1. Introduction
The global transition toward sustainable energy solutions has ignited a profound exploration of renewable technologies among researchers, aiming to minimize its environmental impact. Fossil fuels, the backbone of the energy infrastructure for decades, have exacted a heavy toll on the environment, prompting a reevaluation of power generation [
1,
2]. This pivotal change, transitioning from fossil fuel predominance in 2015 to a forecasted 98% dependence on renewables by 2040 and aiming for zero greenhouse gas (GHG) emissions by 2050, highlights the imperative nature of the transition [
3].
Climate change, air pollution, and resource consumption amplify the imperative to reassess power generation methods [
4,
5]. In the quest for sustainable solutions, myriad renewable energy sources emerged. Nevertheless, many of these sustainable solutions suffer from irregularity, resulting in mismatches between the available electrical energy and consumption by end users. To mitigate this fluctuation, the development of efficient energy storage systems becomes essential as a priority to develop suitable energy conversion or storage systems for the power grid [
6]. Simultaneously, the unsustainability of non-renewable energy technologies dependent on fossil fuels such as gasoline, diesel, and coal necessitates a critical assessment [
7].
Hydrogen promises to evolve into a principal conduit for chemical energy in the trajectory toward a more sustainable energy ecosystem, with the expectation that hydrogen and its derivatives constitute 12% of total energy consumption by 2050 [
8]. This transformation holds profound implications for global energy systems, particularly for reshaping the balance between conventional energy sources and innovative solutions [
9]. The aim of this review is to illustrate the role of hydrogen and its complex interaction with renewable energy frameworks, fostering a trajectory toward cleaner and more sustainable energy practices.
Hydrogen, a fundamental element, has emerged as a beacon of promise amidst the challenges posed by traditional energy sources [
10,
11,
12,
13,
14]. Unlike its counterparts derived solely from non-renewable sources, hydrogen stands out for its capability to be produced using renewable energy sources, such as solar and wind energy, geothermal power, and others.
Hydrogen is characterized as a versatile and environmentally friendly energy carrier, especially when it is produced through water electrolysis powered by renewables [
1,
15,
16,
17]. The synergy between hydrogen and green production methods represents a pivotal step toward a sustainable future, offering a pathway that aligns seamlessly with environmental preservation and innovation principles. This eco-friendly variant of hydrogen, aptly termed “green hydrogen”, has catalyzed the reshaping of the energy landscape [
15,
18,
19,
20]. Additionally, this growth underscores the crucial role of low-carbon hydrogen, with predictions suggesting that, by 2050, roughly two-thirds of total hydrogen production will be derived from renewable electricity, with the remaining third generated through natural gas in conjunction with carbon capture and storage [
21].
It is evident that the pursuit of sustainable energy sources continues and, consequently, the demand for comprehensive information has increased, which means that when it comes to assessing the efficiency trends, environmental impact, and costs of hydrogen production, readers may find that such data are unavailable in the literature from one source. This review provides the efficiency trends of various production methods over the years. While individual efficiency figures have been extracted and analyzed [
22,
23,
24,
25,
26,
27], the absence of a unified table makes it challenging to perform a side-by-side evaluation of the different methods. When examining available reviews on greenhouse gas emissions and the costs associated with hydrogen production methods, it is apparent that many sources concentrate on a single production method and its related emissions and costs [
23,
24,
25,
26,
27,
28,
29,
30,
31]. Recognizing this knowledge gap, this review takes on the task of unearthing and presenting this elusive data, seeking to provide a robust foundation for informed decision-making, innovation, and holistic energy transformation.
In this context, the following sections explore the details of renewable and non-renewable energy technologies, the attributes of hydrogen production processes, and the potential integration of hydrogen and fuel cell technologies into the energy sector. This study endeavors to contribute meaningfully to the discourse surrounding sustainable energy, thereby facilitating a more conscientious and responsible energy trajectory.
Section 2 provides an overview of energy production methods, hydrogen technologies, and environmental sustainability.
Section 3 outlines the methodology employed in the review, detailing the systematic steps that are followed.
Section 4 discusses the role of hydrogen as a sustainable solution for electricity production, mainly focusing on its potential energy and transportation medium. In
Section 5, we present the principal characteristics of the hydrogen production processes, emphasizing the significance of electrolysis as a key player in shaping the trajectory of hydrogen applications.
Section 6 explores efficiency trends in hydrogen electrolysis and fuel cell advances and challenges. Recent advancements in hydrogen production and storage technologies are reported in
Section 7. Finally,
Section 8 and
Section 9 summarize the essential findings and implications of the study.
2. Energy Production, Hydrogen Technologies, and Environmental Sustainability
The expansive landscape of energy production methods, hydrogen production techniques, and the resultant global greenhouse gas (GHG) emissions necessitates a theoretical overview. In this section, the focus is directed to developing comprehension of the fundamental principles supporting these essential components. The investigation offers a comprehensive basis for this task, highlighting the core concepts that contribute to our understanding of critical elements in the broader context of energy and environmental sustainability.
The first of these topics involves energy production methods, focusing on the global energy landscape division between non-renewable and renewable sources, emphasizing the need for innovative solutions such as hydrogen-based energy carriers. After exploring how energy production can provide sustainable and innovative solutions based on hydrogen, the second part of the section talks about hydrogen production methods, examining the role that each method plays in the global energy landscape and shaping the pursuit of hydrogen as a sustainable energy carrier. Thermochemical processes utilize diverse resources, whereas water electrolysis, specifically proton exchange membrane fuel cells (PEMFC) and solid oxide electrolysis (SOE), arise as promising avenues for clean and sustainable energy production. This exploration highlights the need for a strategic approach in selecting the optimal hydrogen production technique. Finally, this section employs statistical data to emphasize how hydrogen plays a pivotal role in mitigating GHG emissions and fostering sustainable energy practices.
2.1. Energy Production Methods
The technology of renewable energy causes minimal harm to the environment. These encompass, among others, solar photovoltaic and thermal, wind, geothermal, tidal, low-head hydropower (small-scale), biomass and biogas, and hydrogen fuel cells (hydrogen generated from renewable resources) [
32].
Non-renewable energy technologies encompass those that rely on fossil fuels such as gasoline, diesel, oil, propane, methane, natural gas, or coal for energy generation. Distributed generators (DGs), operating on fossil fuels, are not regarded as sustainable sources of energy generation, given the non-renewable nature of their energy sources [
33]. These include the internal combustion engine (ICE), combustion turbine, gas turbine, microturbine, and fuel cells (using some form of fossil fuel, such as natural gas, to generate hydrogen).
In the current global energy landscape, energy production is notably stratified, with a considerable portion still reliant on non-renewable sources such as fossil fuels. However, a growing emphasis on sustainability has led to a parallel surge in adopting renewable energy technologies. This dichotomy underscores the multifaceted challenge of harmonizing energy demand with environmental stewardship, driving a critical reassessment of energy systems and necessitating innovative solutions such as hydrogen-based energy carriers.
In terms of hydrogen energy carriers, hydrogen fuel cells are anticipated to assume a pivotal role as an energy carrier in prospective global energy systems. With fossil fuel resources vanishing and environmental apprehensions mounting, hydrogen is positioned to ascend as an increasingly significant chemical energy carrier, potentially evolving into the primary chemical energy carrier of the future [
34].
2.2. Hydrogen Production Methods
Each method involves specific considerations regarding efficiency, cost, resource availability, and potential environmental impact. This array of choices reinforces the imperative for an informed and strategic approach when opting for the most suitable hydrogen production technique in each context.
The production of hydrogen encompasses a diverse spectrum of methodologies [
35]. Thermochemical processes, which rely on the collaboration of heat and chemical reactions, enable the extraction of hydrogen from organic sources like fossil fuels, biomass, and water [
36,
37]. The intriguing capability to disassociate water (H
2O) into its elemental constituents, hydrogen (H
2) and oxygen (O
2), prevails through the application of electrolysis or harnessed solar energy [
27,
31,
38,
39,
40]. Biological pathways orchestrated by microorganisms such as bacteria and algae offer an alternative avenue for hydrogen generation. This rich tapestry of production techniques illuminates the manifold strategies for acquiring hydrogen. It underscores the nuanced imperative of judiciously selecting the optimal approach [
41] in alignment with contextual nuances and sustainability aspirations.
In specific thermal processes, the energy inherent in diverse resources like natural gas, coal, or biomass is utilized to extract hydrogen from their molecular composition. In other approaches, heat, coupled with closed chemical cycles, produces hydrogen from raw materials such as water [
42]. The various thermochemical processes are described in further detail in
Table 1.
Another way to generate hydrogen uses electrolyzers, which employ energy to split water molecules into hydrogen and oxygen. This technology is already developed and is available in the market, with systems capable of efficiently utilizing renewable energies for this separation process.
Following a detailed exploration of diverse hydrogen production methods, a comprehensive view of the global energy landscape further contextualizes the significance of these approaches. When exploring the separate pathways for acquiring hydrogen, it is noteworthy to analyze these methods according to the existing composition of energy sources worldwide. The following distribution of energy sources exemplifies this division [
43].
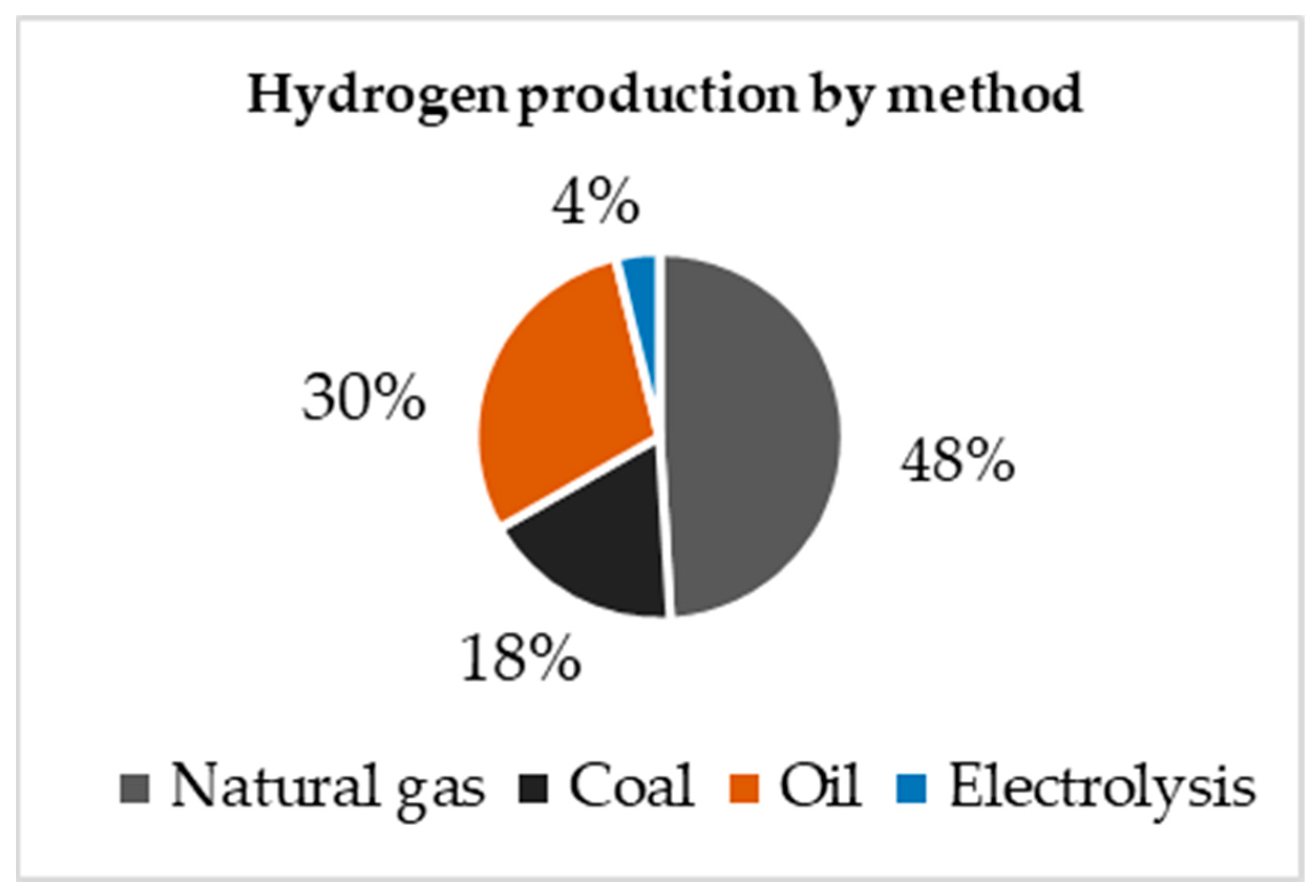
As depicted in
Figure 1, hydrogen production methods comprise the following processes: natural gas steam reforming, which is the most widely used process but leads to significant greenhouse gas (GHG) emissions. Approximately 48% of the global hydrogen demand is met through natural gas steam reforming, with 30% coming from oil reforming, 18% from coal gasification, 3.9% from water electrolysis, and 0.1% from other sources [
18]. Despite the emissions produced, at present, natural gas is widely used due to its abundance, efficiency, and low cost. Conversely, only 3.9% of hydrogen is produced by electrolysis; this is the only method mentioned that can be used with renewable energy and that does not generate a substantial amount of greenhouse gas (GHG) emissions, positioning itself as the best alternative for moving toward a transition to sustainable energy.
