Integrated Impacts of Soil Salinity and Drought Stresses on the Decomposition of Plant Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Preparation
2.2. Soil Incubation
2.3. Soil Respiration
2.4. Soil Microbial Biomass Carbon
2.5. Enzyme Assays
2.6. Statistical Analysis
3. Results
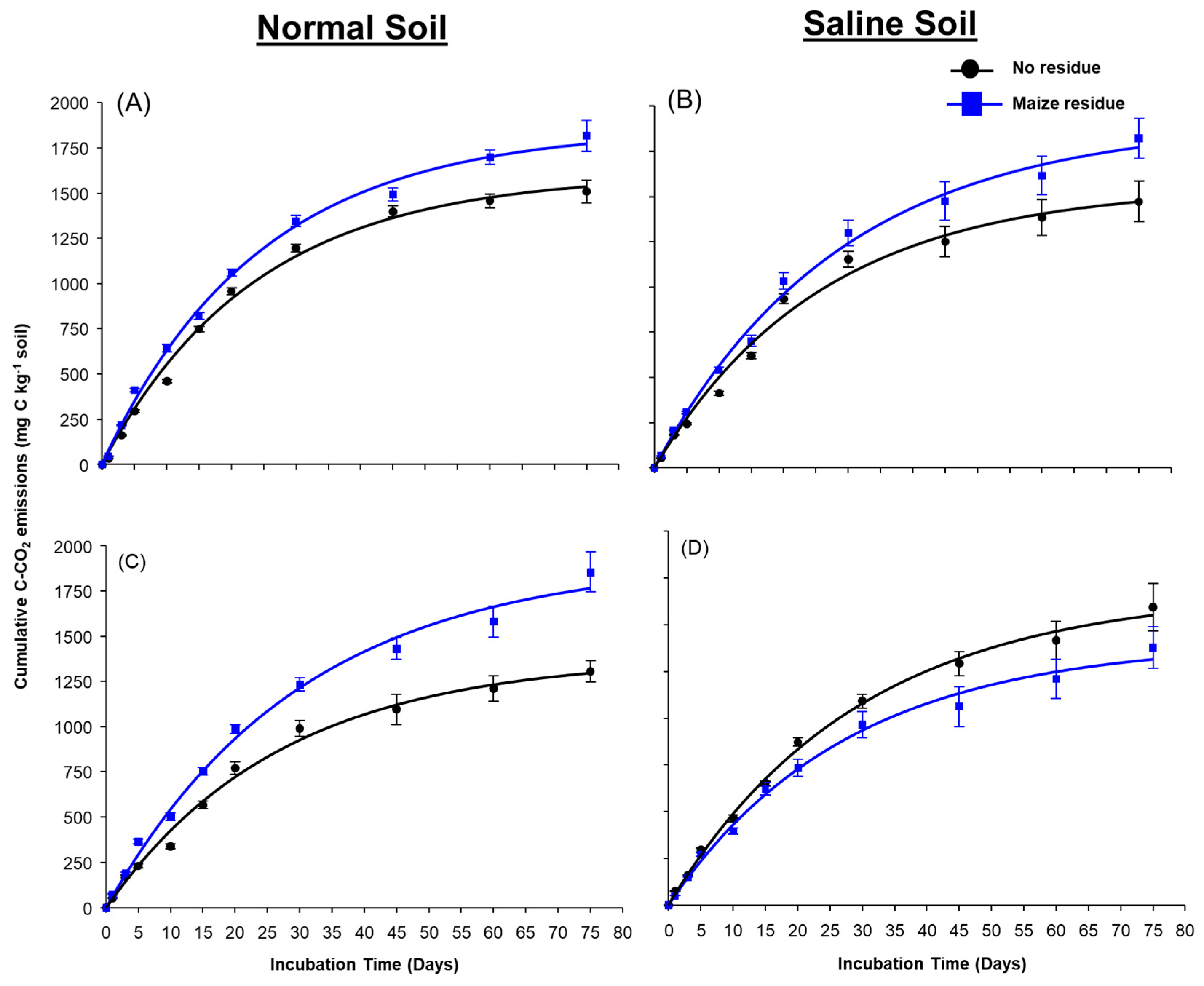
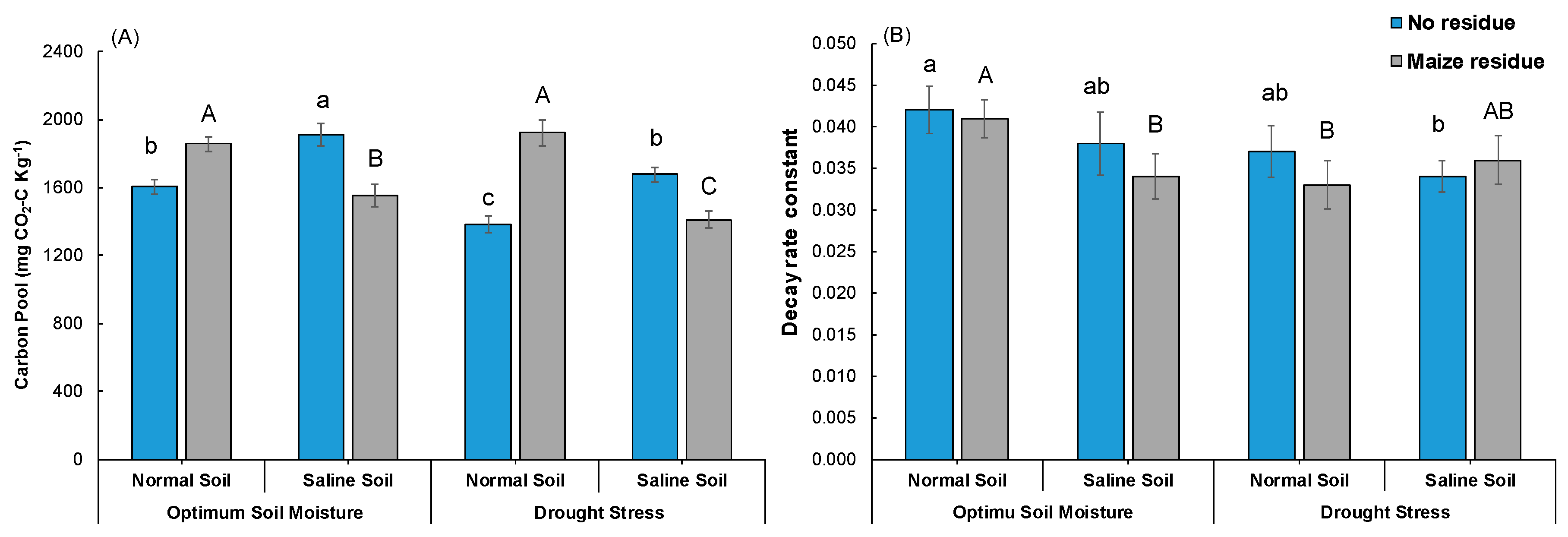
3.1. Soil Respiration
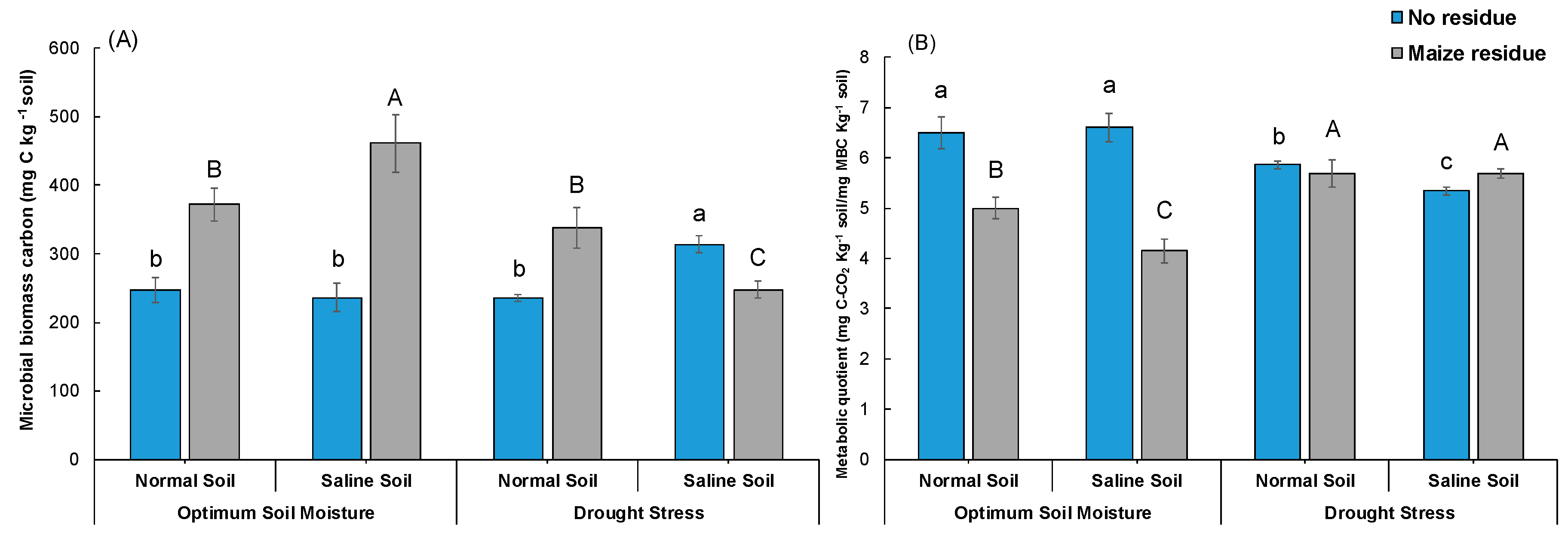
3.2. Soil Microbial Biomass Carbon and Metabolic Quotient
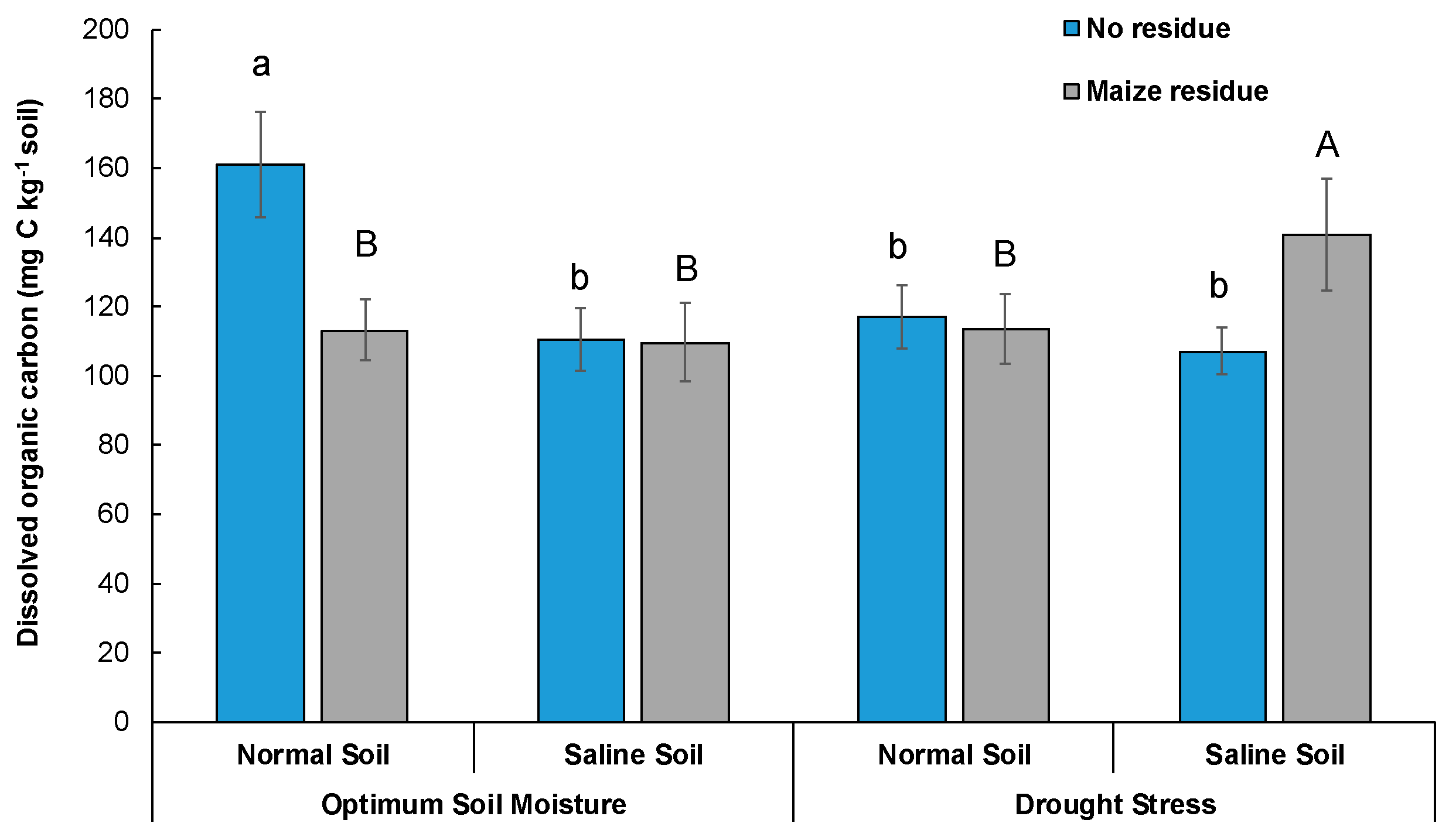
3.3. Dissolved Organic Carbon
3.4. Soil Extracellular Enzyme Activities
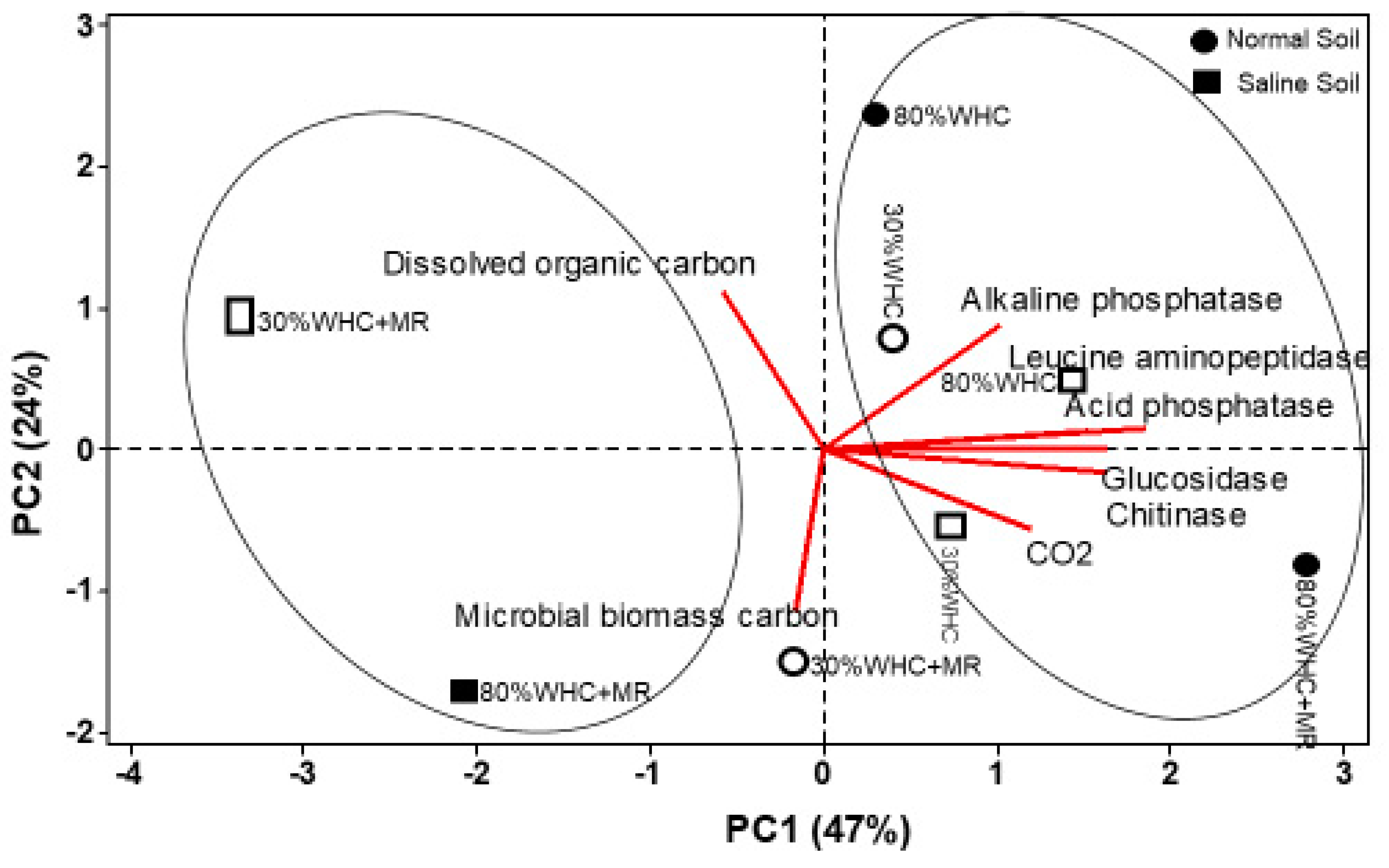
3.5. Principal Component Analysis
4. Discussion
4.1. Maize Residue Decomposition under Drought Stress
4.2. Maize Residue Decomposition under Salinity Stress
4.3. Maize Residue Decomposition under the Combined Stresses of Drought and Salinity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydin-Kandemir, F.; Erlat, E. Assessment of the relationship of the salt-covered area and the groundwater storage/drought indicators in the disappearing Lake Tuz in Turkey (1985–2021). Environ. Monit. Assess. 