Secondary Succession in Fallow Agroforestry Systems Managed in Tropical Dry Forest in Western Mexico
Abstract
1. Introduction
2. Materials and Methods
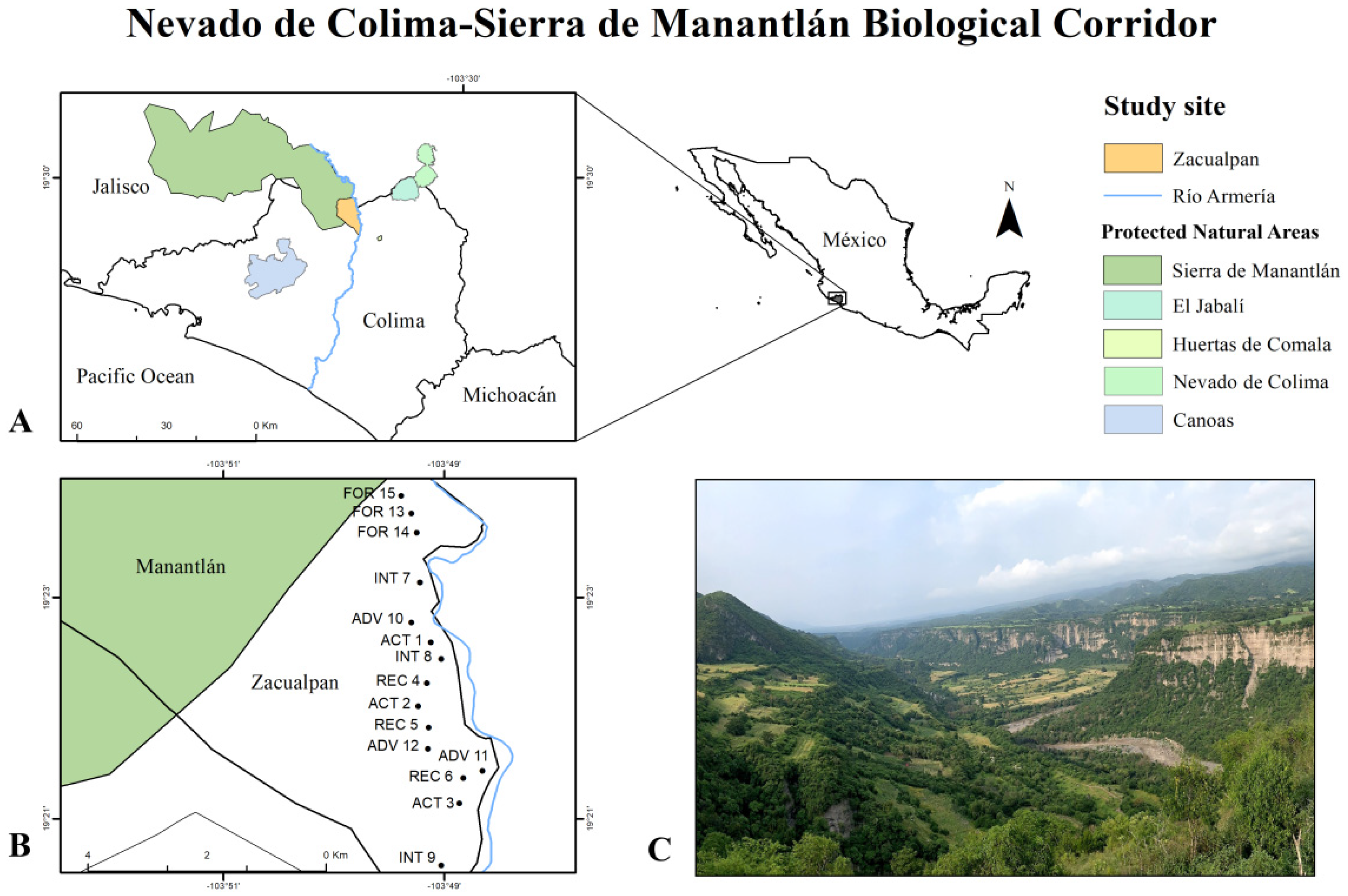
2.1. Study Site
2.2. Research Design
Vegetation Sampling
2.3. Biodiversity Conservation Capacity Data Analysis
2.3.1. Species Diversity and Composition
2.3.2. Vegetation Structure
3. Results
3.1. AFS Characterization
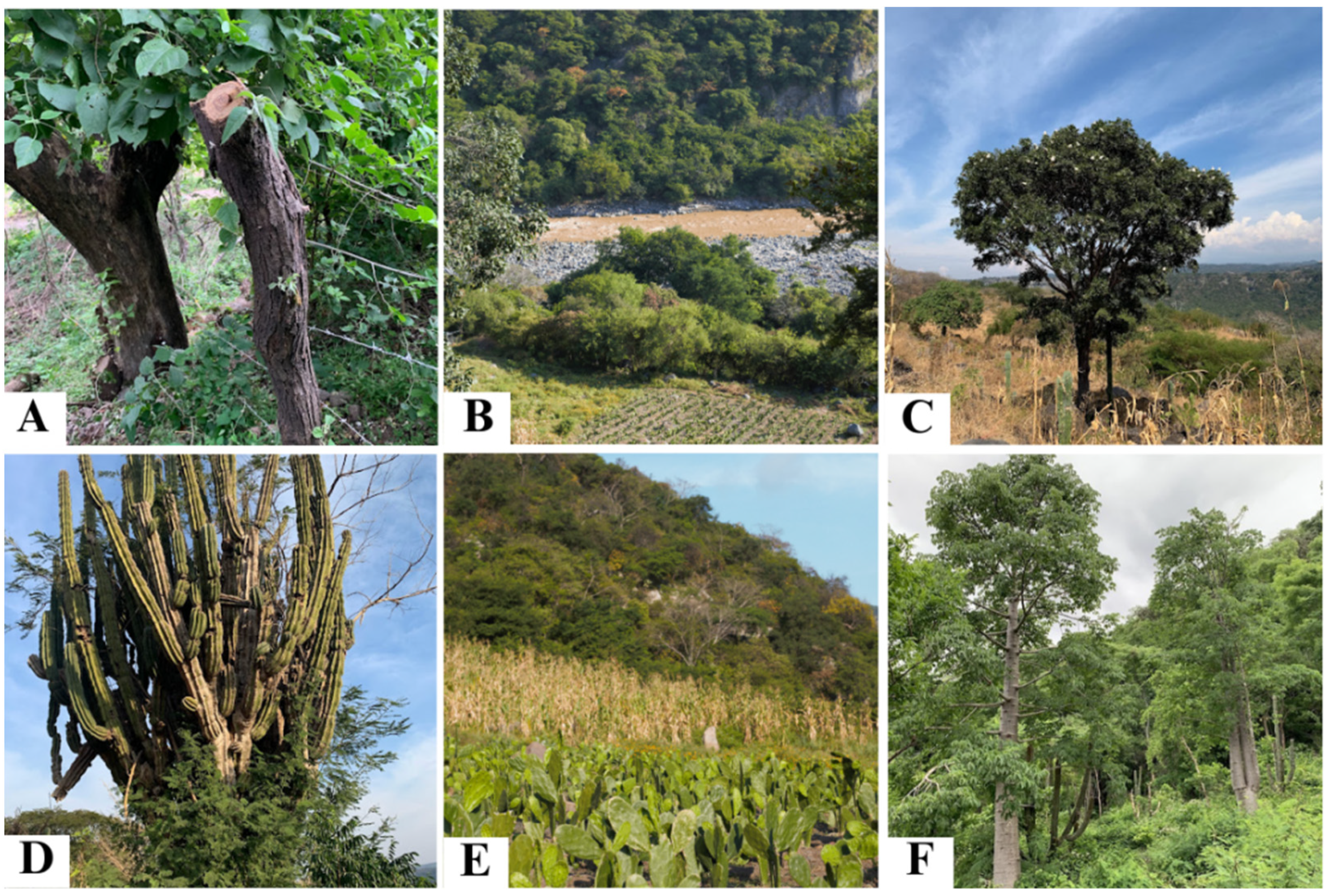
Agroforestry Landscape, Plant Management and Agroforestry Practices
- (A)
- Live fences. This is one of the most common practices because it delimits parcels and prevents livestock from entering. Live fences may include large diameter trees that have been protected for long periods of time, which not only delineate the boundaries of parcels but also provide shade for trails. The components of live fences mostly provide useful products to their managers and have a high resprouting capacity. The most commonly used species were chacalcahuitl (Senegalia macilenta), guamúchil (Pithecellobium dulce), papelillos (Bursera spp.), and zolocuahuitl (Cordia elaeagnoides).
- (B)
- Vegetation fringes. Borders of native vegetation in different states of conservation are intentionally left in parcels cleared for agriculture, especially in areas with pronounced slopes, which helps to prevent soil erosion. The most common species in these fringes were Crateva palmeri, granjeno (Celtis iguanaea), Coursetia glandulosa, vainilla (Senna atomaria), and San Antonio (Tabernaemontana tomentosa).
- (C)
- Scattered or isolated trees. These are generally useful species left to grow in the midst of crops, which is usually to provide shade and food. People used to leave tree species that do not shed most of their canopy during the dry season. These are the cases of majahua (Heliocarpus terebinthinaceus) and P. dulce as well as tall trees such as parota (Enterolobium cyclocarpum), and capire (Sideroxylon capiri).