2.3. Global Greenhouse Gas (GHG) Emissions
Greenhouse gas (GHG) emissions arise from several key economic sectors, including energy, industry, buildings, and transport. In 2021, the total global GHG emissions amounted to 44 gigatons of CO
2 equivalent (GtCO
2eq). This measurement represents the combined impact of all greenhouse gases, expressed in terms of the equivalent amount of carbon dioxide (CO
2). Notably, the largest contributor to these emissions was the energy systems sector, responsible for 34% (15 GtCO
2eq) of the total emissions, followed by transport (16%; 7.2 GtCO
2eq), industry (14%; 6.2 GtCO
2eq), and buildings operation (6%; 2.9 GtCO
2eq).
Figure 2,
Figure 3,
Figure 4 and
Figure 5 show the direct emissions produced within each sector, providing insight into their respective contributions to global GHG emissions. The data shown in this section were extracted from previous publications [
53,
54,
55,
56].
The CO
2 global emission graph illustrates the trajectory of emissions from 1970 to 2021, with a notable recurring pattern: the power industry consistently ranks at the top of the emission list. This trend underscores the significant impact of the power sector on greenhouse gas emissions. This dominance in emission rankings can be attributed to several factors but is significantly driven by the necessity of using fossil fuels such as coal, natural gas, and oil, which release large amounts of CO
2 when burned. In addition, the power sector often faces challenges in adopting cleaner and more sustainable technologies as transitioning to renewable energy sources requires substantial investments in infrastructure and may encounter regulatory barriers. That is the reason why, despite efforts to reduce emissions through efficiency improvements and the adoption of cleaner technologies, the power industry continues to be a major contributor to GHG emissions [
53,
58].
Also, the data in
Figure 3 confirm that the production of electrical energy significantly contributes to the emission of greenhouse gases, accounting for approximately 34% of these emissions. This substantial environmental impact is primarily associated with generating electricity, with various sources responsible for these emissions.
Figure 3 provides a detailed representation of global energy production sources. The data presented show that more than 50% of the worldwide energy supply comes from non-renewable sources, which are known to produce significant greenhouse gas emissions. In contrast, only a small fraction, approximately 5%, is generated from renewable sources, including solar, wind, and hydropower. This energy landscape presents a critical challenge in terms of alignment with the Sustainable Development Goals for 2030, especially Goals 7 and 13, which emphasize affordable and clean energy in Goal 7 and climate action in Goal 13 [
59]. Acknowledging this disparity, the expanding energy sector is actively seeking alternative solutions to meet the rising global energy demand, with hydrogen emerging as a promising contender. Its potential to mitigate greenhouse gas emissions and improve the sustainability of energy generation positions hydrogen as a viable option for addressing environmental concerns and meeting future energy needs [
60,
61].
Figure 3.
Representation of the total energy supply by different sources (figure elaborated by the authors, based on the data published in [
57,
61]).
Figure 3.
Representation of the total energy supply by different sources (figure elaborated by the authors, based on the data published in [
57,
61]).
Based on the previous figures, the total amount of GHG generated from the main fossil fuels used for electricity generation worldwide is shown in
Figure 4 [
53,
54,
62].
Figure 4.
Total GHG emissions from fuel combustion per product around the world (figure elaborated by the authors, based on the data published in [
53,
54,
62]).
Figure 4.
Total GHG emissions from fuel combustion per product around the world (figure elaborated by the authors, based on the data published in [
53,
54,
62]).
Following the annual reports issued by the International Energy Agency (IEA), 2019 witnessed the emission of 14 gigatons of CO
2 equivalent (GtCO
2eq) within the energy sector. Particularly harmful agents were emphasized, with coal, peat, and oil ranking highest, followed by natural gas. Additionally, biofuels and waste, while contributing to this emission profile to a lesser extent, also play a role (
Figure 3) [
53,
54,
57,
60]. It must be pointed out that the data presented from 2019 has been chosen because it represents the most recent year from which we can obtain the relevant information without it being distorted by abnormal conditions, as seen in 2020 and 2021 due to the global pandemic (COVID-19).
Figure 5 illustrates the percentage of global emissions emitted in 2019; however, the total energy supplied to the global electrical system amounted to 607,000 petajoules (PJ) [
54,
57]. The same figure shows that the energy derived from nuclear or renewable sources releases no pollutant gases into the atmosphere. Concerning nuclear energy, this does produce nuclear waste, which incurs high costs and has an extended treatment process. Power sourced from green or renewable sources does not encounter these issues. Nevertheless, its primary challenge lies in storage, as the availability of some clean energy sources is uncontrollable, as exemplified by solar energy, wherein natural occurrences, such as varying sunlight levels, cannot be regulated. This fact presents a challenge when meeting energy demand since, for a robust energy system, there must be the capacity to store energy during periods of low demand and supply additional power during periods of increased energy demand [
12,
37,
51,
54,
55,
57,
58,
59,
60,
61,
62,
63,
64].
Figure 5.
Representation of the total greenhouse gas emission by different methods of energy production (figure elaborated by the authors, based on the data published in [
54,
65]).
Figure 5.
Representation of the total greenhouse gas emission by different methods of energy production (figure elaborated by the authors, based on the data published in [
54,
65]).
Figure 5 highlights that renewable energy sources have a negligible impact on greenhouse gas emissions, effectively contributing 0% to the problem. However, despite this environmental advantage, the adoption of renewable energy has been limited, as shown in
Figure 4, which can mainly be attributed to the associated costs, including investment in infrastructure, energy storage, and the grid upgrades needed to accommodate the intermittent nature of some renewable sources. In contrast to the environmental benefits of renewables, their implementation faces economic and infrastructural challenges that contribute to the difficulties seen in adopting these sources, even considering the United Nations’ sustainable development goals’ targets.
3. Methodology
For the development of this review, the authors followed a precise path based on the following steps (see
Figure 6):
Data collection: Data were extracted from specialized scientific study reports in the literature, as cited in the bibliography: [
1,
2,
3,
10,
11,
12,
13,
14,
15,
16,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
30,
31,
34,
35,
36,
37,
38,
39,
40,
42,
47,
50,
51,
53,
55,
60,
62,
63,
64,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90,
91,
92,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102,
103,
104,
105,
106,
107,
108,
109,
110,
111,
112,
113,
114,
115,
116,
117,
118,
119,
120,
121,
122,
123,
124,
125,
126,
127,
128,
129,
130,
131,
132,
133,
134,
135,
136,
137].
Information authentication: The objective was to provide greater clarity on the advances made in the field of green hydrogen based on electrolysis. In this context, the rise of a relatively new technology promoted research and the development of articles in reputable journals, books, and academic institutions, allowing the identification of reports that added value to the field.
Organization and distribution of topics: A close relationship was found among different authors, confirming the findings discussed in the literature.
Table 2,
Table 3 and
Table 4 summarize the relevant information collected, showing the most accurate results in each statement.
Analysis of selected information: The most relevant and accurate information was chosen, making it possible to carry out the analysis and discussion presented later in this work. It was very useful to make a table of reference sources through the years and map the trend of the energy sector and the evolution of renewable technology.
4. Hydrogen as a Sustainable Solution for Electricity Production
When evaluating the quantity of GHG released into the atmosphere, based on the electricity production method, and recognizing the need for sustainability in renewable energy sources, it is necessary to utilize elements such as hydrogen, which has emerged as a viable option due to its environmentally friendly production process and its ability to capture surplus energy from natural energy generation methods [
24,
26,
28,
32,
40,
60,
133,
134,
136].
Because of the transition from fossil fuels, countries and international institutions have established protocols and methodologies for calculating greenhouse gas emissions, focusing on the main detractor: CO
2. Based on this focus, various methodologies have been employed for the calculation of greenhouse gas emission factors [
24,
26,
28,
40,
66,
120]. These methodologies categorize greenhouse gas emissions into two branches: direct emissions and indirect emissions. Direct emissions refer to the amount of CO
2eq emitted directly into the atmosphere in an area or through a productive sector, while indirect emissions are based on those derived from the transportation, loss, or secondary use of fuels or elements that generate greenhouse gases. In both cases, the use of methodologies to estimate the emission factor varies, depending on their scope, using the GHG Protocol, which can be divided into Scope 1 for direct emissions and Scope 2 for indirect emissions. Emission factors (EFs) can be obtained following various methodologies, such as the comprehensive environmental data archive (CEDA), MoEW, or the new stoichiometric method [
134], which use equations to estimate the amounts of CO
2eq emitted; alternatively, databases such as those provided by IPCC, BEIS, IEA or EPA, among other entities [
61,
92,
133], represent the average of all available data for the estimation of the EFs to be used in each of the various production activities.
The methodology that is generally followed to calculate emissions involves a systematic process with several key steps. It begins with gathering detailed activity data related to greenhouse gas (GHG) sources, like energy consumption and production processes. Quality control measures are then applied to ensure data accuracy and completeness. Following this process, emissions are estimated using appropriate factors or methodologies, as mentioned before. As a result of all these dependencies, it is essential to adhere to recognized standards, such as the GHG Protocol’s Corporate Accounting and Reporting Standard, for guidance throughout the process. Based on this approach, EFs help countries and companies develop reliable emission inventories, which are crucial for understanding, managing, and reducing emissions effectively.
Table 2 illustrates the most widely used GHG factors in the process of generating electricity, based on the IPCC, GHG Protocol, and IPHE regarding the main sources of EFs for most countries and companies [
61,
92,
94,
96,
97].
The methodology used by these countries to calculate the EFs and the GHG impact on the environment are based on Equations (1) and (2), which are used to calculate the efficiency and GHG emissions of plant generation, either according to country or depending on the source [
55,
62,
67,
68,
92,
138].
