Pesticidal Plant Treatments Combined with Improved Soil Fertility Can Reduce Damage Caused by Fusarium Wilt (Fusarium oxysporum f.sp. phaseoli) and Bean Fly (Ophiomyia phaseoli) in Common Bean Production (Phaseolus vulgaris L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fusarium Inoculum for Use in the Screenhouse Trials
2.2. Preparation of Pesticidal Plant Materials
2.3. Evaluating Pesticidal Plant Efficacy to Control Common Bean Fusarium Wilt
2.4. Establishment of Laboratory Bean Fly (Ophiomyia phaseoli) Colony
2.5. Preparation of Pesticidal Plants for Bean Fly (Ophiomyia phaseoli) Experiment
2.6. Evaluating Pesticidal Plant Efficacy to Control Bean Fly (Ophiomyia phaseoli) Damage to Common Bean
2.7. Evaluation of Pesticidal Plant Treatments on Beans Planted in Two Soil Types on Bean Fly Damage
2.8. Assessment of Pest and Soil Fertility Management on Bean Fly (Ophiomyia phaseoli) Damage
2.9. Data Analysis
3. Results
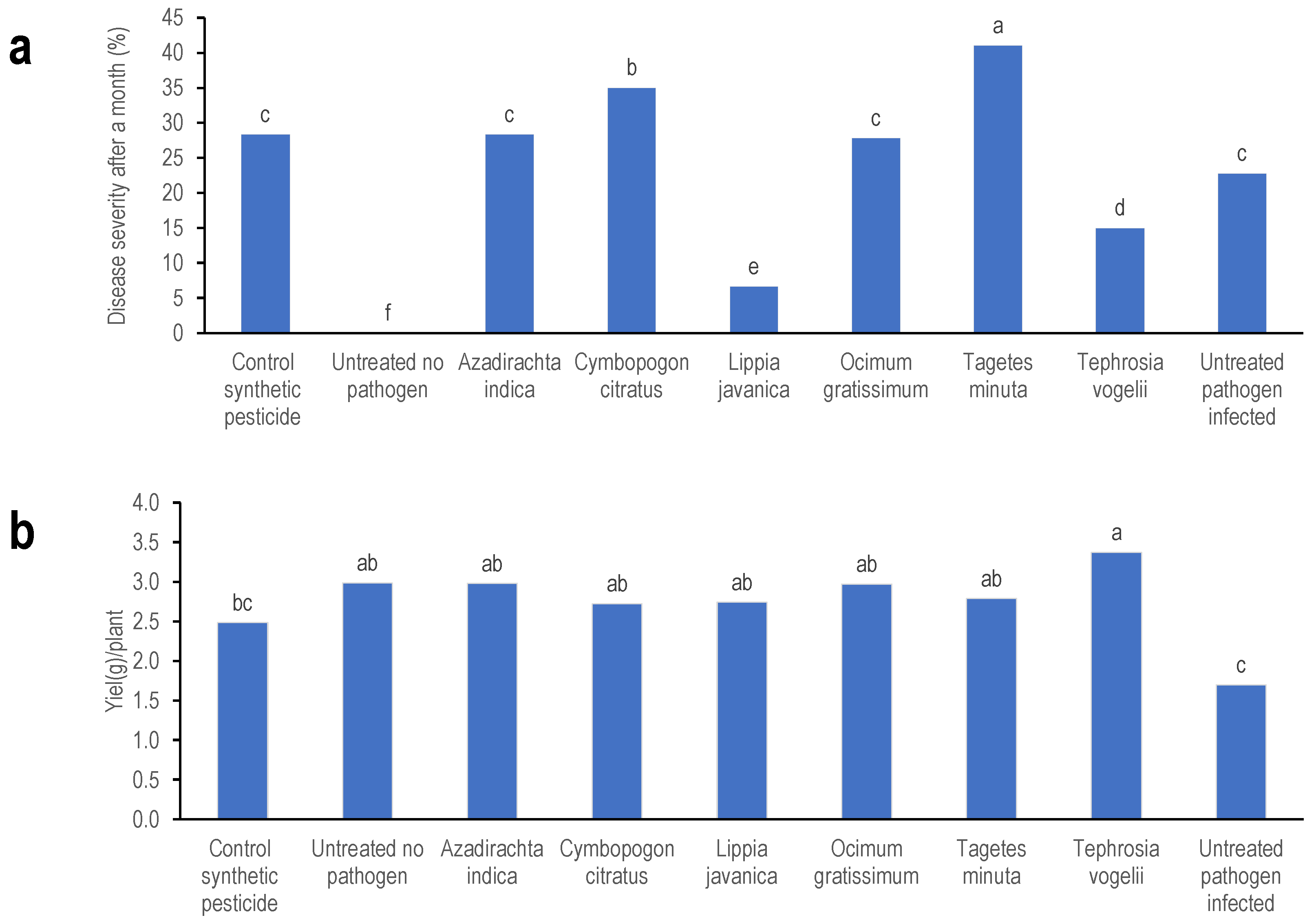
3.1. Evaluating Pesticidal Plant Efficacy to Control Common Bean Fusarium Wilt
3.2. Evaluating Pesticidal Plant Efficacy to Control Bean Fly (Ophiomyia phaseoli) Damage to Common Bean
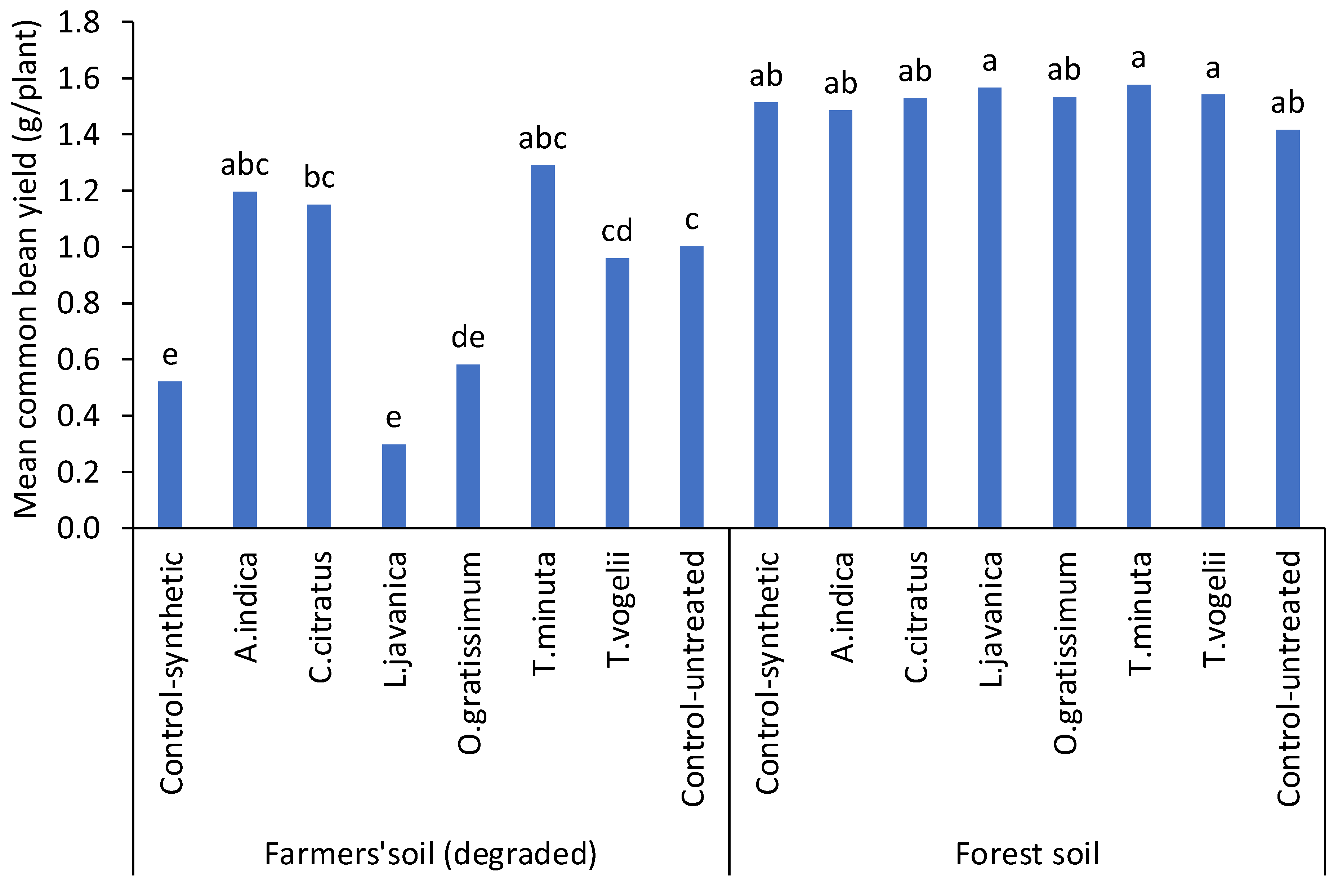
3.3. Evaluation of Pesticidal Plant Treatments on Beans Planted in Two Soil Types on Bean Fly (Ophiomyia phaseoli) Damage
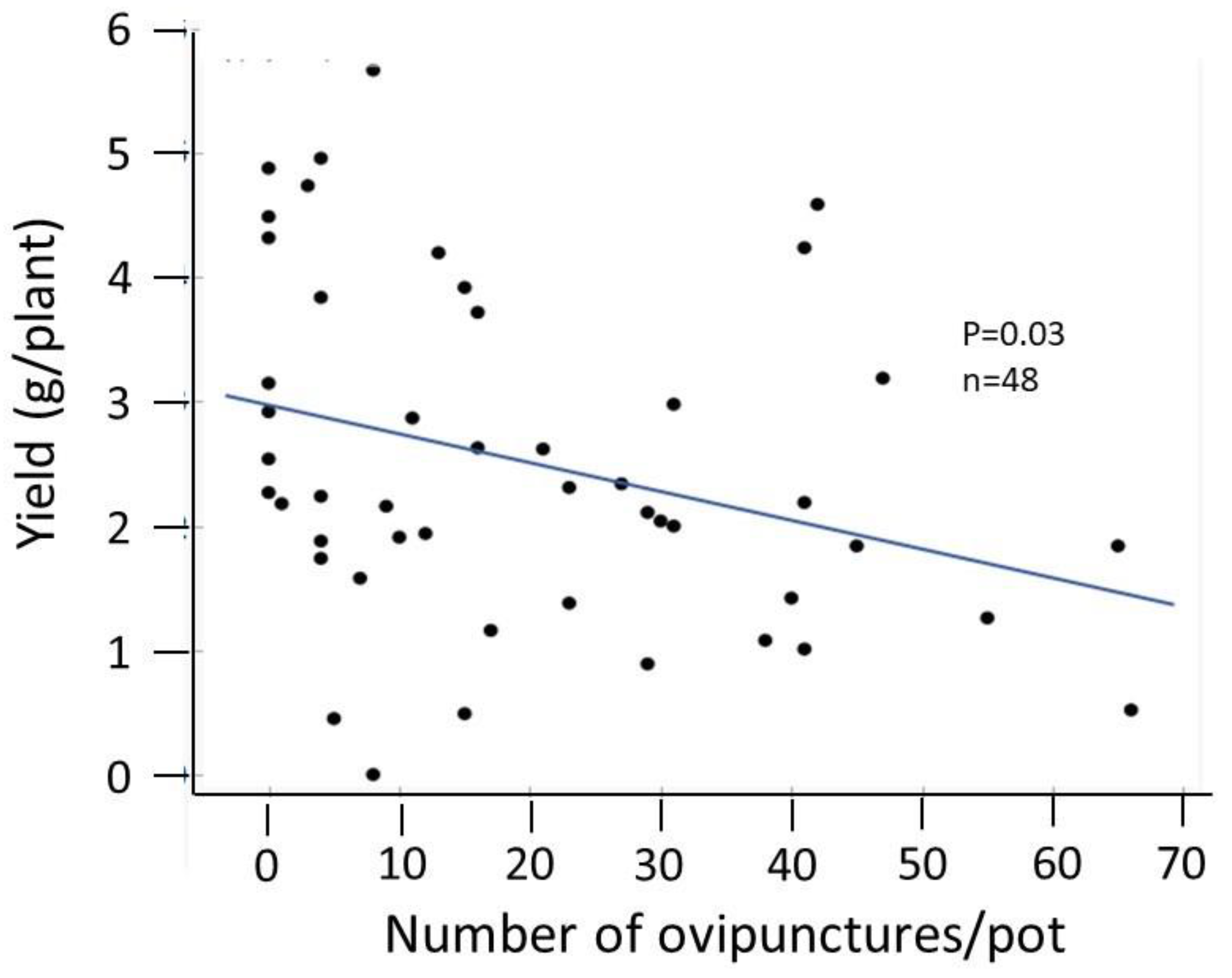
3.4. Assessment of Pest and Soil Fertility Management on Bean Fly (Ophiomyia phaseoli) Damage
4. Discussion
4.1. Pesticidal Plant Efficacy on Fusarium Wilt Disease
4.2. Effects of Plant Extract Delivery Mode on Fusarium Wilt Disease
4.3. Response of Bean Fly to Plant Extracts
4.4. Interactions of Plant Extracts with Fertility Inputs for Bean Fly
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okungbowa, F.I.; Shittu, H. Fusarium Wilts: An Overview. Environ. Res. J. 2014, 6, 1935–3049. [Google Scholar]
- Yadav, A.; Chandrasekhar Reddy, D.; Yadav, A.; Yadav, T.; Singh, H.; Abhishek Yadav, C. Stem Fly, Ophiomyia phaseoli (Tryon) (Insecta: Diptera: Agromyzidae) a Major Insect: A Review. J. Entomol. Zool. Stud. 2019, 7, 1200–1205. [Google Scholar]
- Morris, M.M. Effect of Soil Nutrients and Intercropping on Soil Borne Diseases and Seed Quality of Common Bean in Busia County. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2017. [Google Scholar]
- Hillocks, R.J.; Madata, C.S.; Chirwa, R.; Minja, E.M.; Msolla, S. Phaseolus Bean Improvement in Tanzania, 1959–2005. Euphytica 2006, 150, 215–231. [Google Scholar] [CrossRef]
- Papias, H.B.; Conrad, K.B.; Susan, N.M.; Inocent, I.R. Morphological and Molecular Identification of Pythium spp. Isolated from Common Beans (Phaseolus vulgaris) Infected with Root Rot Disease. Afr. J. Plant Sci. 2016, 10, 1–9. [Google Scholar] [CrossRef]