- (D)
- Vegetation islands. These are small groups of trees composed of one or more tree species such as pitayo (Stenocereus queretaroensis), chacalcahuitl (S. macilenta), and ciruelo (Spondias purpurea).
- (E)
- Remnants of native vegetation. In this category, we include large areas of vegetation in different stages of conservation that are left to stand within the parcels. These remnants offer a variety of resources, including firewood (S. macilenta) and timber such as tepemezquite (Lysiloma divaricatum) and guásima (Guazuma ulmifolia). They also produce edible forest products such as ilama fruits (Annona macroprophyllata), embiona flower buds (Ledenbergia macrantha), and naznaca, which is a mushroom that grows on Sapranthus violaceus.
- (F)
- Vegetation patches. These are represented by small orchards of native fruit trees growing together with some wild plant species. Vegetation patches are one of the most common practices on parcels that continue to be used and protected even after the milpa is no longer maintained and the parcels begin to regenerate. The most common fruit trees in these orchards were the ciruelos (S. purpurea), bonetes (Jacaratia mexicana), guamúchil (P. dulce), and pitayos (S. queretaroensis).
- (G)
- Corral. We found only one corral in an active AFS. It consisted of an array of S. macilenta and huizaches (Vachellia spp.) trees used as a corral for livestock. This practice combines the use of trees for living fences, shade, and forage.
3.2. Biodiversity Conservation Capacity
3.2.1. Species Richness and Composition
3.2.2. Species Diversity
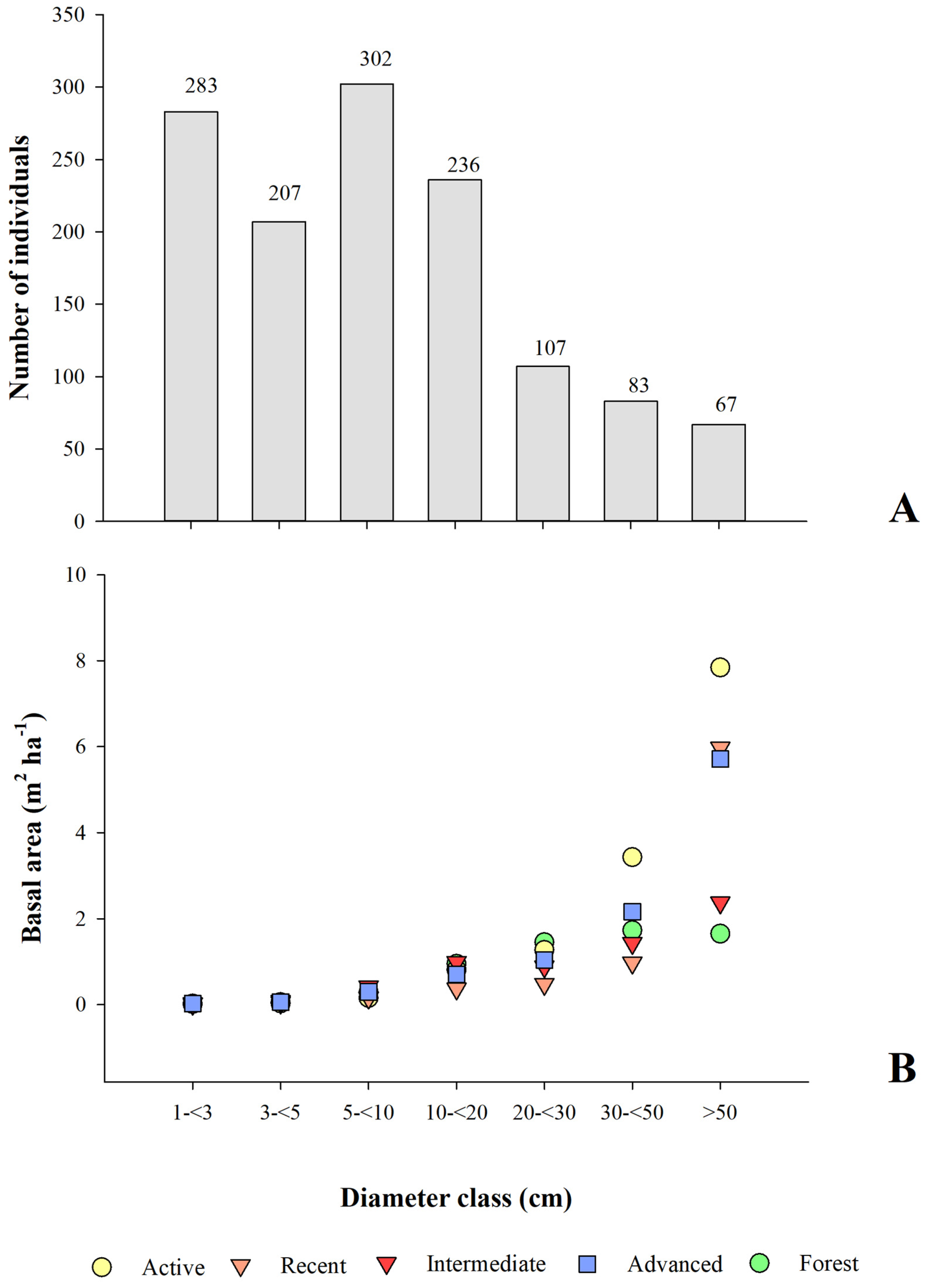
3.2.3. Vegetation Structure
4. Discussion
4.1. Agroforestry Practices Characterization
4.2. Biodiversity Conservation Capacity
Species Composition and Diversity
4.3. Vegetation Structure
4.4. Conservation and Restoration Implications of Vegetation Succession Management
- (1)
- Leaving tree stumps. The way in which trees are felled down allows the persistence of some tree stumps with high coppicing capacity. Local managers recognize this practice as a way to prevent soil erosion and also as a way to promote the rapid recovery of fallen trees.
