Effects of Pyrolysis Temperature on Biochar Physicochemical and Microbial Properties for H2S Removal from Biogas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Completion of the Sewage Sludge Pyrolysis Process
2.2. The Determination of the Significant Physicochemical Properties of the Biofilter Packing Material
2.3. The Preparation for Biofiltration Processes
3. Results and Discussion
3.1. The Results of the Physicochemical Properties of Pyrolyzed Biochar
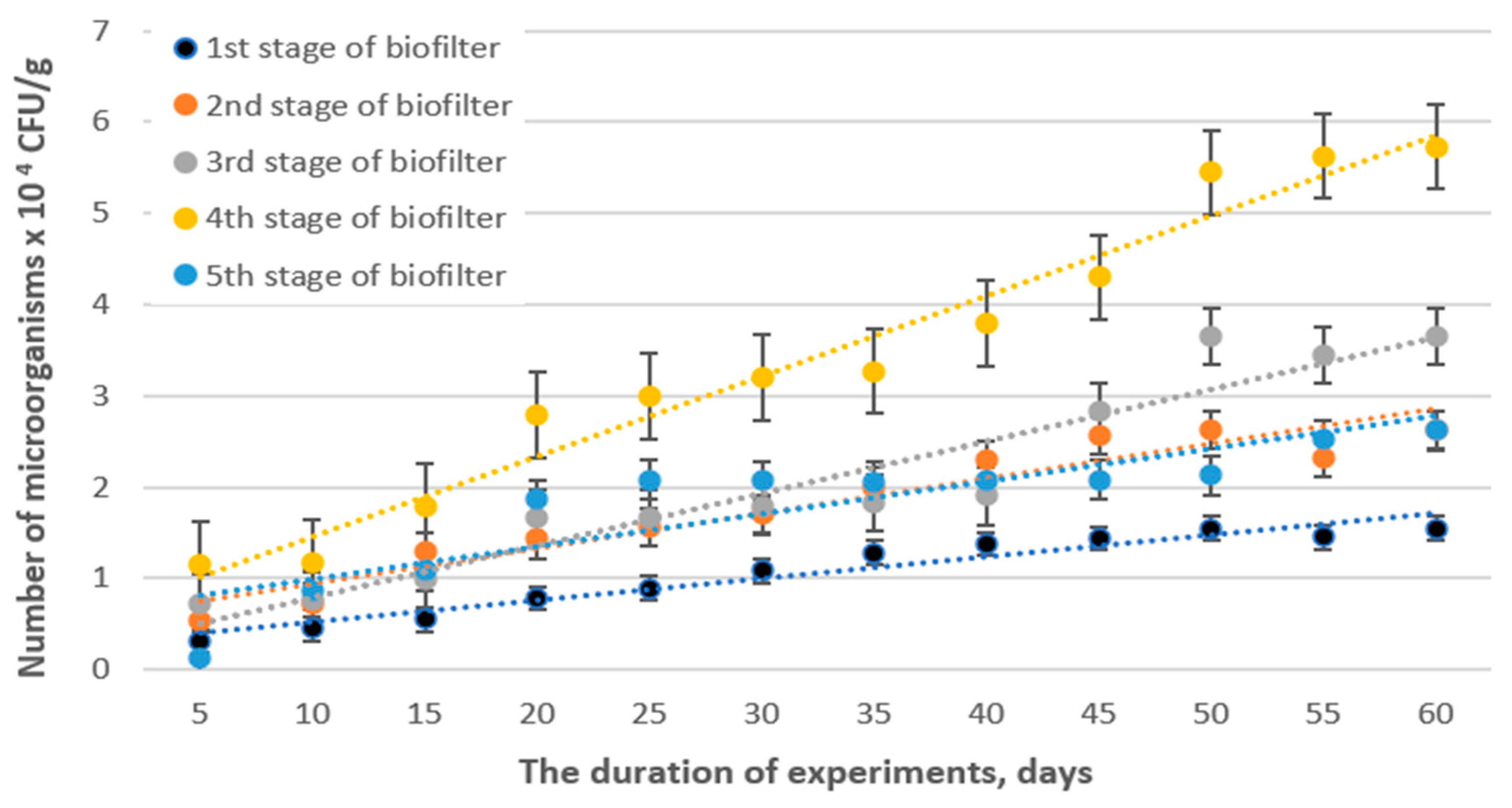
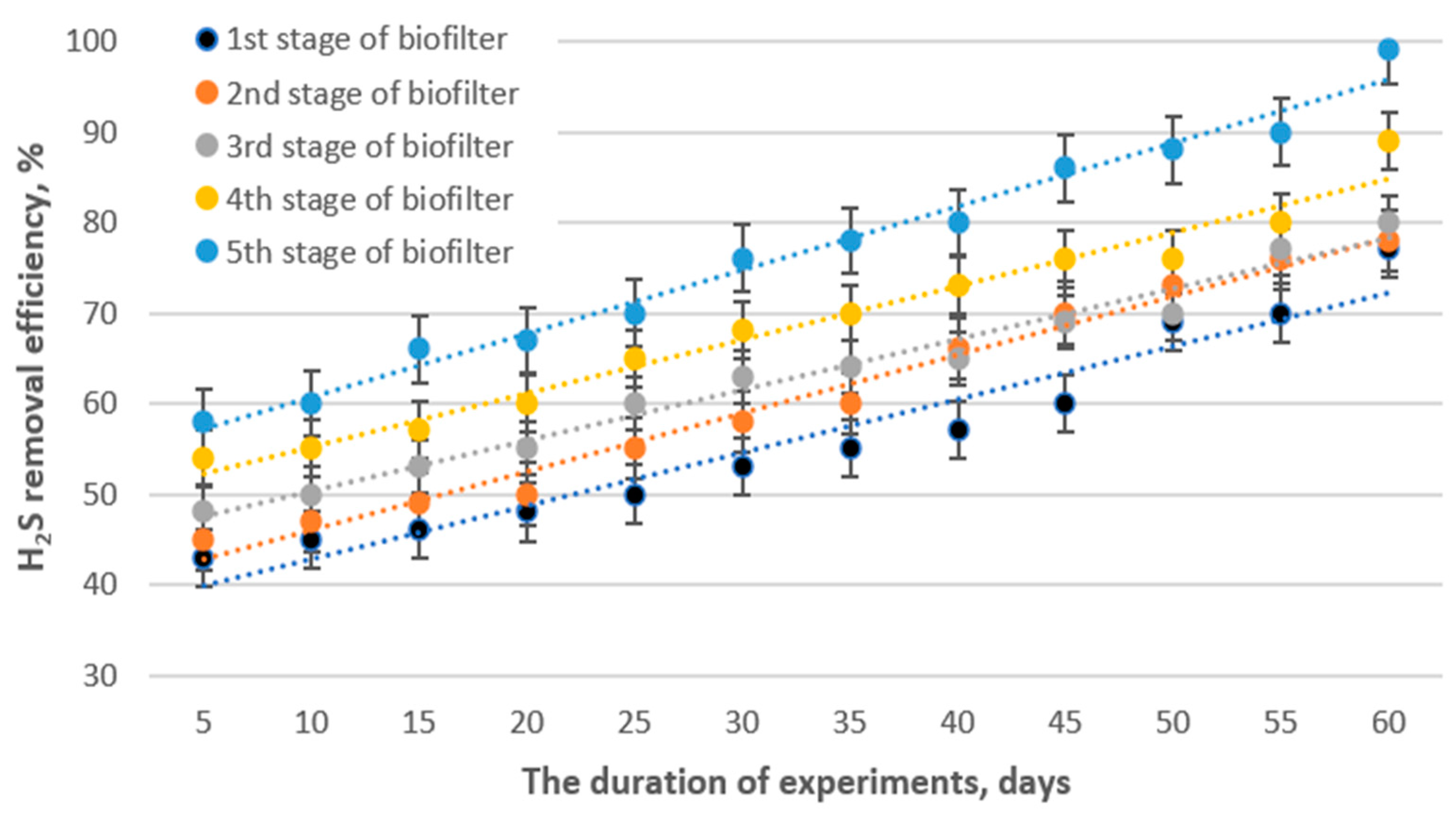
3.2. Biofiltration Performance and Kinetic Study of Hydrogen Sulfide Removal
- Negligible turbulence: Large turbulence is assumed to be negligible based on experimental data indicating a laminar flow pattern (with a Reynolds number between 0.2 and 0.5 and 0.5 for full-scale operations) in typical biofilters;
- Homogeneous filter materials: The composition of the filter material, including porosity and water content, is assumed to be homogeneous;
- Zero initial H2S concentration: It is assumed that the initial concentration of hydrogen sulfide (H2S) in the biofilter is zero;
- Stoichiometric hydrogen sulfide production is assumed to follow a stoichiometric relationship, as described by the following equation: S + NaOH + Al + HCl → 3H2S + Al2O3;
- Biomass distribution and density within the biofilter are assumed to be homogeneous.
