Bituminous Soil Remediation in the Thermal Plasma Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Ultimate and Proximate Material Assessment
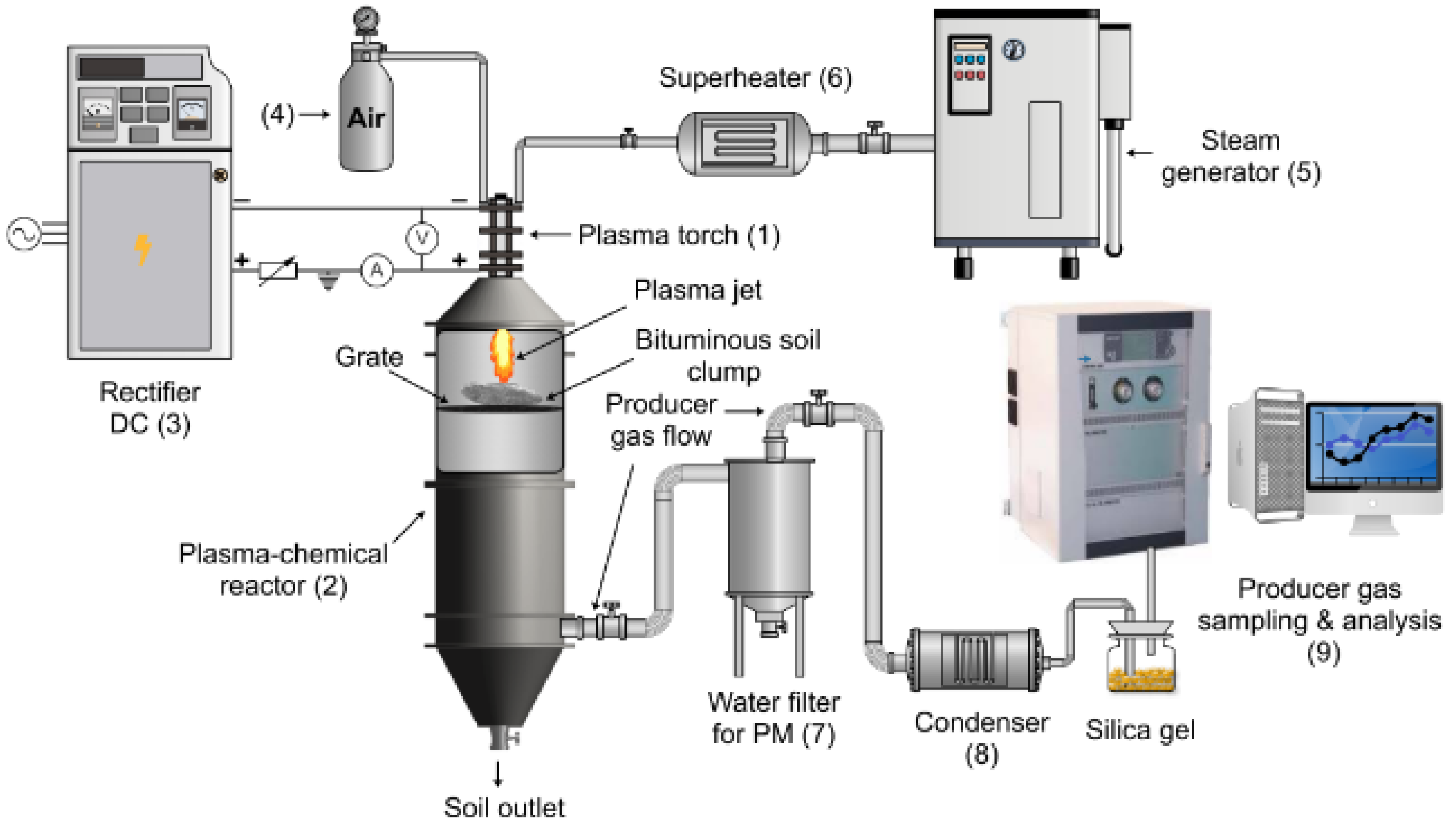
2.2. The Experimental Set-Up of Bituminous Soil De-Pollution with Plasma
2.3. Facilities Used for the Soil and Produced Gas Analysis
2.4. Plasma Formation and Bitumen De-Pollution Process
3. Results and Discussion
3.1. Analysis of the Surface Morphology of Bituminous and De-Polluted Soil
3.2. Assessment of Soil’s Elemental Content
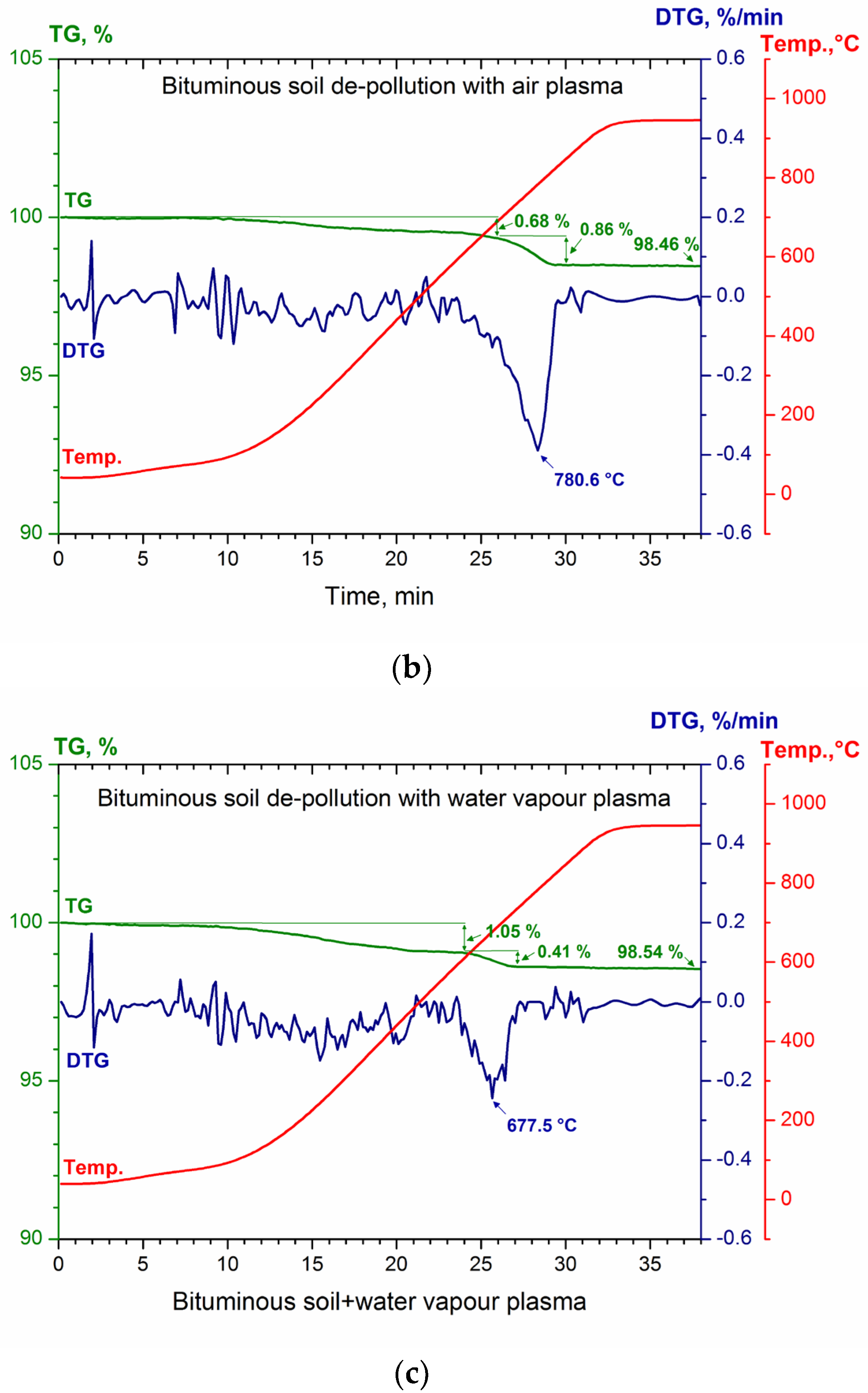
3.3. Thermal Analysis of Bituminous and De-Polluted Soil
3.4. Assessment of the Produced Gaseous Compounds
3.5. The Thermal Arc Plasma’s Capacity to De-Pollute Bituminous Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivagami, K.; Padmanabhan, K.; Joy, A.C.; Nambi, I.M. Microwave (MW) Remediation of Hydrocarbon Contaminated Soil Using Spent Graphite—An Approach for Waste as a Resource. J. Environ. Manag. 2019, 230, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K.; Hussein, Z.S.; Mohamed, N.H.; Safwat, G.; El-Dessouky, M.A.; Imbrea, I.; Imbrea, F. Assessment of Vinca Rosea (Apocynaceae) Potentiality for Remediation of Crude Petroleum Oil Pollution of Soil. Sustainability 2023, 15, 11046. [Google Scholar] [CrossRef]
- Karthick, A.; Roy, B.; Chattopadhyay, P. A Review on the Application of Chemical Surfactant and Surfactant Foam for Remediation of Petroleum Oil Contaminated Soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-Assisted Remediation of Hydrocarbons in Water and Soil: Application, Mechanisms, Challenges and Opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- National Academies of Sciences; Division on Earth, Life