Abstract
There are different phosphorus (P) sources of varied concentrations in aquatic ecosystems. The sensing of P by cyanobacteria in the environment is predominantly regulated by two-component signal transduction systems in which the phosphate (Pho) regulon plays a crucial role in maintaining phosphate homeostasis. It responds rapidly and connects to metabolic processes through cross-talk mechanisms. However, the physiological and biochemical mechanisms of the cyanobacterial response to different P sources remain unclear. This review article aims to integrate the physiological and molecular information on the regulatory mechanisms of the cyanobacterial response to different P sources in terms of hydrolysis, transport, and inorganic P (DIP) utilization strategies. Topics covered include enzymatic utilization of DOP (C-O-P, C-P), phosphate transport systems, and exploring the potential P metabolic pathways that might occur in cyanobacteria. This is of great significance for mitigating eutrophication and maintaining the sustainable development of aquatic systems.
1. Introduction
Lake eutrophication and cyanobacterial bloom have become challenges to sustainable water resource management. Cyanobacteria are the only known prokaryotes on Earth capable of photosynthesis and oxygen production [1]. Cyanobacterial blooms arise in aquatic environments due to distinct physiological traits and environmental conditions [2]. Cyanobacterial blooms have an impact on the aquatic environment’s biological balance and biodiversity. Bloom-forming organisms negatively impact water quality and natural landscapes. Dead and decaying cyanobacterial cells create volatile chemicals and secondary metabolites that may threaten human and aquatic health [3,4,5]. Phosphorus (P) is an essential element for cellular structure, energy, and information storage, as well as energy and information transfer, which directly affects the physiological and ecological characteristics of cyanobacteria [6,7,8]. It is crucial for cellular metabolism, gene storage, replication, and transcription. Understanding the response mechanism of cyanobacteria to dissolved phosphorus (DP) in the water body at the cellular and molecular level is of great ecological significance. It will assist with developing an improved understanding of the uptake and use of P in cyanobacteria.
DP in the water column can be classified into dissolved inorganic phosphorus (DIP) and dissolved organic phosphorus (DOP). Among the DIP sources, soluble orthophosphate (Pi) with +5 valence is a P source that is directly and preferentially used by cyanobacteria. However, the concentration of Pi is usually low in freshwater ecosystems, and it is scarce in aqueous ecosystems [9,10]. In contrast, DOP accounts for 70–80% of dissolved total phosphorus (DTP) and is the main P source in the water body [11]. DOP can be used as a biological alternative source of P in cases of Pi deficiency [12]. Phosphorus (Pho) regulons, the core of the phosphorus assimilation pathway in cyanobacteria, mainly control the uptake of phosphate and regulate its metabolism in cyanobacterial cells. It coordinates the sensing of environmental phosphate levels and the co-regulation of gene expression to regulate adaptive responses to P limitation [13,14]. The most common Pho regulons include extracellular enzymes capable of obtaining Pi from organophosphate esters, Pi-specific transport proteins, and enzymes involved in nutrient storage [15]. Cyanobacteria can also convert Pi into polyphosphate (poly-P) and store it in intracellular polyphosphate bodies (PPBs), which act as a reservoir of Pi and an energy supply for fueling cellular metabolism [16]. Poly-P is a linear polymer of phosphate residues linked by high-energy phosphoanhydride bonds, which contain three to hundreds of orthophosphate units [17,18].
Under P deficiency, the cells hydrolyze poly-Ps and release Pi to meet the cellular demand [19,20]. Meanwhile, cyanobacteria can induce the secretion of hydrolases to enzymatically convert the substrate DOP into DIP, which can be directly absorbed and used [21]. In addition, cyanobacteria upregulate the expression of high-affinity phosphate transporters to enhance the rate of Pi uptake and promote Pi transport, ensuring normal growth under low Pi concentrations [22,23,24,25].
The response of cyanobacteria to P is a complex process. The physiological and biochemical mechanisms as well as the pathway related to the hydrolysis, transport, and intracellular uptake and utilization of P in cyanobacteria remain unknown. In this paper, from the molecular mechanism of P use by cyanobacteria, we focused on reviewing (1) the use of DOP by cyanobacteria, including hydrolysis pathways and transport mechanisms of DOP; and (2) the use of DIP by cyanobacteria, including molecular mechanisms regulating intracellular poly-P accumulation. Based on previous studies, we systematically summarize the molecular regulatory mechanisms of cyanobacteria in response to different P sources. This review is essential for understanding the mechanisms behind cyanobacterial blooms and is crucial for protecting aquatic ecosystems and promoting sustainable development.
2. P Forms in Water
Human activities lead to the eutrophication of water bodies and an increase in the content of DOP, which is a non-negligible nutrient source in aquatic ecosystems and an important nutrient source for cyanobacteria. DOP is the dominant form in most freshwater systems, accounting for 25–50% of total phosphorus (TP) [26]. DOP originates from various sources, including exudation from phytoplankton cells, autolysis of dead cells, and decomposition of particulate organic debris, in addition to DOP caused by human activities such as agricultural and industrial emissions and wastewater discharge. These activities release waste and pollutants containing organic P compounds, which eventually enter water bodies [27,28]. DOP can be categorized into phosphoesters and phosphonates. Liquid-state phosphorus nuclear magnetic resonance (31P NMR) spectroscopy indicates that DOP in aquatic environments can be classified into two primary groups: phosphoesters with C-O-P bonds and phosphonates with C-P bonds [29,30]. Phosphoesters, which serve as a significant source of P for organisms, are categorized as phosphomonoesters or phosphodiesters. Phosphomonoesters, constituting the primary component of DOP in phytoplankton at an average content of approximately 83%, are frequently encountered in the forms of mononucleotides, phosphosugar, phosphoric acid ester, inositol hexaphosphate, and cholinephosphate [31,32]. Phosphodiesters are produced via the decomposition of bacteria and aquatic organisms, including the degradation products of DNA, RNA, and aquatic plants [33].
Phosphonates, characterized by their +3-oxidation state and stable C-P covalent bonds, are derivatives of phosphoric acid [34]. Despite their prevalence in the freshwater system, up to 35%, of phosphonates are not readily absorbed and utilized directly [11,35,36]. These compounds have diverse environmental sources, including cellular components such as phospholipids, nucleic acids, proteins, and polysaccharides released through cell lysis and anthropogenic inputs such as pesticides, herbicides, fungicides, cleaning additives, and flame retardants [28,37,38]. Methylphosphonate (MPN), glyphosate, and 2-aminoethyl phosphate (2-AEP) are common C-P-bonded DOP compounds that cyanobacteria can assimilate.
3. Use of DOP by Cyanobacteria
Variations in cyanobacterial strains or ecotypes are determined by distinct gene factors, influencing their utilization of various P sources within aquatic ecosystems [13]. The hydrolysis and transportation of DOP are essential processes for cyanobacteria to acquire and metabolize DOP, primarily controlled through the regulation of hydrolysis- and transport-associated enzymes. This bioavailability of DOP is influenced by various processes, including the hydrolysis pathways of C-P-bonded phosphonate and C-P-bonded phosphoester. Elucidating these two processes at the molecular level is crucial for comprehending the utilization of DOP by cyanobacteria in aquatic ecosystems.
3.1. Hydrolysis of DOP
The different methods of hydrolysis for various types of DOP lead to variations in bioavailability for cyanobacteria. This review focuses on elucidating the hydrolysis pathways of C-P bonded phosphonate and C-O-P bonded phosphoester. Although C-P bonds in phosphate esters exhibit stability and resistance to various degradation processes, numerous cyanobacteria strains have the ability to uptake and utilize phosphonate compounds containing C-P bonds [3,37,39,40,41]. Phosphoesters with C-O-P bonds are prevalent constituents of DOP in aquatic environments. The genes associated with the hydrolysis and utilization of DOP by cyanobacteria, such as alkaline phosphatases (AP) and nucleases that break down C-O-P bonds, as well as C-P lyases responsible for the degradation of C-P bonds in phosphonates, are summarized in Table 1.
