspects in the Development of Anodes for Use in Seawater Electrolysis. Int. J. Hydrog. Energy 2022, 47, 3532–3549. [Google Scholar] [CrossRef]
- Abdel-Aal, H.K.; Hussein, I.A. Parametric Study for Saline Water Electrolysis: Part III—Precipitate Formation and Recovery of Magnesium Salts. Int. J. Hydrog. Energy 1993, 18, 553–556. [Google Scholar] [CrossRef]
- Sale, T.; Petersen, M.; Gilbert, D. Electrically Induced Redox Barriers for Treatment of Groundwater. 2005. 187p. Available online: https://www.clu-in.org/download/contaminantfocus/dnapl/Treatment_Technologies/CU-0112-fr-01.pdf (accessed on 22 February 2024).
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Drewniak, L.; Krawczyk, P.S.; Mielnicki, S.; Adamska, D.; Sobczak, A.; Lipinski, L.; Burec-Drewniak, W.; Sklodowska, A. Physiological and Metagenomic Analyses of Microbial Mats Involved in Self-Purification of Mine Waters Contaminated with Heavy Metals. Front. Microbiol. 2016, 7, 1252. [Google Scholar] [CrossRef]
- Ozer, T.B.; Erkaya, I.A.; Udoh, A.U.; Duygu, D.Y.; Akbulut, A.; Bayramoglu, G.; Arica, M.Y. Biosorption of Cr(VI) by Free and Immobilized Pediastrum Boryanum Biomass: Equilibrium, Kinetic, and Thermodynamic Studies. Environ. Sci. Pollut. Res. 2012, 19, 2983–2993. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of Cyanobacteria/Microalgae and Bacteria: Biotechnological Potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Kotb, M.M.A. Coral Translocation and Farming as Mitigation and Conservation Measures for Coastal Development in the Red Sea: Aqaba Case Study, Jordan. Environ. Earth Sci 2016, 75, 439. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Mohan, S.V. Critical Parametric Influence on Microalgae Cultivation towards Maximizing Biomass Growth with Simultaneous Lipid Productivity. Renew. Energy 2016, 98, 64–71. [Google Scholar] [CrossRef]
- Gorny, J.; Billon, G.; Noiriel, C.; Dumoulin, D.; Lesven, L.; Madé, B. Chromium Behavior in Aquatic Environments: A Review. Environ. Rev. 2016, 24, 503–516. [Google Scholar] [CrossRef]
- Ma, L.; Xu, J.; Chen, N.; Li, M.; Feng, C. Microbial Reduction Fate of Chromium (Cr) in Aqueous Solution by Mixed Bacterial Consortium. Ecotoxicol. Environ. Saf. 2019, 170, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Vankar, P.S.; Srivastava, S.K. Bioremediation of Hexavalent Chromium by a Cyanobacterial Mat. Appl. Water Sci. 2012, 2, 245–251. [Google Scholar] [CrossRef]
- Zhang, W.; Majidi, V. Monitoring the Cellular Response of Stichococcus Bacillaris to Exposure of Several Different Metals Using in Vivo 31P NMR and Other Spectroscopic Techniques. Environ. Sci. Technol. 1994, 28, 1577–1581. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Cui, C. Remediation of Heavy Metal-Contaminated Soils by Electrokinetic Technology: Mechanisms and Applicability. Chemosphere 2021, 265, 129071. [Google Scholar] [CrossRef] [PubMed]
- Habibul, N.; Hu, Y.; Sheng, G.-P. Microbial Fuel Cell Driving Electrokinetic Remediation of Toxic Metal Contaminated Soils. J. Hazard. Mater. 2016, 318, 9–14. [Google Scholar] [CrossRef]
- Hansen, H.K.; Ottosen, L.M.; Ribeiro, A.B. Electrokinetic Soil Remediation: An Overview. In Electrokinetics Across Disciplines and Continents: New Strategies for Sustainable Development; Ribeiro, A.B., Mateus, E.P., Couto, N., Eds.; Springer: Cham, Switzerland, 2016; pp. 3–18. ISBN 978-3-319-20179-5. [Google Scholar]