Studies; Board on Chemical Sciences; Committee on the Effects of Diluted Bitumen on the Environment. Spills of Diluted Bitumen from Pipelines: A Comparative Study of Environmental Fate, Effects, and Response; National Academies Press: Washington, DC, USA, 2016; ISBN 0309380103. [Google Scholar]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of Soil and Water Contaminated with Petroleum Hydrocarbon: A Review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Diao, Z.H.; Zhang, W.X.; Liang, J.Y.; Huang, S.T.; Dong, F.X.; Yan, L.; Qian, W.; Chu, W. Removal of Herbicide Atrazine by a Novel Biochar Based Iron Composite Coupling with Peroxymonosulfate Process from Soil: Synergistic Effect and Mechanism. Chem. Eng. J. 2021, 409, 127684. [Google Scholar] [CrossRef]
- Diao, Z.H.; Yan, L.; Dong, F.X.; Qian, W.; Deng, Q.H.; Kong, L.J.; Yang, J.W.; Lei, Z.X.; Du, J.J.; Chu, W. Degradation of 2,4-Dichlorophenol by a Novel Iron Based System and Its Synergism with Cd(II) Immobilization in a Contaminated Soil. Chem. Eng. J. 2020, 379, 122313. [Google Scholar] [CrossRef]
- Li, D.C.; Xu, W.F.; Mu, Y.; Yu, H.Q.; Jiang, H.; Crittenden, J.C. Remediation of Petroleum-Contaminated Soil and Simultaneous Recovery of Oil by Fast Pyrolysis. Environ. Sci. Technol. 2018, 52, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Vidonish, J.E.; Alvarez, P.J.J.; Zygourakis, K. Pyrolytic Remediation of Oil-Contaminated Soils: Reaction Mechanisms, Soil Changes, and Implications for Treated Soil Fertility. Ind. Eng. Chem. Res. 2018, 57, 3489–3500. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Wu, B.; Li, X.; Ma, F.; Li, F.; Gu, Q. Hematite-Facilitated Pyrolysis: An Innovative Method for Remediating Soils Contaminated with Heavy Hydrocarbons. J. Hazard. Mater. 2020, 383, 121165. [Google Scholar] [CrossRef]