3.1.1. Hydrolysis Pathways of C-O-P-Bonded DOP
When DIP availability is limited, cyanobacteria produce AP to hydrolyze and utilize extracellular DOP to release Pi for cyanobacterial uptake [12,42]. AP enzymes are nonspecific phosphomonoesterases that play a crucial role in Pho regulons, functioning most effectively under alkaline conditions. AP enzymes can be categorized into four major groups based on their localization and sites of action: (1) secreted or extracellular AP, (2) periplasmic AP, (3) membrane-bound AP, and (4) cytoplasmic AP [43]. The subcellular localization of AP in 3733 bacteria has been investigated, revealing that AP are predominantly found in the cytoplasmic (41%) and extracellular (30%) compartments, with smaller proportions in the periplasm (17%), outer membrane-associated (12%), and inner membrane-associated (0.9%) regions [44]. Different types of AP, such as phoA, phoD, phoX, and phoV, exhibit distinct ecological distributions and sources in the environment. Additionally, AP enzymes can be classified based on substrate specificity into phosphomonoesterases, phosphodiesterases, and phosphotriesterases [43]. The active sites of AP facilitate the binding of various metal ions [45,46]. Among these, the phoA gene was the first to be identified as encoding an alkaline phosphatase, initially observed to be synthesized and expressed by Escherichia coli. The enzyme functions as a homodimer capable of hydrolyzing phosphate monoesters, with its active site containing auxiliary groups consisting of magnesium and zinc. Additionally, phoD and phoX were initially characterized in Bucillus subtilis and Vibrio cholerae [47]; phoD and phoX are monomeric enzymes capable of hydrolyzing both phosphomonoesters and phosphodiesters, activated by calcium and iron [48], while phoV is a non-specific phosphomonoesterase activated by zinc [49]. In terms of the secretion pathways of AP, phoD and phoX are exported to the periplasm via the twin-arginine translocation (TAT) pathway, whereas phoA is secreted via the general secretion pathway (Sec). PhoA is reliant on the presence of disulfide bonds in the cytoplasm for proper folding, whereas the TAT pathway is limited to transporting fully folded proteins. The genes phoA, phoD, and phoX responsible for encoding AP demonstrate significant sequence variability [48]. The phoD gene exhibits greater sequence abundance and diversity in both aquatic and soil environments compared to phoA and phoX [44]. Within the Cyanobacteria phylum, the phoX gene is more prevalent than phoA and phoD [44]. It is noteworthy that more than 50% of PhoX is found extracellularly, while over 40% of phoA and phoD sequences are distributed in the cytoplasm. This suggests that different types of AP may have different pathways for DOP mineralization [47].
3.1.2. Hydrolysis Pathway of C-P Bonded DOP
The presence of residual unconverted phosphates in DOP can serve as a source of available P for certain cyanobacterial strains, necessitating specialized enzymatic machinery to break the C-P bond [50,51]. This adaptation represents an evolutionary advantage for cyanobacteria in a low-P environment [52]. Phosphonate catabolism features include the broad specificity of the C-P lyase pathway, as well as substrate oxidation and hydrolysis pathways with broad specificity. The oxidative cleavage pathway with broad specificity is denoted by the 2-aminoethylphosphonic acid (2-AEP) phosphonate oxygenase (phnY*Z) pathway [28,53,54]. C-P lyases, which consist of phosphonate degradation gene clusters (phn), are induced in response to low P conditions and primarily regulated by a two-component system. The C-P lyase pathway is currently the most extensively researched pathway, with phnCDE serving as an ABC transporter complex, phnF acting as the regulator of the phn operon (also functioning as a repressor), and the C-P complex (phnG-M) is participating in the cleavage of C-P bonds. The phn gene cluster is comprised of the membrane-associated C-P lyase complex and the C-P transcriptional regulatory accessory genes (phnN-P) [28,39,53,54]. In the presence of phnGHL, the nucleoside phosphorylase phnI facilitates the substitution of the phosphonate group in adenosine triphosphate (ATP) with a phosphonate moiety, resulting in the formation of a triphosphate ester. PhnM functions as a phosphonate hydrolase, leading to the production of inorganic pyrophosphate and 5-phosphoribosyl-1-phosphate. Subsequently, phnJ further degrades 5-phosphoribosyl-1-phosphate, and adenosylmethionine cleaves the C-P bond, producing an alkane and 5-phosphoribosyl-1,2-cyclic phosphate [27].
C-P lyases in E. coli have been the subject of investigation, particularly in relation to a cluster of 14 genes (phnC-phnP) responsible for the uptake and catabolism of phosphonates. This gene cluster is found in the genome and is transcribed together by a single promoter. Initially classified as a Psi locus, these genes were subsequently determined to be a component of the Pho regulon [55].
Studies have found that a deficiency of P in Trichodesmium erythraeum induces the expression of C-P lyase genes. This demonstrates that phosphonates serve as alternative P sources. Additionally, similar C-P lyases, such as phnJ, phnX, and the hydrolase-encoding gene phnA, have been identified in the metagenomic library [56,57]. The presence of C-P-bonded DOPs not only fulfills the P requirements of cyanobacterial cells but also plays an important role in the P redox cycle within the ecosystem. However, P metabolism in cyanobacteria is a complex issue since organisms of the same genus may decompose the same substance in different ways [37].

Table 1.
Genes related to the hydrolysis of DOP by cyanobacteria and their functions (adapted from [43,58]. Permission has been obtained from [43]. Copyright 2015 Microbiology).
Table 1.
Genes related to the hydrolysis of DOP by cyanobacteria and their functions (adapted from [43,58]. Permission has been obtained from [43]. Copyright 2015 Microbiology).
| System | Gene | Function |
|---|---|---|
| C-O-P hydrolysate | phoX | Alkaline phosphatase |
| phoA | Alkaline phosphatase | |
| phoD | Alkaline phosphatase | |
| phoV | Alkaline phosphatase | |
| nucH | Extracellular nuclease | |
| C-P lyase | phnF | Repressor proteins |
| PhnG | C-P lyase complex protein | |
| PhnK | C-P lyase complex protein | |
| PhnL | C-P lyase complex protein | |
| phnM | C-P lyase complex protein | |
| phnO | C-P lyase complex protein | |
| phnJ | C-P lyase complex protein | |
| phnI | C-P lyase complex protein | |
| phnH | C-P lyase complex protein | |
| phnF | C-P lyase complex protein |
3.2. P Transport System
The gene cluster associated with P metabolism belongs to the Pho regulon and is controlled by the two-component regulatory system [59]. Three distinct transport processes have been identified in bacteria: (1) a low affinity-high velocity phosphate inorganic transport (Pit) system; (2) a low affinity-high velocity Na-dependent phosphate transport (Npt) system; and (3) a high affinity-low velocity phosphate-specific transport (Pst) system [60,61,62]. The primary phosphate transport system utilized by cyanobacteria is the Pst system [63]. In instances where DIP levels are adequate, degradation of poly-P leads to a rise in intracellular Pi concentration. Phosphate chelates with metal ions and is exported from the cell through the Pit system via co-transport. This process created a proton gradient that can be coupled with other reactions to support a series of metabolic activities such as the production of ATP, the formation of Na+ pumps, and the transport of the neutral metal-phosphate complex [8]. The low-affinity inorganic phosphate system is not universal in cyanobacteria [64]. The Pit transport system has been found in Raphidiopsis raciborskii, which favors Pi uptake under high-P conditions and thus can enhance adaptations to different environmental changes [58]. In P-resource-constrained environments, the Pst transport system is commonly responsible for phosphate uptake [22]. Genes involved in P transport are listed in Table 2, including the two-component regulatory system for sensing extracellular Pi, the Pit system, the Pst system, and genes related to the transport complex for DOP.

Table 2.
P transport-related genes in cyanobacteria and their functions (adapted from [43,58]. Permission has been obtained from [43]. Copyright 2015 Microbiology).