- Hansen, H.K.; Rojo, A. Testing Pulsed Electric Fields in Electroremediation of Copper Mine Tailings. Electrochim. Acta 2007, 52, 3399–3405. [Google Scholar] [CrossRef]
- Hassan, I.; Mohamedelhassan, E.; Yanful, E.K. Solar Powered Electrokinetic Remediation of Cu Polluted Soil Using a Novel Anode Configuration. Electrochim. Acta 2015, 181, 58–67. [Google Scholar] [CrossRef]
- Jeon, E.-K.; Jung, J.-M.; Kim, W.-S.; Ko, S.-H.; Baek, K. In Situ Electrokinetic Remediation of As-, Cu-, and Pb-Contaminated Paddy Soil Using Hexagonal Electrode Configuration: A Full Scale Study. Environ. Sci. Pollut. Res. 2015, 22, 711–720. [Google Scholar] [CrossRef]
- Ryu, B.-G.; Yang, J.-S.; Kim, D.H.; Baek, K. Pulsed Electrokinetic Removal of Cd and Zn from Fine-Grained Soil. J. Appl. Electrochem. 2010, 40, 1039–1047. [Google Scholar] [CrossRef]
- Sun, T.R.; Ottosen, L.M. Effects of Pulse Current on Energy Consumption and Removal of Heavy Metals during Electrodialytic Soil Remediation. Electrochim. Acta 2012, 86, 28–35. [Google Scholar] [CrossRef]
- Sun, T.R.; Ottosen, L.M.; Jensen, P.E.; Kirkelund, G.M. Effect of Pulse Current on Acidification and Removal of Cu, Cd, and as during Suspended Electrodialytic Soil Remediation. Electrochim. Acta 2013, 107, 187–193. [Google Scholar] [CrossRef]
- Yuan, S.; Zheng, Z.; Chen, J.; Lu, X. Use of Solar Cell in Electrokinetic Remediation of Cadmium-Contaminated Soil. J. Hazard. Mater. 2009, 162, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Xu, X.; Li, H.; Wang, Q.; Wang, N.; Yu, H. The Influence of Macroelements on Energy Consumption during Periodic Power Electrokinetic Remediation of Heavy Metals Contaminated Black Soil. Electrochim. Acta 2017, 235, 604–612. [Google Scholar] [CrossRef]
- Yuan, C.; Hung, C.-H.; Chen, K.-C. Electrokinetic Remediation of Arsenate Spiked Soil Assisted by CNT-Co Barrier—The Effect of Barrier Position and Processing Fluid. J. Hazard. Mater. 2009, 171, 563–570. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Zhou, D.-M.; Cang, L.; Li, L.-Z.; Wang, P. Solid/Solution Cu Fractionations/Speciation of a Cu Contaminated Soil after Pilot-Scale Electrokinetic Remediation and Their Relationships with Soil Microbial and Enzyme Activities. Environ. Pollut. 2009, 157, 2203–2208. [Google Scholar] [CrossRef]
- Tchounwou, P.; Yedjou, C.; Patlolla, A.; Sutton, D. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Annamalai, S.; Sundaram, M. Electro-Bioremediation: An Advanced Remediation Technology for the Treatment and Management of Contaminated Soil. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-981-13-3425-2. [Google Scholar]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-Noble Metal-Nitride Based Electrocatalysts for High-Performance Alkaline Seawater Electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef]
- Virkutyte, J.; Sillanpää, M.; Latostenmaa, P. Electrokinetic Soil Remediation—Critical Overview. Sci. Total Environ. 2002, 289, 97–121. [Google Scholar] [CrossRef]
- Méndez, E.; Pérez, M.; Romero, O.; Beltrán, E.D.; Castro, S.; Corona, J.L.; Corona, A.; Cuevas, M.C.; Bustos, E. Effects of Electrode Material on the Efficiency of Hydrocarbon Removal by an Electrokinetic Remediation Process. Electrochim. Acta 2012, 86, 148–156. [Google Scholar] [CrossRef]