- Mwaipopo, B.; Nchimbi-Msolla, S.; Njau, P.; Tairo, F.; William, M.; Binagwa, P.; Kweka, E.; Kilango, M.; Mbanzibwa, D. Viruses Infecting Common Bean (Phaseolus vulgaris L.) in Tanzania: A Review on Molecular Characterization, Detection and Disease Management Options. Afr. J. Agric. Res. 2017, 12, 1486–1500. [Google Scholar] [CrossRef] [PubMed]
- Sekamatte, M.B.; Okwakol, M.J.N. The Present Knowledge on Soil Pests and Pathogens in Uganda. Afr. J. Ecol. 2007, 45, 9–19. [Google Scholar] [CrossRef]
- CIAT. Bean Production Problems in the Tropics, 2nd ed.; Schwartz, H.F., Pastor-Corrales, M., Eds.; Press Run: Cali, Columbia, 1989; ISBN 9589183042. [Google Scholar]
- Srinivasan, R. Insect and Mite Pests on Vegetable Legumes: A Field Guide for Identification and Management; Mecozzi, M., Ed.; AVRDC-The World Vegetable Center: Shanhua, Taiwan, 2014; Publication #14–778; ISBN 9290582065. [Google Scholar]
- Greathead, D.J. A Study in East Africa of the Bean Flies (Dipt., Agromyzidae) Affecting Phaseolus vulgaris and of Their Natural Enemies, with the Description of a New Species of Melanagromyza Hend. Bull. Entomol. Res. 1969, 59, 541–561. [Google Scholar] [CrossRef]
- Tengecho, B.; Coulson, C.L.; d’Souza, H.A. Distribution and Effect of Bean Flies, Ophiomyia phaseoli and O. spencerella, on Beans at Kabete, Kenya. Int. J. Trop. Insect Sci. 1988, 9, 505–508. [Google Scholar] [CrossRef]
- Sariah, J.B.; Makundi, R.H. Effect of Sowing Time on Infestation of Beans (Phaseolus vulgaris L.) by Two Species of the Bean Stem Maggot, Ophiomyia spencerella and Ophiomyia phaseoli (Diptera: Agromyzidae). Arch. Phytopathol. Plant Prot. 2007, 40, 45–51. [Google Scholar] [CrossRef]
- Buruchara, R.; Ampofo, K.; Mukankusi, C. Bean Disease and Pest Identification and Management. Int. Cent. Trop. Agric. 2010, 371, 6–8. [Google Scholar]
- Mukankusi, C.; Derera, J.; Melis, R.; Gibson, P.T.; Buruchara, R. Genetic Analysis of Resistance to Fusarium Root Rot in Common Bean. Euphytica 2011, 182, 11–23. [Google Scholar] [CrossRef]
- Clare, M.M.; Melis, R.; Derera, J.; Laing, M.; Buruchara, R. Identification of Sources of Resistance to Fusarium Root Rot among Selected Common Bean Lines in Uganda. J. Anim. Plant Sci. 2010, 7, 876–891. [Google Scholar]
- Rusuku, G.; Buruchara, R.A.; Gatabazi, M.; Pastor-Corrales, M.A. Occurrence and Distribution in Rwanda of Soilborne Fungi Pathogenic to the Common Bean. Plant Dis. 1997, 81, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Mukankusi, C.M.; Melis, R.J.; Derera, J.; Buruchara, R.A.; Mark, D. A Screening Technique for Resistance to Fusarium Root Rot of Common Bean. Afr. J. Plant Sci. 2011, 5, 152–161. [Google Scholar]
- Valenciano, J.B.; Casquero, P.A.; Boto, J.A.; Marcelo, V. Evaluation of the Occurrence of Root Rots on Bean Plants (Phaseolus vulgaris) Using Different Sowing Methods and with Different Techniques of Pesticide Application. N. Z. J. Crop Hortic. Sci. 2006, 34, 291–298. [Google Scholar] [CrossRef]
