Phosphate Removal from Polluted Water via Lanthanum-Modified Sludge Biochar
Abstract
:1. Introduction
2. Materials and Method
2.1. Preparation of Materials
2.2. Material Characterization
2.3. Batch Adsorption
2.4. Adsorption and Desorption Experiments
2.5. Fixed-Bed Column Experiments
3. Results and Discussion
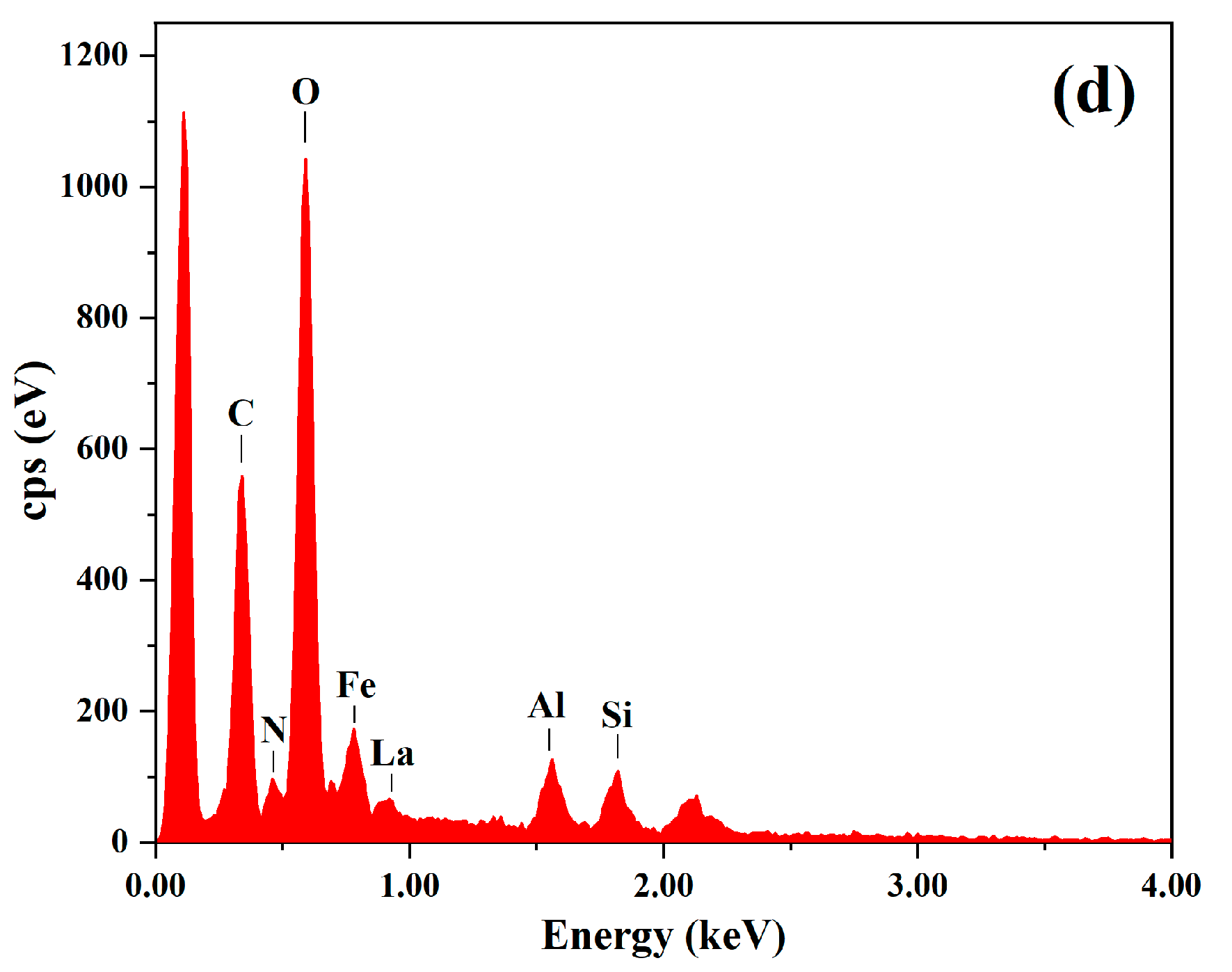
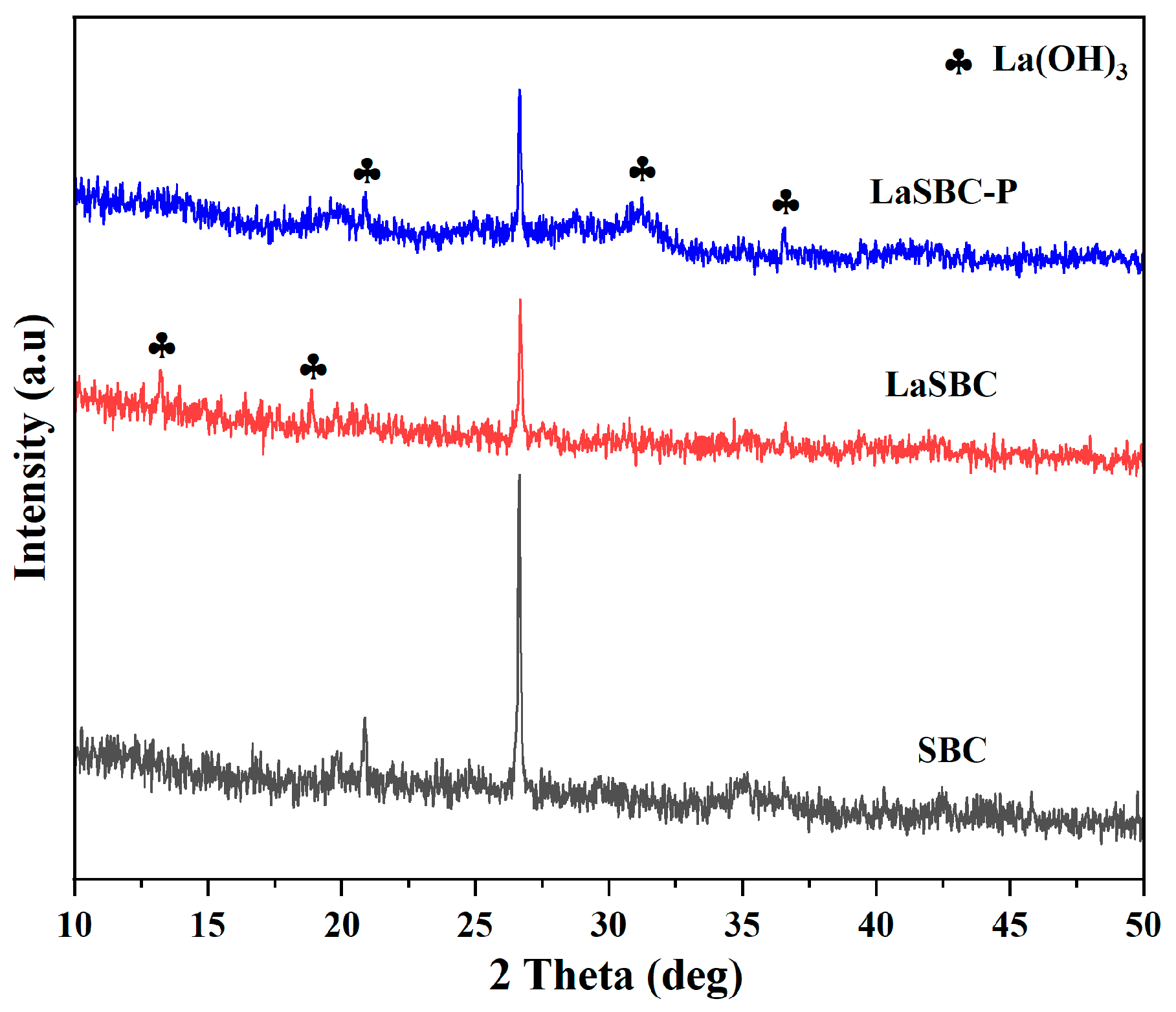
3.1. Material Characterization
3.2. Effect of Environmental Parameters
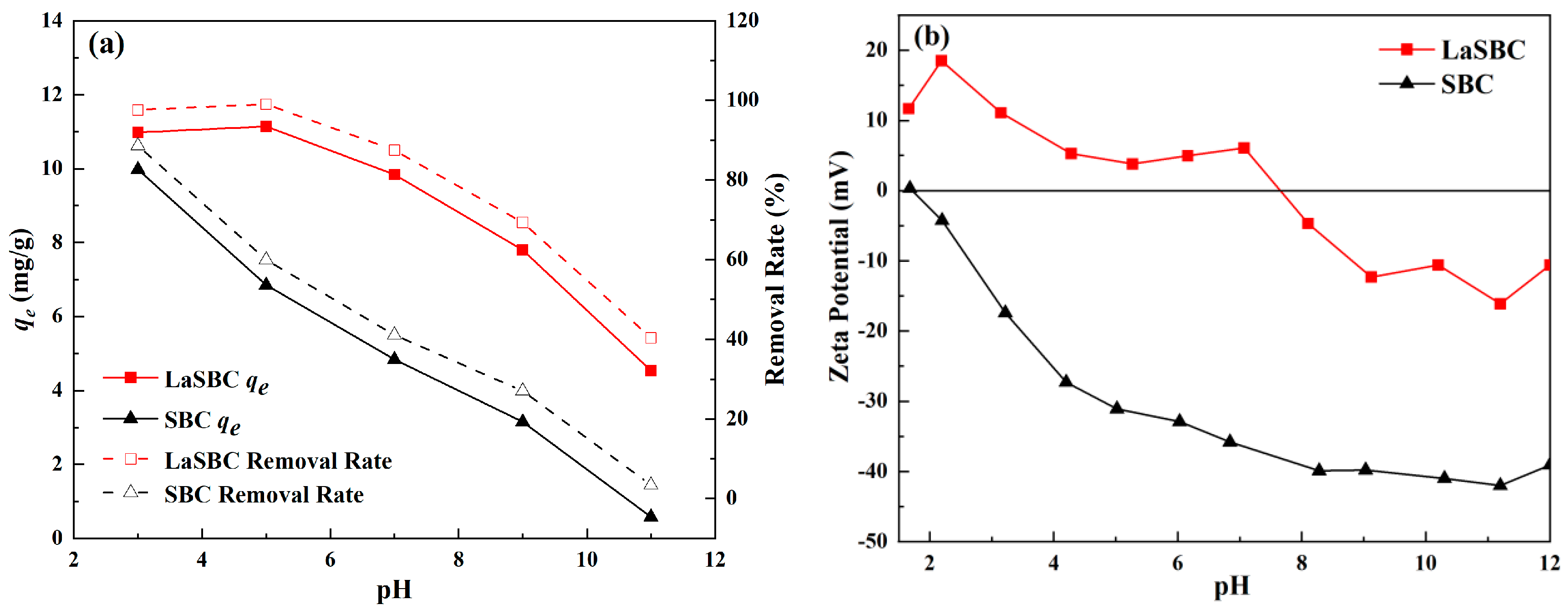
3.2.1. Effect of pH
3.2.2. Effect of Adsorbent Dosages
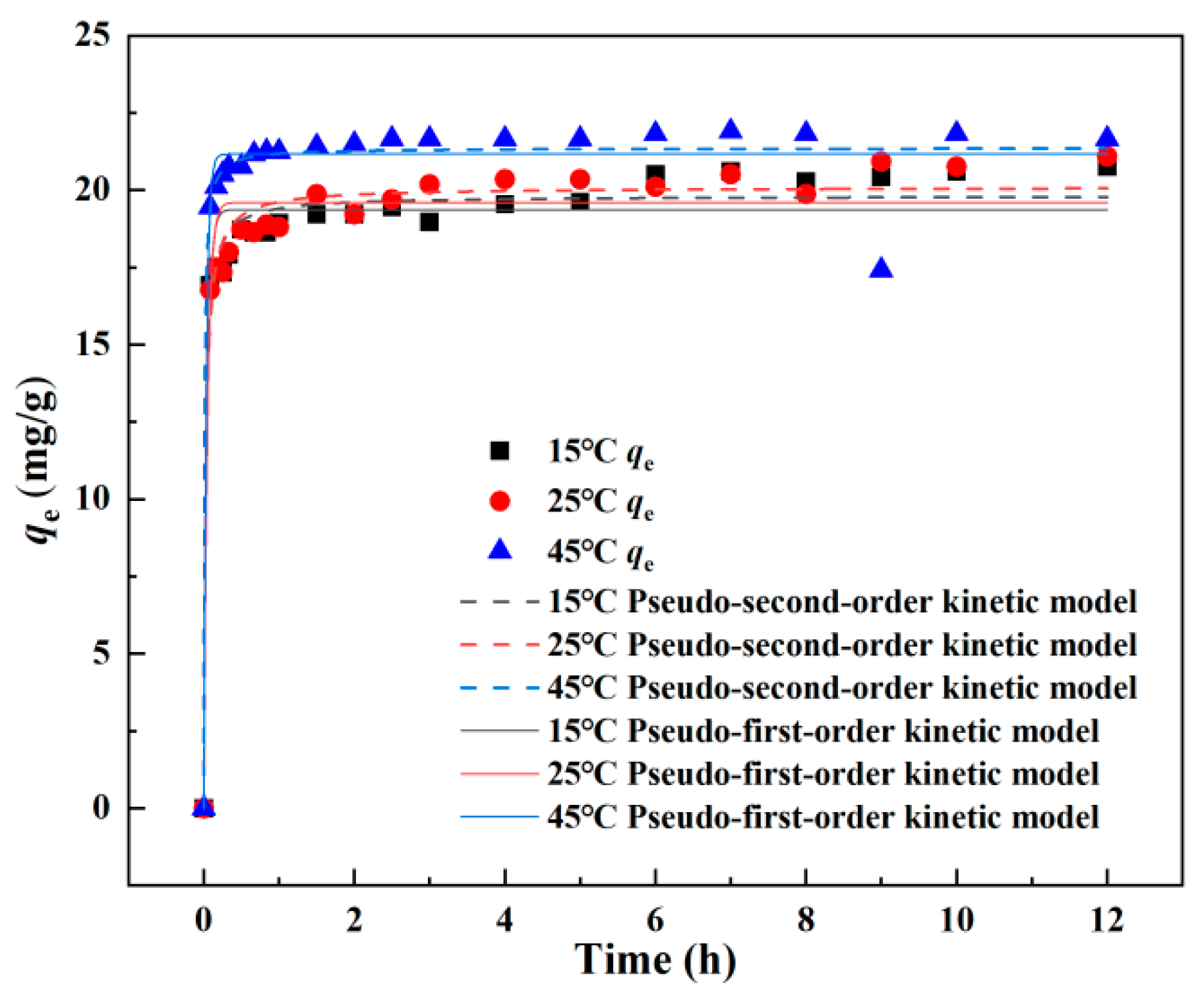
3.3. Adsorption Kinetics
3.4. Adsorption Isotherms
3.5. Adsorption Thermodynamics
3.6. Adsorption and Desorption Studies
3.7. Dynamic Adsorption Studies
3.8. Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.; Yang, Q.; Wang, D.; Wang, S.; Chen, F.; Zhong, Y.; Yi, K.; Yao, F.; Jiang, C.; Li, S.; et al. Nickel toxicity to the performance and microbial community of enhanced biological phosphorus removal system. Chem. Eng. J. 2017, 313, 415–423. [Google Scholar] [CrossRef]
- Wang, D.; Yang, G.; Li, X.; Zheng, W.; Wu, Y.; Yang, Q.; Zeng, G. Inducing mechanism of biological phosphorus removal driven by the aerobic/extended-idle regime. Biotechnol. Bioeng. 2012, 109, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, X.; Wang, D.; Wu, Y.; Wang, Q.; Liu, Y.; Li, X.; An, H.; Zhao, J.; Chen, F.; et al. Free ammonia-based pretreatment enhances phosphorus release and recovery from waste activated sludge. Chemosphere 2018, 213, 276–284. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xu, X.; Qi, S.; Zhao, Y.; Ren, Z.; Gao, B. Preferable uptake of phosphate by hydrous zirconium oxide nanoparticles embedded in quaternary-ammonium Chinese reed. J. Colloid Interface Sci. 2017, 496, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Lee, S.; Lee, Y.J. Synthesis of novel magnesium ferrite (MgFe2O4)/biochar magnetic composites and its adsorption behavior for phosphate in aqueous solutions. Bioresour. Technol. 2017, 245, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Mo, C.; Li, X.; Zhang, J.; An, H.; Yang, Q.; Wang, D.; Zhao, J.; Zhong, Y.; Zeng, G. Effects of different ratios of glucose to acetate on phosphorus removal and microbial community of enhanced biological phosphorus removal (EBPR) system. Environ. Sci. Pollut. Res. 2017, 24, 4494–4505. