Phytoremediation of Tungsten Tailings under Conditions of Adding Clean Soil: Microbiological Research by Metagenomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Experimental Methods and Data Analysis
2.3.1. Sample Collection and Analysis
2.3.2. Metagenomic Analysis of Tailing Soils
2.3.3. Data Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Overview of Variation in Agricultural Indicators
3.2. Analysis of Microbial Diversity
3.3. Variation in the Microbial Composition and Structure
3.4. Functional Prediction
3.5. Nitrogen and Cycling-Related Genes
3.5.1. Nitrogen Cycling-Related Genes
3.5.2. Phosphorus Cycling-Related Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Qin, W.; Jiao, F.; Dong, L.; Guo, J.; Zhang, J.; Yang, C. Review of Tungsten Resource Reserves, Tungsten Concentrate Production and Tungsten Beneficiation Technology in China. Trans. Nonferrous Met. Soc. China 2022, 32, 2318–2338. [Google Scholar] [CrossRef]
- Lin, C.; Li, R.; Cheng, H.; Wang, J.; Shao, X. Tungsten Distribution in Soil and Rice in the Vicinity of the World’s Largest and Longest-Operating Tungsten Mine in China. PLoS ONE. 2014, 9, e91981. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, B.; Chen, X.; Dong, Y.; Lin, H. Insight into Soilless Revegetation of Oligotrophic and Heavy Metal Contaminated Gold Tailing Pond by Metagenomic Analysis. J. Hazard. Mater. 2022, 435, 128881. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, M.; Fu, X.; Ouyang, L.; Chen, R.; Wu, X. Ecological Remediation Effect of Organic Bacterium Manure-Fast Growing Tree Species on a Manganese Tailing Site. China Environ. Sci. 2019, 39, 5219–5227. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, X.; Guo, Y.; Li, H.; Li, X.; Liu, J. Ecological Evolution during the Three-Year Restoration Using Rhizosphere Soil Cover Method at a Lead-Zinc Tailing Pond in Karst Areas. Sci. Total Environ. 2022, 853, 158291. [Google Scholar] [CrossRef] [PubMed]
