Abstract
In the discussion about sustainable forestry, a key role is played by the development of ecosystem services, including ecological, social, and economic ones, in which biodiversity and carbon (C) sequestration are among the most important. Afforestation of disturbed and post-mining sites is one of the ways to minimize the negative impact of civilization on the environment. Optimizing C sequestration strategies at post-mining sites plays a crucial role in promoting ecosystem recovery, supporting climate change mitigation, and enabling C offsetting. In this study, we compared the C storage in the soil and plant biomass of forest ecosystems developed on coal-mine heaps for different scenarios of reclamation and succession. We tested combinations of sites (i.e., non-reclaimed sites on bare carboniferous rock [BR] and sites reclaimed by applying topsoil [TS]) and successional woodland and tree plantation. The estimated potential for total C storage (in the soil + biomass) for TS sites ranged from 68.13 to 121.08 Mg ha−1, of which 52.20–102.89 Mg ha−1 was stored in the soil and 12.09–20.15 Mg ha−1 in the biomass. In the non-reclaimed sites on BR, the total C storage was much higher, amounting to 523.14 Mg ha−1 (507.66 Mg ha−1 being in the soil), which was due to the geogenic coal content in the BR. However, the C storage in the biomass (15.48 Mg ha−1) and litter (5.91 Mg ha−1) was similar to the amounts obtained from the reclaimed sites. The number of species did not differ statistically significantly between the analyzed variants. On average, 14 species were recorded in the plots. The average Shannon–Wiener index (H’) value was higher for sites with BR (1.99) than TS variants on reclaimed plots (1.71). The lowest H’ value was for those plots with Robinia pseudacacia in the stand. One of the main implications of the obtained results for sustainable forestry is the perspective of using succession in the recovery of a disturbed ecosystem. We noted that woodlands from succession on BR are highly biodiverse, have high C sequestration potential, and do not require time-consuming reclamation treatments.
1. Introduction
Despite efforts to transition economies and embrace alternative energy sources, post-mining areas present a significant environmental challenge [1]. The impacts of mining activities, both active and abandoned, have manifested in diverse geomorphological effects, such as waste-rock spoil heaps, contaminated and brownfield sites, and underutilized fallow land. These disturbances extend to all components of the ecosystem due to mining operations affecting both the above- and below ground [2]. The spoil material from coal mining, deposited in heaps, often hinders the establishment of vegetation due to its high skeletal content, low pH from its pyrite content, and relatively high salinity [3,4]. Furthermore, the weathered materials typically exhibit low nutrient availability, especially phosphorus and nitrogen (N) [5], emphasizing the extensive ecological impact of mining activities on post-mining landscapes.
Given the heterogeneity and challenging conditions of post-mining sites, a range of reclamation methods can be utilized to expedite the establishment of vegetation, soil development, and ecosystem growth. These methods include topsoiling, which entails the application of fertile material to spoil heaps, in conjunction with the use of mineral or organic fertilizers, and the introduction of suitable plant species [6,7]. Topsoiling plays a crucial role in enhancing the properties of post-mining soils, leading to improved growth conditions for plants [8]. Despite its benefits, topsoiling is labor-intensive and costly. Topsoil suffers from limited availability and is often of substandard quality, requiring meticulous processing and storage before application [6,9]. Directly introducing suitable vegetation to dumped spoil material can serve as a viable alternative to topsoiling in supporting the establishment of vegetation at restored sites [6]. Another approach involves allowing post-mining heaps or sections to undergo succession to reduce reclamation expenses and foster the development of more resilient, self-sustaining ecosystems over time [10,11]. Succession is a natural process that allows for the spontaneous recolonization of vegetation and the restoration of diverse ecosystems [12]. Studies have shown that post-mining sites can be reclaimed effectively through succession or passive restoration methods, leading to increased biodiversity and ecosystem services [13,14]. By allowing natural processes to unfold and only assisting them when necessary, these sites can recover and continue to support diverse plant communities over time [15,16].
The novel ecosystems that emerge on spoil heaps are considered fragile and require meticulous restoration and management. A comprehensive understanding of the technical aspects involved in handling spoil heaps, and a recognition of their ecological significance in urban and industrial settings, is essential. This knowledge empowers individuals and communities to actively engage in supporting and restoring ecological functions, such as plant community succession, dynamic soil processes, soil microbiological activity, afforestation efficiency, biomass production, carbon (C) sequestration [3], the development of biodiversity [17], as well as the response of the plants to environmental stress factors [18]. A large body of research has shown that biodiversity loss can reduce ecosystem functioning. Novel ecosystems, such as hard-coal mines, increase diversity, allowing the scope and functions of developing ecosystems to expand [19], depending on the type of reclamation. The biodiversity of the plants in post-industrial areas is mainly influenced by substrate parameters and the availability of propagules and is expressed as the average species diversity in individual locations of a post-industrial ecosystem. Moreover, due to the extreme habitat conditions prevailing in such heaps [5,6] and their mosaic arrangement, to assess diversity, it is necessary to compare the vegetation between these post-industrial ecosystems [15]. The use of various types of reclamation leads to the colonization of areas by vegetation within the same dump differently, depending on the type of reclamation method used [6]. This is most often expressed as the value of the Shannon–Wiener index (H’) as a way of measuring the diversity of the species in a community and of defining the alpha diversity [20].
Proper reclamation of post-mining sites is of key importance for subsequent forest management, including the performance of basic ecological functions that can increase the resistance of the human environment to climate change. They also provide valuable ecosystem services, including significant C sequestration capabilities [19,21]. This highlights the importance of proactive management and restoration efforts in post-mining landscapes to promote ecological sustainability and environmental resilience.
Vegetation restoration has been recognized as a key and sustainable approach to enhancing post-mining soil quality and functions. It serves as an effective method for increasing the organic matter input to soils and promoting soil C sequestration [22]. The dynamic response of soil organic C varies depending on the type of vegetation restoration, which directly impacts the litter mass [23]. Research has found that the choice of vegetation significantly influences changes in the soil C pool and its dynamics by affecting the physical and chemical protection of the organic matter provided by soil aggregates [24]. The nutrient cycling facilitated by plant–soil interactions and the C sink function of ecosystems may exhibit temporal and spatial variations. Consequently, the timing of introducing different vegetation types, the duration of the vegetation restoration, and the plant combinations can profoundly influence the C sequestration potential in post-mining areas.