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Yang, Q.; Wang, D.; Xu, Q.; Yao, F.; Chen, F.; Tao, Z.; Huang, X. Hydrated lanthanum oxide-modified diatomite as highly efficient adsorbent for low-concentration phosphate removal from secondary effluents. J. Environ. Manag. 2019, 231, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yu, X.; Tang, J.; Zhu, Y.; Zhang, Y.; Li, D. Enhanced adsorption of phosphate by flower-like mesoporous silica spheres loaded with lanthanum. Microporous Mesoporous Mater. 2015, 217, 225–232. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Z.; Wu, Z.; Xu, F.; Yang, D.; He, Q.; Li, G.; Chen, Y. Novel lanthanum doped biochars derived from lignocellulosic wastes for efficient phosphate removal and regeneration. Bioresour. Technol. 2019, 289, 121600. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jin, P.; Wang, X.; Zhang, Q.; Chen, X. Phosphate recovery through adsorption assisted precipitation using novel precipitation material developed from building waste: Behavior and mechanism. Chem. Eng. J. 2016, 292, 246–254. [Google Scholar] [CrossRef]
- Shang, Y.; Kan, Y.; Xu, X. Stability and regeneration of metal catalytic sites with different sizes in Fenton-like system. Chin. Chem. Lett. 2023, 34, 108278. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Lahori, A.H.; Mahar, A. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour. Technol. 2016, 215, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liang, C.; Yu, J.; Zhang, Q.; Song, M.; Chen, F. Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration. Chem. Eng. J. 2017, 315, 345–354. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium. Bioresour. Technol. 2008, 99, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Bai, S.; Shan, H. Mechanisms of phosphate removal from aqueous solution by blast furnace slag and steel furnace slag. J. Zhejiang Univ.-Sci. A 2008, 9, 125–132. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, R.; Duan, X.; Wang, S.; Ren, N.; Ho, S.-H. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation. Water Res. 2020, 187, 116390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium chain carboxylic acids production from waste biomass: Current advances and perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef]