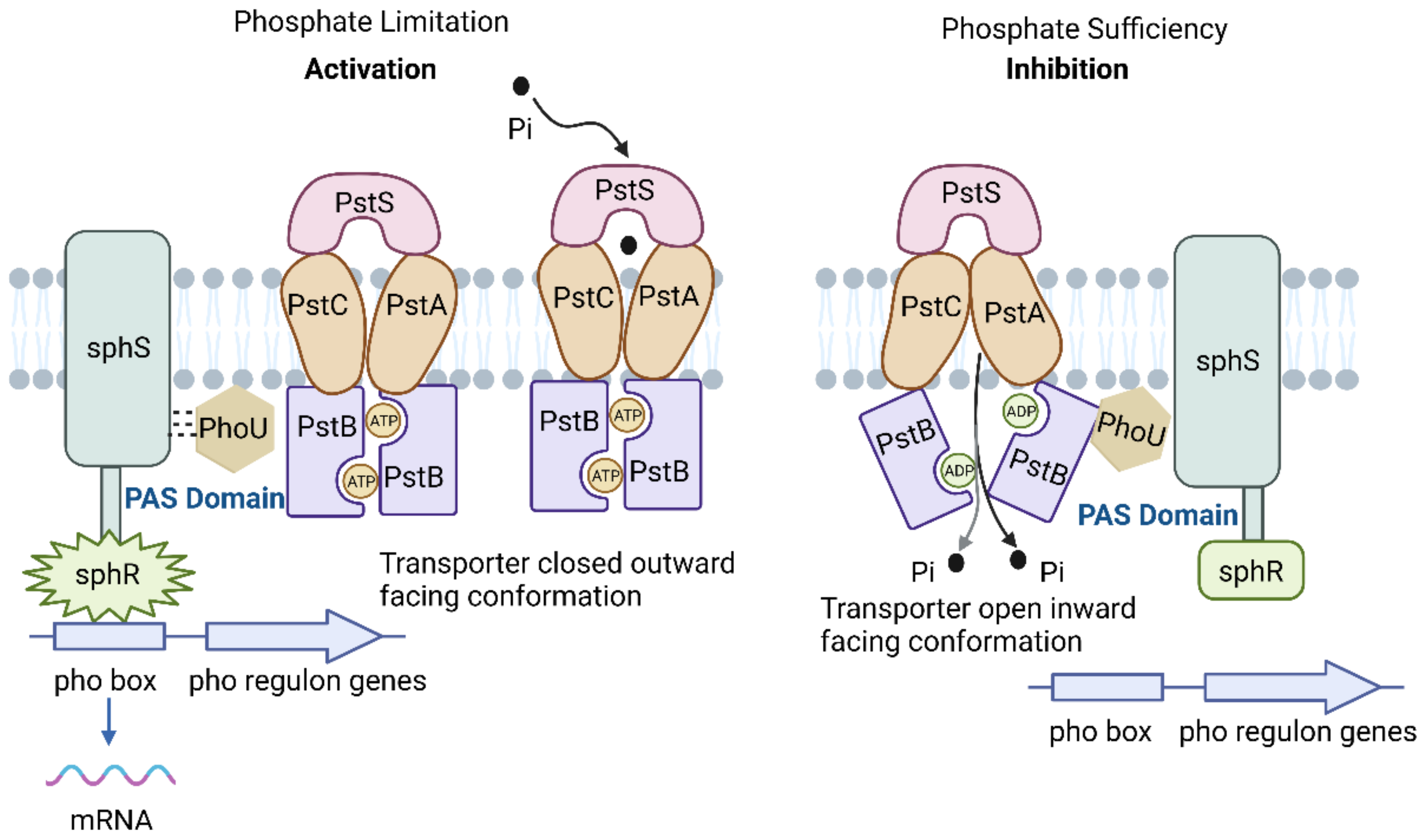
Extracellular Pi sensing and cellular responses of cyanobacteria are regulated by the two-component regulatory system sphS-sphR, which is homologous to phoB-phoR in E. coli [65]. Figure 1 illustrates the Pst system in cyanobacteria. ATP-binding cassette (ABC) proteins comprise a protein family actively transporting various molecules across cell membranes. The Pst system is a specific type of ABC transport system that plays an important role in phosphate metabolism in cyanobacteria. The Pst system contains four structural domains, including two membrane-binding domains and two nucleotide-binding domains, and consists of a periplasmic phosphate-binding protein (pstS), two membrane proteins (pstC and pstA), an ATP-binding protein (pstB), and a regulatory protein (phoU) [28]. The pstS protein facilitates the transfer of captured Pi to pstC and pstA, thereby contributing to the formation of a membrane channel protein. PstB functions as a membrane-binding protein that also acts as a site for ATP hydrolysis. The phosphate-specific transport system accessory protein, PhoU, is responsible for sensing Pi levels in the environment [66]. Under Pi limitation, the dissociation of pstS from pstA and pstC results in a conformational alteration of the pstSCAB complex. Following the conformational change, the signal is transmitted to the histidine kinase sphS (homologous to phoR in E. coli), which phosphorylates and activates sphR (homologous to E. coli phoB). This activation upregulates the expression of genes associated with the pho regulon. This includes regulation of AP, the inorganic phosphate transport system, and transcription of other related genes to enhance Pi transport efficiency and facilitate the utilization of DOP [43,67]. Under conditions of Pi-sufficient, the phosphorylation of the response regulator sphR is disturbed, while sphS remains dephosphorylated (inactive) [28,43]. The PAS domain functions as an internal sensor of redox potential, oxygen level, and cell energy status, facilitating protein-protein interactions upon activation [68]. The phoU protein and the Pst system cooperatively control protein activities through the processes of phosphorylation and dephosphorylation. The regulatory mechanism of Pi varies across different cyanobacterial strains due to differences in their evolutionary adaptations. The absence of pho box in the gene sequences of the marine cyanobacterial strains Synechococcus spp. and Prochlorococcus spp. suggests a potential evolutionary loss [13]. Furthermore, the lack of periplasmic domains in the genes responsible for signal transmission complicates the identification of regulatory components involved in sensing Pi scarcity [15].
Figure 1.
Phosphate-specific transport system (adapted with permission from [43]. Copyright 2015 Microbiology, created with BioRender.com, accessed on 26 June 2024).
The Phn operon encodes various proteins, including phnK and phnL, which function as transport proteins for phosphates, and phnCDE, a complex of ABC-type transport proteins [69]. Numerous cyanobacterial strains, including Trichodesmium spp., Synechococus spp., and Prochlorococus spp., have been identified as utilizing phosphonates as a source of phosphorus. These strains possess the Phn transport system and C-P lyases, enabling them to thrive by utilizing phosphonates as their exclusive P source [37]. In the Baltic Sea, Teikari et al. found that Nodularia spumigena used phosphonates, and expression of phnJ was found to be significantly upregulated based on bioinformatics analysis [41]. Shah et al. found that the phnD1-binding proteins of four Synechococcus strains selectively bound organophosphate compounds and that the PhnCDE transport protein is a constitutive phosphate transporter [70]. The complete P metabolism pathway of Cylindrospermopsis raciborskii, as revealed through whole-genome sequencing, allows for efficient utilization of phosphonates from the environment [58]. Reviewing and summarizing the molecular regulatory mechanisms of the three P transport systems will help enhance our understanding of the Pi transport mode in cyanobacteria.
4. Use of DIP by Cyanobacteria
There are several forms of P, with only Pi and poly-P being directly used by phytoplankton. Cyanobacteria store Pi when DIP is sufficient in the aquatic environment and break down poly-P to release Pi to maintain normal physiological activities during conditions of limited P. Poly-P not only provides Pi to cells during P deficiency but also provides energy for various intracellular biochemical processes. This section focuses on the molecular regulatory mechanisms of intracellular poly-P accumulation and use in cyanobacteria.