Spontaneous succession is a slow process, and it is not always able to restore the forest ecosystem in a comparable time compared to the use of technical reclamation [25]. Although the biodiversity of ecosystems from succession compared to afforestation is often similar [26,27], sites with succession are characterized by a lower C sequestration potential than reclaimed sites [28]. For example, Pietrzykowski and Krzaklewski [28] determined the accumulation rate of C and N to be three times and five higher, respectively, in sand-mine soils under afforestation than under succession communities. A similar tendency has been observed by Frouz et al. [29] in clayey soils in areas after lignite mining. In reclaimed mine soils (under 22- to 32-year-old stands of different tree species), higher C accumulation has been observed [29]. Reclamation leads to increased humus accumulation, humification, and humus transformation rates than succession on spoil heaps after lignite mining. However, the differences between reclaimed and non-reclaimed sites decrease with site age and are very small in 40-year-old sites [30]. The advantages of the reclamation and afforestation of post-mining sites also include both higher biomass and higher C sequestration in the biomass compared to areas left to succession [31,32]. However, Frouz et al. [33] found higher woody biomass in reclaimed sites in younger sites (<20 years) compared to sites with succession. In older sites, this difference disappears [33]. Determining the potential for C sequestration in biomass is particularly important in the first stages of ecosystem development because it is found that, in this period, the impact of different plant communities on the potential for C sequestration is manifested primarily in the biomass and the upper organic horizons of the soils [31,34].
In this work, we aimed to contribute to the discussion on the usefulness of natural regeneration by spontaneous succession versus technical reclamation using topsoiling and planting trees on a post-mining site in the context of biodiversity versus C sequestration potential as the crucial ecosystem services of restored post-mining sites. We hypothesized that tree communities from spontaneous succession on carboniferous substrates in the first phase of ecosystem development would result in a similar soil C storage potential and biodiversity as full reclamation treatments and tree plantation.
2. Materials and Methods
2.1. Study Site
The study was carried out on the “Sośnica” spoil heap after hard-coal mining in Zabrze and Gliwice (50°16′22″ N, 18°44′43″ E), Upper Silesia, Poland. The climate of the area is temperate, with a mean annual precipitation of 737 mm and annual temperature of 8.8 °C (data for 1990–2022 from the Katowice meteorological station, source: www.tutiempo.net, accessed 15 May 2023). The heap had an area of approximately 170 ha and a height of more than 30 m. It comprised carboniferous rocks, which were primarily shale, sandstone, and conglomerates made from these [35].
Our study involved two types of substrates: the bare carboniferous rock (BR) and the bare rock covered by approximately 50 cm of topsoil (TS). Research plots were established with four variants: (i) in communities with succession dominated by Betula pendula, Populus tremula, and Pinus sylvestris on BR (S-BR); (ii) in communities with succession dominated by Populus tremula on TS (S-TS); (iii) at sites with Robinia pseudoacacia from planting on TS (Rb-TS); and (iv) with a mixture of Betula pendula and Alnus glutinosa from planting on TS (Re-TS). Soils in the S-BR variant were characterized by a higher sand fraction than the S-TS soils at 0–10 cm depth and a higher clay fraction than all the other variants at 10–30 cm depth. A higher silt fraction was observed in the S-TS than in the S-BR variant at 0–10 cm depth. The S-BR soils had lower pH values than the others at both depths. At 0–10 cm depth, the bulk density (BD) was higher in the S-TS than the S-BR and Re-TS soils, while at 10–30 cm depth, the BD was higher in the Re-TS than the Rb-TS and S-BR soils (Table 1). The stand age was 10–15 years. In total, 16 research plots, with four replications of each variant of 10 × 10 m, were randomly established on the identified experimental patches on the spoil heap.

Table 1.
Site characteristics.
2.2. Soil Sampling and Analysis
Samples from the organic (Oi + Oe) and mineral (0–10 and 10–30 cm depth) horizons were collected from each plot in October 2021. A composite soil sample representing each plot was collected from five subsamples (four from the corners and one from the middle of each plot). For the BD calculation, two independent samples from depths of 0–10 cm and 10–30 cm per plot were taken from the center, keeping their structures intact via collection in 100 cm3 cylinders. The organic horizon (Oi + Oe) samples were collected from five 20 × 20 cm squares in each plot. Each sample of the litter layer in the fresh state was weighed using an laborathory scale, and the mixed samples were considered representative of each test area.
For the mineral layer samples (0–10 cm and 10–30 cm depth), the following parameters were determined: the granulometric composition (texture) was determined using a Fritsch GmbH laser particle sizer ANALYSETTE 22; the pH was determined potentiometrically in water at a 1:2.5 soil–solution ratio; and the total organic C contents were measured using a LECO TruMac® CNS. The intact samples collected in cylinders were sieved (2-mm mesh size), weighed, dried at 105 °C, and reweighed. The weight was used to calculate the dry weight of the original sample, which was then divided by the volume to obtain the BD of the fine fraction (<2 mm). The organic horizon samples (Oi + Oe) were oven-dried, and the measured moisture loss was used to calculate the dry mass. The dried samples were grounded, and the C content was measured using a LECO TruMac® CNS, with the pH determined potentiometrically in water at a 1:5 ratio.
2.3. Biomass Study
For the aboveground understory vegetation (forest floor herbaceous plants + shrubs) biomass analysis, samples were taken from 1 m2 subplots. In the laboratory, the biomass was oven-dried, weighed, and ground. The measured loss of moisture was used to calculate the dry mass.
Tree measurements on each plot were conducted by measuring the tree diameter at 1.3 m (Dbh) and then the height. The most common method of determining the biomass of various tree components is to use empirical allometric equations, in which simple-to-measure tree characteristics, such as Dbh and height, are used as predictors [36,37]. The equations need to be developed for each specific population based on a random sample of the trees destructively separated into individual components, which is very expensive and labor-intensive. Because of these inconveniences, biomass estimates are often obtained from equations developed for similar populations. However, it should be noted that the key to the selection of existing equations is to take into account the similarity of the populations; otherwise, systematic errors in the biomass estimates may occur [38,39].