- Benchimol-Reis, L.L.; Bueno, C.J.; Carbonell, S.A.M.; Chiorato, A.F. Fusarium Wilt–Common Bean Pathosystem: Pathogen Variability and Genetic Control. Crop Sci. 2023, 63, 2609–2622. [Google Scholar] [CrossRef]
- Karel, A.K.; Ashimogo, G.C. Economics of Insect Control on Common Beans and Soybeans in Tanzania. J. Econ. Entomol. 1991, 84, 996–1000. [Google Scholar] [CrossRef]
- Gayon, F.; Grimm, C. Methods for Control of Soil-Dwelling Pests and/or Soil-borne Diseases. U.S. Patent 8,765,160 B2, 1 July 2014. [Google Scholar]
- Prieto, J.A.; Patiño, O.J.; Plazas, E.A.; Pabón, L.C.; Ávila, M.C.; Guzmán, J.D.; Delgado, W.A.; Cuca, L.E. Natural Products from Plants as Potential Source Agents for Controlling Fusarium. In Fungicides—Showcases of Integrated Plant Disease Management from Around the World; Nita, M., Ed.; Intech: Rijeka, Croacia, 2013; ISBN 9789537619992. [Google Scholar]
- Obongoya, B.; Wagai, S.; Odhiambo, G. Phytotoxic Effect of Selected Crude Plant Extracts on Soil-Borne Fungi of Common Bean. Afr. Crop Sci. Soc. 2010, 18, 15–22. [Google Scholar] [CrossRef]
- Shimira, F.; Uğur, S.; Özdemir, Ş.M.; Yalçın Mendi, Y. Future and Prospect Use of Pyrethrum (Chrysanthemum cinerariifolium) as Part of the Integrated Pest and Disease Management (IPDM) Tool in Turkey. Turk. J. Agric. Food Sci. Technol. 2021, 9, 150–158. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Klingen, I.; Eilenberg, J.; Meadow, R. Effects of Farming System, Field Margins and Bait Insect on the Occurrence of Insect Pathogenic Fungi in Soils. Agric. Ecosyst. Environ. 2002, 91, 191–198. [Google Scholar] [CrossRef]
- Sun, B.D.; Liu, X.Z. Occurrence and Diversity of Insect-Associated Fungi in Natural Soils in China. Appl. Soil Ecol. 2008, 39, 100–108. [Google Scholar] [CrossRef]
- Hirt, H. Healthy Soils for Healthy Plants for Healthy Humans. EMBO Rep. 2020, 21, e51069. [Google Scholar] [CrossRef] [PubMed]
- Akullo, D.; Kanzikwera, R.; Birungi, P. Indigenous Knowledge in Agriculture: A Case Study of the Challenges in Sharing Knowledge of Past Generations in a Globalized Context in Uganda. Indilinga Afr. J. Indig. Knowl. Syst. 2007, 4, 249–263. [Google Scholar]
- Lwoga, E.T. Knowledge Management Approaches in Managing Agricultural Indigenous and Exogenous Knowledge in Tanzania. J. Doc. 2011, 67, 407–430. [Google Scholar] [CrossRef]
- Ampofo, K.; Said, M.; Ulciky, E. Participatory IPM Development and Extension: The Case of Bean Foliage Beetles in Hai, Northern Tanzania. In Proceedings of the Pan-Africa Bean Research Alliance Millennium Workshop, Arusha, Tanzania, 28 May–1 June 2001; pp. 87–95. [Google Scholar]
- Stevenson, P.C.; Belmain, S.R. Pesticidal Plants in African Agriculture: Local Uses and Global Perspectives. Outlooks Pest Manag. 2016, 27, 226–230. [Google Scholar] [CrossRef]
- Karel, A.K.; Rweyemamu, C.L. Yield Losses in Field Beans Following Foliar Damage by Ootheca bennigseni (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1984, 77, 762–765. [Google Scholar] [CrossRef]
