Abstract
The recovery concept of cleaning solutions, based on single-phase detergents from cleaning-in-place (CIP) effluents from the dairy industry, is presented. The first step consists of ultrafiltration (UF) (with a cut-off of 5 or 10 kDa) to reduce the high load of milk proteins, followed by nanofiltration (NF) (with a cut-off of 200 Da) to separate low molecular weight lactose. Membrane steps were performed in the concentration mode, achieving a recovery of 75% of the solutions. UF modules reduced 70–85% of chemical oxygen demand (COD), 99% of milk proteins, and 45–70% of lactose, limiting the susceptibility of NF modules to fouling. Combined with nanofiltration, the efficiency of the purification system is 100% for proteins and more than 99% for lactose. The solutions recovered in the proposed purification variants are recognized as sodium hydroxide solutions with a surfactant admixture, and they can be successfully re-used for cleaning processes in the production plant.
1. Introduction
The future of industrial plants is to operate within a circular economy model. This concept promotes the use of materials from existing industrial processes to minimize the consumption of raw materials in production processes and the secondary pollution of the natural environment. When creating a closed-loop pattern, water deserves special attention as it is a unique element. It has no equivalent in the economic system and, depending on the context, it can be a resource, product, and service [1]. The transformation process towards a circular economy must include a stage of waste prevention practices, waste processing, and recycling operations. With regard to industrial wastewater, the focus should be on its treatment to limit the release of harmful substances into the environment and to recover water as an alternative source for production processes. The implementation of a circular economy model has particularly great potential in the food industry, which includes the dairy industry. These activities focus primarily on reducing the amount of wastewater generated, effective wastewater treatment to recover valuable components, closing water cycles and reusing the water taken from the distribution system, and recovering chemicals used in the hygienization and washing processes at production plants. These latter operations are considered to be the main source of wastewater in the dairy sector since washing processes account for 70% of water consumption [2].
One of the most important steps leading to the rationalization of water and wastewater management in dairy plants was the implementation of CIP technology and replacing the conventional cleaning of production lines and equipment using alkalis and acids with single-phase detergents. New detergent formulas are mainly composed of alkalis or acids, surfactants, complex disinfecting agents, and defoamers [3]. This change in the pattern of cleaning eliminates two steps from the washing procedure (the intermediate rinse and the second circulation of detergent solution) and results in time and water savings, as well as a considerable reduction in energy costs for heating the washing solution and for recirculation pumps [3,4,5,6]. However, wastewater from CIP operations is still not being properly managed and constitutes a major environmental and economic problem for industrial plants [7,8]. These CIP streams contain rinsing water, specific organic residues for the production profile expressed with a high level of chemical oxygen demand (COD) and biochemical oxygen demand (BOD), and chemical cleaning agents [9]. Traditionally, CIP solutions are discharged after one or several uses [10]. The multiuse of cleaning solutions without proper treatment creates favorable conditions for the multiplication of microorganisms, which constitutes a significant sanitary threat, especially in the case of food production [11].
Pressure-driven membrane processes have particularly great potential for recycling CIP effluents. Despite the significant popularity of single-phase detergents for use in washing operations in the food industry in the last decade, few reports in the literature discuss the possibility of recovering or recycling cleaning compounds from CIP wastewater. The feasibility of recovering contaminated single-phase detergents used in a clean-in-place system in a yogurt industrial plant was verified by Fernández et al. [12]. The membrane (KOCH MPS-34, Koch Membrane Systems, Wilmington, MA, USA) operating at a high recovery rate (75%) achieved a stable permeate flux and retention. The NF permeate exhibited a very good cleaning efficiency (related to a high pH value, low surface tension, and low amount of suspended solids, which are considered the most important parameters) to wash UHT equipment and an industrial yogurt filling machine. A nanofiltration unit (300 Da) was used by Suarez et al. [13] to recover a spent single-phase detergent in a yogurt factory. Membranes retained around 90% of the organic matter (COD); however, this did not reduce the cleaning efficiency, which affected the fresh detergent savings at the production plant by about 18%. Previous research carried out by the author [14] on model solutions, also demonstrated the usefulness of pressure-driven membrane processes for cleaning and concentrating using single-phase detergent solutions. Integrated purification processes provided a very high separation of the milk components and organic compounds of single-phase detergent, resulting in a partial loss of the detergent properties of the solutions. An interesting contribution to this research area is the work of Kim et al. [15] in which the authors analyzed a sequential system that combined ultrafiltration (4 kDa) and nanofiltration (200 Da) for the separation of organic compounds, followed by forward osmosis (FO) as a concentration step for the cleaning agents. UF-NF reduced the protein concentration by 96% and lactose concentration below the detection limit (below 1.5 ppm). The concentration of active alkali content (NaOH) decreased from 0.31% to 0.22%. Reducing the concentration of organic compounds effectively reduced the fouling of the FO membranes. The concentration using thin-film composite FO membranes resulted in an increase in NaOH concentration by 0.5–0.6%.