Here, the biomass of the aboveground tree components (i.e., stems, branches, and leaves) was estimated based on equations already published in the literature [40,41,42,43,44,45,46,47]. To minimize the risk of bias, we used equations built for populations of trees growing under similar growth conditions. Thus, the choice of equations considered various tree and site characteristics, such as tree species, age, Dbh and height range, geographical region, and habitat type, so that they corresponded as closely as possible to those observed in the present study. In addition, we chose equations that were developed using a large sample and preferred those that used two predictors––Dbh and height. The aboveground biomass of the tree components (stems, branches, leaves) for each species was determined based on the equations summarized in Table 2. The belowground (i.e., roots) biomass was estimated as 25% of the total biomass [32].

Table 2.
Equations used to determine the tree biomass fractions [40,41,42,43,44,45,46,47].
2.4. Plant Diversity
Indicators of the plant biodiversity in the study plots with introduced trees and spontaneously encroaching trees were based on the composition of the communities using the number of plant species in the study plot for each taxon separately. The number of species and the Shannon–Wiener index (H’ index) were used to represent the alpha diversity [20], the latter calculated using the species abundance data using Kovach Computing Services’ MVSP 3.13.p. software.
The H’ index was calculated as follows:
where pi is the proportion of the entire community made up of species i.
H = −Σpi × ln(pi)
2.5. Statistical Analysis
The datasets were statistically analyzed using Statistica 13.1 software. The statistical significance between the tested variants was determined using the non-parametric Kruskal–Wallis test for several groups at p < 0.05 [48].
3. Results
3.1. Vegetation Biomass
In the Rb-TS variant, there was a higher herbaceous forest floor plant biomass compared to S-BR and S-TS. Higher shrub biomass was also observed in Re-TS compared to Rb-TS. No differences were found among the variants in tree density, Dbh, height, stem biomass, branch biomass, aboveground or belowground tree biomass, and total biomass (Table 3).

Table 3.
Tree parameters and plant biomass on spoil heaps after hard-coal mining.
3.2. Plant Biodiversity
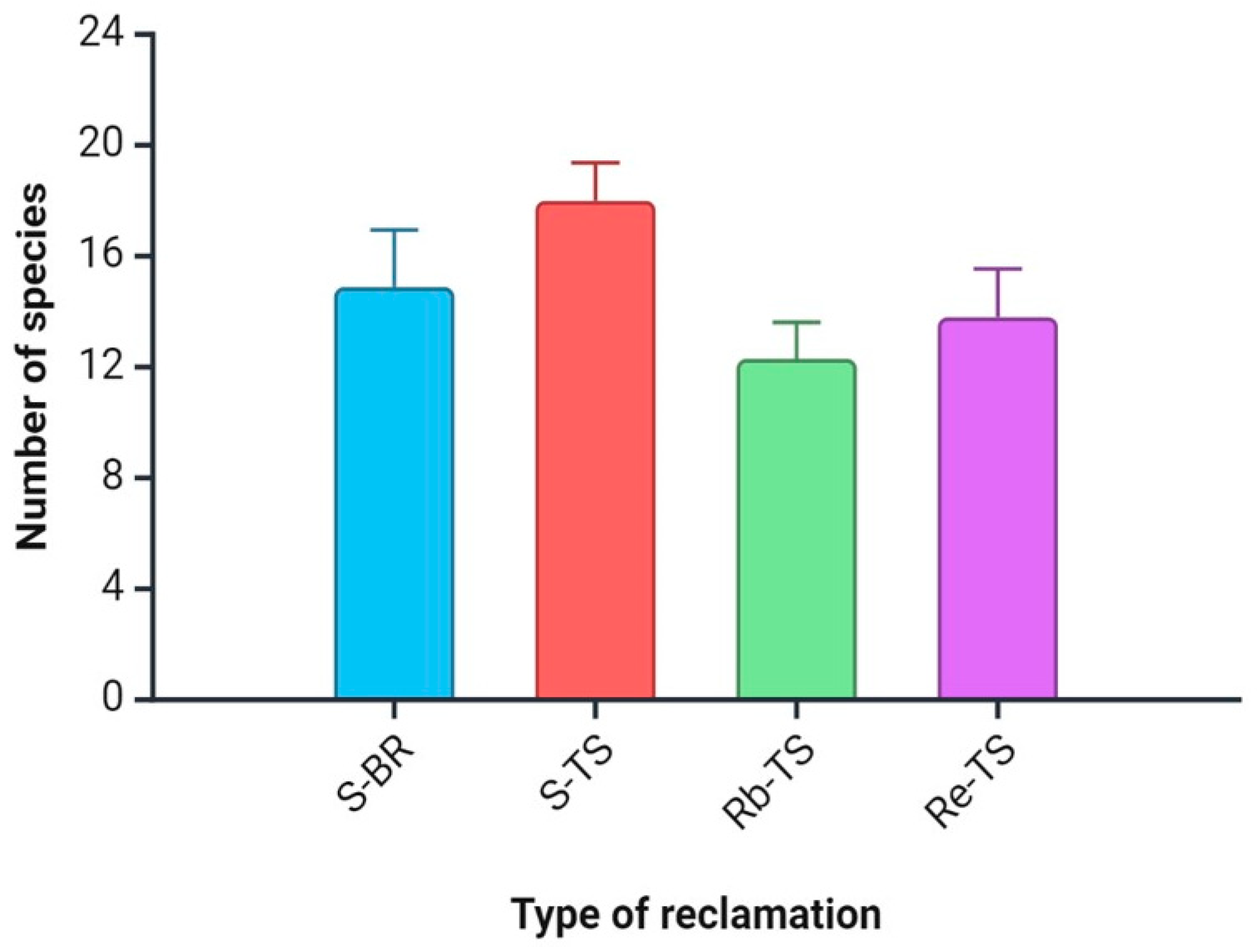
The results of the analysis showed no statistically significant differences between the number of species on the study plot variants. The average number of species was between 12.11 (Rb-TS) and 18.11 (S-TS; Figure 1).