3.3. Microbiological Studies of the Packing Material and Removal Efficiency of Hydrogen Sulfide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagheri, M.; Torben Bauer, T.; Ekman Burgman, L.; Wetterlund, E. Fifty years of sewage sludge management research: Mapping researchers’ motivations and concerns. J. Environ. Manag. 2023, 325, 116412. [Google Scholar] [CrossRef] [PubMed]
- Kirchmann, H.; Börjesson, G.; Kätterer, T.; Cohen, Y. From agricultural use of sewage sludge to nutrient extraction: A soil science outlook. Ambio 2017, 46, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Styszko, K.; Durak, J.; Kończak, B.; Głodniok, M.; Borgulat, A. The impact of sewage sludge processing on the safety of its use. Sci. Rep. 2022, 12, 12227. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Gyawali, R.; Lens, P.N.L.; Lohani, S.P. Chapter 11—Technologies for removal of hydrogen sulfide (H2S) from biogas. In Emerging Technologies and Biological Systems for Biogas Upgrading; Aryal, N., Ottosen, L.D.M., Kofoed, M.V.W., Pant, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 295–320. [Google Scholar] [CrossRef]
- Kulawong, S.; Artkla, R.; Sriprapakhan, P.; Maneechot, P. Biogas purification by adsorption of hydrogen sulfide on NaX and Ag-exchanged NaX zeolites. Biomass Bioenergy 2022, 159, 106417. [Google Scholar] [CrossRef]
- Nhut, H.H.; Thanh, V.T.; Le, L.T. Removal of H2S in biogas using biotrickling filter: Recent development. Process Saf. Environ. Prot. 2020, 144, 297–309. [Google Scholar] [CrossRef]
- Cortés, J.J.G.; Almenglo, F.; Ramírez, M.; Cantero, D. Simultaneous removal of ammonium from landfill leachate and hydrogen sulfide from biogas using a novel two-stage oxic-anoxic system. Sci. Total Environ. 2021, 750, 141664. [Google Scholar] [CrossRef] [PubMed]
- Danila, V.; Zagorskis, A.; Januševičius, T. Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review. Process 2022, 10, 1304. [Google Scholar] [CrossRef]
- Khan, M.U.; EnLee, J.T.; Bashir, M.A.; Dissanayake, P.D.; WahTong, Y.S.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Mohammadi, K.; Vaiskunaite, R. Analysis and evaluation of the biogas purification technologies from H2S. Environ. Prot. Technol. Manag. VGTU 2023, 15, 1–9. [Google Scholar] [CrossRef]
- Gaga, Y.; Benmessaoud, S.; Kara, M.; Assouguem, A.; Al-Ghamdi, A.A.; Al-Hemaid, F.M.; Elshikh, M.S.; Ullah, R.; Banach, A.; Bahhou, J. New Margin-Based Biochar for Removing Hydrogen Sulfide Generated during the Anaerobic Wastewater Treatment. Water 2022, 14, 3319. [Google Scholar] [CrossRef]
- Haosagul, S.; Prommeenate, P.; Hobbs, G.; Pisutpaisal, N. Sulfur-oxidizing bacteria in full-scale biogas cleanup system of ethanol industry. Renew. Energy 2020, 150, 965–972. [Google Scholar] [CrossRef]
- Juntarachat, N.; Onthong, U. Removal of hydrogen sulfide from biogas using banana peel and banana empty fruit bunch biochars as alternative adsorbents. Biomass Convers. Biorefinery 2022, 26, 1–2. Available online: https://link.springer.com/article/10.1007/s13399-022-03430-z (accessed on 21 May 2024). [CrossRef]
- Choudhury, A.; Lansing, S. Adsorption of hydrogen sulfide in biogas using a novel iron-impregnated biochar scrubbing system. J. Environ. Chem. Eng. 2021, 9, 104837. [Google Scholar] [CrossRef]