2023, 195, 333. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Pal, S.C.; Chakrabortty, R.; Saha, A.; Chowdhuri, I. A systematic review on climate change and geo-environmental factors induced land degradation: Processes, policy-practice gap and its management strategies. Geol. J. 2023, 58, 3487–3514. [Google Scholar] [CrossRef]
- FAOSTAT, Food and Agriculture Organization of the United Nations, Rome, Italy. 2020. Available online: http://faostat.fao.org/ (accessed on 24 June 2023).
- Röthig, T.; Trevathan-Tackett, S.M.; Voolstra, C.R.; Ross, C.; Chaffron, S.; Durack, P.J.; Warmuth, L.M.; Sweet, M. Human-induced salinity changes impact marine organisms and ecosystems. Glob. Chang. Biol. 2023, 29, 4731–4749. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Wu, Y.; Núñez-Delgado, A.; Kuzyakov, Y.; Peng, Q.-A.; Lin, S.; Hu, R. Enzyme activities and organic matter mineralization in response to application of gypsum, manure and rice straw in saline and sodic soils. Environ. Res. 2023, 224, 115393. [Google Scholar] [CrossRef] [PubMed]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Sharaya, R.; Gill, R.; Kalwan, G.; Naeem, M.; Tuteja, N.; Gill, S.S. Plant-microbe interaction mediated salinity stress tolerance for sustainable crop production. S. Afr. J. Bot. 2023, 161, 454–471. [Google Scholar] [CrossRef]
- Qadeer, A.; Wakeel, A.; Cheema, S.A.; Sanaullah, M. Interactive effects of salinity and drought stresses on soil respiration and microbial activities. Pak. J. Agric. Sci. 2022, 59, 35–42. [Google Scholar]
- Hui, D.; Deng, Q.; Tian, H.; Luo, Y. Global climate change and greenhouse gases emissions in terrestrial ecosystems. In Handbook of Climate Change Mitigation and Adaptation; Springer: Cham, Switzerland, 2022; pp. 23–76. [Google Scholar]