- (2)
- Maintaining stands of abundant species with high coppicing capacity. These species are often and can be used as fuelwood and good quality poles [21]. In Zacualpan, the endemic species Senegalia macilenta is recognized as having these characteristics.
- (3)
- Species protection. In active and in fallow AFSs, the management aimed at protecting plants of interest and eliminating plant species that may pause or arrest vegetation succession—as previously reported for V. farnesiana and Mimosa arenosa [55]—could significantly promote vegetation recovery.
- (4)
- (5)
- To continue the practice of fallow. Maintaining landscapes with patches at different successional stages can ensure the presence of habitats used by a diversity of species, soil fertility, and sources of propagules for the recovery of the used land.
- (6)
- Maintaining long-term fallows parcels. These parcels can be enriched by planting trees and to recover soil fertility and species diversity.
- (7)
- Maintaining high-quality matrix. It is possible and recommendable to maintain forest patches with 30% of cover in dispersed patches and 10% of cover in continuous vegetation areas, which can be achieved through the various agroforestry practices [58].
- (8)
- Ensuring the maintenance of forest patches within the agricultural landscape matrix. This action would promote the protection of species and propagules as well as the preservation of key functions for the recovery of ecosystems.
- (9)
- Promoting the traditional way of managing the milpa system and agrobiodiversity. The milpa system allows for obtaining the benefits of an ancient polyculture and favors the conservation of local biodiversity through agroforestry practices.
- (10)
- Encouraging the conservation of local ecological knowledge and biocultural memory. Local or traditional ecological knowledge (LEK or TEK) encompasses accumulated knowledge, practices, and beliefs that are culturally transmitted across generations and relate to human interactions with nature. Thus, local knowledge and biocultural memory are critical for protecting, promoting, conserving and restoring biodiversity [60,61].
- (11)
- Conserving communal land tenure. Community agreements on vegetation management as well as the inheritance of agroforestry practices can promote the conservation of biodiversity.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casas, A.; Caballero, J.; Mapes, C.; Zárate, S. Manejo de La Vegetación, Domesticación de Plantas y Origen de La Agricultura En Mesoamérica. Bot. Sci. 2017, 61, 31–47. [Google Scholar] [CrossRef]
- Kareiva, P.; Watts, S.; McDonald, R.; Boucher, T. Domesticated Nature: Shaping Landscapes and Ecosystems for Human Welfare. Science 2007, 316, 1866–1869. [Google Scholar] [CrossRef] [PubMed]
- Levis, C.; Flores, B.M.; Moreira, P.A.; Luize, B.G.; Alves, R.P.; Franco-Moraes, J.; Lins, J.; Konings, E.; Peña-Claros, M.; Bongers, F.; et al. How People Domesticated Amazonian Forests. Front. Ecol. Evol. 2018, 5, 1–21. [Google Scholar] [CrossRef]
- Peters, C.M. Economic Botany and Management Potential of Neotropical Seasonally Dry Forests. In Seasonally Dry Tropical Forests; Dirzo, R., Young, H.S., Mooney, H.A., Ceballos, G., Eds.; Island Press: Washington, DC, USA, 2011; pp. 239–257. [Google Scholar] [CrossRef]