- Sarathchandra, S.S.; Rengel, Z.; Solaiman, Z.M. Remediation of Heavy Metal-Contaminated Iron Ore Tailings by Applying Compost and Growing Perennial Ryegrass (Lolium Perenne L.). Chemosphere 2022, 288, 132573. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Ding, Z.; Sun, Q.; Wang, N. Effects of alien soil improvement of copper tailings on physiological characteristics and heavy metals uptake of Vetiveria zizanioides. J. Agro⁃Environ. Sci. 2021, 40, 83–91. (In Chinese) [Google Scholar]
- Bigot, M.; Guterres, J.; Rossato, L.; Pudmenzky, A.; Doley, D.; Whittaker, M.; Pillai-McGarry, U.; Schmidt, S. Metal-Binding Hydrogel Particles Alleviate Soil Toxicity and Facilitate Healthy Plant Establishment of the Native Metallophyte Grass Astrebla Lappacea in Mine Waste Rock and Tailings. J. Hazard. Mater. 2013, 248–249, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, C.; Zhang, Q.; Jiang, X.; Liu, C.; Lin, H.; Li, B. Selected Rhizobacteria Facilitated Phytoremediation of Barren and Heavy Metal Contaminated Gold Mine Tailings by Festuca Arundinacea. Chemosphere 2023, 337, 139297. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Qian, J.; Zhang, F.; Kong, X.; Li, H.; Luan, S.; Zhang, Q.; Kang, Z.; Han, Z.; Zhang, Z. An Ecological Remediation Model Combining Optimal Substrate Amelioration and Native Hyperaccumulator Colonization in Non-Ferrous Metal Tailings Pond. J. Environ. Manag. 2022, 322, 116141. [Google Scholar] [CrossRef]