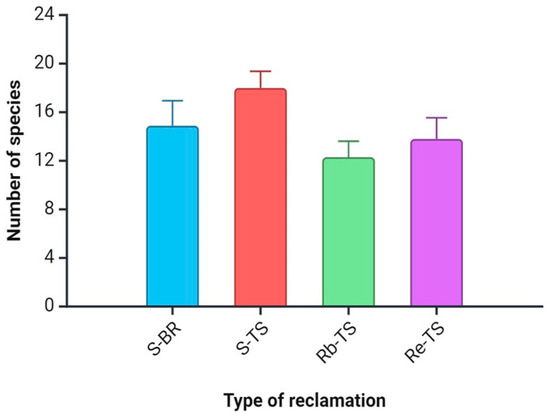
Figure 1.
Differences between the number of species on study sites. Explanation: S-BR = succession on bare carboniferous rock; S-TS = succession on topsoil; Rb-TS = Robinia from planting on topsoil; and Re = the mixture of tree species from planting on topsoil. Values are means ± SE for each parameter and each variant.
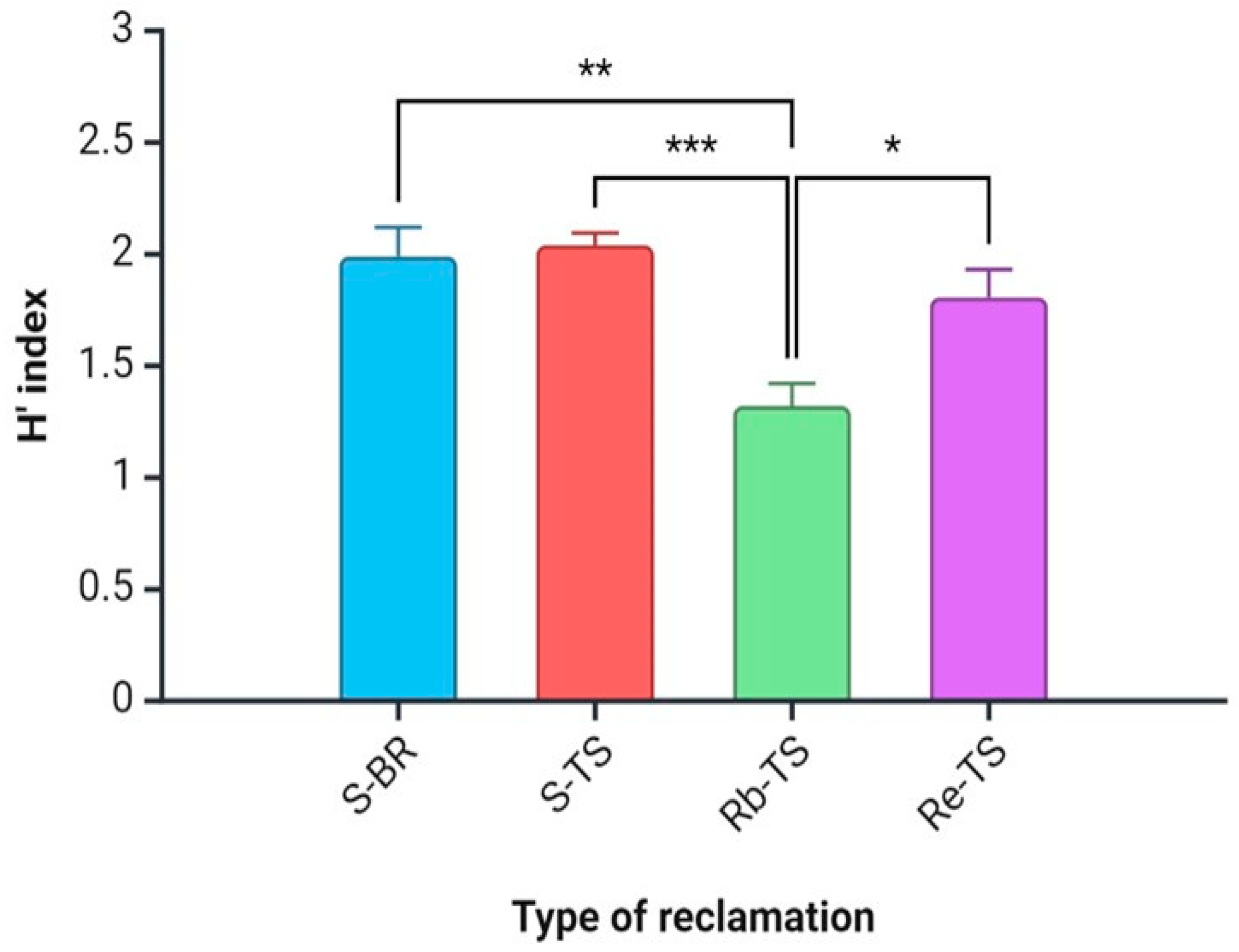
The average H’ index value was higher for the phytocoenoses on the BR (1.99) than for the TS variants (1.80) and was different and statistically significant. The average H’ index value was different in the TS group, where Rb-TS stood out as being statistically significant with dominant Robinia pseudoacacia (Figure 2).
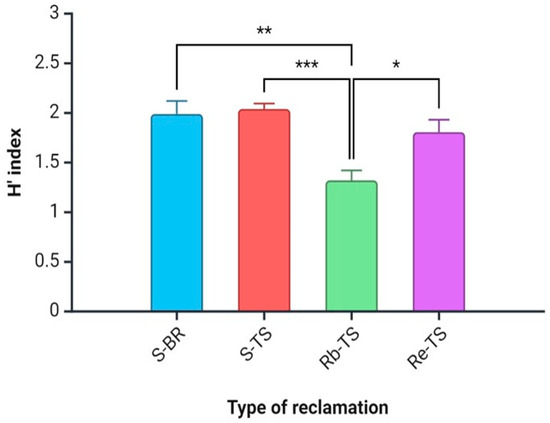
Figure 2.