- Kang, C.-U.; Kim, D.-H.; Khan, M.A.; Kumar, R.; Ji, S.-E.; Choi, K.-W.; Paeng, K.-J.; Park, S.; Jeon, B.-H. Pyrolytic Remediation of Crude Oil-Contaminated Soil. Sci. Total Environ. 2020, 713, 136498. [Google Scholar] [CrossRef]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification. A Review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Ouda, O.K.M.; Raza, S.A.; Nizami, A.S.; Rehan, M.; Al-Waked, R.; Korres, N.E. Waste to Energy Potential: A Case Study of Saudi Arabia. Renew. Sustain. Energy Rev. 2016, 61, 328–340. [Google Scholar] [CrossRef]
- AlQattan, N.; Acheampong, M.; Jaward, F.M.; Ertem, F.C.; Vijayakumar, N.; Bello, T. Reviewing the Potential of Waste-to-Energy (WTE) Technologies for Sustainable Development Goal (SDG) Numbers Seven and Eleven. Renew. Energy Focus 2018, 27, 97–110. [Google Scholar] [CrossRef]
- Perna, A.; Minutillo, M.; Lubrano Lavadera, A.; Jannelli, E. Combining Plasma Gasification and Solid Oxide Cell Technologies in Advanced Power Plants for Waste to Energy and Electric Energy Storage Applications. Waste Manag. 2018, 73, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Song, X.; Wei, C.; LaChance, J. A Review on the Sustainability of Thermal Treatment for Contaminated Soils. Environ. Pollut. 2019, 253, 449–463. [Google Scholar] [CrossRef]
- Gimžauskaitė, D.; Aikas, M.; Tamošiūnas, A. Recent Progress in Thermal Plasma Gasification of Liquid and Solid Wastes. In Recent Advances in Renewable Energy Technologies; Elsevier, Academic Press: London, UK, 2022; Volume 2, pp. 155–196. [Google Scholar]