4.1. Intracellular Poly-P Accumulation
The accumulation of poly-P in cyanobacteria is strongly dynamic, mainly depending on phosphate availability and the growth phase [71]. In the case of sufficient external Pi, Pi is diffused into the periplasm via outer membrane pore proteins and subsequently transported to cellular PPBs by an inorganic phosphate transporter. The phenomenon of taking up more Pi than is needed for growth is commonly known as “luxury uptake” [72]. Cyanobacteria exhibit fluctuations in their Pi uptake pattern, leading to the enlargement of poly-P particles and disruption of cell structure [73]. In addition to luxury uptake, the synthesis of poly-P involves an “overplus response” and a “deficiency response” [71,74]. In the overplus response, cells overproduce polyP in excess of luxury uptake levels when phosphate is resupplied to P-starved cells [75]. In the P-deficiency response, poly-P accumulation occurs even in conditions of limited Pi availability [74]. Luxury uptake of P by cyanobacteria improves the ecological adaptability of cyanobacteria and limits the ability of their competitors to undergo normal uptake and use nutrients [76].
Poly-P has been observed in the center of cells, near the carboxysome. Poly-P has also been localized to the nuclear ribosomal DNA region and thylakoid [77,78]. Poly-Ps are linear or cyclic biological macromolecules that are synthesized through the polymerization of tens to thousands of phosphates via high-energy phosphate bonds [18,79]. These molecules are stored as high-energy phosphate bonds in adenosine triphosphate (ATP) for short periods of time (seconds to minutes) and stay as high-energy phosphate bonds for long periods of time (hours to days).
4.2. Use of Intracellular Poly-Ps
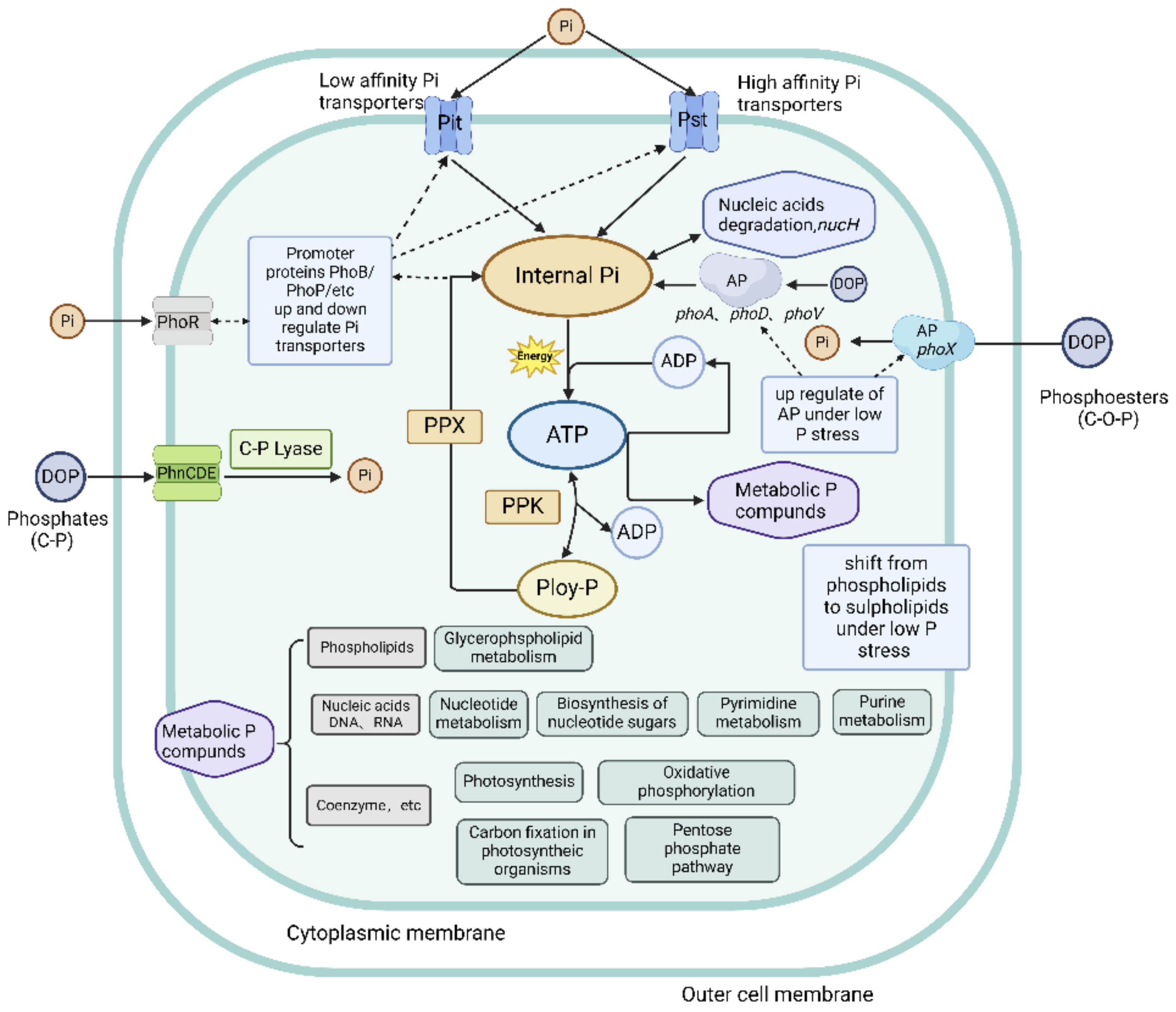
In the conditions of P starvation, poly-Ps are hydrolyzed by polyphosphate kinase (PPK) and exopolyphosphatase (PPX) enzymes, encoded by the ppk and ppx genes, respectively, to produce Pi, which is used for replication and division [20,80]. Poly-P is the major intracellular phosphate that provides energy, driving biological processes [81]. ATP can be converted by PPK to poly-P and release ADP in the process, which can then be regenerated back into ATP by PPK (Figure 2).
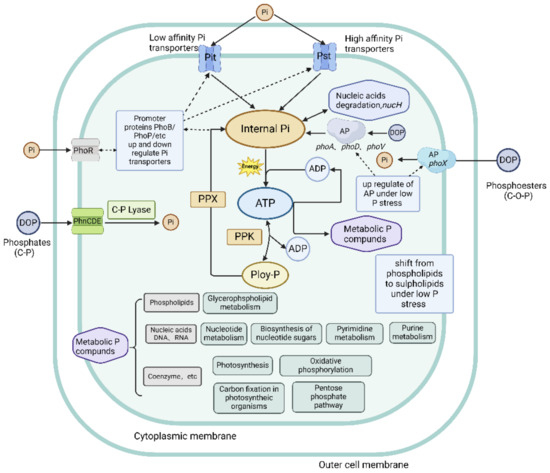
Figure 2.
Molecular mechanisms of P uptake, metabolism, and storage. Solid black lines indicate P transport or metabolic pathways; dashed black lines represent upregulation or downregulation of proteins or protein complexes involved in P metabolism (adapted with permission from [20]. Copyright 2016 Journal of Phycology, created with BioRender.com, accessed on 26 June 2024).
In prokaryotes, the regulation of poly-P biosynthesis is also mediated by Pho regulons. The synthesis and accumulation of poly-Ps have a significant impact on various cellular functions, and currently known functions of poly-Ps include: (1) an energy storage reservoir for driving biological processes; (2) maintaining homeostasis of adenylate and metal cations; (3) a scaffold for sequestering isolating cations; (4) chaperone function; (5) modifying protein activity by covalent binding and providing substrates for nucleic acids; and (6) assisting in cellular adaptation to environmental stresses [16]. If a starvation state continues for extended periods of time, DNA and RNA can also be hydrolyzed by nucleases to generate Pi, which is essential for sustaining cellular metabolism [67,82]. Jentzsch et al. found that the P in cyanobacterial DNA was redistributed into poly-P, implying intracellular biosynthesis shifted from growth to maintenance [83]. The above review of the literature results on the potential effects of poly-P on cellular biological processes will help improve our comprehension of the approaches that P uses inside cyanobacterial cells, revealing the molecular mechanisms of intracellular P use.
4.3. Intracellular Phosphate Metabolism Pathway
P is a vital nutrient that constitutes nucleic acids (DNA and RNA), cell membrane, ATP, and various coenzyme synthesis [20,84]. This study provides a comprehensive overview of the key metabolic pathways involved in the intracellular utilization of P. Specifically, in glycerophospholipid metabolism, Pi plays a critical role in the biosynthesis and degradation of phospholipids, which are essential components of cellular membranes and also play important roles in signal transduction pathways such as phosphatidic acid (PA), diacylglycerol (DAG), and phosphatidylinositol-4, 5-biosphospphate (PIP2) [85].