The presented study is a case study on the recovery of spent single-phase detergents from the dairy industry, in order to re-use the cleaning agents. The proposed treatment systems are intended to constitute the basis for the implementation of the technology at the industrial plant. Studies reflecting the technical conditions were performed in the long-term filtration runs, at a 75% recovery rate for the feed solutions. The effectiveness of sequential purification systems that combine ultrafiltration and nanofiltration was verified. The role of ultrafiltration was to remove a large load of organic pollutants, in order to protect nanofiltration membranes against fouling. The final quality of the recovered cleaning agent depended on the nanofiltration process.
2. Materials and Methods
The experiments used a spent detergent, obtained from a single-phase washing procedure, from a dairy plant located in Wielkopolska Voivodeship, Poland. This washing protocol used a commercial chlorine-free alkaline detergent whose composition consisted primarily of sodium hydroxide, together with complexing agents and low-foaming surfactants. The cleaning cycle comprised the following stages: (i) recovery of milk residues by drainage and expulsion with water, (ii) pre-rinsing with water to remove components from devices more thoroughly, (iii) cleaning with detergent, and (iv) rinsing with clean water. The main washing stage was carried out for 20 min, with a 1.5% detergent solution at a temperature of 70 °C. The effluents were separated into three main streams and stored in separate tanks. One of the tanks collected sewage from stages (i) and (ii), another from stage (iii) and the last from stage (iv). This strategy allowed for a significant reduction in the contamination of the detergents used with milk components coming from production processes and prevented a detergent dilution with rinsing water.
The averaged effluents of stage (iii), with the composition given in Table 1, were subjected to purification and concentration processes to recover the detergent formula in order to re-use them in the following cleaning cycles. On the basis of the preliminary tests, for the purification of contaminated detergent solutions, a sequence of two pressure-driven membrane processes was selected. The effluents were pre-treated by ultrafiltration and then the collected permeate was transferred to nanofiltration as a feed solution (Figure 1). Each stage was carried out for solutions that were cooled to a room temperature of 22 °C, until 75% recovery rate was achieved.

Table 1.
Characteristics of the feed solution and analytical procedures.
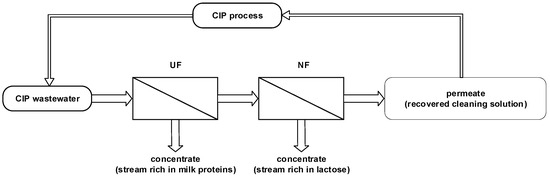
Figure 1.
Schematic diagram of the recovery of CIP solutions.
Filtration experiments were performed in a semi-pilot cross-flow plant in concentration mode with continuous recirculation of the retentate to the feed tank and the separate collection of the permeate. The installation was equipped with a thermostatic system that allowed the temperature of the solution to be maintained at a set level.
The studies used commercially available ultrafiltration modules (ceramic type C5 and C10, polymeric type PM5) and a nanofiltration module (polymeric type AFC30) of different characteristics (Table 2). As a result of the alkaline nature of the purified solutions, modules with high resistance to chemical agents were selected for the tests.

Table 2.
Characteristics of the UF and NF modules [16,17,18].
