Improvement and Stability of Soil Organic Carbon: The Effect of Earthworm Mucus Organo-Mineral Associations with Montmorillonite and Hematite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Composition Characterization of EM
2.3. EM–Mineral Sorption Experiment
2.4. The Oxidation Resistance of EM–Mineral Associations
3. Results and Discussion
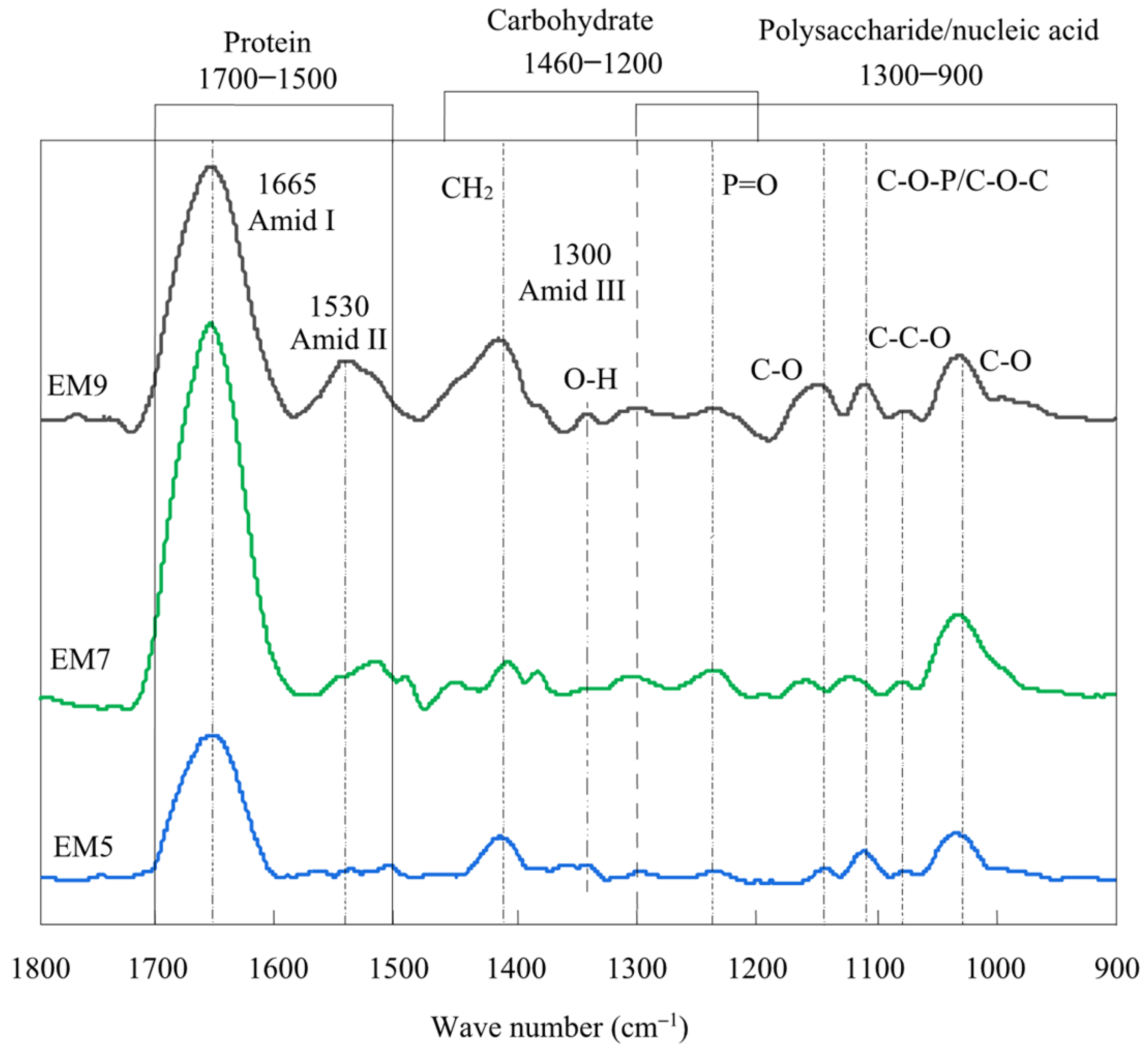
3.1. Characterization of Various pH Stimuli-Responsive EM
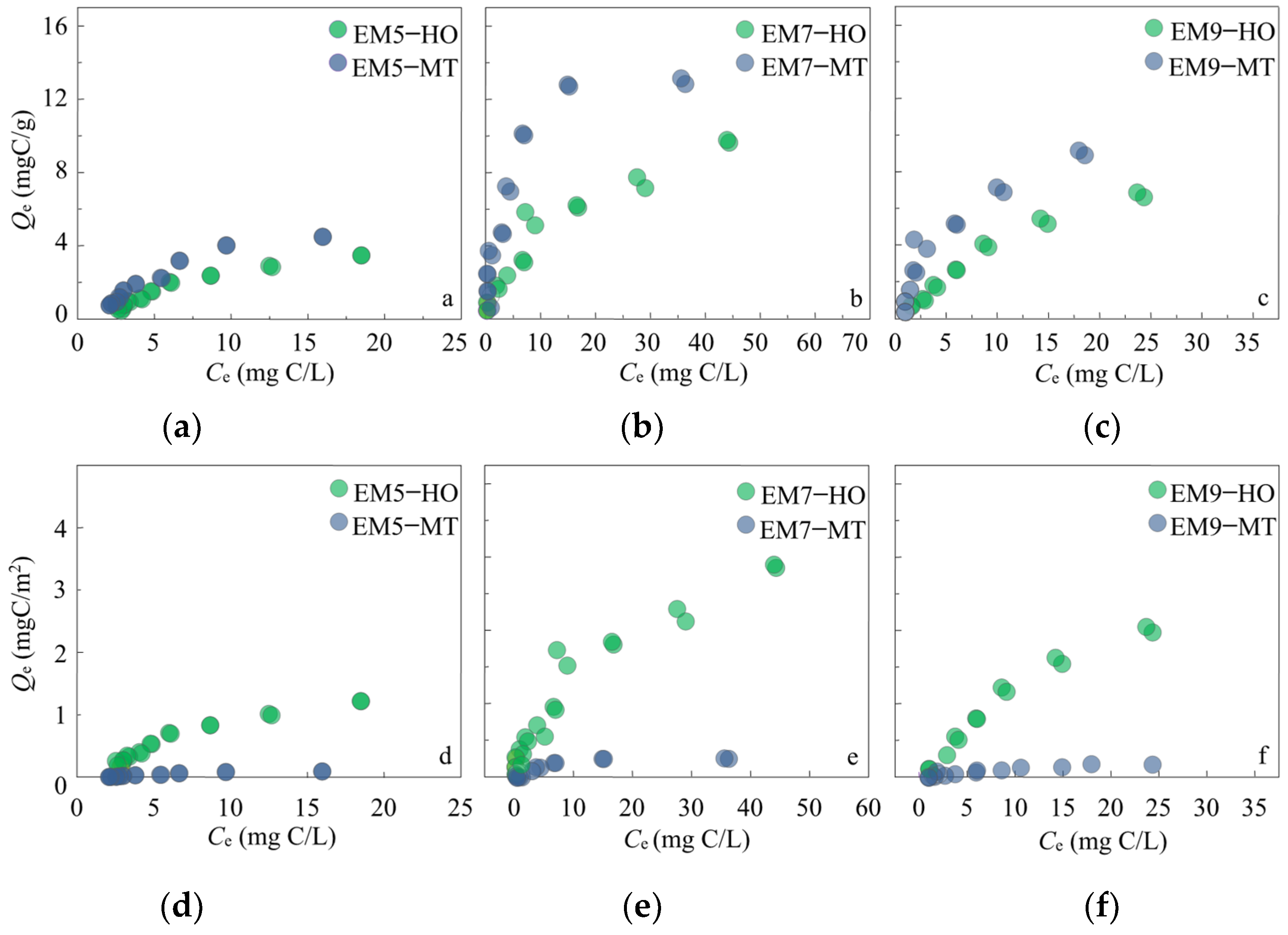
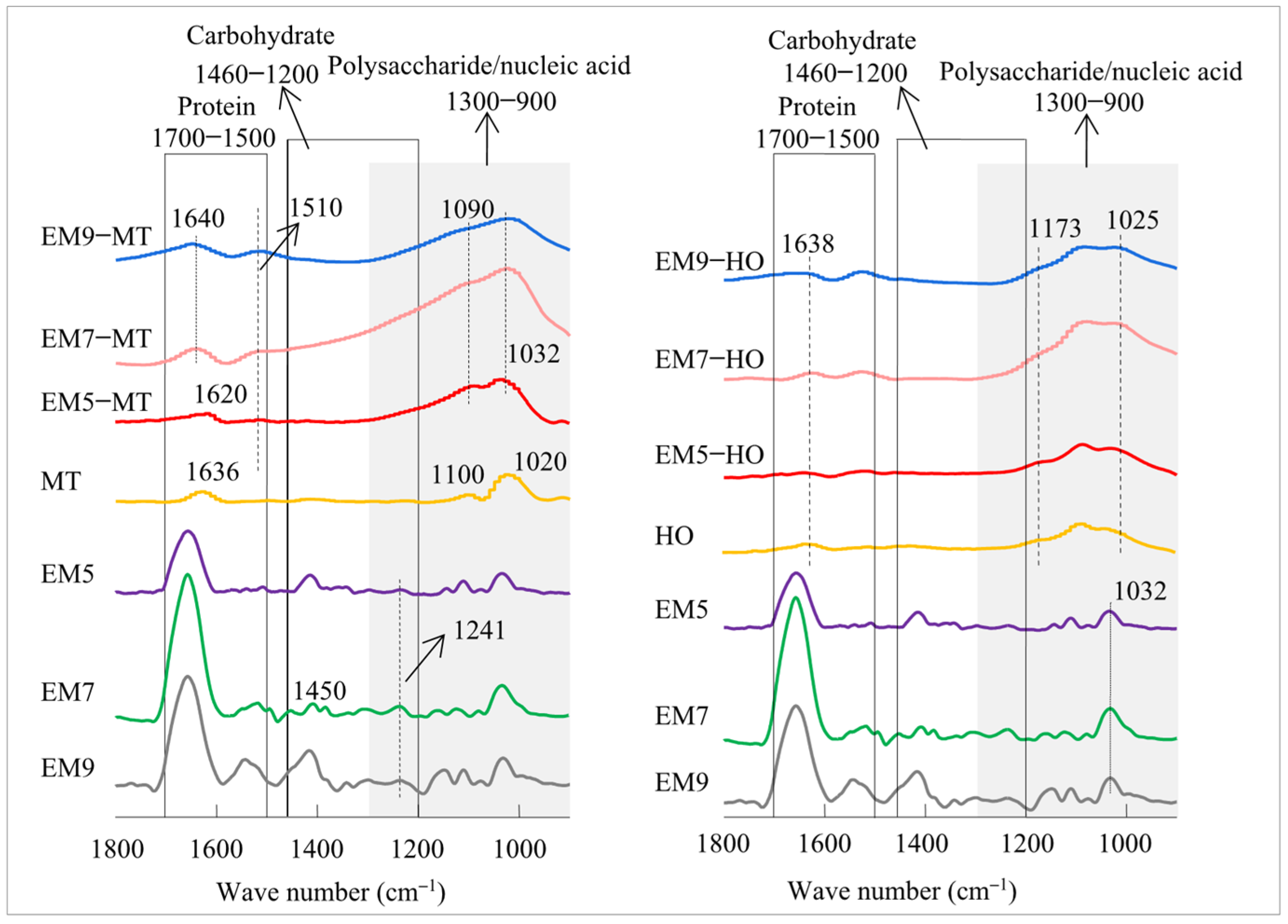
3.2. The Formation of EM–Mineral Associations
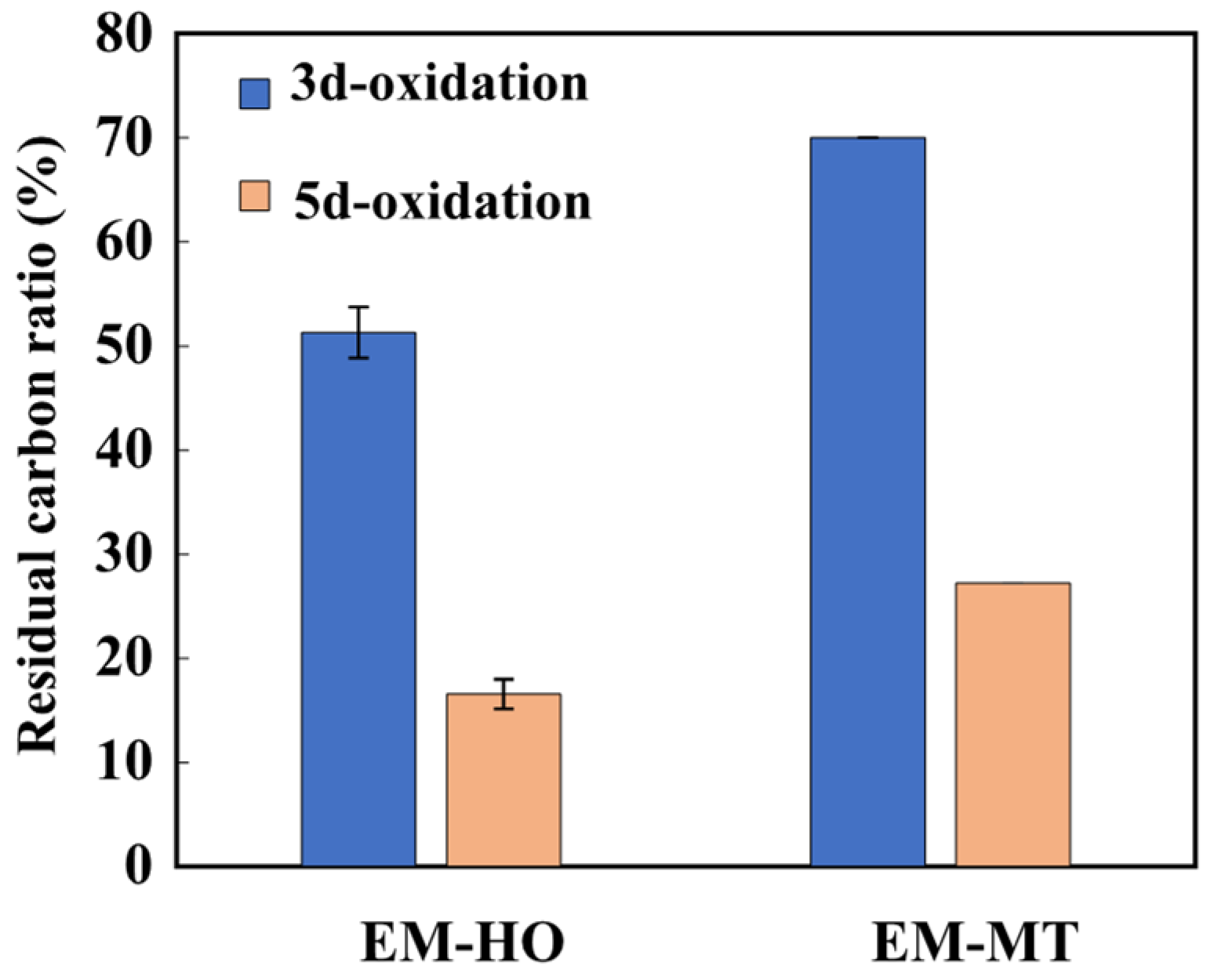
3.3. Carbon Stability of EM–Mineral Associations for Oxidation Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J.; Vig, A.P. Earthworm as ecological engineers to change the physico-chemical properties of soil: Soil vs. vermicast. Ecol. Eng. 2016, 90, 1–5. [Google Scholar] [CrossRef]
- Bohlen, P.J.; Parmelee, R.W.; Allen, M.F.; Ketterings, Q.M. Differential Effects of Earthworms on Nitrogen Cycling from Various Nitrogen-15-Labeled Substrates. Soilence Soc. Am. J. 1999, 63, 882–890. [Google Scholar] [CrossRef]
- Abail, Z.; Sampedro, L.; Whalen, J.K. Short-term carbon mineralization from endogeic earthworm casts as influenced by properties of the ingested soil material. Appl. Soil Ecol. 2017, 116, 79–86. [Google Scholar] [CrossRef]
- Pathma, J.; Sakthivel, N.J.S. Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. SpringerPlus 2012, 1, 26. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S. Mucus excretion and carbon turnover of endogeic earthworms. Biol. Fertil. Soils 1991, 12, 217–220. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Pevear, D.R.; Hill, R.J. Mineral surface control of organic carbon in black shale. Science 2002, 295, 657–660. [Google Scholar] [CrossRef]