- Qu, Z.; Huang, L.; Guo, M.; Sun, T.; Xu, X.; Gao, Z. Application of Novel Polypyrrole/Melamine Foam Auxiliary Electrode in Promoting Electrokinetic Remediation of Cr(VI)-Contaminated Soil. Sci. Total Environ. 2023, 876, 162840. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, J.; Sun, X.; Cheng, Z.; Liu, Y.; Wang, Y. High Density of Free-Standing Holey Graphene/PPy Films for Superior Volumetric Capacitance of Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 21763–21772. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.B.; Kirkelund, G.M.; Ottosen, L.M.; Jensen, P.E.; Lejon, T. Multivariate Methods for Evaluating the Efficiency of Electrodialytic Removal of Heavy Metals from Polluted Harbour Sediments. J. Hazard. Mater. 2015, 283, 712–720. [Google Scholar] [CrossRef]
- Bellagamba, M.; Cruz Viggi, C.; Ademollo, N.; Rossetti, S.; Aulenta, F. Electrolysis-Driven Bioremediation of Crude Oil-Contaminated Marine Sediments. New Biotechnol. 2017, 38, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Cappello, S.; Cruz Viggi, C.; Yakimov, M.; Rossetti, S.; Matturro, B.; Molina, L.; Segura, A.; Marqués, S.; Yuste, L.; Sevilla, E.; et al. Combining Electrokinetic Transport and Bioremediation for Enhanced Removal of Crude Oil from Contaminated Marine Sediments: Results of a Long-Term, Mesocosm-Scale Experiment. Water Res. 2019, 157, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.B.; Simões, M.; Simões, L.C. The Effects of Sodium Hypochlorite against Selected Drinking Water-Isolated Bacteria in Planktonic and Sessile States. Sci. Total Environ. 2016, 565, 40–48. [Google Scholar] [CrossRef]
- Roeßler, M.; Sewald, X.; Müller, V. Chloride Dependence of Growth in Bacteria. FEMS Microbiol. Lett. 2003, 225, 161–165. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.-H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L.; et al. Solar-Driven, Highly Sustained Splitting of Seawater into Hydrogen and Oxygen Fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef]
- Han, D.; Wu, X.; Li, R.; Tang, X.; Xiao, S.; Scholz, M. Critical Review of Electro-Kinetic Remediation of Contaminated Soils and Sediments: Mechanisms, Performances and Technologies. Water Air Soil Pollut. 2021, 232, 335. [Google Scholar] [CrossRef]
- Block, S.S. Disinfection, Sterilization, and Preservation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; ISBN 0-683-30740-1. [Google Scholar]
- Ho, S.; Mittal, G.; Cross, J. Effects of High Field Electric Pulses on the Activity of Selected Enzymes. J. Food Eng. 1997, 31, 69–84. [Google Scholar] [CrossRef]
- Mao, X.; Wang, J.; Ciblak, A.; Cox, E.E.; Riis, C.; Terkelsen, M.; Gent, D.B.; Alshawabkeh, A.N. Electrokinetic-Enhanced Bioaugmentation for Remediation of Chlorinated Solvents Contaminated Clay. J. Hazard. Mater. 2012, 213–214, 311–317. [Google Scholar] [CrossRef]
- Simpson, R.K.; Whittington, R.; Earnshaw, R.G.; Russell, N.J. Pulsed High Electric Field Causes ‘All or Nothing’ Membrane Damage in Listeria Monocytogenes and Salmonella Typhimurium, but Membrane H+–ATPase Is Not a Primary Target. Int. J. Food Microbiol. 1999, 48, 1–10. [Google Scholar] [CrossRef]