- Dobslaw, C.; Glocker, B. Plasma Technology and Its Relevance in Waste Air and Waste Gas Treatment. Sustainability 2020, 12, 8981. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- Arshad, M.Y.; Saeed, M.A.; Tahir, M.W.; Raza, A.; Ahmad, A.S.; Tahir, F.; Borkowski, B.; Mączka, T.; Niedzwiecki, L. Role of Experimental, Modeling, and Simulation Studies of Plasma in Sustainable Green Energy. Sustainability 2023, 15, 14193. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Gimžauskaitė, D.; Uscila, R.; Aikas, M. Thermal Arc Plasma Gasification of Waste Glycerol to Syngas. Appl. Energy 2019, 251, 113306. [Google Scholar] [CrossRef]
- Agon, N.; Hrabovský, M.; Chumak, O.; Hlína, M.; Kopecký, V.; Mašláni, A.; Bosmans, A.; Helsen, L.; Skoblja, S.; Van Oost, G.; et al. Plasma Gasification of Refuse Derived Fuel in a Single-Stage System Using Different Gasifying Agents. Waste Manag. 2016, 47, 246–255. [Google Scholar] [CrossRef]
- Aikas, M.; Gimžauskaitė, D.; Tamošiūnas, A.; Uscila, R.; Snapkauskienė, V. Thermal Arc Air Plasma Application for Biomass (Wood Pellets) Gasification. Clean Technol. Environ. Policy 2024, 26, 31–43. [Google Scholar] [CrossRef]
- Kuo, P.C.; Illathukandy, B.; Wu, W.; Chang, J.S. Plasma Gasification Performances of Various Raw and Torrefied Biomass Materials Using Different Gasifying Agents. Bioresour. Technol. 2020, 314, 123740. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.F.S.; Petraconi Filho, G.; Couto, A.A.; da Silva Sobrinho, A.S.; Miranda, F.S.; Massi, M. Evaluation of Thermal Plasma Process for Treatment Disposal of Solid Radioactive Waste. J. Environ. Manag. 2022, 311, 114895. [Google Scholar] [CrossRef] [PubMed]
- LST EN ISO 16968:2015; Solid Biofuels—Determination of Minor Elements. Lithuanian Standards Board: Vilnius, Lithuania, 2015.