- Chen, Y.; Ho, S.-H.; Nagarajan, D.; Ren, N.; Chang, J.-S. Waste biorefineries—Integrating anaerobic digestion and microalgae cultivation for bioenergy production. Curr. Opin. Biotechnol. 2018, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, S.; Ho, S.-H.; Wang, C.; Lin, Y.-C.; Nagarajan, D.; Chang, J.-S.; Ren, N. Integration of sludge digestion and microalgae cultivation for enhancing bioenergy and biorefinery. Renew. Sustain. Energy Rev. 2018, 96, 76–90. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Ren, N.; Ho, S.-H. Optimizing the production of short and medium chain fatty acids (SCFAs and MCFAs) from waste activated sludge using different alkyl polyglucose surfactants, through bacterial metabolic analysis. J. Hazard. Mater. 2020, 384, 121384. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xia, S.; Lyu, J.; Tang, J. Highly efficient removal of aqueous Hg2+ and CH3Hg+ by selective modification of biochar with 3-mercaptopropyltrimethoxysilane. Chem. Eng. J. 2019, 360, 1646–1655. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2018, 247, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Li, G.; Tang, Y.-T.; Reid, B.J.; Ngwabie, N.M.; Sun, G.-X. The removal of arsenic from solution through biochar-enhanced precipitation of calcium-arsenic derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liu, M.; Li, Y.; Li, H.; Wen, Z. Computational study of phosphate adsorption on Mg/Ca modified biochar structure in aqueous solution. Chemosphere 2021, 269, 129374. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Li, T.; Su, X.; Yu, X.; Song, H.; Zhu, Y.; Zhang, Y. La(OH)3-modified magnetic pineapple biochar as novel adsorbents for efficient phosphate removal. Bioresour. Technol. 2018, 263, 207–213. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Sun, F.; Shi, W.; Qi, D.; Wang, K.; Shi, R.; Cui, F.; Wang, C.; Chen, X. Highly Efficient Phosphate Scavenger Based on Well-Dispersed La(OH)3 Nanorods in Polyacrylonitrile Nanofibers for Nutrient-Starvation Antibacteria. ACS Nano 2015, 9, 9292–9302. [Google Scholar] [CrossRef]