Both DNA and RNA are made up of basic building blocks called nucleotides, which are vectors of genetic information that encode proteins. Ribose phosphate is used in the synthesis of nucleotides and nucleic acids. The synthesis of nucleotides is the primary way in which cells utilize Pi in cyanobacteria’s life activities [67,86]. Nucleotide metabolism, the biosynthesis of nucleotides, pyrimidine metabolism, and purine metabolism pathways are all important components of nucleotide metabolism. In Microcystis aeruginosa (Chao 1910), the pentose phosphate pathway initiates the generation of ribose 5-phosphate, which serves as a precursor molecule for purine and pyrimidine synthesis. Subsequent enzymatic reactions convert ribose 5-phosphate into ribose 5-phosphate pyrophosphate, which undergoes further transformations to synthesize various purine nucleotides [67].
Pi is also crucial in photosynthesis, where accessory pigments capture the energy from impinging photons and funnel it to the reaction centers. Water molecules are spilt photochemically and release electrons that are transported to reduce NADPH. This results in a gradient of a proton across the membrane, which is used to drive ATP synthesis. Both NADPH and ATP are essential for carbon fixation and various cellular metabolic processes [87,88]. Pi is also involved in the synthesis of some enzymes related to carbon fixation. Such as ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), which is the primary enzyme in nature for inorganic carbon into organic compounds, catalyzing the primary reactions in both photosynthesis and photorespiration [89]. Pi is considered an activator of Rubisco [90]. Phosphoketolase is an ATP-sensitive enzyme that is responsible for regulating carbon fixation in cyanobacteria [91]. Furthermore, Shinde et al. have demonstrated that the pentose phosphate pathway can activate enzymes in the Calvin cycle [92].
Cyanobacteria are important primary producers by metabolizing different forms of P in the environment, thereby influencing the global phosphorus cycle [93]. Future research could benefit from integrating molecular biology experimental techniques to investigate the localization, environmental conditions, mechanisms of polyphosphate synthesis, and its interactions with other cellular organelles. The intracellular metabolism of Pi is a complex regulatory network where cells regulate the absorption, transportation, utilization, and storage of Pi through two-component signaling systems, phosphate transporters, and a series of enzymes. Pi enters the cell and mainly supports essential biological processes, including nucleic acid synthesis, energy metabolism, and membrane phospholipid synthesis. Nevertheless, these processes are also mediated by numerous key enzymes and regulatory factors, necessitating further investigation into their cellular biological functions. Techniques such as gene knockout can be used to verify these key genes and regulatory factors, revealing the mechanisms of regulation and signal transduction in intracellular Pi metabolism in cyanobacteria. By grooming the cellular processes related to Poly-P and the metabolic pathways highly associated with P metabolism, it helps to reveal the mechanism of intracellular Pi utilization.
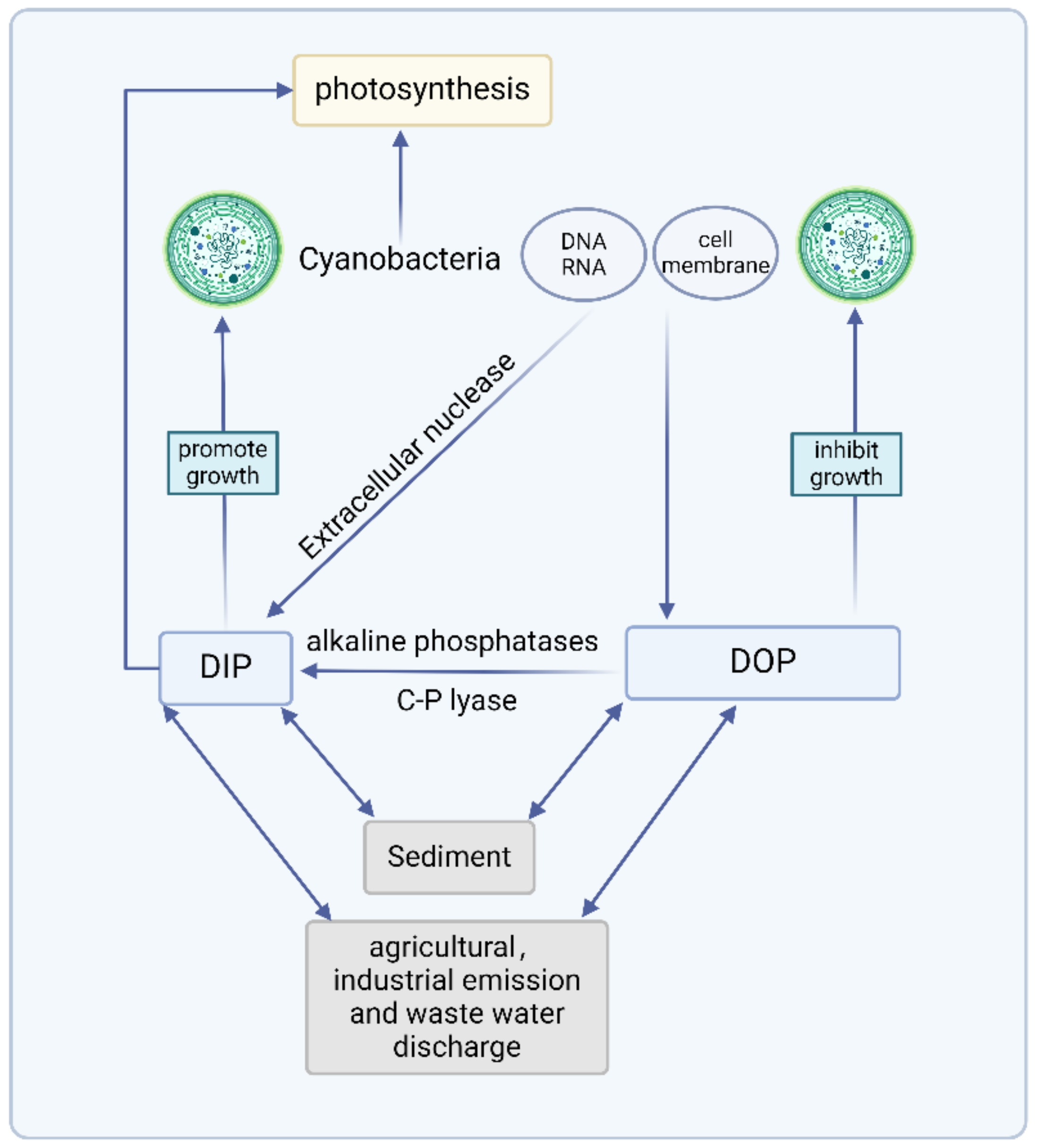
In natural water, there are multiple forms of Pi. The response mechanism of cyanobacteria to DOP and DIP is illustrated in Figure 3. The response mechanisms of cyanobacteria to different P sources need to be further intensively studied by combining molecular biology methods. Advances in gene sequencing technology will allow authors to complete the sequencing of cyanobacterial genes and study how hydrolysis-related enzymes, P transporters, and intracellular poly-P respond to different environmental P sources. This review provides a theoretical basis for understanding important scientific issues such as P cycling in freshwater ecosystems and cyanobacterial ecology.
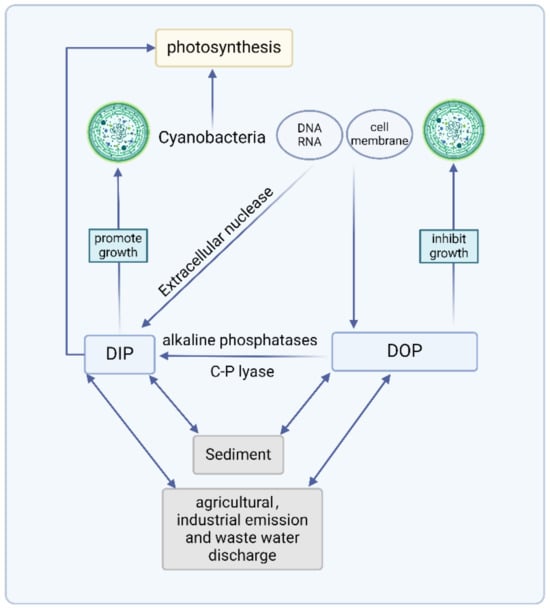
Figure 3.