- Sandhu, O.S.; Jat, M.L.; Gupta, R.K.; Thind, H.S.; Sidhu, H.S.; Singh, Y. Influence of residue type and method of placement on dynamics of decomposition and nitrogen release in maize-wheat-mungbean cropping on permanent raised beds: A litterbag study. Sustainability 2022, 14, 864. [Google Scholar] [CrossRef]
- Yemadje, P.L.; Chevallier, T.; Guibert, H.; Bertrand, I.; Bernoux, M. Wetting-drying cycles do not increase organic carbon and nitrogen mineralization in soils with straw amendment. Geoderma 2017, 304, 68–75. [Google Scholar] [CrossRef]
- Bogati, K.; Sewerniak, P.; Walczak, M. Effect of changes in soil moisture on agriculture soils: Response of microbial community, enzymatic and physiological diversity. Ecol. Quest. 2023, 34, 1–33. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Wilson, G.W.; Guo, Y.; Bi, Y.; Xiong, X.; Liu, N. Transformation of litter carbon to stable soil organic matter is facilitated by ungulate trampling. Geoderma 2021, 385, 114828. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.; Simpson, M.J. Plant-or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Liang, A.; Duan, Y.; Chen, H.; Yu, Z.; Fan, R.; Liu, H.; Pan, H. Chemical Composition of Plant Residues Regulates Soil Organic Carbon Turnover in Typical Soils with Contrasting Textures in Northeast China Plain. Agronomy 2022, 12, 747. [Google Scholar] [CrossRef]
- Surey, R.; Schimpf, C.M.; Sauheitl, L.; Mueller, C.W.; Rummel, P.S.; Dittert, K.; Kaiser, K.; Böttcher, J.; Mikutta, R. Potential denitrification stimulated by water-soluble organic carbon from plant residues during initial decomposition. Soil Biol. Biochem. 2020, 147, 107841. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agronomy 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Tome Jr, J.; Dechen, A.R. Microwave oven-drying of soil samples for chemical testing in Brazil. Commun. Soil Sci. Plant Anal. 1995, 26, 515–529. [Google Scholar] [CrossRef]
- Allison, L.; Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. No.60; Soil and Water Conservative Research Branch, Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1954; pp. 8–12.