- Liu, B.; Nan, J.; Zu, X.; Zhang, X.; Huang, W.; Wang, W. La-based-adsorbents for efficient biological phosphorus treatment of wastewater: Synergistically strengthen of chemical and biological removal. Chemosphere 2020, 255, 127010. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiang, C.; Zhang, G.; Wang, H.; Ji, Q.; Qu, J. Activation of Lattice Oxygen in LaFe (Oxy)hydroxides for Efficient Phosphorus Removal. Environ. Sci. Technol. 2019, 53, 9073–9080. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Huang, H.; Liang, W.; et al. An overview of carbothermal synthesis of metal–biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Li, J.; Xu, C.; Huang, L.-Z.; Wang, D. Magnetite/Lanthanum hydroxide for phosphate sequestration and recovery from lake and the attenuation effects of sediment particles. Water Res. 2018, 130, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Wu, X.; Shao, P.; Ren, Z.; Ding, L.; Luo, X. Ultra-high capacity of lanthanum-doped UiO-66 for phosphate capture: Unusual doping of lanthanum by the reduction of coordination number. Chem. Eng. J. 2019, 358, 321–330. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate adsorption on lanthanum loaded biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Huang, H.; Zhao, N.; Zhang, M.; Cao, L. Investigation into lanthanum-coated biochar obtained from urban dewatered sewage sludge for enhanced phosphate adsorption. Sci. Total Environ. 2020, 714, 136839. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, B. Insights on the Molecular Mechanism for the Recalcitrance of Biochars: Interactive Effects of Carbon and Silicon Components. Environ. Sci. Technol. 2014, 48, 9103–9112. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar. Process Saf. Environ. Prot. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Zhang, J.; Wang, Z.; Guo, Z.; Ali, J.; Wang, L.; Yu, Z.; Zhang, X.; Sun, Y. Effective removal of microplastics by filamentous algae and its magnetic biochar: Performance and mechanism. Chemosphere 2024, 358, 142152. [Google Scholar] [CrossRef]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using sewage sludge with high ash content for biochar production and Cu(II) sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef] [PubMed]