Table 2.
Greenhouse gas (GHG) conversion factors (based on data from References [
62,
138]).
Table 2.
Greenhouse gas (GHG) conversion factors (based on data from References [
62,
138]).
| Type | Fuel | Unit | Factor |
|---|
| Gaseous Fuels | LPG | Liters [L] | 1.55709 |
| Natural gas | Cubic meter [m3] | 2.02135 |
| Liquid Fuels | Diesel | Liters [L] | 2.70553 |
| Fuel oil | Liters [L] | 3.17522 |
| Solid Fuels | Coal | Tons [Tn] | 2252.34 |
| Biogas | Biogas | Tons [Tn] | 1.21518 |
| Biofuel | Biodiesel | Liters [L] | 0.16751 |
| Renewable | Solar PV | N/A | 0.00 |
| Wind | N/A |
| Hydropower | N/A |
| Geothermal | N/A |
Equations (1) and (2) show the formulas used to estimate the efficiency and GHG emissions of electrolyzers [
55,
67,
69]:
where the
the total energy input, and the total energy consumed are expressed in MWh. GHG is expressed in CO
2eq, and the emission factor is expressed in CO
2eq/MWh.
Table 3 provides valuable insights into different feedstocks and their respective methods of hydrogen production, highlighting the topics of efficiency, GHG emissions, and production costs. This demonstrates that water-based methods, mainly photolysis driven by solar energy, offer the advantage of zero GHG emissions, but their efficiency remains unspecified [
24]. Conversely, electrolysis, powered by electricity, demonstrates a relatively high-efficiency range of 60–90%, with low to moderate GHG emissions and variable production costs [
103]. Thermal methods, such as thermochemical water splitting (thermolysis), exhibit a moderate efficiency of 50% but have relatively high GHG emissions and competitive production costs. Biomass-based approaches, including gasification and microbial electrolysis cells, offer moderate efficiency levels and GHG emissions, with economical production costs. Hydrocarbon-based techniques, such as steam reforming and partial oxidation, deliver greater efficiency but generate substantial GHG emissions [
104,
108]. Overall, the choice of feedstock and production process involves a trade-off between efficiency, environmental impact, and economic factors, underscoring the importance of selecting the most suitable method based on specific objectives and constraints within the hydrogen production sector.
Table 3.
Summary of the principal data of hydrogen production techniques, grouped according to the production process (table elaborated by the authors, based on the data published in [
104], with substantial modifications).
Table 3.
Summary of the principal data of hydrogen production techniques, grouped according to the production process (table elaborated by the authors, based on the data published in [
104], with substantial modifications).
| Feedstocks | Energy | Production
Process | Efficiency 1 (%) | GHG Emissions 2
(kg CO2 per kg of Hydrogen) | Price of Production 3 (USD per kg) | Reference |
|---|
| Water | Solar | Photolysis | N/A | 0 | 10.36 | [54,57,60,76,77,132] |
| Electricity | Alkaline electrolysis | 60–80% | 2.93 | 1.84–2.88 | [37,49,52,57,60,77,98,122,126,139] |
| Proton exchange membrane electrolysis (PEM) | 70–90% | 2.37 | 4–6 |
| Solid oxidant estate electrolysis (SOE) | 80–98% | 1.49 | 3.6 |
| Thermal | Thermochemical water splitting (thermolysis) | 50 | 9–20 | 2.17–2.63 | [47,48,76,124,140] |
| Biomass | Thermal | Gasification | 35–50 | 2–3 | 1.77–2.05 | [55,124,128,130,141] |
| Electricity | Microbial electrolysis cell | 78 | 1–2 | N/A | [41,55,74,123,134] |
| Hydrocarbons | Thermal | Steam reforming | 70–85 | 8–10 | 2.27 | [55,125,131] |
| Partial oxidation | 60–75 | 9–12 | N/A | [36,37,125,126] |
| Autothermal reforming | 60–75 | 9–12 | 2.08 | [37,55,125] |
| Thermal decomposition (pyrolysis) | 58 | 10.9 | 2.6–3.2 | [37,47,55,124] |
| Steam-iron process | N/A | 1–2 | N/A | [55,124,125,131] |
Based on the data shown in
Table 3, electrolysis (alkaline, PEM, and SOE) stands out as a compelling choice for hydrogen production when considering the data presented. First, it boasts a relatively high efficiency range of 60–90%, making it one of the most energy-efficient methods available. This efficiency translates into effectively utilizing the energy input, making it an attractive option for clean hydrogen production. Additionally, the low to moderate emissions from electrolysis further enhance its environmental appeal. Compared to some other methods with higher emissions, such as hydrocarbon-based techniques, electrolysis contributes to a smaller carbon footprint in the hydrogen production process [
53,
54,
55,
125,
128,
129]. Similarly, there are variations within each of the technologies used in the electrolysis process; the data show that alkaline electrolysis offers advantages due to its low cost, but the lower level of efficiency achieved and the GHG emissions make this technology less preferable when compared to others like PEM or SOE. PEM technology has an efficiency range of between 70 and 90%, while SOE technology ranges between 80 and 98%, particularly in specific cases where system feedback is present and at high temperatures making this more acceptable in terms of energy efficiency and lower pollutant levels.
At present, proton exchange membrane (PEM) electrolysis stands out as one of the most prevalent methods for hydrogen production. However, despite its popularity, PEM electrolysis faces challenges due to its high cost and system complexity, while alkaline electrolysis is facing difficulties with the integration of renewable energy sources because they are less responsive to fluctuations in power supply. All these challenges underscore the importance of ongoing research and development efforts aimed at energy integration to enhance the economic viability and sustainability of hydrogen production methods.
In general terms, electrolysis is versatile and adaptable to various energy sources, including electricity from renewable sources, ensuring that electrolysis can align with green energy initiatives by harnessing clean electricity to produce hydrogen with minimal environmental impact. Additionally, electrolysis offers variable production costs, allowing flexibility in its production strategies. It can operate efficiently with low-cost electricity, making it economically competitive in the evolving energy landscape [
70,
71,
72,
73,
77,
141,
142,
143].
5. The Principal Characteristics of Energy Depend on the Production Process of Hydrogen
The pursuit of a sustainable energy future has elevated hydrogen as a vital clean and efficient energy carrier, which is essential for combating climate change and advancing global energy independence. Among the myriad methods of hydrogen production, electrolysis emerges as a pivotal player, driven by its unique attributes and key role in shaping the trajectory of hydrogen applications [
122]. Beyond its technological advancements, electrolysis holds the potential to dictate the course of hydrogen utilization in diverse industries and regions worldwide, ensuring secure energy supplies for the future.
Notably, solar-powered water electrolysis, utilizing photovoltaic energy, has garnered significant attention as a green hydrogen production method. However, a comprehensive evaluation of its environmental impacts and net energy balance is imperative to ascertain its contribution to global decarbonization efforts [
23,
38,
114].
This is why ensuring a sustainable and green hydrogen industry requires a thorough assessment of the efficiency, emissions, and economic viability of various hydrogen production methods.
Figure 7,
Figure 8 and
Figure 9 serve as a comprehensive foundation for objectively evaluating and distinguishing among these methods, based on a review of a wide range of books and scientific papers [
12,
31,
39,
55,
63,
112,
126,
127].
Gasification, electrolysis, and steam reforming are the most widely used methods for hydrogen generation. However, as demonstrated in
Figure 7, not all these methods offer the highest achievable efficiency regarding hydrogen output per kilowatt hour of energy consumed (kWh). Hydrogen generation is highly energy-intensive, making efficiency a critical factor when selecting the hydrogen production method. Electrolysis is the most efficient method, achieving an efficiency rate of up to 90%, surpassing all other options. In contrast, the second most efficient method, steam reforming, achieves only 85% efficiency. However, as illustrated in
Figure 8, when transitioning to a more sustainable economy, the environmental impact of steam reforming is not favorable.
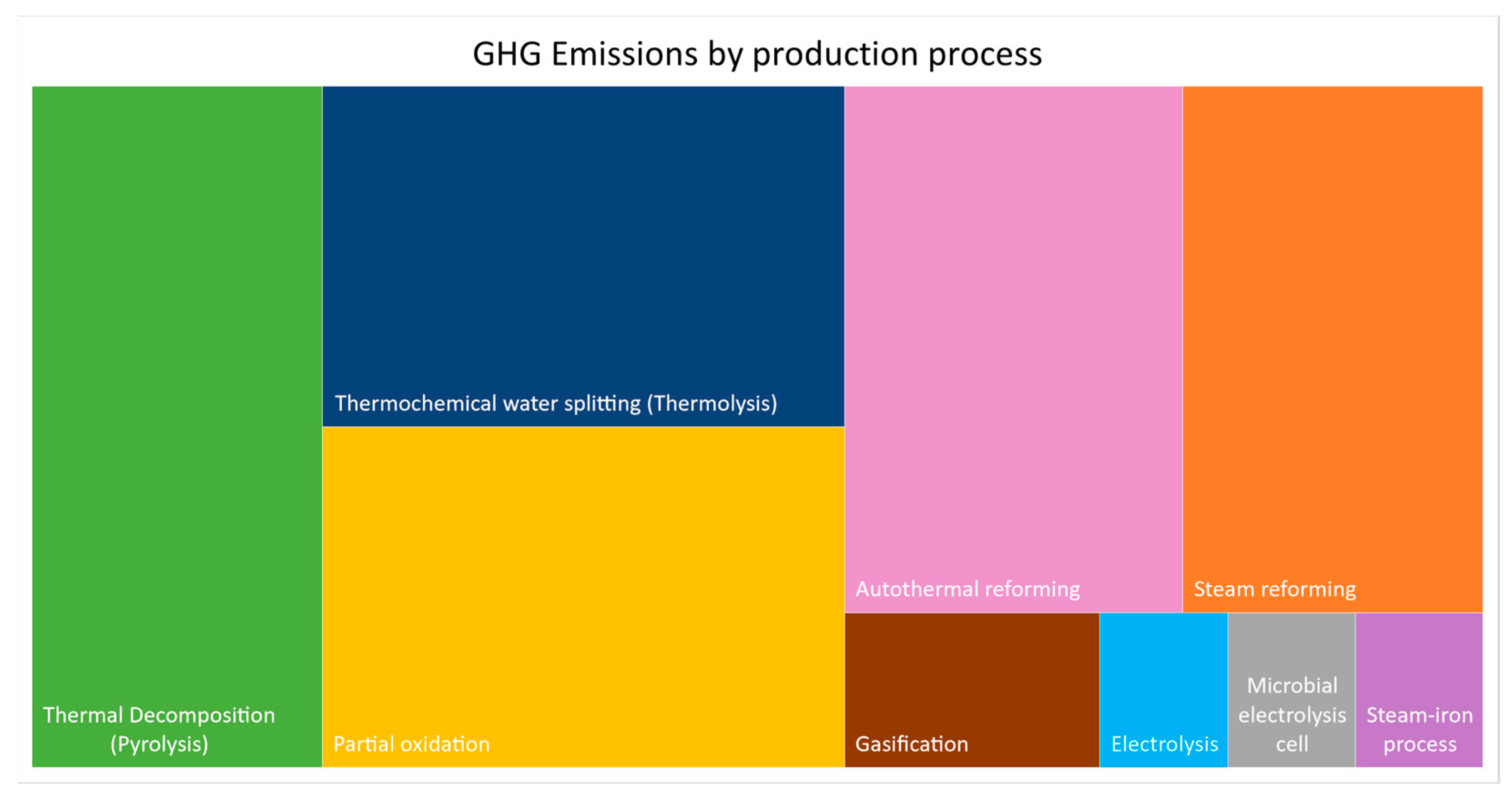
The chart in
Figure 8 provides a comparative analysis of the greenhouse gas emissions resulting from the methods discussed in this article. The larger areas on the chart, particularly steam reforming, autothermal reforming, thermochemical water splitting (thermolysis), pyrolysis, and partial oxidation, represent the most environmentally harmful methods among those presented. In contrast, electrolysis and the steam-iron process are the processes that contribute the least to this type of emission.