- Ulmer, H.M.; Heinz, V.; Gänzle, M.G.; Knorr, D.; Vogel, R.F. Effects of Pulsed Electric Fields on Inactivation and Metabolic Activity of Lactobacillus Plantarum in Model Beer. J. Appl. Microbiol. 2002, 93, 326–335. [Google Scholar] [CrossRef]
- Vega-Mercado, H.; Powers, J.R.; Barbosa-Cànovas, G.V.; Swanson, B.G. Plasmin Inactivation with Pulsed Electric Fields. J. Food Sci. 1995, 60, 1143–1146. [Google Scholar] [CrossRef]
- Wick, L.Y.; Buchholz, F.; Fetzer, I.; Kleinsteuber, S.; Härtig, C.; Shi, L.; Miltner, A.; Harms, H.; Pucci, G.N. Responses of Soil Microbial Communities to Weak Electric Fields. Sci. Total Environ. 2010, 408, 4886–4893. [Google Scholar] [CrossRef]
- Yeom, H.W.; Streaker, C.B.; Zhang, Q.H.; Min, D.B. Effects of Pulsed Electric Fields on the Activities of Microorganisms and Pectin Methyl Esterase in Orange Juice. J. Food Sci. 2000, 65, 1359–1363. [Google Scholar] [CrossRef]
- Lear, G.; Harbottle, M.J.; van der Gast, C.J.; Jackman, S.A.; Knowles, C.J.; Sills, G.; Thompson, I.P. The Effect of Electrokinetics on Soil Microbial Communities. Soil Biol. Biochem. 2004, 36, 1751–1760. [Google Scholar] [CrossRef]
- Cang, L.; Zhou, D.-M.; Alshawabkeh, A.N.; Chen, H.-F. Effects of Sodium Hypochlorite and High pH Buffer Solution in Electrokinetic Soil Treatment on Soil Chromium Removal and the Functional Diversity of Soil Microbial Community. J. Hazard. Mater. 2007, 142, 111–117. [Google Scholar] [CrossRef]
- Lear, G.; Harbottle, M.J.; Sills, G.; Knowles, C.J.; Semple, K.T.; Thompson, I.P. Impact of Electrokinetic Remediation on Microbial Communities within PCP Contaminated Soil. Environ. Pollut. 2007, 146, 139–146. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, H.-Y.; Lee, Y.-J.; Kim, C.W.; Yang, J.-W. Effect of Electrokinetic Remediation on Indigenous Microbial Activity and Community within Diesel Contaminated Soil. Sci. Total Environ. 2010, 408, 3162–3168. [Google Scholar] [CrossRef]
- Wan, C.; Du, M.; Lee, D.-J.; Yang, X.; Ma, W.; Zheng, L. Electrokinetic Remediation and Microbial Community Shift of β-Cyclodextrin-Dissolved Petroleum Hydrocarbon-Contaminated Soil. Appl. Microbiol. Biotechnol. 2011, 89, 2019–2025. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Zhang, Z.; Zhu, C.; Tian, Y.; Ye, J. Evolution of Microbial Communities during Electrokinetic Treatment of Antibiotic-Polluted Soil. Ecotoxicol. Environ. Saf. 2018, 148, 842–850. [Google Scholar] [CrossRef]
- Jackman, S.A.; Maini, G.; Sharman, A.K.; Knowles, C.J. The Effects of Direct Electric Current on the Viability and Metabolism of Acidophilic Bacteria. Enzym. Microb. Technol. 1999, 24, 316–324. [Google Scholar] [CrossRef]
- Scheunert, I.; Attar, A.; Zelles, L. Ecotoxicological Effects of Soil-Bound Pentachlorophenol Residues on the Microflora of Soils. Chemosphere 1995, 30, 1995–2009. [Google Scholar] [CrossRef]
- Harbottle, M.; Sills, G.C.; Jackman, S.; Thompson, I.P. Movement of Pentachlorophenol in Unsaturated Soil by Electrokinetics. In Proceedings of the 3rd Symposium on Electrokinetic Remediation, Karlsruhe, Germany, 18–20 April 2001. [Google Scholar]
- Genovese, M.; Crisafi, F.; Denaro, R.; Cappello, S.; Russo, D.; Calogero, R.; Santisi, S.; Catalfamo, M.; Modica, A.; Smedile, F.; et al. Effective Bioremediation Strategy for Rapid in Situ Cleanup of Anoxic Marine Sediments in Mesocosm Oil Spill Simulation. Front. Microbiol. 2014, 5, 162. [Google Scholar] [CrossRef]