- Malé, Q.; Barléon, N.; Shcherbanev, S.; Dharmaputra, B.; Noiray, N. Numerical Study of Nitrogen Oxides Chemistry during Plasma Assisted Combustion in a Sequential Combustor. Combust. Flame 2024, 260, 113206. [Google Scholar] [CrossRef]
- Zhou, R.; Rezaeimotlagh, A.; Zhou, R.; Zhang, T.; Wang, P.; Hong, J.; Soltani, B.; Mai-Prochnow, A.; Liao, X.; Ding, T.; et al. In-Package Plasma: From Reactive Chemistry to Innovative Food Preservation Technologies. Trends Food Sci. Technol. 2022, 120, 59–74. [Google Scholar] [CrossRef]
- Topolovec, B.; Škoro, N.; Puač, N.; Petrovic, M. Pathways of Organic Micropollutants Degradation in Atmospheric Pressure Plasma Processing—A Review. Chemosphere 2022, 294, 133606. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, H.; Xu, Z.; Zhang, Z.; Cheng, C.; Ni, G.; Lan, Y.; Meng, Y.; Xia, W.; Chu, P.K. Preferential Production of Reactive Species and Bactericidal Efficacy of Gas-Liquid Plasma Discharge. Chem. Eng. J. 2019, 362, 402–412. [Google Scholar] [CrossRef]
- Sanito, R.C.; You, S.J.; Wang, Y.F. Degradation of Contaminants in Plasma Technology: An Overview. J. Hazard. Mater. 2022, 424, 127390. [Google Scholar] [CrossRef]
- Wang, W.; Snoeckx, R.; Zhang, X.; Cha, M.S.; Bogaerts, A. Modeling Plasma-Based CO2 and CH4 Conversion in Mixtures with N2, O2, and H2O: The Bigger Plasma Chemistry Picture. J. Phys. Chem. C 2018, 122, 8704–8723. [Google Scholar] [CrossRef]
- Kapaldo, J.; Han, X.; Ptasinska, S. Shielding-Gas-Controlled Atmospheric Pressure Plasma Jets: Optical Emission, Reactive Oxygen Species, and the Effect on Cancer Cells. Plasma Process. Polym. 2019, 16, 1800169. [Google Scholar] [CrossRef]
- Jaworski, Z.; Pianko-Oprych, P. A Comparative Thermodynamic Study of Equilibrium Conditions for Carbon Deposition from Catalytic C-H-O Reformates. Energies 2018, 11, 1177. [Google Scholar] [CrossRef]
- Gao, Y.; Deng, L. Converting Carbon Dioxide into Alkanes via Alkane Reverse Combustion Reaction. Sci. Bull. 2016, 61, 1160–1162. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, C.; Huang, S.; Zhang, S.; Tan, X.; Lian, Z.; Zou, J.J.; Zhang, X.; Li, G.; Wang, D. A Comprehensive Review on Steam Reforming of Liquid Hydrocarbon Fuels: Research Advances and Prospects. Fuel 2024, 368, 131596. [Google Scholar] [CrossRef]
- Kim, M.J.; Gong, J.H.; Jeon, K.W.; Shim, J.O.; Jang, W.J. Thermodynamic Approach for Hydrogen Production from the Steam Reforming Reaction of Aromatic Hydrocarbons (BTX). Int. J. Hydrogen Energy 2024, 49, 1215–1225. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Martirosyan, O.V.; Kuzmin, D.V.; Isaenko, S.I.; Makeev, B.A.; Antonets, I.V.; Utkin, A.A. Transformations of Natural Bitumens of Different Degrees of Metamorphism at a Low Vacuum Heating in the Temperature Range of 400–1000 °C. J. Pet Sci. Eng. 2019, 173, 315–325. [Google Scholar] [CrossRef]
- Werkovits, S.; Bacher, M.; Theiner, J.; Rosenau, T.; Grothe, H. Multi-Spectroscopic Characterization of Bitumen and Its Polarity-Based Fractions. Constr. Build. Mater. 2022, 352, 128992. [Google Scholar] [CrossRef]
- Agarry, S.E. Biodegradation of Bitumen in Soil and Its Enhancement by Inorganic Fertilizer and Oxygen Release Compound: Experimental Analysis and Kinetic Modelling. J. Microb. Biochem. Technol. 2014, 4, 2. [Google Scholar] [CrossRef]
- Kanaujia, P.K. Gas Chromatography | Petroleum and Petrochemical Applications. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–231. ISBN 9780081019832. [Google Scholar]