- Sánchez-Vélez, A.; García-Núñez, R.M. The Dying Mexican Tropical Dry Forest: Finding Treasures among the Ruins. Biodiversity 2000, 1, 16–26. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A Global Overview of the Conservation Status of Tropical Dry Forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Melo, F.P.L.; Martínez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple Successional Pathways in Human-Modified Tropical Landscapes: New Insights from Forest Succession, Forest Fragmentation and Landscape Ecology Research: Multiple Successional Pathways. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass Resilience of Neotropical Secondary Forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Amissah, L.; Bongers, F.; Hordijk, I.; Kok, J.; Laurance, S.G.W.; Lohbeck, M.; Martínez-Ramos, M.; Matsuo, T.; Meave, J.A.; et al. Successional Theories. Biol. Rev. 2023, 98, 2049–2077. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Rozendaal, D.M.A.; Bongers, F.; Almeida, d.J.S.; Álvarez, F.S.; Andrade, J.L.; Arreola-Villa, F.; Becknell, J.M.; Bhaskar, R.; Boukili, V.; et al. Functional Recovery of Secondary Tropical Forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2003405118. [Google Scholar] [CrossRef] [PubMed]
- Gavito, M.E.; Paz, H.; Barragán, F.; Siddique, I.; Arreola-Villa, F.; Pineda-García, F.; Balvanera, P. Indicators of Integrative Recovery of Vegetation, Soil and Microclimate in Successional Fields of a Tropical Dry Forest. For. Ecol. Manag. 2021, 479, 118526. [Google Scholar] [CrossRef]
- Siddique, I.; Gavito, M.E.; Mora, F.; del C. Godínez Contreras, M.; Arreola-Villa, F.; Pérez-Salicrup, D.; Martínez-Ramos, M.; Balvanera, P. Woody Species Richness Drives Synergistic Recovery of Socio-Ecological Multifunctionality along Early Tropical Dry Forest Regeneration. For. Ecol. Manag. 2021, 482, 118848. [Google Scholar] [CrossRef]
- Pérez-Cárdenas, N.; Mora, F.; Arreola-Villa, F.; Arroyo-Rodríguez, V.; Balvanera, P.; Flores-Casas, R.; Navarrete-Pacheco, A.; Ortega-Huerta, M.A. Effects of Landscape Composition and Site Land-Use Intensity on Secondary Succession in a Tropical Dry Forest. For. Ecol. Manag. 2021, 482, 118818. [Google Scholar] [CrossRef]
- Miller, P.M.; Kauffman, J.B. Effects of Slash and Burn Agriculture on Species Abundance and Composition of a Tropical Deciduous Forest. For. Ecol. Manag. 1998, 103, 191–201. [Google Scholar] [CrossRef]
- Miller, P.M. Coppice Shoot and Foliar Crown Growth after Disturbance of a Tropical Deciduous Forest in Mexico. For. Ecol. Manag. 1999, 116, 163–173. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Bongers, F.; Pérez-García, E.A.; Meave, J.A. Successional Change and Resilience of a Very Dry Tropical Deciduous Forest Following Shifting Agriculture. Biotropica 2008, 40, 422–431. [Google Scholar] [CrossRef]
- Ceccon, E.; Huante, P.; Rincón, E. Abiotic Factors Influencing Tropical Dry Forests Regeneration. Braz. Arch. Biol. Technol. 2006, 49, 305–312. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental Changes during Secondary Succession in a Tropical Dry Forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar] [CrossRef]
- Ceccon, E.; Hernández, P. Seed Rain Dynamics Following Disturbance Exclusion in a Secondary Tropical Dry Forest in Morelos, Mexico. Rev. Biol. Trop. 2009, 57, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Quisehuatl-Medina, A.; Averett, J.P.; Endress, B.A.; Lopez-Toledo, L. Removal of Cattle Accelerates Tropical Dry Forest Succession in Northwestern Mexico. Biotropica 2020, 52, 457–469. [Google Scholar] [CrossRef]
- Rendón-Carmona, H.; Martínez-Yrízar, A.; Balvanera, P.; Pérez-Salicrup, D. Selective Cutting of Woody Species in a Mexican Tropical Dry Forest: Incompatibility between Use and Conservation. For. Ecol. Manag. 2009, 257, 567–579. [Google Scholar] [CrossRef]
- González-Insuasti, M.S.; Martorell, C.; Caballero, J. Factors That Influence the Intensity of Non-Agricultural Management of Plant Resources. Agrofor. Syst. 2008, 74, 1–15. [Google Scholar] [CrossRef]
- Lambert, D.P. Crop Diversity and Fallow Management in a Tropical Deciduous Forest Shifting Cultivation System. Hum. Ecol. 1996, 24, 427–453. [Google Scholar] [CrossRef]
- Rico-Gray, V.; Chemás, A.; Mandujano, S. Uses of Tropical Deciduous Forest Species by the Yucatecan Maya. Agrofor. Syst. 1991, 14, 149–161. [Google Scholar] [CrossRef]