- Morales Pontet, N.G.; Fernandez, C.; Perillo, V.; La Colla, N.; Serra, A.; Botté, S. Preliminary Assessment of Microbial Mats in Seawater Metal Remediation. Environ. Monit. Assess. 2023, 195, 516. [Google Scholar] [CrossRef]
- Velizarov, S. Electric and Magnetic Fields in Microbial Biotechnology: Possibilities, Limitations, and Perspectives. Electromagn. Biol. Med. 1999, 18, 185–212. [Google Scholar] [CrossRef]
- Beschkov, V.N.; Peeva, L.G. Effects of Electric Current Passing through the Fermentation Broth of a Strain Acetobacter Suboxydans. Bioelectrochem. Bioenerg. 1994, 34, 185–188. [Google Scholar] [CrossRef]
- Blake, R.; Howard, G.; McGinness, S. Enhanced Yields of Iron-Oxidizing Bacteria by In Situ Electrochemical Reduction of Soluble Iron in the Growth Medium. Appl. Environ. Microbiol. 1994, 60, 2704–2710. [Google Scholar] [CrossRef]
- Chang, Y.-H.D.; Grodzinsky, A.J.; Wang, D.I.C. Augmentation of Mass Transfer through Electrical Means for Hydrogel-Entrapped Escherichia Coli Cultivation. Biotechnol. Bioeng. 1995, 48, 149–157. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tokuda, H.; Soga, T.; Yoshinaga, T.; Takeda, M. Effect of Electric Current on Growth and Alcohol Production by Yeast Cells. J. Ferment. Bioeng. 1998, 85, 250–253. [Google Scholar] [CrossRef]
- Nakasono, S.; Matsumoto, N.; Saiki, H. Electrochemical Cultivation of Thiobacillus Ferrooxidans by Potential Control. Bioelectrochem. Bioenerg. 1997, 43, 61–66. [Google Scholar] [CrossRef]
- Wang, Q.; Zhong, S.; Xie, Y.; Zhang, Z.; Yang, G. Electrochemical Reactions During Constant DC Current Stimulation: An In Vitro Experiment with Cultured Rat Calvarial Cells. Electro-Magnetobiology 1995, 14, 31–40. [Google Scholar] [CrossRef]
- Chen, C.; Yang, X.; Luo, H.; Zeng, D.; Sima, M.; Huang, S. Linking Microbial Community and Biological Functions to Redox Potential during Black-Odor River Sediment Remediation. Environ. Sci. Pollut. Res. 2020, 27, 40392–40404. [Google Scholar] [CrossRef]
- Liu, C.; Shen, Q.; Zhou, Q.; Fan, C.; Shao, S. Precontrol of Algae-Induced Black Blooms through Sediment Dredging at Appropriate Depth in a Typical Eutrophic Shallow Lake. Ecol. Eng. 2015, 77, 139–145. [Google Scholar] [CrossRef]
- Song, C.; Liu, X.; Song, Y.; Liu, R.; Gao, H.; Han, L.; Peng, J. Key Blackening and Stinking Pollutants in Dongsha River of Beijing: Spatial Distribution and Source Identification. J. Environ. Manag. 2017, 200, 335–346. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Fang, Y.; Huang, R. Analysis on the Formation Condition of the Algae-Induced Odorous Black Water Agglomerate. Saudi J. Biol. Sci. 2014, 21, 597–604. [Google Scholar] [CrossRef]
- Watson, S.B. Cyanobacterial and Eukaryotic Algal Odour Compounds: Signals or by-Products? A Review of Their Biological Activity. Phycologia 2003, 42, 332–350. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A Review of Nitrogen Enrichment Effects on Three Biogenic GHGs: The CO2 Sink May Be Largely Offset by Stimulated N2O and CH4 Emission. Ecol. Lett. 2009, 12, 1103–1117. [Google Scholar] [CrossRef]