Membrane processes were continuously monitored to assess the transport properties of the modules (including their susceptibility to fouling) and the degree of recovery rate using the following parameters:
- Flux (L·m−2·h−1):
- Recovery rate (%):
Vp—volume of the permeate (L) at time t, V0—initial volume of the feed (treated) solution,
- Normalized flux (%):
The quality of the permeates was assessed in averaged samples, taking into account the same parameters, as characterized in Table 1 for raw wastewater. The separation ability of the modules was assessed on the rejection (%):
where Cf—compound concentration in the feed (mg·L−1), Cp—compound concentration in the permeate (mg·L−1).
Each filtration run was examined under selected operating conditions (transmembrane pressure (TMP) and cross-flow velocity (CFV)).
3. Results and Discussion
Ultrafiltration concentration of the purified solutions was carried out to obtain a 75% recovery rate. For each module, a decrease in flux was observed, compared to the pure water permeability (FN value). This decrease deepened with the volume of the collected permeate and, thus, resulted in a more concentrated feed solution (Figure 2). The average values of flux decline (100-FN) determined for the filtration cycle were 55%, 64%, and 61% for the C5, C10, and PM5 modules, respectively (Table 3). This indicates that membranes with a larger cut-off value (and therefore pore size) were more susceptible to fouling and the components of the purified solution penetrated the membrane structure more easily. When comparing the flux of 5 kDa modules, it is observed that the flux of the PM5 module decreases more than that of the C5 module. The main reason for this phenomenon is the greater hydrophilicity of a ceramic module compared to an organic module made of unmodified polysulfone, thus being more susceptible to fouling caused by organic compounds [19].
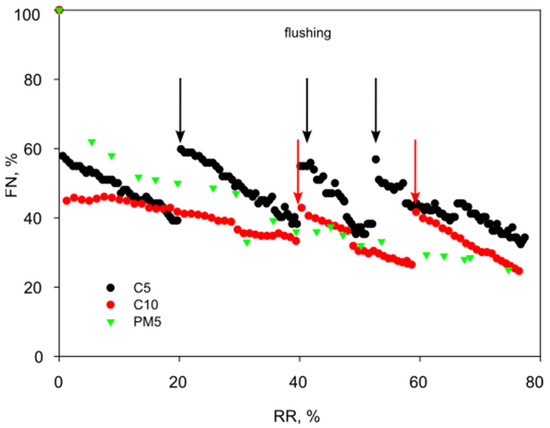
Figure 2.
Normalized flux of ultrafiltration modules depending on the recovery rate (C5: TMP = 3.0 bar, CFV = 4.4 m/s; C10: TMP = 3.0 bar, CFV = 3.8 m/s; PM5: TMP = 1.0 bar, CFV = 2.2 m/s).

Table 3.
Recovery rate and average values of flux decline (100-FN) for unit and integrated membrane processes.
The high-flux characteristics of these membranes intensify the disadvantageous effects of concentration polarization, due to an accumulation of retained solutes at the membrane surface on the feed side, leading to its progressive fouling. In order to restore satisfactory hydraulic efficiency of the ultrafiltration modules, intermediate stages of flushing with water were carried out.
The pre-treatment of spent detergent solutions on UF modules significantly reduced the concentration of organic matter, contributing to a reduction in the fouling of NF modules and its relatively stable flux throughout the concentration cycle. Moreover, the smaller pores of nanofiltration membranes were much less susceptible to penetration by the organic compounds, compared to ultrafiltration membranes (Figure 3). The average values of flux decline (100-FN) of AFC30 were 12% and 11% for the integrated C10 + AFC30 and PM5 + AFC30 systems, respectively (Table 3). However, for the integrated C5 + AFC30 system, an increase in flux was obtained in relation to the permeability of pure water. This phenomenon may be related to both the enlargement of the pores of the polyamide layer and the hydrophilization of the polymer as a result of contact with alkalis [20,21,22,23,24]. Data in the literature indicate that, in PA nanofiltration membranes exposed to concentrated NaOH solutions, changes occur in the polymer structure, causing an increase in pore size [20]. At lower NaOH concentrations, morphological changes were insignificant, and hydrophilicity changes dominated [21]. Gul et al. [22] showed that there was an almost twofold increase in the pore size of the PA6 membrane under a low concentration of alkaline cleaning agent (1% NaOH). On the other hand, increasing the concentration of NaOH up to 5% caused a twofold reduction in the PA6 membrane’s pore size. Jun et al. [23] also showed changes in the hydrophilicity of nanofiltration polyamide membranes. Due to contact with the alkaline solution (pH 13.5) a contact angle decreased from 44.9° to 38.6° for the pristine and the treated membrane, respectively, which resulted from the depolymerization of the amide bond. The authors also confirmed that membranes modified under alkaline conditions showed improved flux with similar rejection of the dyes. Pérez-Álvarez et al. [24] in research on alkaline hydrolysis of PAN membranes showed a significant reduction in the contact angle, and therefore strongly hydrophilic properties of the modified material.