- Rzedowski, J. Capítulo 12. Bosque tropical caducifolio. In Vegetación de México, 1st ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2006; Volume 504, pp. 200–214. [Google Scholar]
- Zizumbo-Villarreal, D.; Colunga-GarcíaMarín, P. Origin of Agriculture and Plant Domestication in West Mesoamerica. Genet. Resour. Crop Evol. 2010, 57, 813–825. [Google Scholar] [CrossRef]
- Moreno-Calles, A.; Casas, A.; Blancas, J.; Torres, I.; Masera, O.; Caballero, J.; Garcia-Barrios, L.; Pérez-Negrón, E.; Rangel-Landa, S. Agroforestry Systems and Biodiversity Conservation in Arid Zones: The Case of the Tehuacán Valley, Central México. Agrofor. Syst. 2010, 80, 315–331. [Google Scholar] [CrossRef]
- Rendón-Sandoval, F.J.; Casas, A.; Moreno-Calles, A.I.; Torres-García, I.; García-Frapolli, E. Traditional Agroforestry Systems and Conservation of Native Plant Diversity of Seasonally Dry Tropical Forests. Sustainability 2020, 12, 4600. [Google Scholar] [CrossRef]
- Casas, A.; Vallejo, M. Agroecología y agrobiodiversidad. In Crisis ambiental en México. Ruta para el Cambio; Merino-Pérez, L., Ed.; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2019; pp. 99–113. [Google Scholar]
- Vallejo-Ramos, M.; Moreno-Calles, A.I.; Casas, A. TEK and Biodiversity Management in Agroforestry Systems of Different Socio-Ecological Contexts of the Tehuacán Valley. J. Ethnobiol. Ethnomed. 2016, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Jose, S. Agroforestry for Ecosystem Services and Environmental Benefits: An Overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Whittaker, R.J. Agroforestry: A Refuge for Tropical Biodiversity? Trends Ecol. Evol. 2008, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Montagnini, F. The Contribution Of Agroforestry To Restoration And Conservation: Biodiversity Islands In Degraded Landscapes. In Agroforestry for Degraded Landscapes; Dagar, J.C., Gupta, S.R., Teketay, D., Eds.; Springer: Singapore, 2020; Volume 1, pp. 445–479. [Google Scholar] [CrossRef]
- Montes-Londoño, I. Tropical Dry Forests in Multi-Functional Landscapes: Agroforestry Systems for Conservation and Livelihoods. In Integrating Landscapes: Agroforestry for Biodiversity Conservation and Food Sovereignty. Advances in Agroforestry; Montagnini, F., Ed.; Springer: Cham, Switzerland, 2017; Volume 12, pp. 47–78. [Google Scholar] [CrossRef]
- Silva-Galicia, A.; Valencia, V.; Arroyo-Rodríguez, V.; Ceccon, E. Weight-of-Evidence Approach for Assessing Agroforestry Contributions to Restore Key Ecosystem Services in Tropical Dry Forests. Agrofor. Syst. 2023, 97, 151–161. [Google Scholar] [CrossRef]
- Denevan, W.M.; Padoch, C.; Prance, G.T.; Treacy, J.M.; Unruh, J.; Alcorn, J.B.; Paitán, S.F.; Inuma, J.C. Swidden-Fallow Agroforestry in the Peruvian Amazon. In Advances in Economic Botany; New York Botanical Garden: New York, NY, USA, 1988; Volume 5, pp. 1–107. [Google Scholar]
- Michon, G.; Foresta, H.D. Agroforests: Pre-Domestication of Forest Trees or True Domestication of Forest Ecosystems? Neth. J. Agric. Sci. 1997, 45, 451–462. [Google Scholar] [CrossRef]
- Diemont, S.A.W.; Bohn, J.L.; Rayome, D.D.; Kelsen, S.J.; Cheng, K. Comparisons of Mayan Forest Management, Restoration, and Conservation. For. Ecol. Manag. 2011, 261, 1696–1705. [Google Scholar] [CrossRef]
- Toledo, V.M.; Ortiz-Espejel, B.; Cortés, L.; Moguel, P.; de Jesús Ordoñez, M. The multiple use of tropical forests by indigenous peoples in Mexico: A case of adaptive management. Conserv. Ecol. 2003, 7, 9. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2004. [Google Scholar]
- Zacualpan. Nuestra Vida, Nuestra Tierra. SubVersiones. Available online: https://subversiones.org/archivos/120723 (accessed on 13 July 2023).
- Grupo Xolocuahuitl. Recetario de Cocina Tradicional Rural de la Comunidad Indígena de Zacualpan, Comala, Colima; Programa de Apoyos a las Culturas Municipales y Comunitarias: Colima, Mexico, 2016.