- Pasumarthi, R.; Sawargaonkar, G.; Kale, S.; Kumar, N.V.; Choudhari, P.L.; Singh, R.; Davala, M.S.; Rani, C.S.; Mutnuri, S.; Jat, M.L. Innovative bio-pyrolytic method for efficient biochar production from maize and pigeonpea stalks and their characterization. J. Clean. Prod. 2024, 448, 141573. [Google Scholar] [CrossRef]
- Qian, G.; Xu, L.; Li, N.; Wang, K.; Qu, Y.; Xu, Y. Enhanced arsenic migration in tailings soil with the addition of humic acid, fulvic acid and thiol-modified humic acid. Chemosphere 2022, 286, 131784. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Gu, L.; Wen, H.; Zhu, N. Coupling sludge-based biochar and electrolysis for conditioning and dewatering of sewage sludge: Effect of char properties. Environ. Res. 2022, 214, 113974. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Sun, Y.; Chen, Y.; Qian, J. La(OH)3 loaded magnetic mesoporous nanospheres with highly efficient phosphate removal properties and superior pH stability. Chem. Eng. J. 2019, 360, 342–348. [Google Scholar] [CrossRef]
- Xiao, Y.-F.; Zhang, Y.; Wang, D.-C.; Su, Y.-M.; Wu, J.; Liu, J.-Q.; Yang, L.-L.; Jin, Z. Hydrothermal synthesis of lanthanum oxide nanoparticles modified pumice: High lanthanum oxide loading ratio and efficiency phosphate removal. J. Environ. Chem. Eng. 2024, 12, 111587. [Google Scholar] [CrossRef]
- Chen, K.; Guo, S.; Zeng, Y.; Huang, W.; Peng, J.; Zhang, L.; Yin, S. Facile preparation and characterization of lanthanum oxide powders by the calcination of lanthanum carbonate hydrate in microwave field. Ceram. Int. 2020, 46, 165–170. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Pan, H.; Xu, X.; Tang, Y.; Ming, J.; Xu, Z.; Tang, R. Improved Luminescence of Lanthanide(III)-Doped Nanophosphors by Linear Aggregation. J. Phys. Chem. C 2007, 111, 4111–4115. [Google Scholar] [CrossRef]
- Shan, S.; Chen, Z.; Yuen Koh, K.; Cui, F.; Paul Chen, J. Development and application of lanthanum peroxide loaded sepiolite nanocomposites for simultaneous removal of phosphate and inhibition of cyanobacteria growth. J. Colloid Interface Sci. 2022, 624, 691–703. [Google Scholar] [CrossRef]
- Tang, Q.; Shi, C.; Shi, W.; Huang, X.; Ye, Y.; Jiang, W.; Kang, J.; Liu, D.; Ren, Y.; Li, D. Preferable phosphate removal by nano-La(III) hydroxides modified mesoporous rice husk biochars: Role of the host pore structure and point of zero charge. Sci. Total Environ. 2019, 662, 511–520. [Google Scholar] [CrossRef]