- Mayer, L.M. Relationship between mineral surfaces and organic carbon concentrations in soils and sediments. Chem. Geol. 1994, 114, 347–363. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Rethemeyer, J.; Matzner, E. Stabilization of dissolved organic matter by sorption to the mineral soil. Soil Biol. Biochem. 2005, 37, 1319–1331. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Castellano, M.J.; Vogel, C.; Wiesmeier, M.; Mueller, C.W. Unlocking complex soil systems as carbon sinks: Multi-pool management as the key. Nat. Commun. 2023, 14, 2967. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Jones, K.D.; Tiller, C.L. Effect of solution chemistry on the extent of binding of phenanthrene by a soil humic acid: A comparison of dissolved and clay bound humic. Environ. Sci. Technol. 1999, 33, 580–587. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Whitman, T.; Zhu, Z.; Lehmann, J. Carbon mineralizability determines interactive effects on mineralization of pyrogenic organic matter and soil organic carbon. Environ. Sci. Technol. 2014, 48, 13727–13734. [Google Scholar] [CrossRef]
- Woo, S.H.; Enders, A.; Lehmann, J. Microbial mineralization of pyrogenic organic matter in different mineral systems. Org. Geochem. 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, X.; Cai, P.; Huang, Q.; Rong, X.; Liang, W. Preferential adsorption of extracellular polymeric substances from bacteria on clay minerals and iron oxide. Colloids Surf. B Biointerfaces 2011, 83, 122–127. [Google Scholar] [CrossRef]