The cost of photolysis is exceptionally high compared to other methods, due to the time it takes to produce 1 kg of hydrogen. Following photolysis, electrolysis ranks as the second-highest cost method, potentially reaching a maximum price of USD 6.27 per kilogram produced. The cost of electrolysis is contingent on the energy source employed, which can vary from photovoltaic panels to wind turbines, both of which currently entail substantial expense (see
Figure 9). However, it is expected that these costs will decrease with sustainable development. In contrast, methods such as steam reforming are among the most economical options, due to the cheapness of the source of their energy supply, biomass.
As presented in
Figure 7,
Figure 8 and
Figure 9, evaluating these dimensions will seek to offer a holistic perspective, enabling the identification of the most economically and environmentally advantageous hydrogen production method.
Driven by the increasing focus on sustainability and reduced environmental impact, electrolysis is the most suitable method for hydrogen production. While it may not be the cheapest option, its high efficiency and minimal greenhouse gas emissions align well with the UN’s SDGs for 2030. However, it is important to consider regional variations, energy source availability, and technological advancements in making a well-informed decision regarding hydrogen production.
6. Efficiency Trends in Hydrogen Electrolysis and Fuel Cells Advances and Challenges
The efficiency of different hydrogen production methods through electrolysis is a critical factor in the quest for sustainable energy generation [
112]. Exploring the efficiency trends of various hydrogen electrolysis methods brings to light the need to examine a pivotal aspect of hydrogen production that directly impacts its viability and adoption.
The objective of this section is to offer a detailed account of the efficiency trends of three principal hydrogen electrolysis techniques, PEM, AWE, and SOE, spanning the years from 1998 to 2020 to show the evolution of the different efficiency trends in hydrogen electrolysis methods.
Over the past two decades, the landscape of hydrogen production through electrolysis methods has witnessed remarkable progress. This transformation is illustrated in the efficiency trends of
Table 4 for three primary methods: the proton exchange membrane (PEM) method, AWE, and solid oxide electrolysis (SOE). The hydrogen production journey began in 1998, with PEM boasting an initial efficiency of 55%. Though there was a brief hiatus in the data, after 2010, PEM efficiency showed an impressive upswing, culminating at 90% in 2020. This upward trajectory underscores the substantial technological advancements within PEM electrolysis research, and this progress highlights why it may be considered the most promising technology for hydrogen production.
Table 4.
Efficiency of different hydrogen electrolysis methods.
Table 4.
Efficiency of different hydrogen electrolysis methods.
| Year | PEM | AWE | SOE | Reference |
|---|
| 1998 | 55% | - | - | [115] |
| 2002 | 55% | - | - | [101,113] |
| 2004 | 60% | 60–70 | 65% | [13,27] |
| 2010 | 55% | 61% | 98% 1 | [24,30,119] |
| 2012 | 65% | 60% | | [28,120,121] |
| 2014 | 70% | 70–75% | - | [14,27] |
| 2015 | 70% | 70–75% | 83% 2 | [40,117] |
| 2017 | 74.1% | 73% | 68–66% | [29,69] |
| 2019 | 74% | 80% | - | [26] |
| 2020 | 90% | 80% | 98% 3 | [22,23,116] |
| 2022 |
Similarly, AWE efficiency data, available from 2004 onwards, reveal a steady climb from an efficiency range of 60–70% to a robust 80% in 2020. This incremental improvement in AWE efficiency underscores the continued progress and innovation of this method.
Most striking is the journey of solid oxide electrolysis (SOE), which showcased media of 83% efficiency in 2015. It is important to note that SOE operates at very high temperatures, having been tested at 650 °C in 2010 and using water vapor rather than liquid water. This method is still considered water electrolysis but instead involves converting steam to hydrogen and oxygen, achieving a high efficiency milestone of 98%. However, the economic feasibility of the SOE method is highly sensitive to factors such as the initial cost of the SOE stack, stack lifetime, and local electricity prices. This sensitivity analysis indicated that the long payback time and high investment costs may challenge its economic viability [
110].
The upward trajectory of efficiency trends in hydrogen production by electrolysis methods sets the stage for the immediate and future viability of hydrogen as a clean energy carrier. Substantial technological advancements show that the ongoing global shift toward cleaner and more sustainable energy solutions is gaining momentum. The steady evolution of hydrogen electrolysis methods, in synergy with renewable energy sources such as photovoltaic systems, is primed to catalyze the development of cleaner energy technologies.
6.1. Fuel Cells
Ruled by the same principle as electrolyzers, fuel cells are classified primarily by the kind of electrolyte they use. The classification determines the kind of chemical reactions that occur within the cell, as well as its operational characteristics. The main types of fuel cells based on electrolyte composition include PEMFC, solid oxide fuel cells (SOFC), and alkaline fuel cells (AFC). Each type has its own advantages, limitations, and suitability for different applications, ranging from portable electronics to stationary power generation. Therefore, understanding electrolyte classification is crucial for grasping the functionalities and potential applications of fuel cells.
As a result of the development of new technologies for generating electrical energy from hydrogen, several institutions have been established worldwide, such as the IPHE, IRENA, IEA, EHA, and NREL, among others, which facilitate the transition of knowledge and provide guidance to nations on working toward a common objective, accelerating the transition process toward new green technologies. Furthermore,
Table 5 describes according to country the status of the development of the three main fuel cells at present: PEMFCs, SOFCs, and AFCs.
Table 5 presents the status of fuel cell technology across different countries and showcases a diverse landscape of advancements and priorities. In the area of PEMFCs, the United States and China stand out as leaders in hydrogen production, while countries like Germany, Spain, and France are spearheading the development of green hydrogen through solar energy. SOFCs see Europe implementing new materials and fabrication processes, with China and the United States prioritizing research to enhance the technology’s reliability. Japan leads in the demonstration of large-scale SOFC deployment. AFCs enable the United States to boast the lowest capital cost of implementation, while China focuses on hydrogen production at varying scales. In general terms, the approaches taken by nations to drive fuel cell technology forward emphasize the importance of international collaboration in fostering sustainable energy solutions.
6.2. Current Challenges in Fuel Cell Technology
Fuel cells, while extremely promising in their potential to provide clean and efficient energy, face some critical challenges that may slow down their implementation. These challenges include high manufacturing costs, the relatively low energy density of hydrogen, safety concerns, fuel cell durability issues, inadequate hydrogen refueling infrastructure, and the complexities of hydrogen storage and transportation [
9,
145].
One significant issue is the reduction of platinum in the catalyst, which is essential to lower costs and enhance the scalability of fuel cells. Platinum is a precious metal, meaning that its high cost is one reason for the limitation of the widespread adoption of fuel cell technology [
146,
147]. Research is focused on finding alternative materials or reducing the amount of platinum required to develop a fuel cell without compromising the efficiency of the cell.
Another critical challenge is hydrogen storage and transportation. Efficient and safe storage of hydrogen is crucial for the viability of fuel cells, especially in mobile applications. Current storage methods, such as high-pressure tanks and cryogenic storage, are presenting challenges in terms of safety, cost, and energy density [
148,
149]. Researchers are exploring advanced materials, such as metal–organic frameworks (MOFs) and chemical hydrogen storage, to overcome this hurdle [
150,
151,
152].
6.3. Improvements in Fuel Cell Efficiency
Improving the efficiency of fuel cells is essential for enhancing their viability and broader adoption in various applications. Recent research highlighted several strategies to achieve this goal, focusing on technological advancements [
153,
154,
155].
One significant approach is the cogeneration of heat and power. Cogeneration, also known as combined heat and power, involves the simultaneous production of electricity and useful heat from the same energy source. This approach not only optimizes energy production efficiency but also contributes to a more sustainable energy system [
156,
157]. By capturing and utilizing the waste heat produced during electrochemical processes, the overall efficiency of the fuel cell system may be significantly increased [
158]. This approach is essential to make progress in fuel cell technology and achieve broader adoption.
Water and thermal management for PEMFCs is crucial for maintaining performance and durability. Effective water management ensures that the membrane remains hydrated, which is necessary to maintain proton conductivity in cells. Thermal management involves controlling the temperature within the fuel cell to prevent overheating and ensure optimal operating conditions. Implementing advanced cooling systems and better water management strategies can significantly improve the overall efficiency and reliability of fuel cells [
159,
160,
161].
Moreover, the integration of advanced control systems can help optimize the performance of fuel cells by adjusting operating parameters in real time to match the demand and conditions. Control systems developed using MATLAB and Simulink enable the simulation of complex fuel cell behaviors and could help with the integration of various auxiliary components such as air and hydrogen supply lines, cooling circuits, and the PEMFC stack unit [
162]. These systems could also incorporate advanced algorithms such as adaptive neural fuzzy inference systems and fuzzy logic controllers, which are effective in managing nonlinearities and ensuring optimal operation under varying conditions [
163]. Additionally, distributed intelligence- and agent-based control architectures have been shown to enhance the efficiency and autonomy of PEM fuel cells by enabling real-time adjustments based on sensory and contextual information [
164]. These advancements in control strategies are crucial for reducing degradation, improving energy management, and extending the lifespan of fuel cell systems [
165].
7. Recent Advancements in Hydrogen Production and Storage Technologies
The pursuit of sustainable and efficient hydrogen production methods has led to significant advancements in technology. These cutting-edge innovations are pivotal in addressing the global energy demand while mitigating environmental impacts. This section explores the latest developments in hydrogen production techniques, highlighting their potential to revolutionize the energy sector [
25].
7.1. Hydrogen Production
Advancements in hydrogen production technologies are focused on improving the efficiency of the systems, as well as reducing costs and enhancing sustainability.
Table 6 summarizes some of the most recent and innovative methods developed in the field.
Table 6 provides a detailed comparison of the various advanced hydrogen production technologies, highlighting their unique strengths and key developments. These technologies showcase the diverse approaches that can exist and that are being developed to meet the global demand for sustainable hydrogen. Ongoing research and innovations are crucial for enhancing these technologies and contributing to the advancement of the hydrogen economy.
An important aspect of the newer hydrogen production methods is their integration with carbon capture, utilization, and storage (CCUS). These technologies capture the carbon dioxide emissions produced during hydrogen production and either utilize them in another industrial process or could store them underground. This integration is crucial for minimizing the carbon footprint of hydrogen production [
172,
173,
174]. Recent research focuses on optimizing CCUS processes to enhance their efficiency and feasibility when they are combined with various production methods [
175,
176,
177,
178].
7.2. Hydrogen Storage Technologies
The rapid development of hydrogen production means that an efficient hydrogen storage system is crucial for the viability of hydrogen as an energy carrier. Various hydrogen storage technologies are being explored and developed to meet this necessity.
Table 7 examines recent developments in hydrogen storage technologies.
Overall, these technological advancements are critical for overcoming the existing limitations of hydrogen storage and are essential for the successful integration of hydrogen into the energy landscape. The continuous improvement in storage capacities, release mechanisms, and system controls shows the potential of hydrogen as a key player in achieving sustainable energy goals. This progress not only supports the development of hydrogen infrastructure but also promotes the transition toward a more sustainable and resilient energy ecosystem.