- Li, S.; Luo, C.; Yan, F.; Yang, Y.; Guo, B.; Wang, L.; Xu, S.; Wu, F.; Ji, P. Remediation of Pb(II) and Cd(II) in polluted waters with calcium thioglycolate–modified straw biochar. Environ. Pollut. 2023, 338, 122638. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Z.; Shan, W.; Chen, Z.; Mei, Z.; Lei, Y.; Wang, W. Adsorption behavior of phosphate on Lanthanum(III) doped mesoporous silicates material. J. Environ. Sci. 2010, 22, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.A.; Mpatani, F.M.; Zhang, X.; Kani, A.N.; Dovi, E.; Han, R.; Li, Z.; Qu, L. Iron (III) and iminodiacetic acid functionalized magnetic peanut husk for the removal of phosphate from solution: Characterization, kinetic and equilibrium studies. J. Clean. Prod. 2020, 268, 122191. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Cui, X.; Dai, X.; Khan, K.Y.; Li, T.; Yang, X.; He, Z. Removal of phosphate from aqueous solution using magnesium-alginate/chitosan modified biochar microspheres derived from Thalia dealbata. Bioresour. Technol. 2016, 218, 1123–1132. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef]
- Qu, J.; Akindolie, M.S.; Feng, Y.; Jiang, Z.; Zhang, G.; Jiang, Q.; Deng, F.; Cao, B.; Zhang, Y. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: Kinetics, isotherms, thermodynamics, mechanisms and reusability exploration. Chem. Eng. J. 2020, 394, 124915. [Google Scholar] [CrossRef]
- Meng, J.; Feng, X.; Dai, Z.; Liu, X.; Wu, J.; Xu, J. Adsorption characteristics of Cu(II) from aqueous solution onto biochar derived from swine manure. Environ. Sci. Pollut. Res. 2014, 21, 7035–7046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Zhang, Y.; Lichtfouse, E. Efficient phosphate recycling by adsorption on alkaline sludge biochar. Environ. Chem. Lett. 2023, 21, 21–30. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, R.; Ning, P.; He, L.; Guan, Q. From wastes to functions: A paper mill sludge-based calcium-containing porous biochar adsorbent for phosphorus removal. J. Colloid Interface Sci. 2021, 593, 434–446. [Google Scholar] [CrossRef]