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils-effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Haider, G.; Ghafoor, A. Planning of experiments. In Manual of Salinity Research Methods; International Water Logging and Salinity Research Institute: Lahore, Pakistan, 1992; pp. 1–9. [Google Scholar]
- Soest, P.V. Use of detergents in the analysis of fibrous feeds. I. Preparation of fiber residues of low nitrogen content. J. Assoc. Off. Anal. Chem. 1963, 46, 825–829. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Cheng, W. Photosynthesis controls of rhizosphere respiration and organic matter decomposition. Soil Biol. Biochem. 2001, 33, 1915–1925. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.; Pommerening, B.; Chaussod, R.; Brookes, P. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Wardle, D.; Ghani, A. A critique of the microbial metabolic quotient (qCO2) as a bioindicator of disturbance and ecosystem development. Soil Biol. Biochem. 1995, 27, 1601–1610. [Google Scholar]
- Sanaullah, M.; Blagodatskaya, E.; Chabbi, A.; Rumpel, C.; Kuzyakov, Y. Drought effects on microbial biomass and enzyme activities in the rhizosphere of grasses depend on plant community composition. Appl. Soil Ecol. 2011, 48, 38–44. [Google Scholar] [CrossRef]
- Pritsch, K.; Raidl, S.; Marksteiner, E.; Blaschke, H.; Agerer, R.; Schloter, M.; Hartmann, A. A rapid and highly sensitive method for measuring enzyme activities in single mycorrhizal tips using 4-methylumbelliferone-labelled fluorogenic substrates in a microplate system. J. Microbiol. Methods. 2004, 58, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Salmasnia, A.; Baradaran Kazemzadeh, R.; Niaki, S.T.A. An approach to optimize correlated multiple responses using principal component analysis and desirability function. Int. J. Adv. Manuf. Technol. 2012, 62, 835–846. [Google Scholar] [CrossRef]
- Liao, Z.; Fan, J.; Lai, Z.; Bai, Z.; Wang, H.; Cheng, M.; Zhang, F.; Li, Z. Response network and regulatory measures of plant-soil-rhizosphere environment to drought stress. Adv. Agron. 2023, 180, 94–129. [Google Scholar]
- Bokade, P.; Gaur, V.K.; Tripathi, V.; Bobate, S.; Manickam, N.; Bajaj, A. Bacterial remediation of pesticide polluted soils: Exploring the feasibility of site restoration. J. Hazard. Mater. 2023, 441, 129906. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Chen, L.; Li, F.; Zhou, G.; Zhang, C.; Ma, D.; Zhang, J. Tradeoffs of microbial life history strategies drive the turnover of microbial-derived organic carbon in coastal saline soils. Front. Microbiol. 2023, 14, 1141436. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, H.; Wang, Z.; Shi, J.; Lv, J.; Wang, X. Responses of the content and spectral characteristics of dissolved organic matter in intercropping soil to drought in northeast China. Plant Soil 2023, 1–15. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, B.; Jiao, Y.; Liu, C.; Li, X.; Meng, X.; Shi, J.; Tian, X. Effect of the combined addition of mineral nitrogen and crop residue on soil respiration, organic carbon sequestration, and exogenous nitrogen in stable organic matter. Appl. Soil Ecol. 2022, 171, 104324. [Google Scholar] [CrossRef]
- Santos, S.R.; Schellekens, J.; Buurman, P.; Cornelis, J.-T.; Vancampenhout, K.; da Silva, W.T.L.; de Camargo, P.B.; Vidal-Torrado, P. Selective sorption and desorption of DOM in Podzol horizons-DOC and aluminium contents of leachates from a column experiment. Sci. Total Environ. 2023, 872, 162234. [Google Scholar] [CrossRef]