- Fang, L.; Cao, Y.; Huang, Q.; Walker, S.L.; Cai, P. Reactions between bacterial exopolymers and goethite: A combined macroscopic and spectroscopic investigation. Water Res. 2012, 46, 5613–5620. [Google Scholar] [CrossRef] [PubMed]
- Gerzabek, M.H.; Aquino, A.J.A.; Balboa, Y.I.E.; Galicia-Andrés, E.; Grančič, P.; Oostenbrink, C.; Petrov, D.; Tunega, D. A contribution of molecular modeling to supramolecular structures in soil organic matter. J. Plant Nutr. Soil Sci. 2022, 185, 44–59. [Google Scholar]
- Liu, X.; Lu, X.; Wang, R.; Zhou, H.; Xu, S. Surface complexes of acetate on edge surfaces of 2: 1 type phyllosilicate: Insights from density functional theory calculation. Geochim. Cosmochim. Acta 2008, 72, 5896–5907. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, L.; Putnis, C.V. Molecular-scale investigations reveal noncovalent bonding underlying the adsorption of environmental DNA on mica. Environ. Sci. Technol. 2019, 53, 11251–11259. [Google Scholar] [CrossRef] [PubMed]
- Guhra, T.; Stolze, K.; Schweizer, S.; Totsche, K.U. Earthworm mucus contributes to the formation of organo-mineral associations in soil. Soil Biol. Biochem. 2020, 145, 107785. [Google Scholar] [CrossRef]
- Di Carlo, E.; Boullemant, A.; Poynton, H.; Courtney, R. Exposure of earthworm (Eisenia fetida) to bauxite residue: Implications for future rehabilitation programmes. Sci. Total Environ. 2020, 716, 137126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-E.; Yu, J.; Ouyang, Y. Activity of Earthworm in Latosol Under Simulated Acid Rain Stress. Bull. Environ. Contam. Toxicol. 2015, 94, 108–111. [Google Scholar] [CrossRef]
- Allen, B.; Hajek, B. Mineral occurrence in soil environments. Miner. Soil Environ. 1989, 1, 199–278. [Google Scholar]