- Namayanja, A.; Msolla, S.N.; Buruchara, R.; Namusoke, A. Genetic Analysis of Resistance to Pythium Root Rot Disease in Common Bean (Phaseolus vulgaris L.) Genotypes. J. Crop Improv. 2014, 28, 184–202. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, I.; Ashraf, U.; Shahzad, T.; Hussain, S.; Shahid, M.; Abid, M.; Ullah, S. Effects of Organic and Inorganic Manures on Maize and Their Residual Impact on Soil Physico-Chemical Properties. J. Soil Sci. Plant Nutr. 2017, 17, 22–32. [Google Scholar] [CrossRef]
- Elka, E.; Laekemariam, F. Effects of Organic Nutrient Sources and NPS Fertilizer on the Agronomic and Economic Performance of Haricot Bean (Phaseolus vulgaris L.) in Southern Ethiopia. Appl. Environ. Soil Sci. 2020, 2020, 8853552. [Google Scholar] [CrossRef]
- Naseri, B. Bean Production and Fusarium Root Rot in Diverse Soil Environments in Iran. J. Soil Sci. Plant Nutr. 2014, 14, 177–188. [Google Scholar] [CrossRef]
- Letourneau, D.K. Bean Fly, Management Practices, and Biological Control in Malawian Subsistence Agriculture. Agric. Ecosyst. Env. 1994, 50, 103–111. [Google Scholar] [CrossRef]
- Kato, F.; Lwehabura, J.; Seenga, R.O.; Kabungo, D.; Kilango, M.; Mukankusi, C.M.; Rubyogo, J.C. Effects of Seed Dressing and Fertilizer on the Common Bean Yields, Bean Stem Maggot and Root Rot Diseases in Southern Highlands of Tanzania. Afr. J. Rural Dev. 2021, 6, 128–149. [Google Scholar]
- Nkhata, W.; Shimelis, H.; Melis, R.; Chirwa, R.; Mathew, I.; Shayanowako, A.; Mzengeza, T. Assessment of Smallholder Farmers’ Awareness of Bean Fly (Ophiomyia spp.) and Management Practices in Central and Northern Malawi: Implications for Resistance Breeding. Crop Prot. 2021, 139, 105353. [Google Scholar] [CrossRef]
- Eke, P.; Nana Wakam, L.; Fokom, R.; Ekounda, T.V.; Bedine Boat, M.A.; Keumoe, R.; Fekam Boyom, F. Common Bean (Phaseolus vulgaris L.) Root Rot in Humid Lowland: Occurrence, and Assessment of Biotic and Agronomic Factors for Mitigation Prospects. Rhizosphere 2020, 16. [Google Scholar] [CrossRef]
- Zitnick-Anderson, K.; Oladzadabbasabadi, A.; Jain, S.; Modderman, C.; Osorno, J.M.; McClean, P.E.; Pasche, J.S. Sources of Resistance to Fusarium solani and Associated Genomic Regions in Common Bean Diversity Panels. Front. Genet. 2020, 11, 00475. [Google Scholar] [CrossRef] [PubMed]
- Binagwa, P.H.; Bonsi, K.; Msolla, S. Evaluation of Common Bean (Phaseolus vulgaris) Genotypes for Resistance to Root Rot Disease Caused by Pythium aphanidermatum and Pythium splendens under Screen House Conditions. J. Nat. Sci. Res. 2019, 6, 36–43. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Fernandes, S. Molecular and Culture-Based Assessment of Root/Crown Rot Fungal/Oomycete Complex of Bean in Mozambique. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2017. [Google Scholar]