- Mitchell, K.; Beesley, L.; Šípek, V.; Trakal, L. Chapter 3—Biochar and its potential to increase water, trace element, and nutrient retention in soils. In Biochar in Agriculture for Achieving Sustainable Development Goals; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Paulionyte, J.; Vaiskunaite, R.; Mazeikiene, A. Evaluation of sewage sludge biochar use in wastewater treatment from phosphate. In Proceedings of the 25th Conference for Junior Researchers “Science—Future of Lithuania”, Vilnius, Lithuania, 18 March 2022; pp. 35–39. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, X.; Jin, Z.; Yang, S.; Zhang, J. The performance and microbial community in a slightly alkaline biotrickling filter for the removal of high concentration H2S from biogas. Chemosphere 2020, 249, 126127. [Google Scholar] [CrossRef] [PubMed]
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage sludge for sustainable agriculture: Contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Agric. 2018, 5, 10. [Google Scholar] [CrossRef]
- Sugurbekova, G.; Nagyzbekkyzy, E.; Sarsenova, A.; Danlybayeva, G.; Anuarbekova, S.; Kudaibergenova, R.; Frochot, C.; Acherar, S.; Zhatkanbayev, Y.; Moldagulova, N. Sewage Sludge Management and Application in the Form of Sustainable Fertilizer. Sustainability 2023, 15, 6112. [Google Scholar] [CrossRef]
- Ćwiertniewicz-Wojciechowska, M.; Cema, G.; Ziembińska-Buczyńska, A. Sewage sludge pretreatment: Current status and future prospects. Environ. Sci. Pollut. Res. 2023, 30, 88313–88330. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, S.; Xu, S.; Johnston, C.; Wu, C. Phosphorus Removal from Dirty Farmyard Water by Activated Anaerobic-352 Digestion-Derived Biochar. Ind. Eng. Chem. Res. 2022, 62, 19216–19224. [Google Scholar] [CrossRef] [PubMed]
- Januševičius, T.; Mažeikienė, A.; Stepova, K.; Danila, V.; Paliulis, D. The Removal of Phosphorus from Wastewater Using a Sewage Sludge Biochar: A Column Study. Water 2024, 16, 1104. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef]
- Cao, B.; Jiang, D.; Zheng, Y.; Rupani, P.F.; Yuan, C.; Hu, Y.; Chen, H.; Li, C.; Hu, X.; Wang, S.; et al. Evaluation of biochar-derived carbocatalysts for pyrolytic conversion of sawdust: Life cycle assessment towards monophenol production. Fuel 2022, 330, 125476. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, J.; Zhang, Y.; He, S.; Esakkimuthu, S.; Zhu, K.; Kumar, S.; Lv, G.; Hu, X. Biochar assisted cultivation of Chlorella protothecoides for adsorption of tetracycline and electrochemical study on self-cultured Chlorella protothecoides. Bioresour Technol. 2023, 389, 129810. [Google Scholar] [CrossRef] [PubMed]
- Watsuntorn, W.; Khanongnuch, R.; Chulalaksananuku, W.; Rene, E.R.; Lens, P.N.L. Resilient performance of an anoxic biotrickling filter for hydrogen sulfide removal from a biogas mimic: Steady, transient state and neural network evaluation. J. Clean. Prod. 2020, 249, 119351. [Google Scholar] [CrossRef]
- De Souza, F.M.; Kahol, P.K.; Gupta, K.R. Introduction to Polyurethane Chemistry. In American Chemical Society; ACS Publications: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Lee, J.T.E.; Ok, Y.S.; Song, S.; Dissanayake, P.D.; Tian, H.; Tio, Z.K.; Cui, R.; Lim, E.Y.; Jong, M.C.; Hoy, S.H.; et al. Biochar utilisation in the anaerobic digestion of food waste for the creation of a circular economy via biogas upgrading and digestate treatment. Bioresour. Technol. 2021, 333, 125190. [Google Scholar] [CrossRef] [PubMed]