8. Discussion
Based on previous sections, this evaluation of hydrogen production methods, considering key factors such as efficiency, environmental impact, and associated costs, as discussed earlier, reaches the following conclusions.
Hydrogen production plays a key role in the search for sustainable energy solutions, in accordance with the United Nations’ Sustainable Development Goals for 2030, specifically Goals 7 and 13, addressing affordable and clean energy and climate action.
As mentioned in
Section 2, energy production tops the list of GHG emissions, contributing up to 34% of the total gases emitted into the atmosphere (20 GtCO
2eq). From this evaluation, hydrogen emerges as a sustainable solution for electricity generation. As presented in
Table 3 of
Section 4, hydrogen production methods can be identified that do not cause significant harm to the environment. Photolysis stands out as the least serious offender, producing 0 kg of CO
2 per kg of H
2, while thermolysis emerges as the major contributor, with 20 kg of CO
2 per kg of H
2 produced.
The methodology employed played a significant role in how the findings in this article were presented. The information presented in this study is derived from a wide range of extensive sources. These sources provide detailed specifications for only one of the methods that were presented and lack a comprehensive comparison of the most commonly used hydrogen production methods.
Table 1 summarizes the three key factors influencing hydrogen production: efficiency, environmental impact, and costs.
Similarly,
Figure 7,
Figure 8 and
Figure 9 provide great visibility for important aspects that must be considered to achieve an effective transition toward a more sustainable energy society. The tables and figures presented herein provide a better focus on the topic discussed, given the current scarcity of information. This was possible by accurately and truthfully compiling the data presented in various articles, journals, and books over the years. This is evident in
Table 4, where an analysis of the data from 1998–2022 is provided.
A comparison among different production methods can be achieved through the three most important aspects: efficiency, costs, and the amount of GHG emitted. Here, the efficiency of the PEM electrolysis process stands out (up to 90%), along with how sustainable it can be, with a maximum of 2 kg of CO2 per kg of H2 produced. Together with the steam-iron process and microbial electrolysis cells, these production methods have the lowest environmental impact.
In this same section, the different production costs of each method were finally related, showing that renewable feedstock and energy have the highest prices, led by photolysis (USD 10.36) and electrolysis, from USD 4.22 to USD 6.27. In contrast, the lowest costs are obtained when using fossil fuels as feedstock, such as in pyrolysis (USD 1.25).
Efficiency is crucial, as it directly impacts the amount of hydrogen generated per unit of energy consumed. High efficiency implies a more resource-efficient process, which is essential for achieving sustainable energy production. Environmental impact is another pivotal consideration, especially in terms of addressing climate action. A production method that minimizes greenhouse gas emissions aligns with the global commitment to mitigate climate change. Moreover, cost analysis is integral to making these methods practical and economically viable for broad-scale implementation.
The efficiency of different hydrogen production methods through electrolysis is a critical factor in the quest for sustainable energy generation.
Table 4 in
Section 6 shows the trend of various hydrogen electrolysis methods, wherein SOE stands out with a remarkable efficiency surge; however, the dynamic nature of the SOE developmental phase introduces variability to the data. In addition, other technologies such as PEM have shown significant development over the years. AWE has improved its current efficiency to 80% since it entered the market in 2004 with only 60%.
These data indicate that the upward trajectory of efficiency trends in hydrogen production by electrolysis methods paves the way for the immediate and future viability of hydrogen as a clean energy carrier. Hydrogen production is looking to play a critical role in the global transition to sustainability.
The energy supply system is still years away from achieving 100% sustainable energy. As presented in
Section 6.2, there are still some challenges, such as the high cost of manufacturing, infrastructure, and safety. However, the current investment in research and development, as presented in
Section 7, will contribute greatly to this transition.
9. Conclusions
In this work, an assessment of the various feedstocks and their corresponding hydrogen production methods was undertaken, considering factors such as efficiency, greenhouse gas (GHG) emissions, production costs, and current challenges. This endeavor aimed to reduce the information gap surrounding hydrogen production methods, particularly in costs and GHG emissions. The primary focus was to obtain a relevant comparison contrasting the methods utilizing renewable energy sources with those relying on fossil fuels. Electrolysis stands out as a prime choice for clean hydrogen production. Notably, it contributes low to moderate GHG emissions, substantially diminishing carbon footprints compared to high-emission techniques. Based on the results revealed by the renewable energy sources of photolysis, electrolysis, gasification, and microbial electrolysis cells, it is noteworthy to emphasize that one of the key advantages of electrolysis lies in its adaptability regarding diverse energy sources, including clean electricity from renewables.
The data presented in this study aims to reduce the existing gap in available information on research into hydrogen production by collecting and summarizing the information scattered on the internet. The results shown are expected to facilitate the decision-making process, considering regional variations, energy source availability, and the potential for technological advancements that may further enhance the economic viability of electrolysis. As nations and industries strive to achieve their climate and sustainability targets, understanding the principal characteristics of hydrogen production methods becomes essential in this transformative journey toward a cleaner and more efficient energy future.
Author Contributions
Conceptualization, N.F.G.-R.; methodology, D.A.D.L.R.-L. and R.T.-M.; validation, F.A.R.-R., J.F.-G. and A.B.R.-B.; investigation, N.F.G.-R., D.A.D.L.R.-L. and R.T.-M.; data curation, R.T.-M. and D.A.D.L.R.-L.; writing—original draft preparation, D.A.D.L.R.-L. and R.T.-M.; writing—review and editing, N.F.G.-R., R.T.-M., D.A.D.L.R.-L., F.A.R.-R. and E.R.-A.; visualization, R.T.-M.; supervision, N.F.G.-R., F.A.R.-R., E.R.-A. and J.F.-G.; project administration, N.F.G.-R.; funding acquisition, N.F.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research is part of the ‘Design of control strategies to improve energy quality in grid-connected photovoltaic generators (2020-2021-3C3-072)’ project, funded by the MESCyT (Ministry of Higher Education Science and Technology) in the Dominican Republic, through Fondocyt 2020-2021. Furthermore, this work is part of the research carried out in the project ‘Development of methodologies based on solar-photovoltaic green hydrogen to stabilize the electrical grid and reduce the carbon footprint for electrical generation (2022-3C1-168)’, also funded by MESCyT through Fondocyt 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the MESCyT (Ministry of Higher Education, Science and Technology) for promoting the development of research in the Dominican Republic.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AFOLU | Agriculture, Forestry, and Other Land Uses |
| CCUS | Carbon Capture, Utilization and Storage |
| CHIRS | Compact Heat Integrated Reactor System |
| DGs | Distributed Generators |
| GHG | Greenhouse Gas |
| GtCO2eq | Gigatons of Carbon Dioxide Equivalent |
| H | Hydrogen |
| H2O | Water |
| ICE | Internal Combustion Engine |
| IEA | International Energy Agency |
| KOH | Potassium Hydroxide |
| LOHCs | Liquid Organic Hydrogen Carriers |
| MOFs | Advanced Metal-Organic Frameworks |
| NaOH | Sodium Hydroxide |
| O | Oxygen |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| SMR | Steam Methane Reforming |
| SOE | Solid Oxide Electrolysis |
| STCG | Solar Thermochemical Hydrogen |
References
- Dunn, S. Hydrogen Futures: Toward a Sustainable Energy System. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Žarković, M.; Lakić, S.; Ćetković, J.; Pejović, B.; Redzepagic, S.; Vodenska, I.; Vujadinović, R. Effects of Renewable and Non-Renewable Energy Consumption, GHG, ICT on Sustainable Economic Growth: Evidence from Old and New EU Countries. Sustainability 2022, 14, 9662. [Google Scholar] [CrossRef]
- de León, C.M.; Ríos, C.; Brey, J.J. Cost of Green Hydrogen: Limitations of Production from a Stand-Alone Photovoltaic System. Int. J. Hydrogen Energy 2023, 48, 11885–11898. [Google Scholar] [CrossRef]
- Gabric, A.J. The Climate Change Crisis: A Review of Its Causes and Possible Responses. Atmosphere 2023, 14, 1081. [Google Scholar] [CrossRef]
- Short, J.R.; Farmer, A. Cities and Climate Change. Earth 2021, 2, 1038–1045. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid Inorganic-Organic Proton-Conducting Membranes Based on SPEEK Doped with WO3 Nanoparticles for Application in Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- He, Y. Renewable and Non-Renewable Energy Consumption and Trade Policy: Do They Matter for Environmental Sustainability? Energies 2022, 15, 3559. [Google Scholar] [CrossRef]
- Geopolitics of the Energy Transformation: The Hydrogen Factor; IRENA: New York, NY, USA, 2022.
- Hassan, Q.; Azzawi, I.D.J.; Sameen, A.Z.; Salman, H.M. Hydrogen Fuel Cell Vehicles: Opportunities and Challenges. Sustainability 2023, 15, 11501. [Google Scholar] [CrossRef]
- Liao, C.H.; Huang, C.W.; Wu, J.C.S. Hydrogen Production from Semiconductor-Based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Yousaf, M.; Mahmood, A.; Elkamel, A.; Rizwan, M.; Zaman, M. Techno-Economic Analysis of Integrated Hydrogen and Methanol Production Process by CO2 Hydrogenation. Int. J. Greenh. Gas Control. 2022, 115, 103615. [Google Scholar] [CrossRef]
- Ball, M.; Weeda, M. The Hydrogen Economy—Vision or Reality? In Compendium of Hydrogen Energy: Hydrogen Use, Safety and the Hydrogen Economy; Woodhead Publishing: Cambridge, UK, 2016; Volume 4, pp. 237–266. [Google Scholar] [CrossRef]
- Levin, D.B.; Chahine, R. Challenges for Renewable Hydrogen Production from Biomass. Int. J. Hydrogen Energy 2010, 35, 4962–4969. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrogen Production by Alkaline Water Electrolysis. Quim. Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Kyung, D.; Yu, C.-H.; Lin, Y.-P.; Yap, J.; Mclellan, B. A Historical Analysis of Hydrogen Economy Research, Development, and Expectations, 1972 to 2020. Environments 2023, 10, 11. [Google Scholar] [CrossRef]
- Brandon, N.P.; Kurban, Z. Clean Energy and the Hydrogen Economy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160400. [Google Scholar] [CrossRef] [PubMed]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics: A Review. Renew. Sustain. Energy Rev. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Scita, R.; Raimondi, P.P.; Noussan, M. Green Hydrogen: The Holy Grail of Decarbonisation? An Analysis of the Technical and Geopolitical Implications of the Future Hydrogen Economy. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Clark, W.W.; Rifkin, J. A Green Hydrogen Economy. Energy Policy 2006, 34, 2630–2639. [Google Scholar] [CrossRef]
- Sarker, A.K.; Azad, A.K.; Rasul, M.G.; Doppalapudi, A.T. Prospect of Green Hydrogen Generation from Hybrid Renewable Energy Sources: A Review. Energies 2023, 16, 1556. [Google Scholar] [CrossRef]
- Marnellos, E.; Zun, M.T.; Mclellan, B.C. Cost Projection of Global Green Hydrogen Production Scenarios. Hydrogen 2023, 4, 932–960. [Google Scholar] [CrossRef]
- Liu, Z.; Han, B.; Lu, Z.; Guan, W.; Li, Y.; Song, C.; Chen, L.; Singhal, S.C. Efficiency and Stability of Hydrogen Production from Seawater Using Solid Oxide Electrolysis Cells. Appl. Energy 2021, 300, 117439. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and Perspectives of Key Materials for PEM Electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Pandian, M.S.; Anwari, M.; Husodo, B.Y.; Hiendro, A. Efficiency and Economics Analysis of Proton Exchange Membrane Fuel Cell. In Proceedings of the 2010 9th International Power and Energy Conference, IPEC 2010, Singapore, 27–29 October 2010; pp. 875–880. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- James, B.D.; Nrel, J.M.M.; Saur, G.; Ramsden, T. Techno-Economic Analysis of PEM Electrolysis for Hydrogen Production; U.S. Department of Energy: Golden, CO, USA, 2014.