- Cheng, S.-B.; Yang, X.-Z.; Zou, L.; Wu, D.-D.; Lu, J.-L.; Cheng, Y.-R.; Wang, Y.; Zeng, J.; Kang, H.-Y.; Sha, L.-N.; et al. Comparative Physiological and Root Transcriptome Analysis of Two Annual Ryegrass Cultivars under Drought Stress. J. Plant Physiol. 2022, 277, 153807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y. Restoration of copper tailings under covering soil by perennial ryegrass. Pratacultural Sci. 2019, 36, 3–10. (In Chinese) [Google Scholar]
- Ming, L.; Hanfei, C.; Zhongyi, A.; Hao, W.; Meng, X.; Yimin, W. Chemical Leaching Combined with Biochar Stabilization Remediate Cadmium Contaminated Soil. Chin. J. Environ. Eng. 2018, 12, 904–913. [Google Scholar]
- Liu, Z.; Lu, B.; Xiao, H.; Liu, D.; Li, X.; Wang, L.; Urbanovich, O.; Nagorskaya, L. Effect of Mixed Solutions of Heavy Metal Eluents on Soil Fertility and Microorganisms. Environ. Pollut. 2019, 254, 112968. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, Y.; Liu, M.; Li, Q.; Xiao, W.; Song, X. Effects of Nitrogen Deposition and Phosphorus Addition on Arbuscular Mycorrhizal Fungi of Chinese Fir (Cunninghamia lanceolata). Sci. Rep. 2020, 10, 12260. [Google Scholar] [CrossRef]
- Hultman, J.; Waldrop, M.P.; Mackelprang, R.; David, M.M.; McFarland, J.; Blazewicz, S.J.; Harden, J.; Turetsky, M.R.; McGuire, A.D.; Shah, M.B.; et al. Multi-Omics of Permafrost, Active Layer and Thermokarst Bog Soil Microbiomes. Nature 2015, 521, 208–212. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, X.; Zhang, S.; Zhang, L.; Li, X.; Hu, X.; Zhao, Q.; Wang, Q.; Yu, C. Distribution of the Microbial Community and Antibiotic Resistance Genes in Farmland Surrounding Gold Tailings: A Metagenomics Approach. Sci. Total Environ. 2021, 779, 146502. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the Influence of Soil Microbial Community on the Long-Term Heavy Metal Pollution of Different Land Use Types and Depth Layers in Mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef]