- Jedynak, K.; Charmas, B. Adsorption properties of biochars obtained by KOH activation. Adsorption 2023, 30, 167–183. [Google Scholar] [CrossRef]
- Vaiskunaite, R. Cleaning of H2S from polluted air using peat biofilter. Environ. Prot. Technol. Manag. VGTU 2020, 12, 1–5. [Google Scholar] [CrossRef]
- Das, J.; Ravishankar, H.; Lens, P.N.L. Biological biogas purification: Recent developments, challenges and future prospects. J. Environ. Manag. 2022, 304, 114198. [Google Scholar] [CrossRef] [PubMed]
- ASTM D6683-19; Standard Practice for Application of Thermoluminescence-Dosimetry (TLD) Systems for Determining Absorbed Dose in Radiation-Hardness Testing of Electronic Devices. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D1293-18; Standard Test Methods for pH of Water. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D1125-23; Standard Test Methods for Electrical Conductivity and Resistivity of Water. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM-D 5231-92; Standard Test Method for Determination of the Composition of Unprocessed Municipal Solid Waste. ASTM International: West Conshohocken, PA, USA, 2003.
- ASTM D2867-99; Standard Test Methods for Moisture in Activated Carbon. ASTM International: West Conshohocken, PA, USA, 1999.
- Altıkat, A.; Alma, M.H.; Altıkat, A.; Bilgili, M.E.; Altıkat, S. A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability 2024, 16, 937. [Google Scholar] [CrossRef]
- ASTM C1069-09; Standard Test Method for Specific Surface Area of Alumina or Quartz by Nitrogen Adsorption. ASTM International: West Conshohocken, PA, USA, 2022.
- Florent, M.; Policicchio, A.; Niewiadomski, S.; Bandosz, T.J. Exploring the options for the improvement of H2S adsorption on sludge derived adsorbents: Building the composite with porous carbons. J. Clean. Prod. 2020, 249, 119412. [Google Scholar] [CrossRef]
- Alkhatib, I.I.I.; Khalifa, O.; Bahamon, D.; Abu-Zahra, M.R.M.; Vega, L.F. Sustainability criteria as a game changer in the search for hybrid solvents for CO2 and H2S removal. Sep. Purif. Technol. 2021, 277, 119516. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, J.; Yin, L.; Liu, H.; Zuo, W.; Tian, Y. Relationship between heavy metalconsolidation and H2S removal by biochar from microwave pyrolysis of municipal sludge: Effect and mechanism. Environ. Sci. Pollut. Res. 2021, 28, 27694–27702. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhao, Y.; Chen, H.; Liu, Y.; Huang, R.; Pan, J. Biochars derived from by-products of microalgae pyrolysis for sorption of gaseous H2S. J. Environ. Chem. Eng. 2022, 10, 107370. [Google Scholar] [CrossRef]
- Yi, K.; Liu, H.; Wang, J.; Lu, G.; Jin, M.; Hu, H.; Yao, H. The adsorption and transformation of SO2, H2S and NH3 by using sludge gasification ash: Effects of Fenton oxidation and CaO pre-conditioning. Chem. Eng. J. 2019, 360, 1498–1508. [Google Scholar] [CrossRef]