- Zhang, H.; Su, S.; Lin, G.; Chen, J. Efficiency Calculation and Configuration Design of a PEM Electrolyzer System for Hydrogen Production. Int. J. Electrochem. Sci 2012, 7, 4143–4157. [Google Scholar] [CrossRef]
- Dobó, Z.; Palotás, Á.B. Impact of the Voltage Fluctuation of the Power Supply on the Efficiency of Alkaline Water Electrolysis. Int. J. Hydrogen Energy 2016, 41, 11849–11856. [Google Scholar] [CrossRef]
- Wang, Z.; Mori, M.; Araki, T. Steam Electrolysis Performance of Intermediate-Temperature Solid Oxide Electrolysis Cell and Efficiency of Hydrogen Production System at 300 Nm3 H−1. Int. J. Hydrogen Energy 2010, 35, 4451–4458. [Google Scholar] [CrossRef]
- Scheepers, F.; Stähler, M.; Stähler, A.; Rauls, E.; Müller, M.; Carmo, M.; Lehnert, W. Improving the Efficiency of PEM Electrolyzers through Membrane-Specific Pressure Optimization. Energies 2020, 13, 612. [Google Scholar] [CrossRef]
- Ang, T.-Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A Comprehensive Study of Renewable Energy Sources: Classifications, Challenges and Suggestions. Energy Strategy Rev. 2022, 43, 2211–2467. [Google Scholar] [CrossRef]
- Nehrir, M.H.; Wang, C. Modeling and Control of Fuel Cells: Distributed Generation Applications; Wiley: New York, NY, USA, 2009; p. 296. [Google Scholar]
- Rosen, M.A.; Koohi-Fayegh, S. The Prospects for Hydrogen as an Energy Carrier: An Overview of Hydrogen Energy and Hydrogen Energy Systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Darko, C.K.; Obiako, P.C.; Kuang, B.; Sun, X.; Jenkins, K. A Comparative Analysis of Different Hydrogen Production Methods and Their Environmental Impact. Clean Technol. 2023, 5, 1344–1380. [Google Scholar] [CrossRef]
- da Rosa, A.V.; Ordóñez, J.C. Hydrogen Production. In Fundamentals of Renewable Energy Processes; Academic Press: Cambridge, MA, USA, 2022; pp. 419–470. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen Production. In Solar Hydrogen Production: Processes, Systems and Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 45–83. [Google Scholar] [CrossRef]
- Ayers, K.E.; Anderson, E.B.; Capuano, C.; Carter, B.; Dalton, L.; Hanlon, G.; Manco, J.; Niedzwiecki, M. Research Advances towards Low Cost, High Efficiency PEM Electrolysis. ECS Trans. 2010, 33, 3–15. [Google Scholar] [CrossRef]
- Hinkley, J.; Hayward, J.; Mcnaughton, R.; Gillespie, R.; Watt, M.; Lovegrove, K. Cost Assessment of Hydrogen Production from PV and Electrolysis Ayako Matsumoto (Mitsui Global Strategic Studies Institute); CSIRO: Perth, Australia, 2016. [Google Scholar]
- Rashid, M.; Khaloofah, M.; Mesfer, A.; Naseem, H.; Danish, M.; Mesfer, M.K. Al Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 2249–8958. [Google Scholar]
- Wang, A.; Shao, P.; Lan, F.; Jin, H. Organic Chemicals in Coal Available to Microbes to Produce Biogenic Coalbed Methane: A Review of Current Knowledge. J. Nat. Gas Sci. Eng. 2018, 60, 40–48. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D. A Comprehensive Review on Recent Advancements in Thermochemical Processes for Clean Hydrogen Production to Decarbonize the Energy Sector. Sustainability 2022, 14, 11206. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. 3.1 Hydrogen Production. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; Volume 3, pp. 1–40. [Google Scholar] [CrossRef]
- Office of Fossil Energy United States Department of Energy. Hydrogen Strategy Enabling A Low-Carbon Economy; United States Department of Energy: Washington, DC, USA, 2020.
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Weiland, N. Systems Analysis on Biomass Gasification to Carbon-Negative Hydrogen; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2023. [Google Scholar]
- Norton, B.; Eames, P.C.; Lo, S.N.G. Full-Energy-Chain Analysis of Greenhouse Gas Emissions for Solar Thermal Electric Power Generation Systems. Renew Energy 1998, 15, 131–136. [Google Scholar] [CrossRef]
- Falter, C.; Sizmann, A. Solar Thermochemical Hydrogen Production in the USA. Sustainability 2021, 13, 7804. [Google Scholar] [CrossRef]
- Acevedo, Y.; Prosser, J.; Huya-Kouadio, J.; McNamara, K.; James, B. Hydrogen Production Cost with Alkaline Electrolysis; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2023. [Google Scholar]
- Kotisaari, M.; Thomann, O.; Montinaro, D.; Kiviaho, J. Evaluation of a SOE Stack for Hydrogen and Syngas Production: A Performance and Durability Analysis. Fuel Cells 2017, 17, 571–580. [Google Scholar] [CrossRef]
- Yu, J.; Ju, F.; Wahab, M.; Agyekum, E.B.; Matasane, C.; Uhunamure, S.E. Estimating the Effects of Economic Complexity and Technological Innovations on CO2 Emissions: Policy Instruments for N-11 Countries. Sustainability 2022, 14, 16856. [Google Scholar] [CrossRef]
- Liu, H.; Clausen, L.R.; Wang, L.; Chen, M. Pathway toward Cost-Effective Green Hydrogen Production by Solid Oxide Electrolyzer. Energy Env. Sci 2023, 16, 2090–2111. [Google Scholar] [CrossRef]
- World Nuclear Association. Carbon Dioxide Emissions from Electricity. Available online: https://world-nuclear.org/information-library/energy-and-the-environment/carbon-dioxide-emissions-from-electricity (accessed on 17 September 2023).
- Greenhouse Gas Emissions from Energy Data Explorer—Data Tools—IEA. Available online: https://www.iea.org/data-and-statistics/data-tools/greenhouse-gas-emissions-from-energy-data-explorer (accessed on 17 September 2023).
- Methodology for Determining the Greenhouse Gas Emissions Associated with the Production of Hydrogen. Available online: https://unece.org/sites/default/files/2023-09/2.%20Laurent%20Antoni%20-2023_09_12_H2_Antoni_IPHE.pdf (accessed on 17 September 2023).
- Our World in Data. Greenhouse Gas Emissions by Sector, World. 2023. Available online: https://ourworldindata.org/grapher/ghg-emissions-by-sector (accessed on 17 September 2023).
- World Energy Balances—Data Product—IEA. Available online: https://www.iea.org/data-and-statistics/data-product/world-energy-balances (accessed on 17 September 2023).
- GHG Protocol. Scope 2 Guidance. Available online: https://ghgprotocol.org/sites/default/files/2023-03/Scope%202%20Guidance.pdf (accessed on 12 November 2023).
- United Nations Sustainable Development Goals. 2020. Available online: https://unstats.un.org/sdgs/report/2020/The-Sustainable-Development-Goals-Report-2020.pdf (accessed on 17 September 2023).
- Executive Summary—Energy Efficiency 2021—Analysis—IEA. Available online: https://www.iea.org/reports/energy-efficiency-2021/executive-summary (accessed on 17 September 2023).
- Ritchie, H.; Roser, M.; Rosado, P. Energy. 2022. Available online: https://ourworldindata.org/energy (accessed on 17 September 2023).
- GHG Protocol. IPCC Emissions Factor Database. Available online: https://ghgprotocol.org/Third-Party-Databases/IPCC-Emissions-Factor-Database (accessed on 4 May 2023).
- Zhou, Y.; Li, R.; Lv, Z.; Liu, J.; Zhou, H.; Xu, C. Green Hydrogen: A Promising Way to the Carbon-Free Society. Chin. J. Chem. Eng. 2022, 43, 2–13. [Google Scholar] [CrossRef]
- Thanapalan, K.K.T.; Williams, J.G.; Liu, G.P.; Rees, D. Modelling of a pem fuel cell system. IFAC Proc. Vol. 2008, 41, 4636–4641. [Google Scholar] [CrossRef]
- Montilla-DJesus, M.; Santos-Martin, D.; Arnaltes, S.; Castronuovo, E.D. Optimal Reactive Power Allocation in an Offshore Wind Farms with LCC-HVdc Link Connection. Renew Energy 2012, 40, 157–166. [Google Scholar] [CrossRef]
- Welcome to the International Association for Hydrogen Energy. Available online: https://www.iahe-hyes.org/ (accessed on 26 June 2023).
- Iliev, I.K.; Beloev, H.I.; Ilieva, D.I.; Badur, J. A Novel Method for Calculating Greenhouse Gas Emissions from the Combustion of Energy Fuels. Arch. Thermodyn. 2023, 43, 3–20. [Google Scholar] [CrossRef]
- Harrison, K.W.; Remick, R.; Martin, G.D. Hydrogen Production: Fundamentals and Case Study Summaries. In Proceedings of the 18th World Hydrogen Energy Conference, Essen, Germany, 16–20 May 2010. [Google Scholar]
- Barelli, L.; Bidini, G.; Cinti, G. Airflow Management in Solid Oxide Electrolyzer (SOE) Operation: Performance Analysis. ChemEngineering 2017, 1, 13. [Google Scholar] [CrossRef]
- Keçebaş, A.; Kayfeci, M. Hydrogen Properties. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–29. [Google Scholar]
- Nastasi, B. Hydrogen Policy, Market, and R&D Projects. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–44. [Google Scholar]
- Karellas, S.; Roumpedakis, T.C. Solar Thermal Power Plants. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–235. [Google Scholar]
- Keçebaş, A.; Kayfeci, M.; Bayat, M. Electrochemical Hydrogen Generation. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–317. [Google Scholar]
- Touloupakis, E.; Torzillo, G. Photobiological Hydrogen Production. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 511–525. [Google Scholar]
- Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Valades-Pelayo, P.J.; Romero-Paredes, H.; Cuentas-Gallegos, A.K.; Arreola-Ramos, C.E. Hydrogen from Solar Thermal Energy. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–363. [Google Scholar]
- Guzmán, H.; Farkhondehfal, M.A.; Tolod, K.R.; Hernández, S.; Russo, N. Photo/Electrocatalytic Hydrogen Exploitation for CO2 Reduction toward Solar Fuels Production. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 365–418. [Google Scholar]
- Narayanan, H.; Viswanathan, B.; Krishnamurthy, K.R.; Nair, H. Hydrogen from Photo-Electrocatalytic Water Splitting. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 419–486. [Google Scholar]
- Harrison, K.W.; Remick, R.; Martin, G.D.; Hoskin, A. Hydrogen Production: Fundamentals and Cas Study Summaries; National Renewable Energy Laboratory: Golden, CO, USA, 2010; preprint. [Google Scholar]
- Möller, M.C.; Krauter, S. Hybrid Energy System Model in Matlab/Simulink Based on Solar Energy, Lithium-Ion Battery and Hydrogen. Energies 2022, 15, 2201. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent Development of Hydrogen and Fuel Cell Technologies: A Review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Ammendola, P.; Yan, X.; Zheng, W.; Wei, Y.; Yan, Z. Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes 2024, 12, 315. [Google Scholar] [CrossRef]
- Report on the Status of the Solid Oxide Fuel Cell Program; United States Department of Energy: Washington, DC, USA, 2019.