- Yan, C.; Wang, F.; Liu, H.; Liu, H.; Pu, S.; Lin, F.; Geng, H.; Ma, S.; Zhang, Y.; Tian, Z.; et al. Deciphering the Toxic Effects of Metals in Gold Mining Area: Microbial Community Tolerance Mechanism and Change of Antibiotic Resistance Genes. Environ. Res. 2020, 189, 109869. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-Term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-Ecosystems across the Globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. Efficacy of Phosphate Solubilizing Actinobacteria to Improve Rock Phosphate Agronomic Effectiveness and Plant Growth Promotion. Rhizosphere 2021, 17, 100284. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, E.; Wu, J.; Ma, D.; Zhang, B.; Zhang, C.; Tian, F.; Zhao, B. Effects of Different Modified Materials on Soil Fungal Community Structure in Saline-Alkali Soil. Environ. Sci. 2024, 45, 3562–3570. [Google Scholar]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The Formation of Biofilms by Pseudomonas Aeruginosa: A Review of the Natural and Synthetic Compounds Interfering with Control Mechanisms. Biomed Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial Resistance to Metals in the Environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Hu, C.; Liu, H.; Bai, Y.; Qu, J. Sulfur-Based Mixotrophic Denitrification Corresponding to Different Electron Donors and Microbial Profiling in Anoxic Fluidized-Bed Membrane Bioreactors. Water Res. 2015, 85, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, W.; Sun, X.; Kong, T.; Li, B.; Liu, Z.; Gao, P. Isolation, Identification and Functional Verification of Sulfur-Oxidizing Microorganisms in Mine Tailing. Ecol. Environ. Sci. 2022, 31, 785–792. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Li, X.; Jiang, Z.; Liu, F.; Shan, Y.; Liu, Y. Fractionation Characteristics of Two-Dimensional Stable Isotopes of Carbon-Chlorine in the Process of Lindane Degradation by Aerobic Microorganisms. Chin. J. Environ. Eng. 2023, 17, 2361–2368. [Google Scholar]