Differences between H’ index values of the study sites. Explanation: see Figure 1. Values are means ± SE for each parameter and each variant. Explanations: *—p < 0.05, **—p < 0.01, ***—p < 0.001.
3.3. Carbon Storage in Ecosystems
The C stock in the Oi + Oe horizon was higher in the S-BR than in the S-TS. The C stock was also higher at both 0–10 cm and 10–30 cm under S-BR compared to all other variants, contributing to a significantly higher total soil C stock in the S-BR variant compared to all other variants. The Rb-TS variant had a higher soil C stock at 0–10 cm and a higher total soil C stock compared to S-TS (Table 4).

Table 4.
C storage in ecosystem components (soil and biomass) on studied spoil heap after coal mining.
Regarding the C stock in the biomass, Rb-TS had a higher forest floor plant C stock than S-BR and S-TS, whereas Re-TS had a significantly higher shrub biomass C stock than Rb-TS. However, there were no differences in the tree aboveground, tree root, tree, or total biomass C stocks among the variants. The ecosystem C stock was the highest under S-BR compared to the other variants, with Rb-TS also exhibiting a higher ecosystem C stock than S-TS (Table 4).
4. Discussion
The much higher C storage value in the soil in the S-BR variant compared to the variants with topsoiling resulted from the presence of geogenic (hard-coal) C in the carboniferous rocks [3]. Geogenic C affects the overall soil C balance in the initial ecosystem and is also important for soil water retention [49,50]. However, the process of its incorporation into the biogeochemical cycle has not been thoroughly studied, although it is known that soil microorganisms play a key role in the process, especially in the case of lignite [49,50,51]. Determining the amount of geogenic C in the total organic C pool in post-mining soils is difficult from a methodological point of view [52]. The content of fossil C has been determined based on the degree of 14C radioactive isotope decay [51] and via formulas that take into account specific post-mining site indicators [53]. Despite the higher C stock in the BR, the literature indicates that soils reclaimed through topsoil application are often characterized by a higher rate of C accumulation (per year) in the soils [54]. This is because the geogenic C may be subject to gradual decomposition, which may be reflected in a decrease in C storage in the soil [55]. Another factor contributing to lower C accumulation in soils developing from carboniferous rock BR may be the so-called soil C saturation [56]. Due to the geogenic C content, it was difficult to compare the C storage in the mineral layers between the BR and TS variants. Reliable results have been obtained by comparing the C stocks in the organic horizons [3], and the BR and TS variants did indeed have similar values in these horizons. However, the soil was tested down to 30 cm, and under the topsoiling, there was a layer of carboniferous waste. Hence, if we had analyzed deeper layers, the amount of C storage might have been similar in both habitat variants.
In sites with topsoiling, no significant differences were found between different types of vegetation. A period of only 10 to 15 years since reclamation seems to be too short to reveal the impact of the various vegetation on C storage in the mineral layers. However, when comparing the values obtained from the shallower (0–10 cm) to deeper (10–30 cm) layers, it can be concluded that the Rb-TS variant is characterized by a higher potential for soil C accumulation compared to the other variants. Nitrogen-fixing species, such as Robinia pseudoacacia, in addition to increasing the N content, also increase the C content in soils [57,58]. This is due to the high decomposition rate of N-fixing species in the litter, which results in low-mass organic horizons and an increase in the amount of organic C reaching the mineral soil [59]. The high N content in the litter increases the decomposition rate during the initial phase while inhibiting the decomposition of the humified soil C [60].
The soil C storage for the sites with topsoiling was within the range given for natural soils under a temperate climate, as well as for post-mining soils. Data from the National Forest Soil Inventory indicate that the C storage at locations throughout Poland has been, on average, 26 Mg ha−1 in the organic horizons and 66 Mg ha−1 in the mineral horizons of the soils (up to 1 m deep) [61]. These values are similar in the organic horizons to those reported in the applicable European forest literature but lower in the mineral horizons (22.1 and 108 Mg C ha−1, respectively) [60,62]. Vindušková and Frouz [63] reported that the C stock in post-mining soils in northern temperate climate zones can range from 4.49 to 93.20 Mg ha−1. Pietrzykowski and Daniels [3] found soil C storage values of 16.8 to 65.0 Mg ha−1 at various post-mining sites in Poland (i.e., sites after lignite, sulfur, and sand mining). Similarly to our findings, much higher values (from 1959.1 to 2975.2 Mg ha−1) were obtained from sites after hard-coal mining [3].
Our results do not support statements concerning the benefits of reclamation and the afforestation of post-mining sites, including higher biomass production and C storage in the tree biomass being greater in reclaimed sites than in those left to succession [64,65]. However, Frouz et al. [33] reported higher woody biomass only in younger stands (<20 years) on reclaimed sites compared to sites left to natural regeneration, although the difference disappeared in older stands [33]. Plant communities from succession are often heterogeneous and significantly different from each other, even over small areas [31]. This observation may explain the differences reported in the literature regarding the growth parameters and biomass achieved by stands from succession and afforestation [66]. For example, in North America, when succession occurs under favorable conditions, some fast-growing timber trees may grow to harvestable size as early as 30 to 40 years after disturbance, while slower-growing hardwoods may require 50 to 60 years or longer. Other sites may remain in the grass–herb–shrub stage, with only scattered trees, for several decades after a disturbance because the soil conditions are unsuitable or the understory vegetation is too competitive for tree growth [66].
Tree stands arising from the process of spontaneous succession (BR) or reclamation through topsoiling (TS) are a priority option for the sustainable forest management of mine spoil heaps because they can provide a wide range of ecosystem services, such as the control of soil erosion, increased biomass and C pool, and restored biodiversity [67]. Our obtained results indicate that biodiversity does not depend on the method of reclamation, which translates into key ecosystem functions, such as CO2 storage and tree productivity [68]. However, this may be due to having access to similar propagules and the extent of the environmental adaptation of the plants to the environmental conditions of spoil heaps [18].