- Li, J.; Cao, L.; Li, B.; Huang, H.; Yu, W.; Sun, C.; Long, K.; Young, B. Utilization of activated sludge and shell wastes for the preparation of Ca-loaded biochar for phosphate removal and recovery. J. Clean. Prod. 2023, 382, 135395. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Huang, H.; Lv, X.; Zhao, N.; Guo, G.; Zhang, D. Removal of phosphate from aqueous solution by dolomite-modified biochar derived from urban dewatered sewage sludge. Sci. Total Environ. 2019, 687, 460–469. [Google Scholar] [CrossRef]
- Cegłowski, M.; Schroeder, G. Preparation of porous resin with Schiff base chelating groups for removal of heavy metal ions from aqueous solutions. Chem. Eng. J. 2015, 263, 402–411. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Zhao, L.; Zhang, H.; Yin, J.; Wei, G.; Qian, K.; Wang, Y.; Yu, C. A designed nanoporous material for phosphate removal with high efficiency. J. Mater. Chem. 2011, 21, 2489. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Li, Q.; Xu, M.; Guo, Y.; Li, J.; Wu, T. pH effect on Re(VII) and Se(IV) diffusion in compacted GMZ bentonite. Appl. Geochem. 2016, 73, 1–7. [Google Scholar] [CrossRef]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xu, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef]
- Hiemstra, T.; Van Riemsdijk, W.H. Surface Structural Ion Adsorption Modeling of Competitive Binding of Oxyanions by Metal (Hydr)oxides. J. Colloid Interface Sci. 1999, 210, 182–193. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Liu, J.; Chang, N.; Wan, L.; Chen, J. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber. Chem. Eng. J. 2012, 185–186, 160–167. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, R.; Li, Y.; Liu, Y.; Wu, Y.; Chen, Y.; Zhang, J.; Chen, S.; Yin, H.; Zeng, Z.; et al. La(OH)3-modified magnetic CoFe2O4 nanocomposites: A novel adsorbent with highly efficient activity and reusability for phosphate removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124870. [Google Scholar] [CrossRef]
- Wu, B.; Fang, L.; Fortner, J.D.; Guan, X.; Lo, I.M.C. Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)3/Fe3O4 nanocomposites. Water Res. 2017, 126, 179–188. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Nasr, M. Regression model, artificial intelligence, and cost estimation for phosphate adsorption using encapsulated nanoscale zero-valent iron. Sep. Sci. Technol. 