- Xu, J.; Tian, G.; Li, Y. Carbon Sources Addition to Promote Degradation of Petroleum Hydrocarbons in Soil by Microbial Community. Environ. Sci. Technol. 2022, 45, 166–177. [Google Scholar]
- Yang, C.; Yin, Z.; Liu, C.; Chang, H.; Xu, Y.; Wang, H. Study on Methane Production of Multiple Agricultural and Rural Organic Solid Wastes by Cooperative Anaerobic Digestion. Acta Sci. Circumstantiae 2023, 33, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Terakado-Tonooka, J.; Fujihara, S.; Ohwaki, Y. Possible Contribution of Bradyrhizobium on Nitrogen Fixation in Sweet Potatoes. Plant Soil. 2013, 367, 639–650. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, N.; Zhang, Z.; Lei, C.; Chen, B.; Qin, G.; Qiu, D.; Lu, T.; Qian, H. Deciphering Microbial Community and Nitrogen Fixation in the Legume Rhizosphere. J. Agric. Food Chem. 2024, 72, 5659–5670. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Ding, J.; Li, C.-Y.; Yan, Z.-Z.; He, J.-Z.; Hu, H.-W. Microbial Functional Attributes, Rather than Taxonomic Attributes, Drive Top Soil Respiration, Nitrification and Denitrification Processes. Sci. Total Environ. 2020, 734, 139479. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Chen, Q.-L.; Zhu, D.; Yang, X.-R.; Qiao, M.; Hu, H.-W.; Zhu, Y.-G. Microbial Functional Traits in Phyllosphere Are More Sensitive to Anthropogenic Disturbance than in Soil. Environ. Pollut. 2020, 265, 114954. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Gutiérrez, M.C.; Siles, J.A.; García-Olmo, J.; Martín, M.A. Chemometric Analysis and NIR Spectroscopy to Evaluate Odorous Impact during the Composting of Different Raw Materials. J. Clean. Prod. 2017, 167, 154–162. [Google Scholar] [CrossRef]