- Wang, H.; Li, X.; Chen, Y.; Li, Z.; Hedding, D.W.; Nel, W.; Ji, J.; Chen, J. Geochemical behavior and potential health risk of heavy metals in basalt-derived agricultural soil and crops: A case study from Xuyi County, eastern China. Sci. Total Environ. 2020, 729, 139058. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Klotzbuecher, T.; Lechtenfeld, O.J.; Hong, H.; Liu, C.; Kaiser, K.; Mikutta, C.; Mikutta, R. Legacy Effects of Sorption Determine the Formation Efficiency of Mineral-Associated Soil Organic Matter. Environ. Sci. Technol. 2022, 56, 2044–2053. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Yang, Y.; Zhang, D.; Wu, M.; Pan, B.; Xing, B. Identifying structural characteristics of humic acid to static and dynamic fluorescence quenching of phenanthrene, 9-phenanthrol, and naphthalene. Water Res. 2017, 122, 337–344. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Ma, Y.; Guo, L.; Sun, J.; Tong, J. Earthworm epidermal mucus: Rheological behavior reveals drag-reducing characteristics in soil. Soil Tillage Res. 2016, 158, 57–66. [Google Scholar] [CrossRef]
- Omoike, A.; Chorover, J. Spectroscopic study of extracellular polymeric substances from Bacillus subtilis: Aqueous chemistry and adsorption effects. Biomacromolecules 2004, 5, 1219–1230. [Google Scholar] [PubMed]
- Lei, J.; Yi, Y.; Lin, X.J.; Ying, L.; Yong, L. Effects of earthworm casts on sorption-desorption, degradation, and bioavailability of nonylphenol in soil. Environ. Sci. Pollut. Res. 2018, 25, 1–10. [Google Scholar]
- Abdulla, H.; Minor, E.C.; Dias, R.F.; Hatcher, P.G. Changes in the compound classes of dissolved organic matter along an estuarine transect: A study using FTIR and 13C NMR. Geochim. Cosmochim. Acta 2010, 74, 3815–3838. [Google Scholar] [CrossRef]