- Sendi, Y.; Pfeiffer, T.; Koch, E.; Mhadhbi, H.; Mrabet, M. Potential of Common Bean (Phaseolus vulgaris L.) Root Microbiome in the Biocontrol of Root Rot Disease and Traits of Performance. J. Plant Dis. Prot. 2020, 127, 453–462. [Google Scholar] [CrossRef]
- Oyen, L.P.A.; Nguyĕn, X.D. Prosea Project. In Essential-Oil Plants; Backhuys Publishers: Leiden, The Netherlands, 1999; ISBN 9057820102. [Google Scholar]
- Anjarwalla, P.; Belmain, S.; Sola, P.; Jamnadass, R.; Stevenson, P. Handbook on Pesticidal Plants; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2016; ISBN 9789290593973. [Google Scholar]
- Nguemtchouin, M.G.M.; Ngassoum, M.B.; Chalier, P.; Kamga, R.; Ngamo, L.S.T.; Cretin, M. Ocimum gratissimum Essential Oil and Modified Montmorillonite Clay, a Means of Controlling Insect Pests in Stored Products. J. Stored Prod. Res. 2013, 52, 57–62. [Google Scholar] [CrossRef]
- Oyourou, J.; Combrinck, S.; Regnier, T.; Marston, A. Purification, Stability and Antifungal Activity of Verbascoside from Lippia javanica and Lantana camara Leaf Extracts. Ind. Crops Prod. 2013, 43, 820–826. [Google Scholar] [CrossRef]
- Madzimure, J.; Nyahangare, E.T.; Hamudikuwanda, H. Acaricidal Efficacy against Cattle Ticks and Acute Oral Toxicity of Lippia javanica (Burm F.) Spreng. Trop. Anim. Health Prod. 2011, 43, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Awadh Ali, N.A.; Sharopov, F.S.; Al-kaf, A.G.; Hill, G.M.; Arnold, N.; Al-Sokari, S.S.; Setzer, W.N.; Wessjohann, L. Composition of Essential Oil from Tagetes minuta and its Cytotoxic, Antioxidant and Antimicrobial Activities. Nat. Prod. Commun. 2014, 9, 265–268. [Google Scholar] [CrossRef]
- Mkindi, A.G.; Tembo, Y.L.B.; Mbega, E.R.; Smith, A.K.; Farrell, I.W.; Ndakidemi, P.A.; Stevenson, P.C.; Belmain, S.R. Extracts of Common Pesticidal Plants Increase Plant Growth and Yield in Common Bean Plants. Plants 2020, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Mann, A. Phytochemical Constituents and Antimicrobial and Grain Protectant Activities of Clove Basil (Ocimum gratissimum L.) Grown in Nigeria. Int. J. Plant Res. 2012, 2, 51–58. [Google Scholar] [CrossRef]
- Dietrich, P.; Cesarz, S.; Eisenhauer, N.; Roscher, C. Effects of Steam Sterilization on Soil Abiotic and Biotic Properties. Soil. Org. 2020, 92, 99–108. [Google Scholar] [CrossRef]
- Moreno-Velandia, C.A.; Izquierdo-García, L.F.; Ongena, M.; Kloepper, J.W.; Cotes, A.M. Soil Sterilization, Pathogen and Antagonist Concentration Affect Biological Control of Fusarium Wilt of Cape Gooseberry by Bacillus velezensis Bs006. Plant Soil 2019, 435, 39–55. [Google Scholar] [CrossRef]
- Buruchara, R.A.; Camacho, L. Common Bean Reaction to Fusarium oxysporum f.sp. phaseoli, the Cause of Severe Vascular Wilt in Central Africa. J. Phytopathol. 2000, 148, 39–45. [Google Scholar]