- Williams, M.C.; Vora, S.D.; Jesionowski, G. Worldwide Status of Solid Oxide Fuel Cell Technology. ECS Trans 2020, 96, 1–10. [Google Scholar] [CrossRef]
- Next Generation Solid Oxide Fuel Cell and Electrolysis Technology|NewSOC|Project|Fact Sheet|H2020|CORDIS|European Commission. Available online: https://www.clean-hydrogen.europa.eu/projects-dashboard/projects-repository/newsoc_en (accessed on 18 March 2023).
- Rathore, S.S.; Biswas, S.; Fini, D.; Kulkarni, A.P.; Giddey, S. Direct Ammonia Solid-Oxide Fuel Cells: A Review of Progress and Prospects. Int. J. Hydrogen Energy 2021, 46, 35365–35384. [Google Scholar] [CrossRef]
- Smolinka, T. Fuels—Hydrogen Production|Water Electrolysis. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 394–413. [Google Scholar] [CrossRef]
- United States Department of Energy. DOE Hydrogen Program Record 23003: Electrolyzer Installations in the United States; United States Department of Energy: Washington, DC, USA, 2023.
- Innovation in Alkaline Electrolysis—Stargate Hydrogen. Available online: https://stargatehydrogen.com/why-us/innovation-in-alkaline-electrolysis/ (accessed on 18 March 2023).
- 100MW Green Hydrogen Plant. Available online: https://www.sinohyenergy.com/100mw-green-hydrogen-plant/ (accessed on 17 March 2023).
- Laurent For More Information on HyDeal Ambition and Its Ecosystem of Production-Supply of Green Hydrogen at Competitive Prices, Please Contact. Available online: https://mcphy.com/en/news/hydeal-ambition/ (accessed on 17 March 2023).
- Wappler, M.; Unguder, D.; Lu, X.; Ohlmeyer, H.; Teschke, H.; Lueke, W. Building the Green Hydrogen Market e Current State and Outlook on Green Hydrogen Demand and Electrolyzer Manufacturing. Int. J. Hydrogen Energy 2022, 47, 33551–33570. [Google Scholar] [CrossRef]
- UNFCCC. Greenhouse Gas Emissions Calculator. Available online: https://unfccc.int/documents/271269 (accessed on 17 March 2023).
- Padro, C.E.G.; Putsche, V. Survey of the Economics of Hydrogen Technologies; National Renewable Energy Laboratory: Golden, CO, USA, 1999. [Google Scholar] [CrossRef]
- Hill, N.; Bramwell, R.; Karagianni, E.; Jones, L.; Maccarthy, J.; Hinton, S.; Walker, C. 2020 Government Greenhouse Gas Conversion Factors for Company Reporting Methodology Paper for Conversion Factors Final Report 2; Department for Business, Energy & Industrial Strategy (BEIS): London, UK, 2020.
- Global Average Levelised Cost of Hydrogen Production by Energy Source and Technology, 2019 and 2050—Charts—Data & Statistics—IEA. Available online: https://www.iea.org/data-and-statistics/charts/global-average-levelised-cost-of-hydrogen-production-by-energy-source-and-technology-2019-and-2050 (accessed on 10 March 2024).
- GHG Protocol. Calculation Tools and Guidance. Available online: https://ghgprotocol.org/calculation-tools-and-guidance (accessed on 7 March 2023).
- Vickers, J.; Peterson, D.; Randolph, K.; Irwin, L.; Desantis, D.; Hamdan, M.; Stetson, N.; Miller, E.; Satyapal, S. DOE Hydrogen and Fuel Cells Program Record Title: Cost of Electrolytic Hydrogen Production with Existing Technology. 2000. Available online: https://www.hydrogen.energy.gov/docs/hydrogenprogramlibraries/pdfs/20004-cost-electrolytic-hydrogen-production.pdf?Status=Master (accessed on 7 March 2023).
- Bossel, U.; Eliasson, B. Energy Hydrogen Economy. Available online: https://afdc.energy.gov/files/pdfs/hyd_economy_bossel_eliasson.pdf (accessed on 17 September 2023).
- Home International Partnership for Hydrogen&Fuel Cells in the Economy. Available online: https://www.energy.gov/eere/fuelcells/international-partnership-hydrogen-and-fuel-cells-economy (accessed on 1 December 2023).
- Hydrogen Fuel Cell Engines Module 4: Fuel Cell Engine Technology Contents. 2022. Available online: https://www.energy.gov/sites/default/files/2014/03/f12/fcm04r0.pdf (accessed on 17 September 2023).
- Janssen, J.L.L.C.C.; Weeda, M.; Detz, R.J.; van der Zwaan, B. Country-Specific Cost Projections for Renewable Hydrogen Production through off-Grid Electricity Systems. Appl. Energy 2022, 309, 118398. [Google Scholar] [CrossRef]
- Global Hydrogen Review 2021; OECD: Paris, France, 2021. [CrossRef]
- Nasser, M.; Megahed, T.F.; Ookawara, S.; Hassan, H. A Review of Water Electrolysis–Based Systems for Hydrogen Production Using Hybrid/Solar/Wind Energy Systems. Environ. Sci. Pollut. Res. 2022, 29, 86994–87018. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Yao, J.G.; Clough, P.T.; Da Costa, J.C.D.; Anthony, E.J.; Fennell, P.S.; Wang, W.; Zhao, M. Enhanced Hydrogen Production from Thermochemical Processes. Energy Environ. Sci 2018, 11, 2647–2672. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for Achieving Carbon Neutrality. Innovation 2021, 35, 48. [Google Scholar] [CrossRef]
- Felseghi, R.A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Ganci, F.; Baguet, T.; Aiello, G.; Cusumano, V.; Mandin, P.; Sunseri, C.; Inguanta, R. Nanostructured Ni Based Anode and Cathode for Alkaline Water Electrolyzers. Energies 2019, 12, 3669. [Google Scholar] [CrossRef]
- Karaca, A.E.; Qureshy, A.M.M.I.; Dincer, I. An Overview and Critical Assessment of Thermochemical Hydrogen Production Methods. J. Clean Prod. 2023, 385, 135706. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Balaji, R.; Ramakrishnan, S. Recent Developments in Hydrogen Fuel Cells: Strengths and Weaknesses. In Sustainable Fuel Technologies Handbook; Academic Press: Cambridge, MA, USA, 2021; pp. 431–456. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; van Herle, J.; Maréchal, F.; Desideri, U. Techno-Economic Optimization of CO2-to-Methanol with Solid-Oxide Electrolyzer. Energies 2019, 12, 3742. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Ozcan, H.; El-Emam, R.S.; Horri, B.A. Recent Advances in High-Temperature Steam Electrolysis with Solid Oxide Electrolysers for Green Hydrogen Production. Energies 2023, 16, 3327. [Google Scholar] [CrossRef]
- Yu, M.; Wang, K.; Vredenburg, H. Insights into Low-Carbon Hydrogen Production Methods: Green, Blue and Aqua Hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273. [Google Scholar] [CrossRef]
- Shapiro, D.S. PEM Electrolyzer and Gas Storage for a Regenerative Fuel Cell System. 2002. Available online: https://www.proquest.com/openview/4268d79e22093d975884c7d7a430714c/1?pq-origsite=gscholar&cbl=18750&diss=y (accessed on 17 September 2023).
- de Jesús Yaritza, M.L. Characterization of Proton Exchange Membrane Fuel Cell and PEM Electrolyzer Using Non-Steady State Electrochemical Techniques. 2004. Available online: https://scholar.uprm.edu/entities/publication/9b4c67f7-6117-474c-a84e-c73937062c8b (accessed on 17 September 2023).
- Knobbe, M.W. Analysis of PEM Electrolyzers; Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 1998. [Google Scholar]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Peters, R.; Deja, R.; Blum, L.; Nguyen, V.N.; Fang, Q.; Stolten, D. Influence of Operating Parameters on Overall System Efficiencies Using Solid Oxide Electrolysis Technology. Int. J. Hydrogen Energy 2015, 40, 7103–7113. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Supporting Information Achieving High Efficiency and Eliminating Degradation in Solid Oxide Electrochemical Cells Using High Oxygen-Capacity Perovskite. 2016. Available online: https://www.researchgate.net/publication/307949925_Achieving_High_Efficiency_and_Eliminating_Degradation_in_Solid_Oxide_Electrochemical_Cells_Using_High_Oxygen-Capacity_Perovskite (accessed on 17 September 2023).
- Laguna-Bercero, M.A. Recent Advances in High Temperature Electrolysis Using Solid Oxide Fuel Cells: A Review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced Alkaline Water Electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Badgett, A.; Ruth, M.; Pivovar, B. Economic Considerations for Hydrogen Production with a Focus on Polymer Electrolyte Membrane Electrolysis. In Electrochemical Power Sources: Fundamentals, Systems, and Applications Hydrogen Production by Water Electrolysis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 327–364. [Google Scholar] [CrossRef]
- Chanthakett, A.; Arif, M.T.; Khan, M.M.K.; Oo, A.M.T. Hydrogen Production from Municipal Solid Waste (MSW) for Cleaner Environment; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. Environmental Impact Assessment of Hydrogen Production via Steam Methane Reforming Based on Emissions Data. Energy Rep. 2022, 8, 13585–13595. [Google Scholar] [CrossRef]
- Uusitalo, V.; Väisänen, S.; Inkeri, E.; Soukka, R. Potential for Greenhouse Gas Emission Reductions Using Surplus Electricity in Hydrogen, Methane and Methanol Production via Electrolysis. Energy Convers. Manag. 2017, 134, 125–134. [Google Scholar] [CrossRef]
- Palmer, G.; Roberts, A.; Hoadley, A.; Dargaville, R.; Honnery, D. Life-Cycle Greenhouse Gas Emissions and Net Energy Assessment of Large-Scale Hydrogen Production via Electrolysis and Solar PV. Energy Environ. Sci 2021, 14, 5113–5131. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, D.P.; Gálvez, J.L.; Moreno, J.; González, A. Hydrogen Production from Fossil Fuels: Life Cycle Assessment of Technologies with Low Greenhouse Gas Emissions. Energy Fuels 2011, 25, 2194–2202. [Google Scholar] [CrossRef]
- Granovskii, M.; Dincer, I.; Rosen, M.A. Economic Aspects of Greenhouse Gas Emissions Reduction by Utilisation of Wind and Solar Energies to Produce Electricity and Hydrogen. In Proceedings of the 2006 IEEE EIC Climate Change Technology Conference, EICCCC 2006, Ottawa, ON, Canada, 10–12 May 2006. [Google Scholar] [CrossRef]
- Gemechu, E.D.; Kumar, A. The Environmental Performance of Hydrogen Production Pathways Based on Renewable Sources. In Renewable-Energy-Driven Future: Technologies, Modelling, Applications, Sustainability and Policies; Academic Press: Cambridge, MA, USA, 2021; pp. 375–406. [Google Scholar] [CrossRef]
- Busch, P.; Kendall, A.; Lipman, T. A Systematic Review of Life Cycle Greenhouse Gas Intensity Values for Hydrogen Production Pathways. Renew. Sustain. Energy Rev. 2023, 184, 113588. [Google Scholar] [CrossRef]
- Fereidooni, M.; Mostafaeipour, A.; Kalantar, V.; Goudarzi, H. A Comprehensive Evaluation of Hydrogen Production from Photovoltaic Power Station. Renew. Sustain. Energy Rev. 2018, 82, 415–423. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroǧlu, T.N. From Hydrocarbon to Hydrogen–Carbon to Hydrogen Economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Gallardo, F.I.; Ferrario, A.M.; Lamagna, M.; Bocci, E.; Garcia, D.A.; Baeza-Jeria, T.E. A Techno-Economic Analysis of Solar Hydrogen Production by Electrolysis in the North of Chile and the Case of Exportation from Atacama Desert to Japan. Int. J. Hydrogen Energy 2021, 46, 13709–13728. [Google Scholar] [CrossRef]
- Ayers, K.E.; Capuano, C.; Anderson, E.B. Recent Advances in Cell Cost and Efficiency for PEM-Based Water Electrolysis. ECS Trans. 2012, 41, 15–22. [Google Scholar] [CrossRef]
- Babic, U.; Suermann, M.; Büchi, F.N.; Gubler, L.; Schmidt, T.J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164, F387–F399. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC: Paris, France, 2006.