- Wu, F.; You, Y.; Werner, D.; Jiao, S.; Hu, J.; Zhang, X.; Wan, Y.; Liu, J.; Wang, B.; Wang, X. Carbon Nanomaterials Affect Carbon Cycle-Related Functions of the Soil Microbial Community and the Coupling of Nutrient Cycles. J. Hazard. Mater. 2020, 390, 122144. [Google Scholar] [CrossRef] [PubMed]
- el Zahar Haichar, F.; Santaella, C.; Heulin, T.; Achouak, W. Root Exudates Mediated Interactions Belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Zhan, J.; Sun, Q. Diversity of Free-Living Nitrogen-Fixing Microorganisms in the Rhizosphere and Non-Rhizosphere of Pioneer Plants Growing on Wastelands of Copper Mine Tailings. Microbiol. Res. 2012, 167, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lv, J. Land-Use Change from Cropland to Plantations Affects the Abundance of Nitrogen Cycle-Related Microorganisms and Genes in the Loess Plateau of China. Appl. Soil Ecol. 2021, 161, 103873. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Jiao, R. Soil Phosphorus Fractions, Phosphatase Activity, and the Abundance of phoC and phoD Genes Vary with Planting Density in Subtropical Chinese Fir Plantations. Soil Tillage Res. 2021, 209, 104946. [Google Scholar] [CrossRef]
- Smart, D.; Callery, S.; Courtney, R. The Potential for Waste-Derived Materials to Form Soil Covers for the Restoration of Mine Tailings in Ireland. Land Degrad. Dev. 2016, 27, 542–549. [Google Scholar] [CrossRef]
- Zheng, X.; Qiu, S.; Zhou, B.; Li, Q.; Chen, M. Leaching of Heavy Metals from Tungsten Mining Tailings: A Case Study Based on Static and Kinetic Leaching Tests. Environ. Pollut. 2024, 342, 123055. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nakase, K.; Tamenishi, T.; Niinae, M. Influence of pH and Cations Contained in Rainwater on Leaching of Cd(II) from Artificially Contaminated Montmorillonite. J. Environ. Chem. Eng. 2020, 8, 104080. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An Assessment of pH-Dependent Release and Mobility of Heavy Metals from Metallurgical Slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]

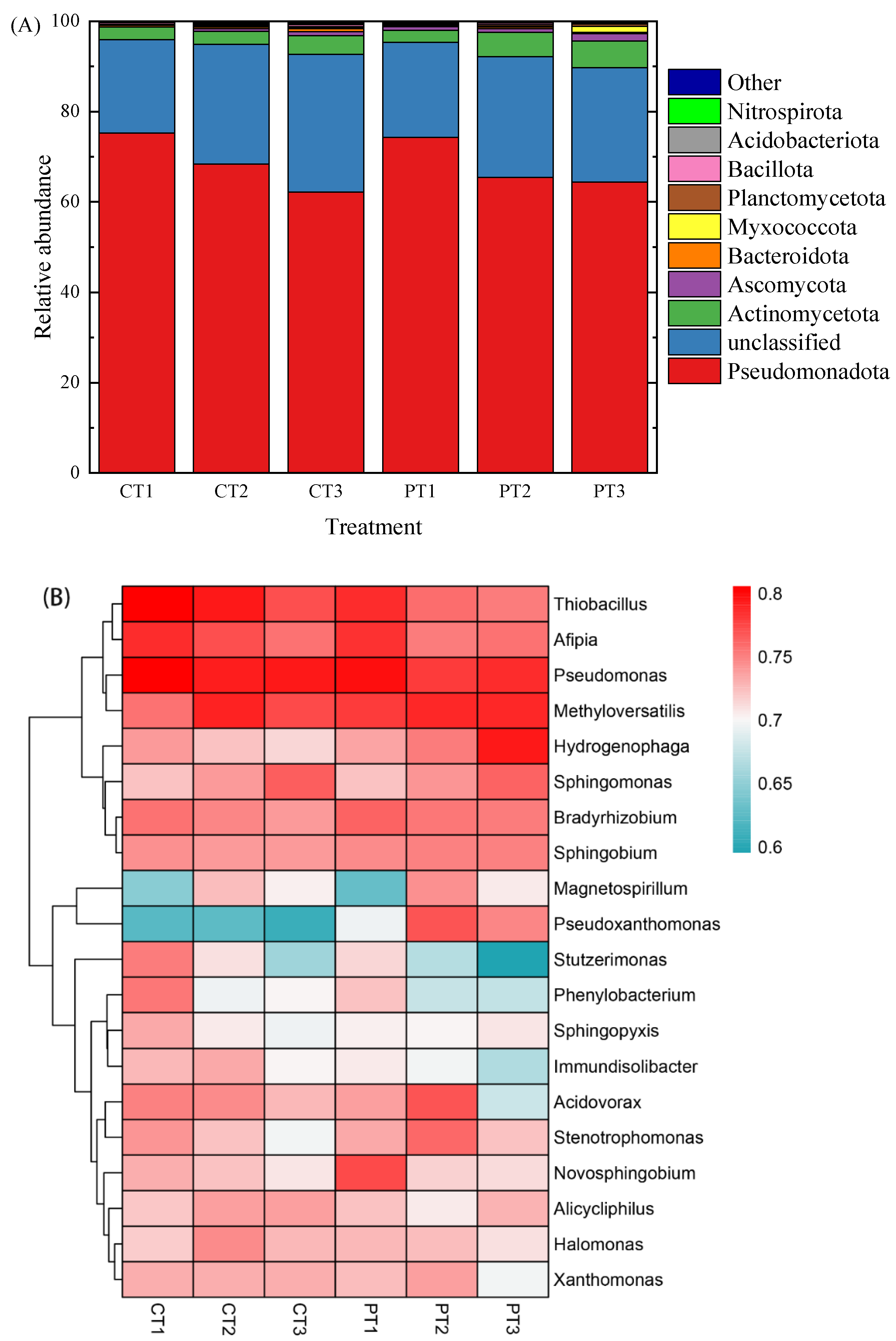
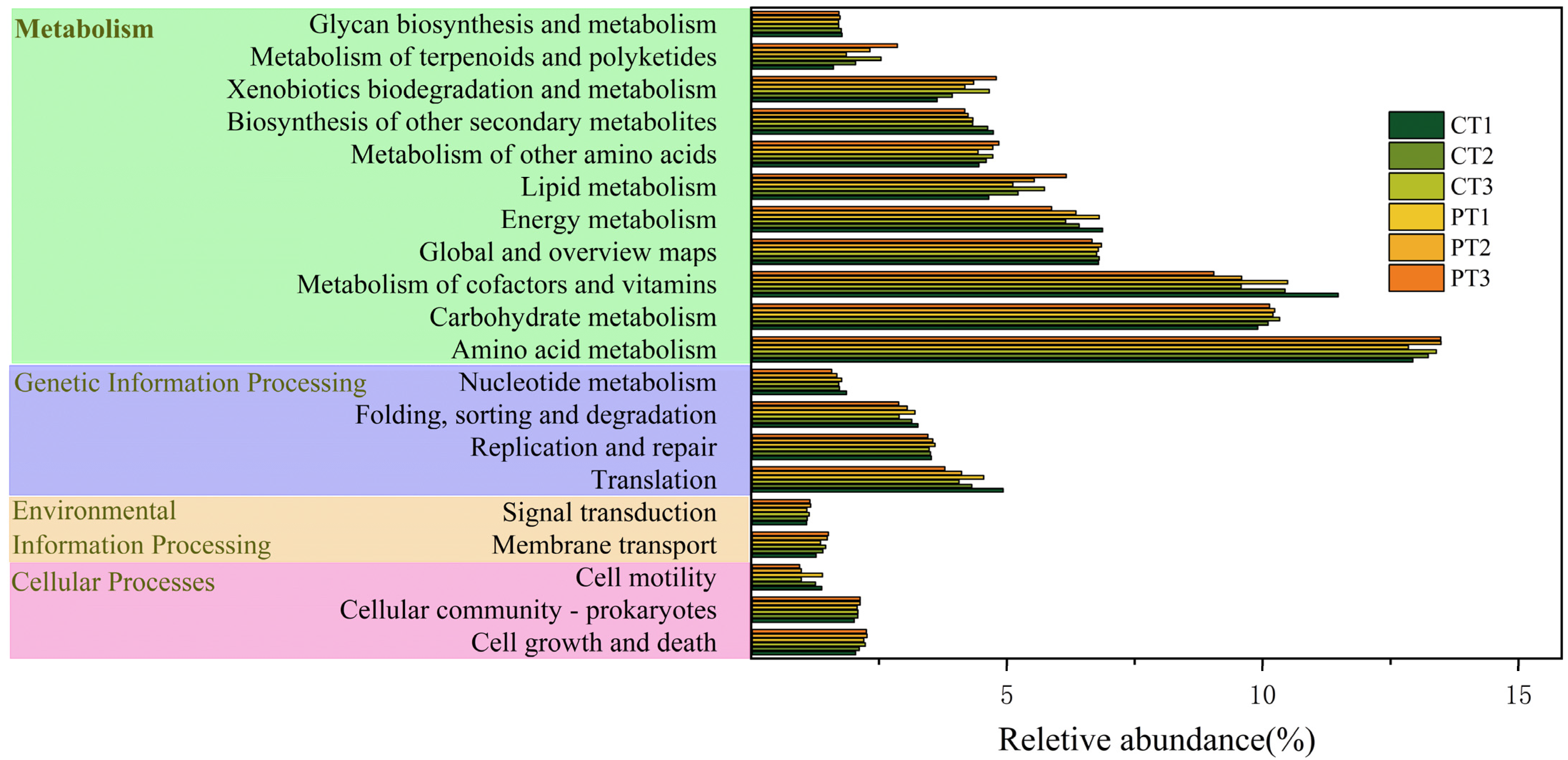
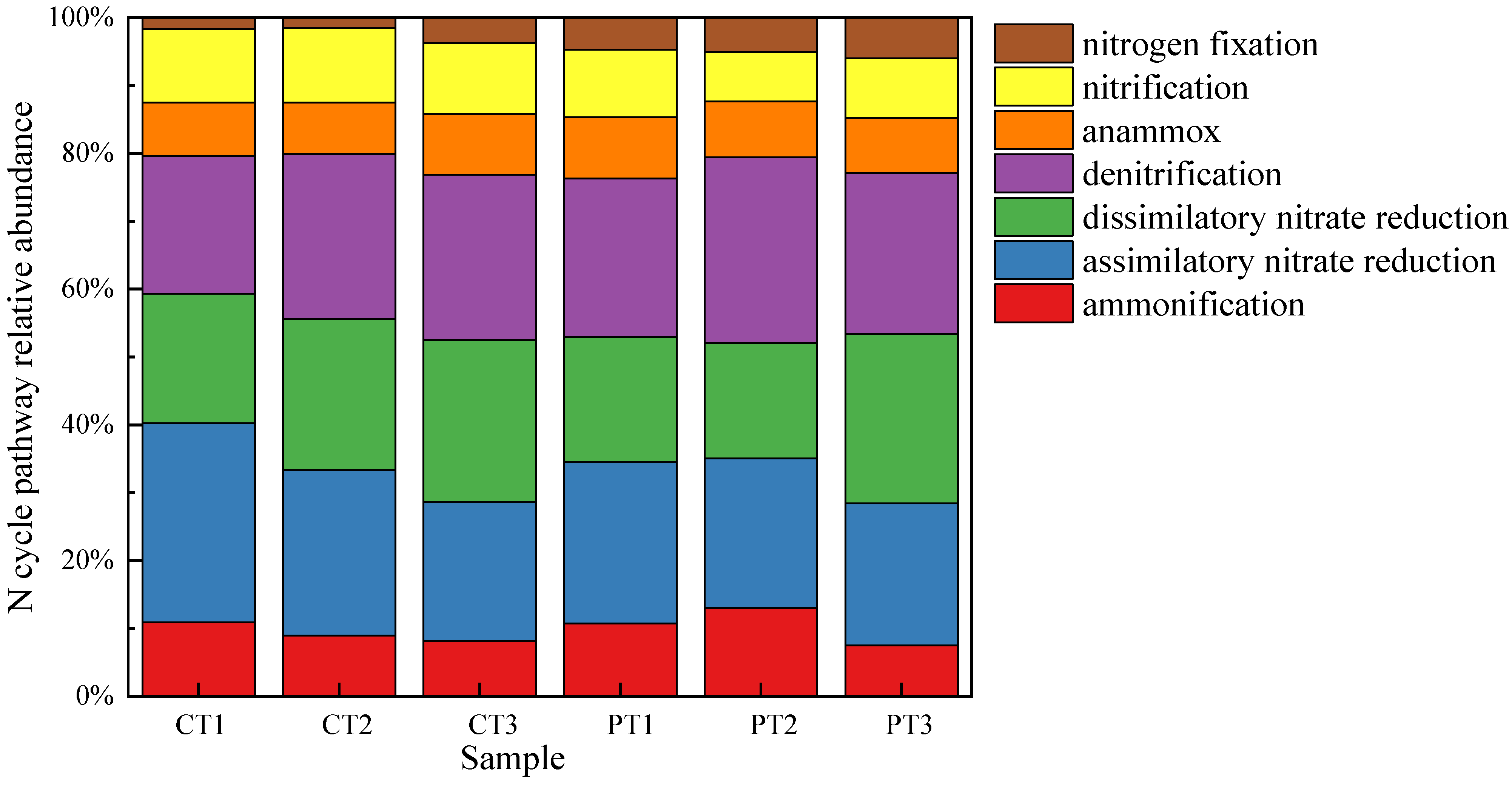

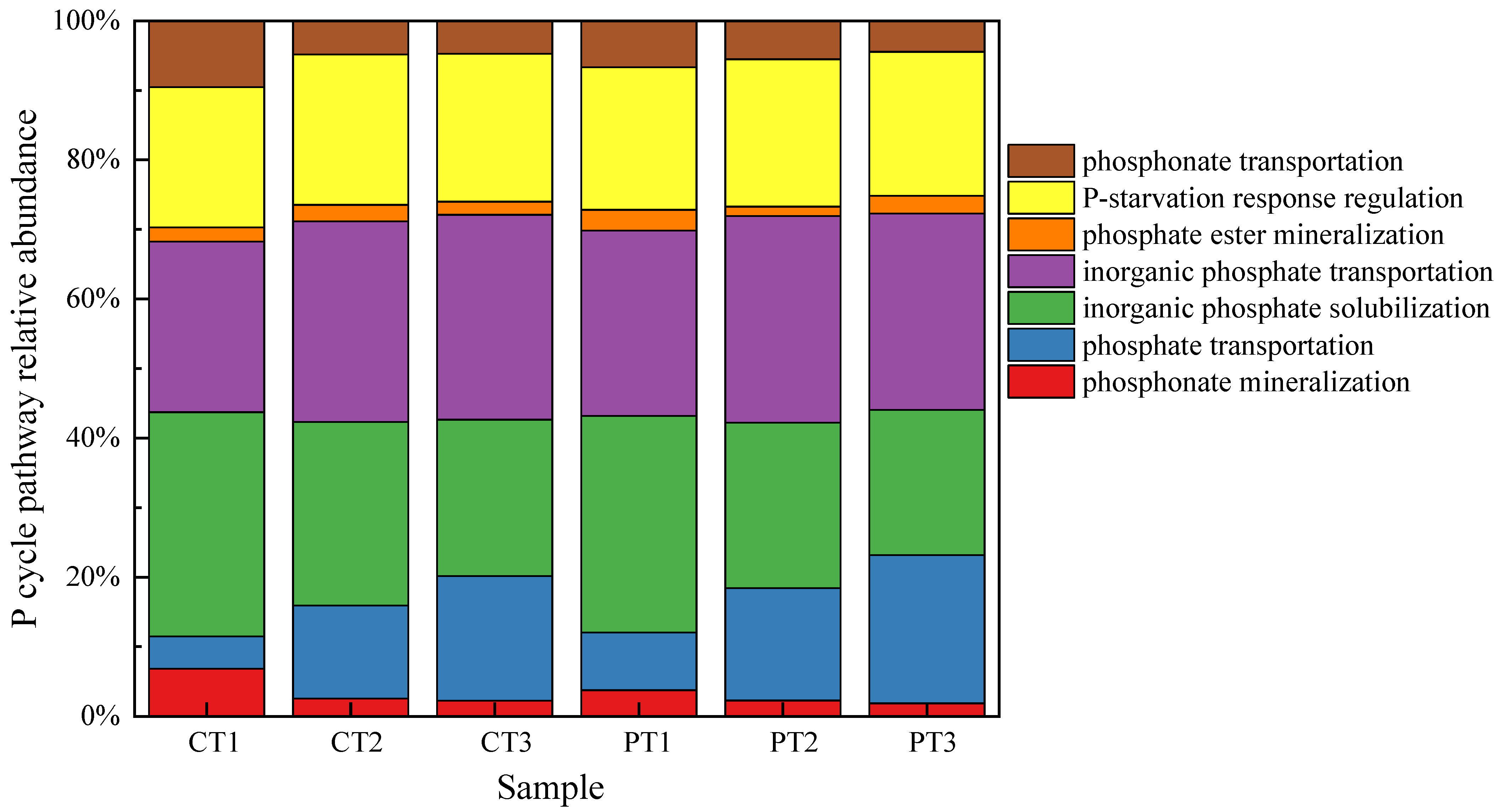

| Samples | Chao | Shannon | Simpson |
|---|---|---|---|
| CT1 | 1503.16 | 5.56 | 0.93 |
| CT2 | 1649.05 | 5.47 | 0.91 |
| CT3 | 1665.66 | 5.35 | 0.89 |
| PT1 | 1661.25 | 5.74 | 0.93 |
| PT2 | 1724.10 | 5.64 | 0.91 |
| PT3 | 1778.38 | 5.56 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Li, Q.; Peng, Y.; Wang, Z.; Chen, M. Phytoremediation of Tungsten Tailings under Conditions of Adding Clean Soil: Microbiological Research by Metagenomic Analysis. Sustainability 2024, 16, 5715. https://doi.org/10.3390/su16135715
Zheng X, Li Q, Peng Y, Wang Z, Chen M. Phytoremediation of Tungsten Tailings under Conditions of Adding Clean Soil: Microbiological Research by Metagenomic Analysis. Sustainability. 2024; 16(13):5715. https://doi.org/10.3390/su16135715
Chicago/Turabian StyleZheng, Xiaojun, Qi Li, Yang Peng, Zongli Wang, and Ming Chen. 2024. "Phytoremediation of Tungsten Tailings under Conditions of Adding Clean Soil: Microbiological Research by Metagenomic Analysis" Sustainability 16, no. 13: 5715. https://doi.org/10.3390/su16135715
APA StyleZheng, X., Li, Q., Peng, Y., Wang, Z., & Chen, M. (2024). Phytoremediation of Tungsten Tailings under Conditions of Adding Clean Soil: Microbiological Research by Metagenomic Analysis. Sustainability, 16(13), 5715. https://doi.org/10.3390/su16135715