- Omoike, A.; Chorover, J. Adsorption to goethite of extracellular polymeric substances from Bacillus subtilis. Geochim. Cosmochim. Acta 2006, 70, 827–838. [Google Scholar]
- Aguiar, N.; Novotny, E.; Oliveira, A.; Rumjanek, V.; Olivares, F.; Canellas, L. Prediction of humic acids bioactivity using spectroscopy and multivariate analysis. J. Geochem. Explor. 2013, 129, 95–102. [Google Scholar] [CrossRef]
- Mikutta, R.; Mikutta, C.; Kalbitz, K.; Scheel, T.; Kaiser, K.; Jahn, R. Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochim. Cosmochim. Acta 2007, 71, 2569–2590. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.R.; Danielsen, K.M. Binding of pyrene to aquatic and commercial humic substances: The role of molecular weight and aromaticity. Environ. Sci. Technol. 1997, 31, 1630–1635. [Google Scholar] [CrossRef]
- Pan, B.; Ghosh, S.; Xing, B. Dissolved organic matter conformation and its interaction with pyrene as affected by water chemistry and concentration. Environ. Sci. Technol. 2008, 42, 1594–1599. [Google Scholar] [CrossRef]
- Elzinga, E.J.; Sparks, D.L. Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation. J. Colloid Interface Sci. 2007, 308, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.; Giesler, R.; Persson, P. Adsorption mechanisms of glucose in aqueous goethite suspensions. J. Colloid Interface Sci. 2011, 353, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Barral, S.; Villa-García, M.; Rendueles, M.; Díaz, M. Interactions between whey proteins and kaolinite surfaces. Acta Mater. 2008, 56, 2784–2790. [Google Scholar] [CrossRef]