5. Conclusions
Our study has demonstrated the high biodiversity and C sequestration potential of vegetation from succession on a BR substrate. Thus, the involvement of this process in the ecosystem restoration of post-mining sites after hard-coal mining is crucial for subsequent forest management. The alpha and beta biodiversities of the plant communities were similar for the reclaimed and non-reclaimed sites. Unpredictable woodlands from succession and woodlands from planting were characterized by similar C storage values in the total tree biomass. The estimated potential for total C storage (soil + biomass) at the sites with topsoiling ranged from 68.13 to 121.08 Mg ha−1, of which 56.05–108.19 Mg ha−1 was stored in the soil and 12.09–20.15 Mg ha−1 in the biomass. In non-reclaimed sites on carboniferous rock BR, the total C storage was much higher, amounting to 523.14 Mg ha−1 (of which 507.66 Mg ha−1 was in the soil), which was due to the geogenic coal content in the carboniferous rock BR. However, the C storage in the biomass (15.48 Mg ha−1) and litter (5.91 Mg ha−1) was similar to the results obtained from the reclaimed sites.
Author Contributions
Conceptualization, B.W., E.S. and M.P. (Marcin Pietrzykowski); methodology, B.W., E.S. and M.P. (Marcin Pietrzykowski); validation, B.W., E.S., A.K.-B., M.B. and W.B.; investigation, B.W., M.P. (Marek Pająk), A.M.M., A.J., J.B., E.S., A.K.-B., M.B. and W.B.; writing—original draft preparation, all authors; writing—review and editing, all authors; visualization, E.S.; supervision, M.P. (Marcin Pietrzykowski); funding acquisition, M.P. (Marcin Pietrzykowski). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland, Grant No. 2020/39/B/ST10/00862.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets are available upon reasonable request from the corresponding author.
Acknowledgments
We would like to express our gratitude to Justyna Sokolowska and Iwona Skowrońska at the Laboratory of Geochemistry and Reclamation, Department of Ecology and Silviculture AUC, for their assistance and their kind collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Skousen, J.; Zipper, C.E. Post-mining policies and practices in the Eastern USA coal region. Int. J. Coal Sci. Technol. 2014, 1, 135–151. [Google Scholar] [CrossRef]
- Bell, F.G.; Donnelly, L.J. Mining and Its Impact on the Environment, 1st ed.; CRC Press: London, UK, 2006. [Google Scholar]
- Pietrzykowski, M.; Daniels, W.L. Estimation of carbon sequestration by pine (Pinus sylvestris L.) ecosystems developed on reforested post-mining sites in Poland on differing mine soil substrates. Ecol. Eng. 2014, 73, 209–218. [Google Scholar] [CrossRef]
- Burghardt, W.; Niggemeyer, M.; Braunersreuther, M. Ways of soil development on stony substrate from hard coal mining spoil. Soil Sci. Ann. 2020, 71, 382–394. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Błońska, A.; Kompała-Bąba, A.; Woźniak, G. Effects of Calamagrostis epigejos, Chamaenerion palustre and Tussilago farfara on nutrient availability and microbial activity in the surface layer of spoil heaps after hard coal mining. Ecol. Eng. 2015, 83, 328–337. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Soil Reclamation of Abandoned Mine Land by Revegetation: A Review. Int. J. Soil Sediment Water 2010, 3, 13. [Google Scholar]
- Kumari, S.; Maiti, S.K. Reclamation of coalmine spoils with topsoil, grass, and legume: A case study from India. Environ. Earth Sci. 2019, 78, 429. [Google Scholar] [CrossRef]
- Kumari, S.; Maiti, S.K. Nitrogen recovery in reclaimed mine soil under different amendment practices in tandem with legume and non-legume revegetation: A review. Soil Use Manag. 2022, 38, 1113–1145. [Google Scholar] [CrossRef]
- Ghose, M.K. Management of topsoil for geo-environmental reclamation of coal mining areas. Environ. Geol. 2001, 40, 1405–1410. [Google Scholar]
- Prach, K.; Hobbs, R.J. Spontaneous succession versus technical reclamation in the restoration of disturbed sites. Restor. Ecol. 2008, 16, 363–366. [Google Scholar] [CrossRef]
- Baasch, A.; Tischew, S.; Bruelheide, H. Twelve years of succession on sandy substrates in a post-mining landscape: A Markov chain analysis. Ecol. Appl. 2010, 20, 1136–1147. [Google Scholar] [CrossRef]
- Prach, K.; Walker, L.R. Four opportunities for studies of ecological succession. Trends Ecol. Evol. 2011, 26, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Peng, S.; Lines, L.R.; Zhu, G.; Hu, Z.; Cui, F. Understanding the capability of an ecosystem nature-restoration in coal mined area. Sci. Rep. 2019, 9, 19690. [Google Scholar] [CrossRef]
- Vachova, P.; Vach, M.; Skalicky, M.; Walmsley, A.; Berka, M.; Kraus, K.; Hnilickova, H.; Vinduskova, O.; Mudrak, O. Reclaimed Mine Sites: Forests and Plant Diversity. Diversity 2022, 14, 13. [Google Scholar] [CrossRef]
- Prach, K.; Tolvanen, A. How can we restore biodiversity and ecosystem services in mining and industrial sites? Environ. Sci. Pollut. Res. 2016, 23, 13587–13590. [Google Scholar] [CrossRef]
- Woźniak, G.; Chmura, D.; Dyderski, M.K.; Błońska, A.; Jagodziński, A.M. How different is the forest on post-coal mine heap regarded as novel ecosystem? For. Ecol. Manag. 2022, 515, 120205. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.M.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hautier, Y.; Hillebrand, H.; Lind, E.M.; Pärtel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Kompała-Bąba, A.; Sierka, E.; Bierza, W.; Błońska, A.; Woźniak, G. Eco-physiological responses of Calamagrostis epigejos L. (Roth) and Solidago gigantea Aition to complex environmental stresses in coal-mine spoil heaps. Land Degrad. Dev. 2021, 32, 5427–5442. [Google Scholar] [CrossRef]