- Ram, V.; Salkuti, S.R. An Overview of Major Synthetic Fuels. Energies 2023, 16, 2834T. [Google Scholar] [CrossRef]
- Zanoni, M.A.B.; Rein, G.; Yermán, L.; Gerhard, J.I. Thermal and Oxidative Decomposition of Bitumen at the Microscale: Kinetic Inverse Modelling. Fuel 2020, 264, 116704. [Google Scholar] [CrossRef]
- Matta, G.; Courtois, N.; Champenois, J.B.; Perrin, S.; Sbirrazzuoli, N. Kinetic Analysis of Pyrolysis and Thermal Oxidation of Bitumen. Thermochim. Acta 2023, 724, 179514. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, A.; Héroux, P.; Sun, Z.; Liu, Y. Remediation of Diesel Fuel Polluted Soil Using Dielectric Barrier Discharge Plasma. Chem. Eng. J. 2021, 417, 128143. [Google Scholar] [CrossRef]
- Abbas, Y.; Lu, W.; Dai, H.; Fu, X.; Ye, R.; Wang, H. Remediation of Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soil with Double Dielectric Barrier Discharge Plasma Technology: Influencing Parameters. Chem. Eng. J. 2020, 394, 124858. [Google Scholar] [CrossRef]
- Redolfi, M.; Makhloufi, C.; Ognier, S.; Cavadias, S.; Tzovolou, D.; Tsakiroglou, C. Kerosene Contaminated Soil Removal by Non-Thermal Atmospheric Plasma Discharge. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2009, 13, 427–437. [Google Scholar] [CrossRef]
- Zhao, J.; Cai, L.; Zhang, A.; Li, G.; Zhang, Y.; Filatova, I.; Liu, Y. Simultaneous Remediation of Diesel-Polluted Soil and Promoted Ryegrass Growth by Non-Thermal Plasma Pretreatment. Sci. Total Environ. 2024, 912, 169295. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Mu, R.; Cheng, W.; Ognier, S. Evaluation of Pulsed Corona Discharge Plasma for the Treatment of Petroleum-Contaminated Soil. Environ. Sci. Pollut. Res. 2017, 24, 1450–1458. [Google Scholar] [CrossRef]
- Lu, N.; Wang, C.; Lou, C. Remediation of PAH-Contaminated Soil by Pulsed Corona Discharge Plasma. J. Soils Sediments 2017, 17, 97–105. [Google Scholar] [CrossRef]
- Acharya, T.R.; Lamichhane, P.; Jaiswal, A.; Amsalu, K.; Hong, Y.J.; Kaushik, N.; Kaushik, N.K.; Choi, E.H. The Potential of Multicylindrical Dielectric Barrier Discharge Plasma for Diesel-Contaminated Soil Remediation and Biocompatibility Assessment. Environ. Res. 2024, 240, 117398. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Zhan, J.; Zhang, A.; Zhu, L.; Sun, Z.; Liu, Y. Contrastive Study on Organic Contaminated Soils Remediated Using Dielectric Barrier Discharge (DBD) Plasma. Sep. Purif. Technol. 2023, 306, 122576. [Google Scholar] [CrossRef]






| Parameter | Bitumen-Polluted Soil |
|---|---|
| Carbon, wt.% | 16.08 |
| Hydrogen, wt.% | 2.16 |
| Nitrogen, wt.% | 0.12 |
| Sulphur, wt.% | 0.49 |
| Oxygen, wt.% 1 | 0.87 |
| Moisture, wt.% | 1.28 |
| Volatiles, wt.% | 11.70 |
| Fixed carbon, wt.% | 6.74 |
| Ashes, wt.% | 80.28 |
| LHV, MJ/kg | 11.96 |
| Bituminous Soil De-Pollution Operating with | ||
|---|---|---|
| Parameter | Air Plasma | Water Vapour Plasma |
| Current of the arc, A | 160 | 160 |
| Voltage of the arc, V | 330 | 350 |
| Power, kW | 52.8 | 56 |
| Bituminous soil content, kg | 1.5 | 1.5 |
| Bitumen amount in the polluted soil, g/kg | 42 | 42 |
| Flow of the gasifying agent, g/s | 4.4 | 4.1 |
| Airflow for cathode protection, g/s | 0.5 | 0.5 |
| Total gas flow, g/s | 4.9 | 4.6 |
| Mean plasma temperature, K | 4100 | 2800 |
| Thermal efficiency of the plasma torch, (η), % | 54 | 74 |