- Stelmach, W.; Szarlip, P.; Trembaczowski, A.; Bieganowski, A.; Kuzyakov, Y. Suppression of Soil Organic Matter Decomposition by Gasoline and Diesel as Assessed by 13C Natural Abundance. Eur. J. Soil Biol. 2016, 73, 8–14. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, Y.; Feng, S.; Ge, Y.; Zhang, W.; He, X.; Wang, K. Soil Organic Carbon Mineralization with Fresh Organic Substrate and Inorganic Carbon Additions in a Red Soil Is Controlled by Fungal Diversity along a pH Gradient. Geoderma 2018, 321, 79–89. [Google Scholar] [CrossRef]
- Zhang, S.; Fang, Y.; Luo, Y.; Li, Y.; Ge, T.; Wang, Y.; Wang, H.; Yu, B.; Song, X.; Chen, J. Linking Soil Carbon Availability, Microbial Community Composition and Enzyme Activities to Organic Carbon Mineralization of a Bamboo Forest Soil Amended with Pyrogenic and Fresh Organic Matter. Sci. Total Environ. 2021, 801, 149717. [Google Scholar] [CrossRef]
- Cai, J.-F.; Jiang, F.; Liu, X.-S.; Sun, K.; Wang, W.; Zhang, M.-X.; Li, H.-L.; Xu, H.-F.; Kong, W.-J.; Yu, F.-H. Biochar-Amended Coastal Wetland Soil Enhances Growth of Suaeda Salsa and Alters Rhizosphere Soil Nutrients and Microbial Communities. Sci. Total Environ. 2021, 788, 147707. [Google Scholar] [CrossRef]
- Ling, L.; Fu, Y.; Jeewani, P.H.; Tang, C.; Pan, S.; Reid, B.J.; Gunina, A.; Li, Y.; Li, Y.; Cai, Y. Organic Matter Chemistry and Bacterial Community Structure Regulate Decomposition Processes in Post-Fire Forest Soils. Soil Biol. Biochem. 2021, 160, 108311. [Google Scholar] [CrossRef]
- Dike, C.C.; Hakeem, I.G.; Rani, A.; Surapaneni, A.; Khudur, L.; Shah, K.; Ball, A.S. The Co-Application of Biochar with Bioremediation for the Removal of Petroleum Hydrocarbons from Contaminated Soil. Sci. Total Environ. 2022, 849, 157753. [Google Scholar] [CrossRef]
- Dike, C.C.; Khudur, L.S.; Hakeem, I.G.; Rani, A.; Shahsavari, E.; Surapaneni, A.; Shah, K.; Ball, A.S. Biosolids-Derived Biochar Enhances the Bioremediation of Diesel-Contaminated Soil. J. Environ. Chem. Eng. 2022, 10, 108633. [Google Scholar] [CrossRef]
- Sarkar, D.; Ferguson, M.; Datta, R.; Birnbaum, S. Bioremediation of Petroleum Hydrocarbons in Contaminated Soils: Comparison of Biosolids Addition, Carbon Supplementation, and Monitored Natural Attenuation. Environ. Pollut. 2005, 136, 187–195. [Google Scholar] [CrossRef]
- Wang, S.; Guo, S. Effects of Soil Organic Carbon Metabolism on Electro-Bioremediation of Petroleum-Contaminated Soil. J. Hazard. Mater. 2023, 459, 132180. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation Technologies for Metal-Contaminated Soils and Groundwater: An Evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of Soils Contaminated with Polycyclic Aromatic Hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation Approaches for Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soils: Technological Constraints, Emerging Trends and Future Directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Pavel, L.V.; Cretescu, I. Characterization and Remediation of Soils Contaminated with Uranium. J. Hazard. Mater. 2009, 163, 475–510. [Google Scholar] [CrossRef]