- Zuccarini, P.; Sardans, J.; Asensio, L.; Peñuelas, J. Altered activities of extracellular soil enzymes by the interacting global environmental changes. Glob. Chang. Biol. 2023, 29, 2067–2091. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Maiti, S.K. Different soil factors influencing dehydrogenase activity in mine degraded lands-state-of-art review. Water Air Soil Pollut. 2021, 232, 360. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.; Wu, X.; Zheng, F.; Song, X.; Lu, J.; Liu, X.; Wang, B.; Abdelrhmana, A.A.; Degré, A. Nitrogen addition mediates the effect of soil microbial diversity on microbial carbon use efficiency under long-term tillage practices. Land Degrad. Dev. 2022, 33, 2258–2275. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhai, Y.; Jia, J.; Zhao, Q.; Wang, W.; Hu, X. Microbial diversity and functions in saline soils: A review from a biogeochemical perspective. J. Adv. Res. 2023; in press. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Q.; Zhang, H.; Zhang, J.; Chen, Y.; Yao, F.; Chen, Y. Effects of exogenous organic matter addition on agricultural soil microbial communities and relevant enzyme activities in southern China. Sci. Rep. 2023, 13, 8045. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Arif, M.S.; Shahzad, S.M.; Yasmeen, T.; Shakoor, A.; Iqbal, S.; Riaz, A.; Zahid, A.; Chapman, S.J. Long-term raw crop residue but not burned residue incorporation improved soil multifunctionality in semi-arid agroecosystems. Soil Tillage Res. 2024, 240, 106073. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sharma, S.; Nisar, S.; Choudhary, O.P. Structural stability and organic matter stabilization in soils: Differential impacts of soil salinity and sodicity. J. Soil Sci. Plant Nutr. 2023, 23, 1751–1773. [Google Scholar] [CrossRef]
- Wichern, F.; Islam, M.R.; Hemkemeyer, M.; Watson, C.; Joergensen, R.G. Organic amendments alleviate salinity effects on soil microorganisms and mineralisation processes in aerobic and anaerobic paddy rice soils. Front. Sustain. Food Syst. 2020, 4, 30. [Google Scholar] [CrossRef]
- Cao, H.; Ding, R.; Kang, S.; Du, T.; Tong, L.; Zhang, Y.; Chen, J.; Shukla, M.K. Drought, salt, and combined stresses in plants: Effects, tolerance mechanisms, and strategies. Adv. Agron. 2023, 178, 107–163. [Google Scholar]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and arid soils: Impact on bacteria, plants, and their interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadeer, A.; Wakeel, A.; Cheema, S.A.; Shahzad, T.; Sanaullah, M. Integrated Impacts of Soil Salinity and Drought Stresses on the Decomposition of Plant Residues. Sustainability 2024, 16, 5368. https://doi.org/10.3390/su16135368
Qadeer A, Wakeel A, Cheema SA, Shahzad T, Sanaullah M. Integrated Impacts of Soil Salinity and Drought Stresses on the Decomposition of Plant Residues. Sustainability. 2024; 16(13):5368. https://doi.org/10.3390/su16135368
Chicago/Turabian StyleQadeer, Abdul, Abdul Wakeel, Sardar Alam Cheema, Tanvir Shahzad, and Muhammad Sanaullah. 2024. "Integrated Impacts of Soil Salinity and Drought Stresses on the Decomposition of Plant Residues" Sustainability 16, no. 13: 5368. https://doi.org/10.3390/su16135368
APA StyleQadeer, A., Wakeel, A., Cheema, S. A., Shahzad, T., & Sanaullah, M. (2024). Integrated Impacts of Soil Salinity and Drought Stresses on the Decomposition of Plant Residues. Sustainability, 16(13), 5368. https://doi.org/10.3390/su16135368