Response mechanism of cyanobacteria to DOP and DIP (created with BioRender.com, accessed on 26 June 2024).
5. Conclusions and Prospects
This review provides an overview of the physiological mechanisms involved in P hydrolysis, transport, and intracellular uptake in cyanobacteria, with a focus on the diverse Pho regulon as a central component of P assimilation pathways. The Pho regulon is highly diverse, plays an important regulatory role in metabolic processes, and is involved in P hydrolysis, transport, and intracellular use in cyanobacteria. However, the different evolutionary adaptations of cyanobacterial strains lead to differences in the ways they transport P under the regulation of different P forms and concentrations. Sequencing of cyanobacterial genes is limited, resulting in a limited understanding of Pho regulon-related genes. For example, the regulatory components that sense P scarcity are unknown, and the hydrolytic role of C-P lyase is only assumed. Future studies need to focus on revealing metabolic pathways related to the hydrolysis of C-P-bonded phosphonates. Furthermore, studies on AP in freshwater ecosystems have mostly focused on extracellular, and there is a lack of research on the mechanism underlying the intracellular alkaline phosphatase response to different DOPs. This study aims to elucidate the role of intracellular alkaline phosphatase in the regulation of P metabolism. Many mechanisms of intracellular P use likely exist and require further exploration, e.g., localization and storage, synthesis conditions, modes of poly-P synthesis, and interactions between poly-P and other organelles. The development of gene sequencing technology will allow complete sequencing of cyanobacterial genes and exploration of how hydrolysis-related enzymes, P transporters, and intracellular poly-P respond to different P sources in the environment. The integration of cell and molecular biology will allow further exploration of cyanobacteria in terms of transcriptional regulation, protein structure and function, and intracellular metabolic pathways at the molecular level. This exploration promises to enhance our comprehension of the physiological regulation of cyanobacteria in response to different P sources. The findings of this review are anticipated to establish a scientific foundation for the mitigation and management of eutrophication, thereby contributing significantly to the sustainable utilization of water resources.
Author Contributions
Q.Z.: conceptualization, writing—original draft, writing—review and editing, visualization. L.J.: conceptualization and supervision. Y.C.: conceptualization, writing—review and editing. H.Y.: conceptualization and supervision. Q.C.: conceptualization, resources. J.Z.: conceptualization and supervision. H.S.: visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been generously funded by the National Natural Science Foundation of China (Grant Nos. 52121006 and 52370205).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Q.C. has been fortunate to receive support from the Xplorer Prize.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, T.; Falkowski, P.G. Genome evolution in cyanobacteria: The stable core and the variable shell. Proc. Natl. Acad. Sci. USA 2008, 105, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Lv, H.; Isabwe, A.; Liu, L.; Yu, X.; Chen, H.; Yang, J. Disturbance-induced phytoplankton regime shifts and recovery of cyanobacteria dominance in two subtropical reservoirs. Water Res. 2017, 120, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Song, L.R.; Jia, Y.L.; Qin, B.Q.; Li, R.H.; Carmichael, W.W.; Gan, N.Q.; Xu, H.; Shan, K.; Sukenik, A. Harmful cyanobacterial blooms: Biological traits, mechanisms, risks, and control strategies. Annu. Rev. Environ. Resour. 2023, 48, 123–147. [Google Scholar] [CrossRef]
- Burford, M.A.; Carey, C.C.; Hamilton, D.P.; Huisman, J.; Paerl, H.W.; Wood, S.A.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef]
- Tanaka, T.; Rassoulzadegan, F.; Thingstad, T.F. Orthophosphate uptake by heterotrophic bacteria, cyanobacteria, and autotrophic nanoflagellates in Villefranche Bay, northwestern Mediterranean: Vertical, seasonal, and short-term variations of the competitive relationship for phosphorus. Limnol. Oceanogr. 2004, 49, 1063–1072. [Google Scholar] [CrossRef]
- Vershinina, O.A.; Znamenskaya, L.V. The Pho regulons of bacteria. Microbiology 2002, 71, 497–511. [Google Scholar] [CrossRef]
- Blank, L.M. The cell and P: From cellular function to biotechnological application. Curr. Opin. Biotech. 2012, 23, 846–851. [Google Scholar] [CrossRef]
- Feng, S.; Qin, B.Q.; Gao, G. The relationships between phosphorus-transmuting bacteria and phosphorus forms in Lake Taihu. J. Lake Sci. 2008, 20, 428–443. (In Chinese) [Google Scholar]
- Halemejko, G.Z.; Chrost, R.J. The role of phosphatases in phosphorus mineralization during decomposition of lake phytoplankton blooms. Arch. Fur Hydrobiol. 1984, 101, 489–502. [Google Scholar]
- Bai, X.L.; Zhou, Y.K.; Sun, J.H.; Ma, J.H.; Zhao, H.Y.; Liu, X.F. Classes of dissolved and particulate phosphorus compounds and their spatial distributions in the water of a eutrophic lake: A 31P NMR study. Biogeochemistry 2015, 126, 227–240. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Ammerman, J.W.; Van Mooy, B.A. Microbes and the marine phosphorus cycle. Oceanography 2007, 20, 110–116. [Google Scholar] [CrossRef]
- Su, Z.; Olman, V.; Xu, Y. Computational prediction of Pho regulons in cyanobacteria. BMC Genom. 2007, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, Y.; Fu, Y.; Bhaya, D. The role of three-tandem Pho Boxes in the control of the C-P lyase operon in a thermophilic cyanobacterium. Environ. Microbiol. 2021, 23, 6433–6449. [Google Scholar] [CrossRef] [PubMed]
- Santos-Beneit, F. The Pho regulon: A huge regulatory network in bacteria. Front. Microbiol. 2015, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Luque, E.; Bhaya, D.; Grossman, A.R. Polyphosphate: A multifunctional metabolite in cyanobacteria and algae. Front. Plant Sci. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Kulaev, I.S.; Vagabov, V.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 1. [Google Scholar]
- Kulaev, I.S.; Vagabov, V.M. Polyphosphate metabolism in micro-organisms. Adv. Microb. Physiol. 1983, 24, 83–171. [Google Scholar] [PubMed]
- Kornberg, A.; Rao, N.N.; Ault-Riche, D. Inorganic polyphosphate: A molecule of many functions. Annu. Rev. Biochem. 1999, 68, 89–125. [Google Scholar] [CrossRef]
- Lin, S.; Litaker, R.W.; Sunda, W.G. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J. Phycol. 2016, 52, 10–36. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.S.; Pandey, V.D.; Mishra, A.K. Factors modulating alkaline phosphatase activity in the diazotrophic rice-field cyanobacterium, Anabaena oryzae. World J. Microbiol. Biot. 2006, 22, 927–935. [Google Scholar] [CrossRef]
- Harke, M.J.; Berry, D.L.; Ammerman, J.W.; Gobler, C.J. Molecular response of the bloom-forming cyanobacterium, Microcystis aeruginosa, to phosphorus limitation. Microb. Ecol. 2012, 63, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Shilova, I.N.; Zehr, J.P. Use of the high-affinity phosphate transporter gene, pstS, as an indicator for phosphorus stress in the marine diazotroph Crocosphaera watsonii (Chroococcales, Cyanobacteria). J. Phycol. 2019, 55, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.C.; Wang, M.; Zhang, J.Y.; Chen, Q.W.; Liu, D.S. Molecular responses to inorganic and organic phosphorus sources in the growth and toxin formation of Microcystis aeruginosa. Water Res. 2021, 196, 117048. [Google Scholar] [CrossRef]