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel Cell and Electrolysis Cell Technologies and Hydrogen Infrastructure Development—A Review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Barone, G.; Buonomano, A.; Forzano, C.; Palombo, A. Solar Thermal Collectors. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 151–178. [Google Scholar]
- Verma, A. Life Cycle Assessment and Greenhouse Gas Abatement Costs of Hydrogen Production from Underground Coal Gasification. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2015. [Google Scholar]
- Buffo, G.; Marocco, P.; Ferrero, D.; Lanzini, A.; Santarelli, M. Power-to-X and Power-to-Power Routes. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 529–557. [Google Scholar]
- Bayod-Rújula, A.A. Solar Photovoltaics (PV). In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–295. [Google Scholar]
- Beijing SinoHy Energy Co., Ltd. Available online: https://www.sinohyenergy.com/ (accessed on 13 March 2024).
- Fakhreddine, O.; Gharbia, Y.; Derakhshandeh, J.F.; Amer, A.M. Challenges and Solutions of Hydrogen Fuel Cells in Transportation Systems: A Review and Prospects. World Electr. Veh. J. 2023, 14, 156. [Google Scholar] [CrossRef]
- Kovtunenko, V.A. The Holby–Morgan Model of Platinum Catalyst Degradation in PEM Fuel Cells: Range of Feasible Parameters Achieved Using Voltage Cycling. Technologies 2023, 11, 184. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H.; Kim, M.P.; Park, C.H.; Tran, Q.N. Recent Developments in Materials for Physical Hydrogen Storage: A Review. Materials 2024, 17, 666. [Google Scholar] [CrossRef] [PubMed]
- Khzouz, M.; Gkanas, E.I. Hydrogen Technologies for Mobility and Stationary Applications: Hydrogen Production, Storage and Infrastructure Development. In Renewable Energy—Resources, Challenges and Applications; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Borja, N.K.; Fabros, C.J.E.; Doma, B.T. Prediction of Hydrogen Adsorption and Moduli of Metal–Organic Frameworks (MOFs) Using Machine Learning Strategies. Energies 2024, 17, 927. [Google Scholar] [CrossRef]
- MOFs for Advanced Applications; MDPI: Basel, Switzerland, 2022; ISBN 978-3-0365-3542-5.
- Zeleňák, V.; Saldan, I. Factors Affecting Hydrogen Adsorption in Metal–Organic Frameworks: A Short Review. Nanomaterials 2021, 11, 1638. [Google Scholar] [CrossRef] [PubMed]
- Dall’Armi, C.; Pivetta, D.; Taccani, R. Hybrid PEM Fuel Cell Power Plants Fuelled by Hydrogen for Improving Sustainability in Shipping: State of the Art and Review on Active Projects. Energies 2023, 16, 2022. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Prospects of Fuel Cell Combined Heat and Power Systems. Energies 2020, 13, 4104. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Renau, J.; García, V.; Domenech, L.; Verdejo, P.; Real, A.; Giménez, A.; Sánchez, F.; Lozano, A.; Barreras, F. Novel Use of Green Hydrogen Fuel Cell-Based Combined Heat and Power Systems to Reduce Primary Energy Intake and Greenhouse Emissions in the Building Sector. Sustainability 2021, 13, 1776. [Google Scholar] [CrossRef]
- Luo, Y.; Meng, Q.; Chi, Y.; Wang, Q.; Zeng, Y.; Deng, Z.; Zou, Y. A Low-Carbon Optimal Operation Method for an Industrial Park Multi-Energy Coupling System Utilizing By-Product Hydrogen. Sustainability 2024, 16, 2354. [Google Scholar] [CrossRef]
- Costa, P.; Pinto, F.; André, R.N.; Marques, P. Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes 2021, 9, 254. [Google Scholar] [CrossRef]
- Chakraborty, S.; Elangovan, D.; Palaniswamy, K.; Fly, A.; Ravi, D.; Seelan, D.A.S.; Rajagopal, T.K.R. A Review on the Numerical Studies on the Performance of Proton Exchange Membrane Fuel Cell (PEMFC) Flow Channel Designs for Automotive Applications. Energies 2022, 15, 9520. [Google Scholar] [CrossRef]
- Yan, S.; Yang, M.; Sun, C.; Xu, S. Liquid Water Characteristics in the Compressed Gradient Porosity Gas Diffusion Layer of Proton Exchange Membrane Fuel Cells Using the Lattice Boltzmann Method. Energies 2023, 16, 6010. [Google Scholar] [CrossRef]
- Tang, X.; Yang, M.; Shi, L.; Hou, Z.; Xu, S.; Sun, C. Adaptive State-of-Health Temperature Sensitivity Characteristics for Durability Improvement of PEM Fuel Cells. Chem. Eng. J. 2024, 491, 151951. [Google Scholar] [CrossRef]
- Corda, G.; Breda, S.; D’Adamo, A. A MATLAB/Simulink Model of a PEM Fuel Cell System Including Ageing Phenomenon; SAE: Warrendale, PA, USA, 2023. [Google Scholar]
- Safarishaal, M.; Sarvi, M. New Hybrid Maximum Power Point Tracking Methods for Fuel Cell Using Artificial Intelligent. AIP Adv. 2023, 13, 045207. [Google Scholar] [CrossRef]
- Rubio, A.; Agila, W.; González, L.; Aviles-Cedeno, J. Distributed Intelligence in Autonomous PEM Fuel Cell Control. Energies 2023, 16, 4830. [Google Scholar] [CrossRef]
- Shuai, Q.; Wang, Y.; Jiang, Z.; Hua, Q. Reinforcement Learning-Based Energy Management for Fuel Cell Electrical Vehicles Considering Fuel Cell Degradation. Energies 2024, 17, 1586. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, K.; Zhang, T.; Xia, J.; Wu, J.; Zhang, Z.; Zhang, B. Surface Modified CoCrFeNiMo High Entropy Alloys for Oxygen Evolution Reaction in Alkaline Seawater. Processes 2023, 11, 245. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J.; Moussab, H. Recent Advances on Hydrogen Production through Seawater Electrolysis. Mater. Sci. Energy Technol. 2020, 3, 780–807. [Google Scholar] [CrossRef]
- Sun, F.; Qin, J.; Wang, Z.; Yu, M.; Wu, X.; Sun, X.; Qiu, J. Energy-Saving Hydrogen Production by Chlorine-Free Hybrid Seawater Splitting Coupling Hydrazine Degradation. Nat. Commun. 2021, 12, 4182. [Google Scholar] [CrossRef] [PubMed]
- Khaodee, W.; Jiwanuruk, T.; Ountaksinkul, K.; Charojrochkul, S.; Charoensuk, J.; Wongsakulphasatch, S.; Assabumrungrat, S. Compact Heat Integrated Reactor System of Steam Reformer, Shift Reactor and Combustor for Hydrogen Production from Ethanol. Processes 2020, 8, 708. [Google Scholar] [CrossRef]
- Slobodkin, I.; Davydova, E.; Sananis, M.; Breytus, A.; Rothschild, A. Electrochemical and Chemical Cycle for High-Efficiency Decoupled Water Splitting in a near-Neutral Electrolyte. Nat. Mater. 2024, 23, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Abdelhamid, H.N.; Fouad, D.M.; El-Bery, H.M. Enhancing Photocatalytic Water Splitting: Comparative Study of TiO2 Decorated Nanocrystals (Pt and Cu) Using Different Synthesis Methods. Fuel 2023, 354, 129248. [Google Scholar] [CrossRef]
- IEA. It Is Time for CCUS to Deliver; IEA: Paris, France, 2024.
- IEA. CCUS in Clean Energy Transitions; IEA: Paris, France, 2020.
- Wang, S.; Wang, J.; Zhang, Y.; Dong, L.; Dong, H.; Du, Q.; Gao, J. Effect of External Mineral Addition on PM Generated from Zhundong Coal Combustion. Energies 2023, 16, 730. [Google Scholar] [CrossRef]
- Edem, D.E.; Abba, M.K.; Nourian, A.; Babaie, M.; Naeem, Z. Experimental Study on the Interplay between Different Brine Types/Concentrations and CO2 Injectivity for Effective CO2 Storage in Deep Saline Aquifers. Sustainability 2022, 14, 986. [Google Scholar] [CrossRef]
- Hasan, M.M.F.; Zantye, M.S.; Kazi, M.-K. Challenges and Opportunities in Carbon Capture, Utilization and Storage: A Process Systems Engineering Perspective. Comput. Chem. Eng. 2022, 166, 107925. [Google Scholar] [CrossRef]
- Nath, F.; Mahmood, M.N.; Yousuf, N. Recent Advances in CCUS: A Critical Review on Technologies, Regulatory Aspects and Economics. Geoenergy Sci. Eng. 2024, 238, 212726. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, X.; Yue, L.; He, Y.; Chen, B. Porous Metal–Organic Frameworks for Hydrogen Storage. Chem. Commun. 2022, 58, 11059–11078. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Kumar, A.; Singh, S. Synthesis of Metal Organic Frameworks (MOFs) and Their Derived Materials for Energy Storage Applications. Clean Technol. 2023, 5, 140–166. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhang, C.; Ding, Z.; Jin, X. Application and Analysis of Liquid Organic Hydrogen Carrier (LOHC) Technology in Practical Projects. Energies 2024, 17, 1940. [Google Scholar] [CrossRef]
- Le, T.-H.; Tran, N.; Lee, H.-J. Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport. Int. J. Mol. Sci. 2024, 25, 1359. [Google Scholar] [CrossRef] [PubMed]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carriers (LOHCs)—Techno-Economic Analysis of LOHCs in a Defined Process Chain. Energy Environ. Sci 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Hartani, M.A.; Hamouda, M.; Abdelkhalek, O.; Benhamou, A.; Ali, B.; Mekhilef, S. Robust Frequency-Decoupling-Based Power Split of Battery/Supercapacitor Hybrid Energy Storage Systems in DC Microgrids. In Proceedings of the 1st International Conference on Physics of Semiconductor Devices, Renewable Energies and Environment, Bechar, Algeria, 14–16 November 2022; MDPI: Basel, Switzerland, 2023; p. 6. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).