- Leštan, D.; Luo, C.; Li, X. The Use of Chelating Agents in the Remediation of Metal-Contaminated Soils: A Review. Environ. Pollut. 2008, 153, 3–13. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.W.; Lau, E.V.; Poh, P.E. A Comprehensive Guide of Remediation Technologies for Oil Contaminated Soil—Present Works and Future Directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhao, D.; Wang, Q. An Overview of Field-Scale Studies on Remediation of Soil Contaminated with Heavy Metals and Metalloids: Technical Progress over the Last Decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Barba, S.; López-Vizcaíno, R.; Saez, C.; Villaseñor, J.; Cañizares, P.; Navarro, V.; Rodrigo, M.A. Electro-Bioremediation at the Prototype Scale: What It Should Be Learned for the Scale-Up. Chem. Eng. J. 2018, 334, 2030–2038. [Google Scholar] [CrossRef]
- Alshawabkeh, A.N.; Yeung, A.T.; Bricka, M.R. Practical Aspects of In-Situ Electrokinetic Extraction. J. Environ. Eng. 1999, 125, 27–35. [Google Scholar] [CrossRef]
- Tang, J.; Qiu, Z.; Tang, H.; Wang, H.; Sima, W.; Liang, C.; Liao, Y.; Li, Z.; Wan, S.; Dong, J. Coupled with EDDS and Approaching Anode Technique Enhanced Electrokinetic Remediation Removal Heavy Metal from Sludge. Environ. Pollut. 2021, 272, 115975. [Google Scholar] [CrossRef]


| Title | Journal | Year | N° Citations | References |
|---|---|---|---|---|
| Remediation technologies for metal-contaminated soils and groundwater: An evaluation | Engineering Geology | 2001 | 1242 | [117] |
| Remediation techniques for heavy metal-contaminated soils: Principles and applicability | Science of the Total Environment | 2018 | 972 | [118] |
| Electrokinetic soil remediation—Critical overview | Science of the Total Environment | 2002 | 761 | [64] |
| Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs) | Journal of Hazardous Materials | 2009 | 678 | [119] |
| Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions | Chemosphere | 2017 | 516 | [120] |
| Characterization and remediation of soils contaminated with uranium | Journal of Hazardous Materials | 2009 | 471 | [121] |
| The use of chelating agents in the remediation of metal-contaminated soils: A review | Environmental Pollution | 2008 | 461 | [122] |
| Polycyclic Aromatic Hydrocarbons: Sources, Toxicity and Remediation Approaches | Frontiers in Microbiology | 2020 | 395 | [123] |
| A comprehensive guide of remediation technologies for oil-contaminated soil—Present works and future directions | Marine Pollution Bulletin | 2016 | 311 | [124] |
| An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade | Water Research | 2018 | 294 | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcino, N.; Crisafi, F.; Catalfamo, M.; Denaro, R.; Smedile, F. Electrokinetic Remediation in Marine Sediment: A Review and a Bibliometric Analysis. Sustainability 2024, 16, 4616. https://doi.org/10.3390/su16114616
Porcino N, Crisafi F, Catalfamo M, Denaro R, Smedile F. Electrokinetic Remediation in Marine Sediment: A Review and a Bibliometric Analysis. Sustainability. 2024; 16(11):4616. https://doi.org/10.3390/su16114616
Chicago/Turabian StylePorcino, Nunziatina, Francesca Crisafi, Maurizio Catalfamo, Renata Denaro, and Francesco Smedile. 2024. "Electrokinetic Remediation in Marine Sediment: A Review and a Bibliometric Analysis" Sustainability 16, no. 11: 4616. https://doi.org/10.3390/su16114616
APA StylePorcino, N., Crisafi, F., Catalfamo, M., Denaro, R., & Smedile, F. (2024). Electrokinetic Remediation in Marine Sediment: A Review and a Bibliometric Analysis. Sustainability, 16(11), 4616. https://doi.org/10.3390/su16114616