- Sierka, E.; Radosz, Ł.; Ryś, K.; Woźniak, G. Ecosystem Services and Post-industrial Areas. In Green Scenarios: Mining Industry Responses to Environmental Challenges of the Anthropocene Epoch, 1st ed.; Dyczko, A., Jagodziński, A., Woźniak, G., Eds.; Taylor & Francis Group: London, UK, 2022; pp. 265–274. [Google Scholar]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Misebo, A.M.; Pietrzykowski, M.; Woś, B. Soil carbon sequestration in novel ecosystems at post-mine sites—A new insight into the determination of key factors in the restoration of terrestrial ecosystems. Forests 2022, 13, 63. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Hu, P.; Zhang, W.; Xiao, L.; Ye, Y.; Xiao, D.; Zhao, J.; Xiao, J.; Wang, K. Bedrock outcrops weakly promote rather than inhibit soil carbon sequestration after vegetation restoration. Sci. Total Environ. 2023, 858, 159470. [Google Scholar] [CrossRef]
- Dong, L.; Fan, J.; Li, J.; Zhang, Y.; Liu, Y.; Wu, J.; Li, A.; Shangguan, Z.; Deng, L. Forests have a higher soil C sequestration benefit due to lower C mineralization efficiency: Evidence from the central loess plateau case. Agric. Ecosyst. Environ. 2022, 339, 108144. [Google Scholar] [CrossRef]
- Reich, P.B.; Hobbie, S.E.; Lee, T.D.; Rich, R.; Pastore, M.A.; Worm, K. Synergistic effects of four climate change drivers on terrestrial carbon cycling. Nat. Geosci. 2020, 13, 787–793. [Google Scholar] [CrossRef]
- Espigares, T.; Moreno-de las Heras, M.; Nicolau, J.M. Performance of Vegetation in Reclaimed Slopes Affected by Soil Erosion. Restor. Ecol. 2011, 19, 35–44. [Google Scholar] [CrossRef]
- Mudrák, O.; Frouz, J.; Velichová, V. Understory vegetation in reclaimed and unreclaimed post-mining forest stands. Ecol. Eng. 2010, 36, 783–790. [Google Scholar] [CrossRef]
- Šebelíková, L.; Řehounková, K.; Prach, K. Spontaneous revegetation vs. forestry reclamation in post-mining sand pits. Environ. Sci. Pollut. Res. 2016, 23, 13598–13605. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowski, M.; Krzaklewski, W. Soil organic matter, C and N accumulation during natural succession and reclamation in an opencast sand quarry (southern Poland). Arch. Agron. Soil Sci. 2007, 53, 473–483. [Google Scholar] [CrossRef]
- Frouz, J.; Pižl, V.; Cienciala, E.; Kalčík, J. Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 2009, 94, 111–121. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Cajthaml, T.; Brus, J.; Frouz, J. Humus accumulation, humification, and humic acid composition in soils of two post-mining chronosequences after coal mining. J. Soils Sediments 2013, 13, 491–500. [Google Scholar] [CrossRef]
- Skousen, J.; Johnson, C.; Garbutt, K. Natural revegetation of 15 abandoned mine land sites in West Virginia. J. Environ. Qual. 1994, 23, 1224–1230. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Vacek, S.; Podrázský, V.; Linda, R.; Kovařík, J. Forest biodiversity and production potential of post-mining landscape: Opting for afforestation or leaving it to spontaneous development? Cent. Eur. For. J. 2018, 64, 116–126. [Google Scholar] [CrossRef]
- Frouz, J.; Dvorščík, P.; Vávrová, A.; Doušová, O.; Kadochová, Š.; Matějíček, L. Development of canopy cover and woody vegetation biomass on reclaimed and unreclaimed post-mining sites. Ecol. Eng. 2015, 84, 233–239. [Google Scholar] [CrossRef]
- Nave, L.E.; Swanston, C.W.; Mishra, U.; Nadelhoffer, K.J. Afforestation Effects on Soil Carbon Storage in the United States: A Synthesis. Soil Sci. Soc. Am. J. 2013, 77, 1035–1047. [Google Scholar] [CrossRef]
- Misebo, A.M.; Szostak, M.; Sierka, E.; Pietrzykowski, M.; Woś, B. The interactive effect of reclamation scenario and vegetation types on physical parameters of soils developed on carboniferous mine spoil heap. Land Degrad. Dev. 2023, 34, 3593–3605. [Google Scholar] [CrossRef]
- Parresol, B.R. Assessing tree and stand biomass: A review with examples and critical comparisons. For. Sci. 1999, 45, 573–593. [Google Scholar] [CrossRef]
- Zianis, D.; Muukkonen, P.; Mäkipää, R.; Mencuccini, M. Biomass and stem volume equations for tree species in Europe. Silva Fenn. 2005, 63, 1–63. [Google Scholar] [CrossRef]
- Neumann, M.; Moreno, A.; Mues, V.; Härkönen, S.; Mura, M.; Bouriaud, O.; Lang, M.; Achten, W.M.J.; Thivolle-Cazat, A.; Bronisz, K.; et al. Comparison of carbon estimation methods for European forests. For. Ecol. Manag. 2016, 361, 397–420. [Google Scholar] [CrossRef]
- Ochał, W.; Socha, J.; Grabczyński, S. Accuracy of empirical formulas for determining aboveground biomass of black alder (Alnus glutinosa (L.) Gaertn.). Sylwan 2014, 158, 431–442. [Google Scholar]
- Annighöfer, P.; Ameztegui, A.; Ammer, C.; Balandier, P.; Bartsch, N.; Bolte, A.; Coll, L.; Collet, C.; Ewald, J.; Frischbier, N.; et al. Species-specific and generic biomass equations for seedlings and saplings of European tree species. Eur. J. For. Res. 2016, 135, 313–329. [Google Scholar] [CrossRef]
- Bronisz, K.; Strub, M.; Cieszewski, C.; Bijak, S.; Bronisz, A.; Tomusiak, R.; Wojtan, R.; Zasada, M. Empirical equations for estimating aboveground biomass of Betula pendula growing on former farmland in central Poland. Silva Fenn. 2016, 50, 1559. [Google Scholar] [CrossRef]
- Johansson, T. Biomass equations for determining fractions of common and grey alders growing on abandoned farmland and some practical implications. Biomass Bioenergy 2000, 18, 147–159. [Google Scholar] [CrossRef]
- Zasada, M.; Bronisz, K.; Bijak, S.; Wojtan, R.; Tomusiak, R.; Dudek, A.; Michalak, K. Empirical formulae for determination of the dry biomass of aboveground parts of the tree. Sylwan 2008, 152, 27–39. [Google Scholar]