- Johnston, C.T.; Premachandra, G.S.; Szabo, T.; Lok, J.; Schoonheydt, R.A. Interaction of Biological Molecules with Clay Minerals: A Combined Spectroscopic and Sorption Study of Lysozyme on Saponite. Langmuir 2012, 28, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Nkoh, J.N.; Hong, Z.; Lu, H.; Li, J.; Xu, R. Adsorption of amino acids by montmorillonite and gibbsite: Adsorption isotherms and spectroscopic analysis. Appl. Clay Sci. 2022, 219, 106437. [Google Scholar] [CrossRef]
- Kolman, K.; Makowski, M.M.; Golriz, A.A.; Kappl, M.; Piglowski, J.; Butt, H.-J.; Kiersnowski, A. Adsorption, Aggregation, and Desorption of Proteins on Smectite Particles. Langmuir 2014, 30, 11650–11659. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.H.; Li, N.; Tong, D.S.; Zhou, C.H.; Lin, C.X.; Xu, C.Y. Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Appl. Clay Sci. 2013, 80–81, 443–452. [Google Scholar] [CrossRef]
- Wang, F.; Jin, L.; Guo, C.; Min, L.; Zhang, P.; Sun, H.; Zhu, H.; Zhang, C. Enhanced heavy metals sorption by modified biochars derived from pig manure. Sci. Total Environ. 2021, 786, 147595. [Google Scholar] [CrossRef]
- Ren, L.; Edwards, P.J.; Perera, C.O.; Hemar, Y. Structural features of a novel polysaccharide isolated from a New Zealand Maori mushroom Iliodiction cibarium. Carbohydr. Res. 2015, 406, 19–26. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Choonpicharn, S.; Tanreuan, K.; Lumyong, S. Characterization of polysaccharides from wild edible mushrooms from Thailand and their antioxidant, antidiabetic, and antihypertensive activities. Int. J. Med. Mushrooms 2020, 22, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Troyer, L.D.; Maillot, F.; Wang, Z.; Wang, Z.; Mehta, V.S.; Giammar, D.E.; Catalano, J.G. Effect of phosphate on U (VI) sorption to montmorillonite: Ternary complexation and precipitation barriers. Geochim. Cosmochim. Acta 2016, 175, 86–99. [Google Scholar] [CrossRef]