- Mutune, B.; Ekesi, S.; Niassy, S.; Matiru, V.; Bii, C.; Maniania, N.K. Fungal Endophytes as Promising Tools for the Management of Bean Stem Maggot Ophiomyia Phaseoli on Beans Phaseolus vulgaris. J. Pest Sci. 2016, 89, 993–1001. [Google Scholar] [CrossRef]
- Yao, Q.; Dong, Y.; Li, W.; Chen, B. The Effects of Non-Host Plant Extracts on the Oviposition Deterrent and Ovicidal Activity of Conopomorpha sinensis Bradley (Lepidoptera: Gracillariidae). Fla. Entomol. 2019, 102, 298–302. [Google Scholar] [CrossRef]
- Al-Charchafchi, F.; Al-Nabhani, I.; Al-Karousi, H.; Al-Qurain, F.; Al-Hanai, A. Effect of Aqueous Extract of Azadirachta indica (Neem) Leaves on Germination and Seedling Growth of Vigna radiata (L.). Pak. J. Biol. Sci. 2007, 10, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Kato-Noguchi, H. Evaluation of Allelopathic Potential of Neem (Azadirachta indica. A. Juss) Against Seed Germination and Seedling Growth of Different Test Plant Species. Int. J. Sustain. Agric. 2010, 2, 20–25. [Google Scholar]
- Appiah, K.S.; Mardani, H.K.; Osivand, A.; Kpabitey, S.; Amoatey, C.A.; Oikawa, Y.; Fujii, Y. Exploring Alternative Use of Medicinal Plants for Sustainable Weed Management. Sustainability 2017, 9, 1468. [Google Scholar] [CrossRef]
- Tlale, O. Bio-Efficacy of Selected Bean Genotypes and Some Plant Powders as Protectant Against the Seed Beetle, Callosobruchus Maculatus (Coleoptera: chrysomelidae). Master’s Thesis, Botswana University of Agriculture and Natural Resources, Gaborone, Botswana, 2009. [Google Scholar]
- Belmain, S.R.; Amoah, B.A.; Nyirenda, S.P.; Kamanula, J.F.; Stevenson, P.C. Highly Variable Insect Control Efficacy of Tephrosia vogelii Chemotypes. J. Agric. Food Chem. 2012, 60, 10055–10063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qin, D.; Chen, J.; Zhang, Z. Plants in the Genus Tephrosia: Valuable Resources for Botanical Insecticides. Insects 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Trouslot, M.-F.; Nef-Campa, C.; Chrestin, É. Production of Rotenoids by Heterotrophic and Photomixotrophic Cell Cultures of Tephrosia vogelii. Phytochemistry 1993, 34, 1515–1520. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between Nutrition, Plant Diseases and Pests. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, H., Marschner, P., Eds.; Academic Press: Chennai, India, 2011; pp. 283–298. ISBN 9780123849052. [Google Scholar]
- Dzenda, T.; Ayo, J.O.; Adelaiye, A.B.; Adaudi, A.O. Ethno-Medical and Veterinary Uses of Tephrosia vogelii Hook.f.: A Review. Niger. Vet. J. 2007, 28, 24–39. [Google Scholar]
- Thembo, K.M.; Vismer, H.F.; Nyazema, N.Z.; Gelderblom, W.C.A.; Katerere, D.R. Antifungal Activity of Four Weedy Plant Extracts against Selected Mycotoxigenic Fungi. J. Appl. Microbiol. 2010, 109, 1479–1486. [Google Scholar] [CrossRef]
- Prakash, M.; Georgin Ophelia, A.; Sathiya Narayanan, G. Cumulative Effect of Botanical Seed Pelleting and Foliar Spray on Morpho Physiological, Leaf Chlorophyll, Gas Exchange and Yield Parameters in Black Gram. Legume Res. 2021, 44, 425–430. [Google Scholar] [CrossRef]