- Torres, R.A.; Marín, D.; Rodero, M.D.R.; Pascual, C.; Sanchez, A.G.; Crespo, I.G.; Lebrero, R.; Torre, R.M. Biogas treatment for H2S, CO2, and other contaminants removal. From Biofiltration to promising Options in Gaseous Fluxes Biotreament. In Biofiltration to Promising Options in Gaseous Fluxes Biotreatment; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Su, J.J.; Hong, Y.Y. Removal of hydrogen sulfide using a photocatalytic livestock biogas desulfurizer. Renew. Energy 2020, 149, 181–188. [Google Scholar] [CrossRef]
- Shang, G.; Liu, L.; Chen, P.; Shen, G.; Li, Q. Kinetics and the mass transfer mechanism of hydrogen sulfide removal by biochar derived from rice hull. J. Air Waste Manag. Assoc. 2016, 66, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Appala, V.N.S.G.; Pandhare, N.N.; Bajpai, S. Mathematical Models for Optimization of Anaerobic Digestion and Biogas Production. In Zero Waste Biorifinery; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Bu, H.; Carvalho, G.; Huang, C.; Sharma, K.R.; Yuan, Z.; Song, Y.; Bond, P.; Keller, J.; Yu, M.; Jiang, G. Evaluation of continuous and intermittent trickling strategies for the removal of hydrogen sulfide in a biotrickling filter. Chemosphere 2021, 291, 132723. [Google Scholar] [CrossRef] [PubMed]
- Clotas, E.S.; Codony, A.C.; Comas, J.; Martín, M.J. Biogas purification through membrane bioreactors: Experimental study on siloxane separation and biodegradation. Sep. Purif. Technol. 2020, 238, 116440. [Google Scholar] [CrossRef]
- Cano, P.I.; Almenglo, F.; Ramírez, M.; Cantero, D. Integration of a nitrification bioreactor and an anoxic biotrickling filter for simultaneous ammonium-rich water treatment and biogas desulfurization. Chemosphere 2021, 284, 131358. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, J.; Jin, Z.; Yang, S.; Shen, L. Enhancing the removal of H2S from biogas through refluxing of outlet gas in biological bubble-column. Bioresour. Technol. 2020, 299, 122621. [Google Scholar] [CrossRef]
- Konkol, D.; Popiela, E.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Moustakas, K.; Opaliński, S.; Korczyński, M.; Krowiak, A.W.; Chojnacka, K. Recent innovations in various methods of harmful gases conversion and its mechanism in poultry farms. Environ. Res. 2022, 214, 113825. [Google Scholar] [CrossRef]
- Pudi, A.; Rezaei, M.; Signorini, V.; Andersson, M.P.; Baschetti, M.G.; Mansouri, S.S. Hydrogen sulfide capture and removal technologies: A comprehensive review of recent developments and emerging trends. Sep. Purif. Technol. 2022, 298, 121448. [Google Scholar] [CrossRef]
- Poser, M.; Silva, L.R.D.E.; Peu, P.; Couvert, A.; Dumont, E. Cellular concrete waste: An efficient new way for H2S removal. Sep. Purif. Technol. 2023, 309, 123014. [Google Scholar] [CrossRef]
- Shi, M.; Xiong, W.; Zhang, X.; Ji, J.; Hu, X.; Tu, Z.; Wu, Y. Highly efficient and selective H2S capture by task-specific deep eutectic solvents through chemical dual-site absorption. Sep. Purif. Technol. 2022, 283, 120167. [Google Scholar] [CrossRef]
- Wang, S.; Nam, H.; Lee, D.; Nam, H. H2S gas adsorption study using copper impregnated on KOH activated carbon from coffee residue for indoor air purification. J. Environ. Chem. Eng. 2022, 10, 108797. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Ma, C.; Qiao, W.; Wang, J.; Ling, L. Functionalization of activated carbon fiber mat with bimetallic active sites for NH3 and H2S adsorption at room temperature. Sep. Purif. Technol. 2022, 303, 122335. [Google Scholar] [CrossRef]