- Ortega-Álvarez, R.; Pacheco-Flores, A.; Casas, A. The “Guamúchil” Cultivation in a Mexican Cultural Landscape: A Wild Food Source for People and Birds. Front. For. Glob. Change 2022, 5, 1020207. [Google Scholar] [CrossRef]
- Jost, L. Partitioning Diversity Into Independent Alpha And Beta Components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (Hill Numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Marcon, E.; Hérault, B. Entropart: An R Package to Measure and Partition Diversity. J. Stat. Soft. 2015, 67, 1–26. [Google Scholar] [CrossRef]
- Pérez-Silva, J.G.; Araujo-Voces, M.; Quesada, V. nVenn: Generalized, Quasi-Proportional Venn and Euler Diagrams. Bioinformatics 2018, 34, 2322–2324. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.R.; Coelho de Souza, F.; Maia, V.A.; de Aguiar-Campos, N.; Coelho, P.A.; Farrapo, C.L.; Santos, A.B.M.; Araújo, F.C.; Gianasi, F.M.; Paula, G.G.P.; et al. Tropical Forests Structure and Diversity: A Comparison of Methodological Choices. Methods Ecol. Evol. 2021, 12, 2017–2027. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley and Sons: New York, NY, USA, 1994; pp. 118–120. [Google Scholar]
- Moreno-Calles, A.I.; Casas, A.; Rivero-Romero, A.D.; Romero-Bautista, Y.A.; Rangel-Landa, S.; Fisher-Ortíz, R.A.; Alvarado-Ramos, F.; Vallejo-Ramos, M.; Santos-Fita, D. Ethnoagroforestry: Integration of Biocultural Diversity for Food Sovereignty in Mexico. J. Ethnobiol. Ethnomed. 2016, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Velarde, E.; Cuevas-Guzmán, R.; Ibarra-Manríquez, G.; Moreno-Gómez, S. Riqueza y Biogeografía de La Flora Arbórea Del Estado de Colima, México. Rev. Mex. Biodivers. 2006, 77, 271–295. [Google Scholar] [CrossRef]
- Ibarra-Manriquez, G.; Urrea-Galeano, L.A.; Cortés-Flores, J.; Hernández-Esquivel, K.B.; Navarrete-Segueda, A.; Ek-Rodríguez, I.L. Plant Community Attributes of a Tropical Dry Forest Physiognomically Dominated by Heteroflorum Sclerocarpum (Fabaceae). Bot. Sci. 2023, 101, 57–75. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C.; Martínez-Yrízar, A.; Búrquez, A.; Lindquist, C. Variation in Vegetation Structure and Soil Properties Related to Land Use History of Old-Growth and Secondary Tropical Dry Forests in Northwestern Mexico. For. Ecol. Manag. 2008, 256, 355–366. [Google Scholar] [CrossRef]
- Romero-Duque, L.P.; Jaramillo, V.J.; Pérez-Jiménez, A. Structure and Diversity of Secondary Tropical Dry Forests in Mexico, Differing in Their Prior Land-Use History. For. Ecol. Manag. 2007, 253, 38–47. [Google Scholar] [CrossRef]
- Gei, M.; Rozendaal, D.M.A.; Poorter, L.; Bongers, F.; Sprent, J.I.; Garner, M.D.; Aide, T.M.; Andrade, J.L.; Balvanera, P.; Becknell, J.M.; et al. Legume Abundance along Successional and Rainfall Gradients in Neotropical Forests. Nat. Ecol. Evol. 2018, 2, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Lebrija-Trejos, E.; Meave, J.A.; Poorter, L.; Pérez-García, E.A.; Bongers, F. Pathways, Mechanisms and Predictability of Vegetation Change during Tropical Dry Forest Succession. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 267–275. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Fahrig, L.; Tabarelli, M.; Watling, J.I.; Tischendorf, L.; Benchimol, M.; Cazetta, E.; Faria, D.; Leal, I.R.; Melo, F.P.L.; et al. Designing Optimal Human-Modified Landscapes for Forest Biodiversity Conservation. Ecol. Lett. 2020, 23, 1404–1420. [Google Scholar] [CrossRef] [PubMed]
- Laurance, S.G. Landscape connectivity and biological corridors. In Agroforestry and Biodiversity Conservation in Tropical Landscapes; Schroth, G., da Fonseca, G.A.B., Harvey, C.A., Gascon, C., Vasconcelos, H.L., Izac, A.M., Eds.; Island Press: Washington, DC, USA, 2004; pp. 50–63. [Google Scholar]
- Berkes, F.; Colding, J.; Folke, C. Rediscovery of Traditional Ecological Knowledge as Adaptive Management. Ecol. Appl. 2000, 10, 1251–1262. [Google Scholar] [CrossRef]