2019, 54, 13–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, B. Modeling batch and column phosphate removal by hydrated ferric oxide-based nanocomposite using response surface methodology and artificial neural network. Chem. Eng. J. 2014, 249, 111–120. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Zhang, X.; Chang, S.; Wang, H.; Wang, M.; Xiang, W.; Gao, B. Microwave biochar produced with activated carbon catalyst: Characterization and adsorption of heavy metals. Environ. Res. 2023, 216, 114732. [Google Scholar] [CrossRef]







| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/g) | R2 | KF (L/g) | 1/n | R2 | |
| LaSBC 15 °C | 84.249 | 0.346 | 0.848 | 22.670 | 0.280 | 0.941 |
| LaSBC 25 °C | 99.910 | 0.213 | 0.871 | 24.876 | 0.288 | 0.940 |
| LaSBC 35 °C | 114.647 | 0.206 | 0.922 | 29.111 | 0.284 | 0.943 |
| LaSBC 45 °C | 130.314 | 0.103 | 0.905 | 28.458 | 0.304 | 0.952 |
| LaSBC 65 °C | 140.237 | 0.077 | 0.924 | 28.347 | 0.310 | 0.960 |
| Biochar | Qm (mg/g) | References |
|---|---|---|
| La-modified sludge biochar (LaSBC) | 140.237 | This study |
| Alkaline sludge biochar | 42.51 | [63] |
| Calcium-containing paper-sludge-based biochar | 68.49 | [64] |
| Iron-modified waste-biochar-sludge-based biochar | 111.0 | [25] |
| Egg shell-modified sludge biochar | 154.18 | [65] |
| Oyster shell-modified sludge biochar | 129.03 | [65] |
| Dolomite-modified sludge biochar | 29.18 | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Sun, X.; Zhang, H.; Li, Q.; Mo, J.; Xing, M.; Dong, B.; Zhu, H. Phosphate Removal from Polluted Water via Lanthanum-Modified Sludge Biochar. Sustainability 2024, 16, 5667. https://doi.org/10.3390/su16135667
Jiang Y, Sun X, Zhang H, Li Q, Mo J, Xing M, Dong B, Zhu H. Phosphate Removal from Polluted Water via Lanthanum-Modified Sludge Biochar. Sustainability. 2024; 16(13):5667. https://doi.org/10.3390/su16135667
Chicago/Turabian StyleJiang, Yufan, Xiaojie Sun, Hongxia Zhang, Qian Li, Jingjing Mo, Meiyan Xing, Bin Dong, and Hongxiang Zhu. 2024. "Phosphate Removal from Polluted Water via Lanthanum-Modified Sludge Biochar" Sustainability 16, no. 13: 5667. https://doi.org/10.3390/su16135667
APA StyleJiang, Y., Sun, X., Zhang, H., Li, Q., Mo, J., Xing, M., Dong, B., & Zhu, H. (2024). Phosphate Removal from Polluted Water via Lanthanum-Modified Sludge Biochar. Sustainability, 16(13), 5667. https://doi.org/10.3390/su16135667