- Xia, G.; Zhou, X.; Hu, J.; Sun, Z.; Yao, J.; Chen, D.; Wang, J. Simultaneous removal of carbon disulfide and hydrogen sulfide from viscose fibre waste gas with a biotrickling filter in pilot scale. J. Clean. Prod. 2019, 230, 21–28. [Google Scholar] [CrossRef]
- Ying, S.; Kong, X.; Cai, Z.; Man, Z.; Xin, Y.; Liu, D. Interactions and microbial variations in a biotrickling filter treating low concentrations of hydrogen sulfide and ammonia. Chemosphere 2020, 255, 126931. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Papurello, D.; Gandiglio, M.; Lanzini, A.; Akpinar, I.; Shearing, P.R.; Manos, G.; Brett, D.J.L.; Zhang, Y.S. Study of H2S Removal Capability from Simulated Biogas by Using Waste-Derived Adsorbent Materials. Processes 2020, 8, 1030. [Google Scholar] [CrossRef]
- Zhang, X.; Lawan, I.; Danhassan, U.A.; He, Y.; Qi, R.; Wu, A.; Sheng, K.; Lin, H. Advances in technologies for in situ desulfurization of biogas. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Kawasaki, Y.; Oshita, K.; Takaoka, M.; Minami, D.; Inoue, G.; Tanaka, T. Economic assessment of biogas purification systems for removal of both H2S and siloxane from biogas. Renew. Energy 2021, 168, 119–130. [Google Scholar] [CrossRef]
- Zhanga, Y.; Oshitaa, K.; Kusakabea, T.; Takaokaa, M.; Kawasakib, Y.; Minamib, D.; Tanaka, T. Simultaneous removal of siloxanes and H2S from biogas using an aerobic biotrickling filter. J. Hazard. Mater. 2020, 391, 122187. [Google Scholar] [CrossRef]
- Choleva, E.; Mitsopoulos, A.; Dimitropoulou, G.; Romanos, G.E.; Kouvelos, E.; Pilatos, G.; Beltsios, K.; Stefanidis, S.; Lappas, A.; Sfetsas, T. Adsorption of Hydrogen Sulfide on Activated Carbon Materials Derived from the Solid Fibrous Digestate. Materials 2023, 16, 5119. [Google Scholar] [CrossRef] [PubMed]


| Type of Method | X-ray Fluorescence | Kjeldahl | Walkley–Black | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of elements | SiO2 | CaO | Al2O3 | P2O5 | Fe2O3 | K2O | MgO | S | N | C |
| Sewage sludge | 18.93% | 11.83% | 3.51% | 9.59% | 4.38% | 1.39% | 2.34% | 0.96% | 4.2% | 30.83% |
| Biochar after 400 °C | 25.67% | 17.45% | 5.23% | 13.43% | 6.38% | 1.78% | 3.39% | 0.78% | 2.1% | 24.17% |
| Biochar after 500 °C | 29.82% | 15.47% | 5.14% | 13.78% | 6.67% | 1.70% | 3.39% | 0.85% | 2.8% | 19.6% |
| Biochar after 600 °C | 30.27% | 15.80% | 5.39% | 14.38% | 6.6% | 1.70% | 3.50% | 0.81% | 2.1% | 15.41% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaiškūnaitė, R.; Mažeikienė, A.; Mohammadi, K. Effects of Pyrolysis Temperature on Biochar Physicochemical and Microbial Properties for H2S Removal from Biogas. Sustainability 2024, 16, 5424. https://doi.org/10.3390/su16135424
Vaiškūnaitė R, Mažeikienė A, Mohammadi K. Effects of Pyrolysis Temperature on Biochar Physicochemical and Microbial Properties for H2S Removal from Biogas. Sustainability. 2024; 16(13):5424. https://doi.org/10.3390/su16135424
Chicago/Turabian StyleVaiškūnaitė, Rasa, Aušra Mažeikienė, and Kamyab Mohammadi. 2024. "Effects of Pyrolysis Temperature on Biochar Physicochemical and Microbial Properties for H2S Removal from Biogas" Sustainability 16, no. 13: 5424. https://doi.org/10.3390/su16135424