- Harawa, R.; Lehmann, J.; Akinnifesi, F.; Fernandes, E.; Kanyama-Phiri, G. Nitrogen Dynamics in Maize-Based Agroforestry Systems as Affected by Landscape Position in Southern Malawi. Nutr. Cycl. Agroecosyst. 2006, 75, 271–284. [Google Scholar] [CrossRef]
- Mashela, P.W.; Shimelis, H.A.; de Waele, D.; Mokgalong, M.N.; Mudau, F.N.; Ngobeni, L.G. Fever Tea (Lippia javanica) as a Root-Knot Nematode Suppressant in Tomato Production. Afr. Plant Prot. 2010, 16, 1–6. [Google Scholar]
- Kiwia, A.; Imo, M.; Jama, B.; Okalebo, J.R. Coppicing Improved Fallows Are Profitable for Maize Production in Striga Infested Soils of Western Kenya. Agrofor. Syst. 2009, 76, 455–465. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; El-Gamal, N.G.; Abdel-Kader, M.M. Control of Wilt and Root Rot Incidence in Phaseolus vulgaris L. by Some Plant Volatile Compounds. J. Plant Prot. Res. 2007, 47, 255–265. [Google Scholar]
- Han, P.; Lavoir, A.-V.; Rodriguez-Saona, C.; Desneux, N. Bottom-Up Forces in Agroecosystems and Their Potential Impact on Arthropod Pest Management. Annu. Rev. Entomol. 2022, 2022, 239–259. [Google Scholar] [CrossRef]
- Murovhi, J.; Phophi, M.M. Mafongoya Efficacy of Plant Materials in Controlling Aphids on Okra (Abelmoschus esculentus L. Moench) in Limpopo Province of South Africa. Agronomy 2020, 10, 1968. [Google Scholar] [CrossRef]
- Andreotti, R.; Garcia, M.V.; Cunha, R.C.; Barros, J.C. Protective Action of Tagetes minuta (Asteraceae) Essential Oil in the Control of Rhipicephalus microplus (Canestrini, 1887) (Acari: Ixodidae) in a Cattle Pen Trial. Vet. Parasitol. 2013, 197, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Molina-Herrera, S.; Romanyà, J. Synergistic and Antagonistic Interactions among Organic Amendments of Contrasted Stability, Nutrient Availability and Soil Organic Matter in the Regulation of C Mineralization. Eur. J. Soil Biol. 2015, 70, 118–125. [Google Scholar] [CrossRef]
- Yang, C.; Du, W.; Zhang, L.; Dong, Z. Effects of Sheep Manure Combined with Chemical Fertilizers on Maize Yield and Quality and Spatial and Temporal Distribution of Soil Inorganic Nitrogen. Complexity 2021, 2021, 4330666. [Google Scholar] [CrossRef]
- Hernández, G.; Ramírez, M.; Valdés-López, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlereth, A.; Wandrey, M.; Erban, A.; Cheung, F.; et al. Phosphorus Stress in Common Bean: Root Transcript and Metabolic Responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef]
- Kirkby, E. Introduction, Definition and Classification of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, H., Marschner, P., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 1–3. ISBN 9780123849052. [Google Scholar]