- Blujdea, V.N.B.; Pilli, R.; Dutca, I.; Ciuvat, L.; Abrudan, I.V. Allometric biomass equations for young broadleaved trees in plantations in Romania. For. Ecol. Manag. 2012, 264, 172–184. [Google Scholar] [CrossRef]
- Johansson, T. Biomass equations for determining fractions of European aspen growing on abandoned farmland and some practical implications. Biomass Bioenergy 1999, 17, 471–480. [Google Scholar] [CrossRef]
- Fortier, J.; Truax, B.; Gagnon, D.; Lambert, F. Allometric equations for estimating compartment biomass and stem volume in mature hybrid poplars: General or site-specific? Forests 2017, 8, 309. [Google Scholar] [CrossRef]
- Annighöfer, P.; Mölder, I.; Zerbe, S.; Kawaletz, H.; Terwei, A.; Ammer, C. Biomass functions for the two alien tree species Prunus serotina Ehrh. and Robinia pseudoacacia L. in floodplain forests of Northern Italy. Eur. J. For. Res. 2012, 131, 1619–1635. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System); Version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Fettweis, U.; Bens, O.; Hüttl, R.F. Accumulation and properties of soil organic carbon at reclaimed sites in the Lusatian lignite mining district afforested with Pinus sp. Geoderma 2005, 129, 81–91. [Google Scholar] [CrossRef]
- Frouz, J. Effects of soil development time and litter quality on soil carbon sequestration: Assessing soil carbon saturation with a field transplant experiment along a post-mining chronosequence. Land Degrad. Dev. 2016, 28, 664–672. [Google Scholar] [CrossRef]
- Rumpel, C.; Kögel-Knabner, I. Characterization of organic matter and carbon cycling in rehabilitated lignite-rich mine soils. Water Air Soil Pollut. 2003, 3, 153–166. [Google Scholar] [CrossRef]
- Chabbi, A.; Sebilo, M.; Rumpel, C.; Schaaf, W.; Mariotti, A. Origin of nitrogen in reforested lignite-rich mine soils revealed by stable isotope analysis. Environ. Sci. Technol. 2008, 42, 2787–2792. [Google Scholar] [CrossRef]
- Amichev, B.Y.; Burger, J.A.; Rodrigue, J.A. Carbon sequestration by forests and soils on mined land in the Midwestern and Appalachian coalfields of the U.S. For. Ecol. Manag. 2008, 256, 1949–1959. [Google Scholar] [CrossRef]
- Akala, V.A.; Lal, R. Soil organic carbon pools and sequestration rates in reclaimed minesoils in Ohio. J. Environ. Qual. 2001, 30, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Frouz, J.; Krištůfek, V.; Livečková, M.; van Loo, D.; Jacobs, P.; Van Hoorebeke, L. Microbial properties of soil aggregates created by earthworms and other factors: Spherical and prismatic soil aggregates from unreclaimed post-mining sites. Folia Microbiol. 2011, 56, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Bartuska, M.; Frouz, J. Carbon accumulation and changes in soil chemistry in reclaimed open-cast coal mining heaps near Sokolov using repeated measurement of chronosequence sites. Eur. J. Soil Sci. 2015, 66, 104–111. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cecillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Woś, B.; Chodak, M.; Józefowska, A.; Pietrzykowski, M. Influence of tree species on carbon, nitrogen, and phosphorus stocks and stoichiometry under different soil regeneration scenarios on reclaimed and afforested mine and post-fire forest sites. Geoderma 2022, 415, 115782. [Google Scholar] [CrossRef]
- Dilly, O.; Munch, J.-C. Microbial biomass content, basal respiration and enzyme activities during the course of decomposition of leaf litter in a black alder (Alnus glutinosa (L.) Gaertn.) forest. Soil Biol. Biochem. 1996, 28, 1073–1081. [Google Scholar] [CrossRef]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Gruba, P.; Socha, J. Exploring the effects of dominant forest tree species, soil texture, altitude, and pHH2O on soil carbon stocks using generalized additive models. For. Ecol. Manag. 2019, 447, 105–114. [Google Scholar] [CrossRef]
- De Vos, B.; Cools, N.; Ilvesniemi, H.; Vesterdal, L.; Vanguelova, E.; Carnicelli, S. Benchmark values for forest soil carbon stocks in Europe: Results from a large scale forest soil survey. Geoderma 2015, 251–252, 33–46. [Google Scholar]
- Vindušková, O.; Frouz, J. Soil carbon accumulation after open-cast coal and oil shale mining in Northern Hemisphere: A quantitative review. Environ. Earth Sci. 2013, 69, 1685–1698. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Krzaklewski, W. Reclamation to Forest in Sand-Filing Mining Exemplified on Szczakowa Sand Mine Cast; Publishing House of the University of Agriculture: Krakow, Poland, 2009. [Google Scholar]
- Vacek, Z.; Linda, R.; Cukor, J.; Vacek, S.; Šimůnek, V.; Gallo, J.; Vančura, K. Scots pine (Pinus sylvestris L.), the suitable pioneer species for afforestation of reclamation sites? For. Ecol. Manag. 2021, 485, 118951. [Google Scholar] [CrossRef]
- Groninger, J.; Skousen, J.; Angel, P.; Barton, C.; Burger, J.; Zipper, C. Mine reclamation practices to enhance forest development through natural succession. In The Forestry Reclamation Approach: Guide to Successful Reforestation of Mined Lands; Adams, M.B., Ed.; General Technical Report NRS-169; Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2017; Chapter 8; pp. 1–7. [Google Scholar]
- Huston, M.A.; Marland, G. Carbon management and biodiversity. J. Environ. Manag. 2003, 67, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, N.; Wright, W.; Loyn, R.H.; Nally, R.M. Ecologically complex carbon’—Linking biodiversity values, carbon storage and habitat structure in some austral temperate forests. Glob. Ecol. Biogeogr. 2011, 20, 260–271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).