- Nazarea, V.D. Local Knowledge and Memory in Biodiversity Conservation. Annu. Rev. Anthropol. 2006, 35, 317–335. [Google Scholar] [CrossRef]

| Category | Agroforestry Practices * | Management ** |
|---|---|---|
| 1 ACTIVE | RV, VP, VI, IT, VF, LF | Milpa, fruit trees, and cultivation of prickle pear (Opuntia spp.). Gathering, promotion, protection and elimination of wild plants. LB of a few goats. |
| 2 ACTIVE | RV, VP, VI, IT, LF | Maize and fruit tree cultivation. Gathering, promotion, protection and elimination of wild plants. LB of a few cows. |
| 3 ACTIVE | RV, VP, VI, IT, VF, LF | Recently cleared cultivation field after 5 years of regeneration. Maize and S. purpurea cultivation. Protection of E. cyclocarpum, Ficus spp., and living fence. LB of a few cows. |
| 4 RECENT REGENERATION | IT, LF | Protection of living fence of P. dulce. Protection of E. cyclocarpum. Elimination of some thorny trees (Vachellia spp). LB of cows. |
| 5 RECENT REGENERATION | LF, VF | Cultivation of P. dulce, protection of C. eleagnoides (living fence) and S. violaceus; and removal of thorny plants (Celtis spp.). Fence to protect land from cow browsing. |
| 6 RECENT REGENERATION | VP, VF, LF | Cultivation of S. purpurea and J. mexicana. Elimination of undesirable wild plants. Protection of isolated E. cyclocarpum trees. |
| 7 INTERMEDIATE REGENERATION | IT, VP, VF | Promotion and gathering of S. macilenta for firewood. Promotion of pasture for fodder. LB of cows. |
| 8 INTERMEDIATE REGENERATION | LF, VP, RV | Promotion and protection of P. dulce. Protection of Ledenbergia macrantha as living fence. Elimination of undesirable trees (H. terebinthinaceus, Celtis spp.). Protection of S. purpurea trees. LB of cows and goats. |
| 9 INTERMEDIATE REGENERATION | LF, VP | Protection of living fence and E. cyclocarpum. LB of goats for consumption and commerce. Gathering of lumber and firewood. |
| 10 ADVANCED REGENERATION | LF, VP, RV, IT | Protection of J. mexicana, Agave sp., and S. queretaroensis. LB of cows and goats. |
| 11 ADVANCED REGENERATION | VP, RV, IT | Protection of S. purpurea and P. dulce. Gathering of plants, lumber and firewood. LB |
| 12 ADVANCED REGENERATION | LF, VP, RV, IT | Protection of P. dulce trees. Gathering of plants, lumber and firewood. LB |
| Diversity | Active | Recent | Intermediate | Advanced | Forest |
|---|---|---|---|---|---|
| 0Dα | 46 | 25 | 34 | 54 | 76 |
| 1Dα | 21.85 | 12.1 | 9.4 | 25.8 | 43.1 |
| 2Dα | 13.05 | 7.09 | 5.01 | 14.85 | 26.11 |
| Evenness factor | 0.28 | 0.28 | 0.15 | 0.28 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Flores, A.; Casas, A.; Moreno-Calles, A.I.; Lindig-Cisneros, R.; Rendón-Sandoval, F.J.; Mora-Ardila, F.; Estañol-Tecuatl, F.; Álvarez-Ríos, G.D.; Ortega-Álvarez, R. Secondary Succession in Fallow Agroforestry Systems Managed in Tropical Dry Forest in Western Mexico. Sustainability 2024, 16, 4760. https://doi.org/10.3390/su16114760
Pacheco-Flores A, Casas A, Moreno-Calles AI, Lindig-Cisneros R, Rendón-Sandoval FJ, Mora-Ardila F, Estañol-Tecuatl F, Álvarez-Ríos GD, Ortega-Álvarez R. Secondary Succession in Fallow Agroforestry Systems Managed in Tropical Dry Forest in Western Mexico. Sustainability. 2024; 16(11):4760. https://doi.org/10.3390/su16114760
Chicago/Turabian StylePacheco-Flores, Alana, Alejandro Casas, Ana I. Moreno-Calles, Roberto Lindig-Cisneros, Francisco Javier Rendón-Sandoval, Francisco Mora-Ardila, Fernando Estañol-Tecuatl, Gonzalo D. Álvarez-Ríos, and Rubén Ortega-Álvarez. 2024. "Secondary Succession in Fallow Agroforestry Systems Managed in Tropical Dry Forest in Western Mexico" Sustainability 16, no. 11: 4760. https://doi.org/10.3390/su16114760
APA StylePacheco-Flores, A., Casas, A., Moreno-Calles, A. I., Lindig-Cisneros, R., Rendón-Sandoval, F. J., Mora-Ardila, F., Estañol-Tecuatl, F., Álvarez-Ríos, G. D., & Ortega-Álvarez, R. (2024). Secondary Succession in Fallow Agroforestry Systems Managed in Tropical Dry Forest in Western Mexico. Sustainability, 16(11), 4760. https://doi.org/10.3390/su16114760