- Samago, T.Y.; Anniye, E.W.; Dakora, F.D. Grain Yield of Common Bean (Phaseolus vulgaris L.) Varieties Is Markedly Increased by Rhizobial Inoculation and Phosphorus Application in Ethiopia. Symbiosis 2018, 75, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Wondimu, W.; Tana, T. Yield Response of Common Bean (Phaseolus vulgaris L.) Varieties to Combined Application of Nitrogen and Phosphorus Fertilizers at Mechara, Eastern Ethiopia. J. Plant Biol. Soil Health 2017, 4, 1–7. [Google Scholar]
- Abdel-Razik, S.A.; Sallam, N.M.A.; Eraky, A.M.I.; Hassan, M.H.A. Integrated Control of Root Rot and Wilt Disease of Faba Bean by Soil Amendment with Suppressive Compost in Combination with Seed Coating with an Antagonistic Yeast. Arch. Phytopathol. Plant Prot. 2012, 45, 1692–1704. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal Plants in Africa: A Global Vision of New Biological Control Products from Local Uses. Ind. Crops Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Abate, T.; Ampofo, J.K.O. Insect Pests of Beans in Africa: Their Ecology and Management. Annu. Rev. Entomol. 1996, 41, 45–73. [Google Scholar] [CrossRef]


| Application of Pesticidal Plants | Oviposition Rate Plant−1 | Seed Yield Plant−1 (g) |
|---|---|---|
| Seed | 4.9 b | 1.5 b |
| Spray only | 16.0 a | 1.3 a |
| Seed and spray | 4.5 b | 1.5 b |
| Pr > F (Model) | 0.001 | 0.001 |
| Significant | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoya, Z.J.; Mkindi, A.G.; Vanek, S.J.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal Plant Treatments Combined with Improved Soil Fertility Can Reduce Damage Caused by Fusarium Wilt (Fusarium oxysporum f.sp. phaseoli) and Bean Fly (Ophiomyia phaseoli) in Common Bean Production (Phaseolus vulgaris L.). Sustainability 2024, 16, 4866. https://doi.org/10.3390/su16114866
Ngoya ZJ, Mkindi AG, Vanek SJ, Stevenson PC, Ndakidemi PA, Belmain SR. Pesticidal Plant Treatments Combined with Improved Soil Fertility Can Reduce Damage Caused by Fusarium Wilt (Fusarium oxysporum f.sp. phaseoli) and Bean Fly (Ophiomyia phaseoli) in Common Bean Production (Phaseolus vulgaris L.). Sustainability. 2024; 16(11):4866. https://doi.org/10.3390/su16114866
Chicago/Turabian StyleNgoya, Zuwena J., Angela G. Mkindi, Steven J. Vanek, Philip C. Stevenson, Patrick A. Ndakidemi, and Steven R. Belmain. 2024. "Pesticidal Plant Treatments Combined with Improved Soil Fertility Can Reduce Damage Caused by Fusarium Wilt (Fusarium oxysporum f.sp. phaseoli) and Bean Fly (Ophiomyia phaseoli) in Common Bean Production (Phaseolus vulgaris L.)" Sustainability 16, no. 11: 4866. https://doi.org/10.3390/su16114866
APA StyleNgoya, Z. J., Mkindi, A. G., Vanek, S. J., Stevenson, P. C., Ndakidemi, P. A., & Belmain, S. R. (2024). Pesticidal Plant Treatments Combined with Improved Soil Fertility Can Reduce Damage Caused by Fusarium Wilt (Fusarium oxysporum f.sp. phaseoli) and Bean Fly (Ophiomyia phaseoli) in Common Bean Production (Phaseolus vulgaris L.). Sustainability, 16(11), 4866. https://doi.org/10.3390/su16114866