- Ranjit, P.; Varkey, D.; Shah, B.S.; Paulsen, I.T. Substrate specificity and ecological significance of PstS homologs in phosphorus uptake in marine Synechococcus sp. WH8102. Microbiol. Spectr. 2024, 12, e02786-23. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, R. Limnology: Lake and River Ecosystems, 3rd ed.; Gulf Professional Publishing: San Diego, CA, USA, 2001. [Google Scholar]
- Peng, S.H.; Huang, S.M.; Zeng, Y.; Zhao, L. Advances on cyanobacteria phosphonate metabolism and its ecological significance. J. Lake Sci. 2023, 35, 43–56. (In Chinese) [Google Scholar]
- McGrath, J.W.; Chin, J.P.; Quinn, J.P. Organophosphonates revealed: New insights into the microbial metabolism of ancient molecules. Nat. Rev. Microbiol. 2013, 11, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.L.; Ingall, E.D.; Benner, R. Marine phosphorus is selectively remineralized. Nature 1998, 393, 426. [Google Scholar] [CrossRef]
- Kolowith, L.C.; Ingall, E.D.; Benner, R. Composition and cycling of marine organic phosphorus. Limnol. Oceanogr. 2001, 46, 309–320. [Google Scholar] [CrossRef]
- Ishii, Y.; Harigae, S.; Tanimoto, S.; Yabe, T.; Yoshida, T.; Taki, K.; Tatsumoto, H. Spatial variation of phosphorus fractions in bottom sediments and the potential contributions to eutrophication in shallow lakes. Limnology 2010, 11, 5–16. [Google Scholar] [CrossRef]
- Reitzel, K.; Ahlgren, J.; DeBrabandere, H.; Waldebäck, M.; Gogoll, A.; Tranvik, L.; Rydin, E. Degradation rates of organic phosphorus in lake sediment. Biogeochemistry 2007, 82, 15–28. [Google Scholar] [CrossRef]
- Ahlgren, J.; Reitzel, K.; Danielsson, R.; Gogoll, A.; Rydin, E. Biogenic phosphorus in oligotrophic mountain lake sediments: Differences in composition measured with NMR spectroscopy. Water Res. 2006, 40, 3705–3712. [Google Scholar] [CrossRef]
- Villarreal-Chiu, J.F.; Quinn, J.P.; McGrath, J.W. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment. Front. Microbiol. 2012, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Mahieu, N.; Condron, L.M. Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Sci. Soc. Am. J. 2003, 67, 497–510. [Google Scholar] [CrossRef]
- Read, E.K.; Ivancic, M.; Hanson, P.; Cade-Menun, B.J.; McMahon, K.D. Phosphorus speciation in a eutrophic lake by 31P NMR spectroscopy. Water Res. 2014, 62, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garcia, M.R.; Davison, M.; Blain-Hartnung, M.; Grossman, A.R.; Bhaya, D. Alternative pathways for phosphonate metabolism in thermophilic cyanobacteria from microbial mats. ISME J. 2011, 5, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Xie, E.; Su, Y.; Deng, S.; Kontopyrgou, M.; Zhang, D. Significant influence of phosphorus resources on the growth and alkaline phosphatase activities of Microcystis aeruginosa. Environ. Pollut. 2021, 268, 115807. [Google Scholar] [CrossRef]
- Kononova, S.V.; Nesmeyanova, M.A. Phosphonates and their degradation by microorganisms. Biochem. Moscow. 2002, 67, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lin, L.Z.; Chen, M.Y.; Teng, W.K.; Zheng, L.L.; Peng, L.; Shu, W.S. The widespread capability of methylphosphonate utilization in filamentous cyanobacteria and its ecological significance. Water Res. 2022, 217, 118385. [Google Scholar] [CrossRef]
- Teikari, J.E.; Fewer, D.P.; Shrestha, R.; Hou, S.; Leikoski, N.; Mäkelä, M.; Sivonen, K. Strains of the toxic and bloom-forming Nodularia spumigena (cyanobacteria) can degrade methylphosphonate and release methane. ISME J. 2018, 12, 1619–1630. [Google Scholar] [CrossRef]
- Zaheer, R.; Morton, R.; Proudfoot, M.; Yakunin, A.; Finan, T.M. Genetic and biochemical properties of an alkaline phosphatase PhoX family protein found in many bacteria. Environ. Microbiol. 2009, 11, 1572–1587. [Google Scholar] [CrossRef]
- Tiwari, B.; Singh, S.; Kaushik, M.S.; Mishra, A.K. Regulation of organophosphate metabolism in cyanobacteria. A review. Microbiology 2015, 84, 291–302. [Google Scholar] [CrossRef]
- Luo, H.; Benner, R.; Long, R.A.; Hu, J. Subcellular localization of marine bacterial alkaline phosphatases. Proc. Natl. Acad. Sci. USA 2009, 106, 21219–21223. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Saito, M.A. Proteomic responses of oceanic Synechococcus WH8102 to phosphate and zinc scarcity and cadmium additions. Front. Microbiol. 2013, 4, 387. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, S.; Diaz, J.M.; Adams, J.C.; Djaoudi, K.; Steck, V.; Waggoner, E.M. Phosphorus as an integral component of global marine biogeochemistry. Nat. Geosci. 2021, 14, 359–368. [Google Scholar] [CrossRef]
- Dai, J.Y.; Gao, G.; Wu, S.; Wu, X.; Zhou, J.; Xue, W.; Chen, D. Bacterial alkaline phosphatases and affiliated encoding genes in natural waters: A review. J. Lake Sci. 2016, 28, 1153–1166. (In Chinese) [Google Scholar]
- Ragot, S.A.; Kertesz, M.A.; Bünemann, E.K. phoD alkaline phosphatase gene diversity in soil. Appl. Environ. Microbiol. 2015, 81, 7281–7289. [Google Scholar] [CrossRef]
- Wagner, K.U.; Masepohl, B.; Pistorius, E.K. The cyanobacterium Synechococcus sp. strain PCC 7942 contains a second alkaline phosphatase encoded by phoV. Microbiology 1995, 141, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Su, Z.; Xu, Y. The evolution of microbial phosphonate degradative pathways. J. Mol. Evol. 2005, 61, 682–690. [Google Scholar] [CrossRef]
- Seweryn, P.; Van, L.B.; Kjeldgaard, M.; Russo, C.J.; Passmore, L.A.; Hove-Jensen, B.; Brodersen, D.E. Structural insights into the bacterial carbon–phosphorus lyase machinery. Nature 2015, 525, 68–72. [Google Scholar] [CrossRef]
- Dyhrman, S.T.; Chappell, P.D.; Haley, S.T.; Moffett, J.W.; Orchard, E.D.; Waterbury, J.B.; Webb, E.A. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature 2006, 439, 68–71. [Google Scholar] [CrossRef]
- Horsman, G.P.; Zechel, D.L. Phosphonate biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef]
- Manav, M.C.; Sofos, N.; Hove-Jensen, B.; Brodersen, D.E. The Abc of phosphonate breakdown: A mechanism for bacterial survival. BioEssays 2018, 40, 1800091. [Google Scholar] [CrossRef] [PubMed]
- White, A.K.; Metcalf, W.W. Microbial metabolism of reduced phosphorus compounds. Annu. Rev. Microbiol. 2007, 61, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Orchard, E.D.; Webb, E.A.; Dyhrman, S.T. Molecular analysis of the phosphorus starvation response in Trichodesmium spp. Environ. Microbiol. 2009, 11, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Tyson, G.W.; DeLong, E.F. Widespread known and novel phosphonate utilization pathways in marine bacteria revealed by functional screening and metagenomic analyses. Environ. Microbiol. 2010, 12, 222–238. [Google Scholar] [CrossRef]
- Chen, Z.J.; Ruan, Z.X.; Cheng, N.; Xiao, L.J.; Peng, L.; Han, B.P.; Lei, L.M. Whole-genome sequencing and phosphorus uptake and transport pathway comparative analysis of Cylindrospermopsis raciborskii N8. Acta Hydrobiol. Sin. 2022, 46, 1130–1141. [Google Scholar]
- Money, V.A.; Moreland, G.M.C.; Lott, J.S.; Edward, N. Crystal Structure of PhnF, a GntR-Family. J. Bacteriol. 2014, 196, 3472. [Google Scholar]