- Borgnino, L.; Giacomelli, C.E.; Avena, M.J.; De Pauli, C.P. Phosphate adsorbed on Fe (III) modified montmorillonite: Surface complexation studied by ATR-FTIR spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 238–244. [Google Scholar] [CrossRef]
- Tertre, E.; Hofmann, A.; Berger, G. Rare earth element sorption by basaltic rock: Experimental data and modeling results using the “Generalised Composite approach”. Geochim. Cosmochim. Acta 2008, 72, 1043–1056. [Google Scholar] [CrossRef]
- Oren, A.; Chefetz, B. Sorptive and desorptive fractionation of dissolved organic matter by mineral soil matrices. J. Environ. Qual. 2012, 41, 526–533. [Google Scholar] [CrossRef]


| Sample | 200–160 ppm | 160–110 ppm | 110–90 ppm | 90–65 ppm | 65–45 ppm | 45–0 ppm |
|---|---|---|---|---|---|---|
| Amides, Carboxyl | Aromatic, Olefinic | Anomeric | O-Alkyl | αC of Amino Acids | Aliphatic | |
| EM5 | 27.6% | 24.6% | 2.0% | 15.9% | 15.5% | 14.4% |
| EM7 | 28.7% | 32.1% | 1.8% | 13.9% | 14.2% | 9.3% |
| EM9 | 27.4% | 27.6% | 2.1% | 14.3% | 13.6% | 15.0% |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmaxa | KL | R2 | Kf | n | R2 | |
| EM5-HO | 7.34 | 4.87 | 0.98 | 0.85 | 0.43 | 0.93 |
| EM7-HO | 11.77 | 7.28 | 0.96 | 1.13 | 0.92 | 0.95 |
| EM9-HO | 8.64 | 6.73 | 0.95 | 0.61 | 0.58 | 0.92 |
| EM5-MT | 9.55 | 6.58 | 0.97 | 1.78 | 0.77 | 0.95 |
| EM7-MT | 15.59 | 13.61 | 0.97 | 3.79 | 1.71 | 0.91 |
| EM9-MT | 11.93 | 8.82 | 0.94 | 2.97 | 1.3 | 0.92 |