| Element | Bituminous Soil, wt.% * | De-Polluted Soil with Air Plasma | De-Polluted Soil with Water Vapour Plasma |
|---|---|---|---|
| Carbon | 70.14 | 5.74 | 7.70 |
| Oxygen | 18.78 | 47.29 | 46.45 |
| Silicon | 4.17 | 27.16 | 27.71 |
| Sulphur | 2.64 | 0.74 | 0.70 |
| Potassium | 0.41 | 2.68 | 2.83 |
| Calcium | 1.09 | 6.12 | 4.49 |
| Magnesium | 0.25 | 1.46 | 1.32 |
| Aluminium | 0.92 | 4.59 | 4.88 |
| Iron | 0.94 | 2.95 | 2.63 |
| Sodium | 0.20 | 0.53 | 0.66 |
| Titanium | 0.38 | 0.63 | 0.47 |
| Phosphorus | 0.08 | 0.12 | 0.15 |
| Parameter | Air Plasma Environment | Water Vapour Plasma Environment |
|---|---|---|
| Bitumen amount in the soil after de-pollution process | <0.089 g/kg | <0.089 g/kg |
| Efficiency of bitumen elimination from the soil | 99.7% | 99.7% |
| Reference | Discharge Type, Power kW | Gasifying Agent | Target Contaminant, g/kg | De-Pollution Time, h | Elimination Efficiency, % |
|---|---|---|---|---|---|
| Present work | Direct current, 52.8 | Air | Bitumen, 42 | 0.28 | 99.7 |
| Present work | Direct current, 56 | Water vapour | Bitumen, 42 | 0.28 | 99.7 |
| [46] | Dielectric barrier discharge, 0.18 | - | Diesel, 5 | 0.67 | 62.0 |
| [46] | Dielectric barrier discharge, 0.18 | - | Diesel, 10 | 0.67 | 74.0 |
| [47] | Double dielectric barrier discharge, 40 kV | Air | Naphthalene, 0.1 | 0.5 | 96.3 |
| [47] | Double dielectric barrier discharge, 40 kV | Air | Phenanthrene, 0.1 | 0.5 | 89.1 |
| [47] | Double dielectric barrier discharge, 40 kV | Air | Pyrene, 0.1 | 0.5 | 88.6 |
| [48] | Dielectric barrier discharge, 0.002 | Air | Kerosene, 0.074 | 0.07 | 25.0 |
| [48] | Dielectric barrier discharge, 0.002 | Air | Kerosene, 0.074 | 0.2 | 88.0 |
| [49] | Dielectric barrier discharge, 0.105 | - | Diesel, 2 | 0.17 | 25–67 |
| [50] | Pulsed corona discharge, 30 kV | - | Gasoline, 2.5 | 1 | 81.0 |
| [50] | Pulsed corona discharge, 30 kV | - | Gasoline, 10 | 1 | 57.0 |
| [51] | Pulsed corona discharge, 18 kV | Air | Phenanthrene, 0.01 | 0.67 | 70.5 |
| [52] | Multi-cylindrical dielectric barrier discharge, 0.03 | Air | Diesel, 10 | 1 | 94.2 |
| [53] | Dielectric barrier discharge, 0.081 | Nitrogen | Gasoline, 5 | 1 | 59.0 |
| [53] | Dielectric barrier discharge, 0.081 | Oxygen | Gasoline, 5 | 1 | 87.0 |
| [53] | Dielectric barrier discharge, 0.081 | Air | Gasoline, 5 | 1 | 92.0 |
| [53] | Dielectric barrier discharge, 0.081 | Argon | Gasoline, 5 | 1 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimžauskaitė, D.; Tamošiūnas, A.; Eimontas, J.; Aikas, M.; Uscila, R.; Snapkauskienė, V. Bituminous Soil Remediation in the Thermal Plasma Environment. Sustainability 2024, 16, 4855. https://doi.org/10.3390/su16114855
Gimžauskaitė D, Tamošiūnas A, Eimontas J, Aikas M, Uscila R, Snapkauskienė V. Bituminous Soil Remediation in the Thermal Plasma Environment. Sustainability. 2024; 16(11):4855. https://doi.org/10.3390/su16114855
Chicago/Turabian StyleGimžauskaitė, Dovilė, Andrius Tamošiūnas, Justas Eimontas, Mindaugas Aikas, Rolandas Uscila, and Vilma Snapkauskienė. 2024. "Bituminous Soil Remediation in the Thermal Plasma Environment" Sustainability 16, no. 11: 4855. https://doi.org/10.3390/su16114855
APA StyleGimžauskaitė, D., Tamošiūnas, A., Eimontas, J., Aikas, M., Uscila, R., & Snapkauskienė, V. (2024). Bituminous Soil Remediation in the Thermal Plasma Environment. Sustainability, 16(11), 4855. https://doi.org/10.3390/su16114855