- Rosenberg, H.; Gerdes, R.; Chegwidden, K. Two systems for the uptake of phosphate in Escherichia coli. J. Bacteriol. 1977, 131, 505–511. [Google Scholar] [CrossRef]
- Willsky, G.R.; Malamy, M.H. Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J. Bacteriol. 1980, 144, 356–365. [Google Scholar] [CrossRef]
- Lebens, M.; Lundquist, P.; Söderlund, L.; Todorovic, M.; Carlin, N.I. The nptA gene of Vibrio cholerae encodes a functional sodium-dependent phosphate cotransporter homologous to the type II cotransporters of eukaryotes. J. Bacteriol. 2002, 184, 4466–4474. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Geraki, K.; Scanlan, D.J.; Zubkov, M.V. Accumulation of ambient phosphate into the periplasm of marine bacteria is proton motive force dependent. Nat. Commun. 2020, 11, 2642. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T.; Haley, S.T. Phosphorus scavenging in the unicellular marine diazotroph Crocosphaera watsonii. Appl. Environ. Microb. 2006, 72, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Hirani, T.A.; Suzuki, I.; Murata, N.; Hayashi, H.; Eaton-Rye, J.J. Characterization of a two-component signal transduction system involved in the induction of alkaline phosphatase under phosphate-limiting conditions in Synechocystis sp. PCC 6803. Plant Mol. Biol. 2001, 45, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Hudek, L.; Premachandra, D.; Webster, W.A.J.; Bräu, L. Role of phosphate transport system component PstB1 in phosphate internalization by Nostoc punctiforme. Appl. Environ. Microb. 2016, 82, 6344–6356. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.Y.; Yu, R.C.; Du, K.; Zhou, Z.C.; Li, X. Genome sequence analysis of phosphorus metabolism pathways of Microcystis aeruginosa Chao 1910 isolated from Chaohu Lake. Microbiol. China 2023, 50, 1491–1510. (In Chinese) [Google Scholar]
- Taylor, B.L.; Zhulin, I.B. PAS domains: Internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. R 1999, 63, 479–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Ye, Q.Z.; Zhu, Z.M.; Wanner, B.L.; Walsh, C.T. Molecular biology of carbon-phosphorus bond cleavage. Cloning and sequencing of the phn (psiD) genes involved in alkylphosphonate uptake and CP lyase activity in Escherichia coli B. J. Biol. Chem. 1990, 265, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.S.; Ford, B.A.; Varkey, D.; Mikolajek, H.; Orr, C.; Mykhaylyk, V.; Paulsen, I.T. Marine picocyanobacterial PhnD1 shows specificity for various phosphorus sources but likely represents a constitutive inorganic phosphate transporter. ISME J. 2023, 17, 1040–1051. [Google Scholar] [CrossRef]
- Li, J.; Dittrich, M. Dynamic polyphosphate metabolism in cyanobacteria responding to phosphorus availability. Environ. Microbiol. 2019, 21, 572–583. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Falkner, G.; Falkner, R. The complex regulation of the phosphate uptake system of cyanobacteria. In Bioenergetic Processes of Cyanobacteria: From Evolutionary Singularity to Ecological Diversity; Springer: Berlin/Heidelberg, Germany, 2011; pp. 109–130. [Google Scholar]
- Martin, P.; Dyhrman, S.T.; Lomas, M.W.; Poulton, N.J.; Van Mooy, B.A. Accumulation and enhanced cycling of polyphosphate by Sargasso Sea plankton in response to low phosphorus. Proc. Natl. Acad. Sci. USA 2014, 111, 8089–8094. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.P.; Amrhein, N.; Freimoser, F.M. Novel method for the quantification of inorganic polyphosphate (iPoP) in Saccharomyces cerevisiae shows dependence of iPoP content on the growth phase. Arch. Microbiol. 2005, 184, 129–136. [Google Scholar] [CrossRef]
- De Mazancourt, C.; Schwartz, M.W. Starve a competitor: Evolution of luxury consumption as a competitive strategy. Theor. Ecol. 2012, 5, 37–49. [Google Scholar] [CrossRef][Green Version]
- Nierzwicki-Bauer, S.A.; Balkwill, D.L.; Stevens, S.E., Jr. Three-dimensional ultrastructure of a unicellular cyanobacterium. J. Cell Biol. 1983, 97, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Liberton, M.; Austin, J.R.; Berg, R.H.; Pakrasi, H.B. Unique thylakoid membrane architecture of a unicellular N2-fixing cyanobacterium revealed by electron tomography. Plant Physiol. 2011, 155, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.H.; Fan, D.D.; Wang, M.M.; Zhao, Q.S.; Yang, L.Y. Structure and function of granular polyphosphate organelle in biological cells. Acta Microbiol. Sin. 2022, 62, 4713–4730. [Google Scholar]
- Schwarz, R.; Forchhammer, K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: Emergence of a complex network of cellular responses. Microbiology 2005, 151, 2503–2514. [Google Scholar] [CrossRef]
- Van Mooy, B.A.; Fredricks, H.F.; Pedler, B.E.; Dyhrman, S.T.; Karl, D.M.; Koblížek, M.; Webb, E.A. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 2009, 458, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Siuda, W.; Chróst, R.J. Utilization of selected dissolved organic phosphorus compounds by bacteria in lake water under non-limiting orthophosphate conditions. Pol. J. Environ. Stud. 2001, 10, 475–484. [Google Scholar]
- Jentzsch, L.; Grossart, H.P.; Plewe, S.; Schulze-Makuch, D.; Goldhammer, T. Response of cyanobacterial mats to ambient phosphate fluctuations: Phosphorus cycling, polyphosphate accumulation and stoichiometric flexibility. ISME Commun. 2023, 3, 6. [Google Scholar] [CrossRef]
- Rychter, A.M.; Rao, I.M. Role of phosphorus in photosynthetic carbon metabolism. Handb. Photosynth. 2005, 2, 123–148. [Google Scholar]
- Liu, Y.N. The Regulation Mechanism of Glycerophosphatide in Ganoderic Acid Biosynthesis under Heat Stress in Ganoderma lucidum; Nanjing Agricultural University: Nanjing, China, 2018. (In Chinese) [Google Scholar]
- Karl, D.M. Microbially mediated transformations of phosphorus in the sea: New views of an old cycle. Annu. Rev. Mar. Sci. 2014, 6, 279–337. [Google Scholar] [CrossRef]
- Haeder, D.P. Photosynthesis in Plants and Algae. Anticancer Res. 2022, 42, 5035–5041. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, C.W. Electron transport and light-harvesting switches in cyanobacteria. Front. Plant Sci. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y.; Altman-Gueta, H.; Wolff, Y.; Gurevitz, M. Rubisco mutagenesis provides new insight into limitations on photosynthesis and growth in Synechocystis PCC6803. J. Exp. Bot. 2011, 62, 4173–4182. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.S. Activation of spinach ribulose 1, 5-bisphosphate carboxylase by inorganic phosphate. Plant Sci. Lett. 1981, 23, 197–206. [Google Scholar] [CrossRef]
- Lu, K.J.; Chang, C.W.; Wang, C.H.; Chen, F.Y.; Huang, I.Y.; Huang, P.H.; Liao, J.C. An ATP-sensitive phosphoketolase regulates carbon fixation in cyanobacteria. Nat. Metab. 2023, 5, 1111–1126. [Google Scholar] [CrossRef]
- Shinde, S.; Zhang, X.; Singapuri, S.P.; Kalra, I.; Liu, X.; Morgan-Kiss, R.M.; Wang, X. Glycogen metabolism supports photosynthesis start through the oxidative pentose phosphate pathway in cyanobacteria. Plant Physiol. 2020, 182, 507–517. [Google Scholar] [CrossRef]
- Tiwari, B. Phosphate metabolism in cyanobacteria: Fundamental prospective and applications. In Cyanobacteria; Academic Press: Cambridge, MA, USA, 2024; pp. 159–175. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).