| Samples | pH | C% | N% | H% | O% | (N+O)/C | H/C |
|---|---|---|---|---|---|---|---|
| EM5-HO | 6.86 | 1.95 | 0.28 | 0.26 | 4.31 | 1.78 | 1.61 |
| EM5-MT | 7.14 | 2.19 | 0.42 | 0.14 | 5.22 | 1.96 | 0.74 |
| EM7-HO | 8.38 | 2.69 | 0.60 | 0.17 | 7.59 | 2.33 | 0.75 |
| EM7-MT | 8.67 | 2.91 | 0.87 | 0.14 | 11.50 | 3.22 | 0.58 |
| EM9-HO | 8.29 | 2.51 | 0.27 | 0.17 | 5.83 | 1.86 | 0.83 |
| EM9-MT | 8.81 | 2.73 | 0.57 | 0.10 | 9.99 | 2.92 | 0.45 |
| Oxidation | Samples | C% | N% | H% | O% | (N+O)/C | H/C |
|---|---|---|---|---|---|---|---|
| Before | EM7-HO | 2.69 | 0.58 | 0.17 | 7.09 | 2.17 | 0.75 |
| EM7-MT | 2.91 | 0.87 | 0.14 | 11.5 | 3.22 | 0.58 | |
| After | EM7-HO | 0.54 | 0.4 | 0.01 | 1.14 | 2.21 | 0.32 |
| EM7-MT | 0.89 | 0.02 | 0.04 | 5.04 | 4.27 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Feng, S.; Wang, L.; Lei, C.; Peng, H.; He, X.; Zhou, D.; Li, F. Improvement and Stability of Soil Organic Carbon: The Effect of Earthworm Mucus Organo-Mineral Associations with Montmorillonite and Hematite. Sustainability 2024, 16, 5458. https://doi.org/10.3390/su16135458
Li Y, Feng S, Wang L, Lei C, Peng H, He X, Zhou D, Li F. Improvement and Stability of Soil Organic Carbon: The Effect of Earthworm Mucus Organo-Mineral Associations with Montmorillonite and Hematite. Sustainability. 2024; 16(13):5458. https://doi.org/10.3390/su16135458
Chicago/Turabian StyleLi, Yuxuan, Siyue Feng, Lin Wang, Chencen Lei, Hongbo Peng, Xinhua He, Dandan Zhou, and Fangfang Li. 2024. "Improvement and Stability of Soil Organic Carbon: The Effect of Earthworm Mucus Organo-Mineral Associations with Montmorillonite and Hematite" Sustainability 16, no. 13: 5458. https://doi.org/10.3390/su16135458