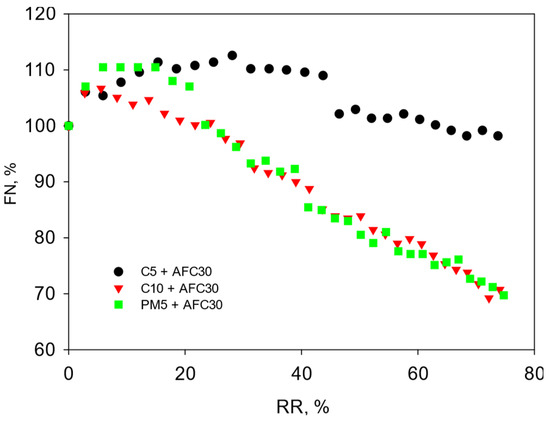
Figure 3.
Normalized flux of nanofiltration modules depending on the recovery rate (AFC30: TMP = 4.0 bar, CFV = 0.6 m/s).
The parameters measured in the averaged samples of the permeates recovered during the concentration cycle are presented in Figure 4 and Table 4.
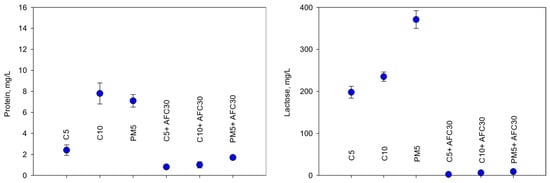
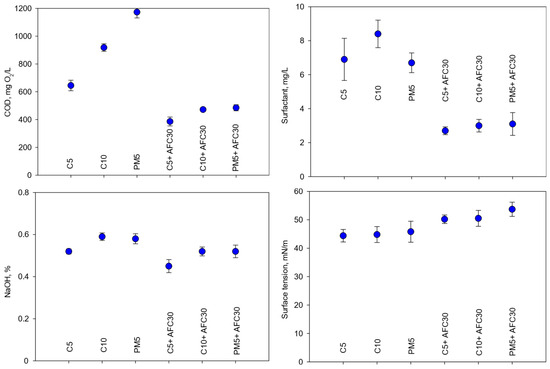
Figure 4.
Composition of the recovered cleaning solutions (C5: TMP = 3.0 bar, CFV = 4.4 m/s; C10: TMP = 3.0 bar, CFV = 3.8 m/s; PM5: TMP = 1.0 bar, CFV = 2.2 m/s; AFC30: TMP = 4.0 bar, CFV = 0.6 m/s).

Table 4.
Composition of the recovered cleaning solutions (C5: TMP = 3.0 bar, CFV = 4.4 m/s; C10: TMP = 3.0 bar, CFV = 3.8 m/s; PM5: TMP = 1.0 bar, CFV = 2.2 m/s; AFC30: TMP = 4.0 bar, CFV = 0.6 m/s).
Ultrafiltration ensured a significant removal of organic compounds: COD reduction was approximately 80%, 85%, and 70% for the C5, C10, and PM5 modules, respectively. This correlated with the removal of compounds of lactic and detergent origin.
The highest separation efficiency (99%) was achieved for proteins present in milk, the main components of which are casein and whey proteins, with shares of 80% and 20%, respectively [25].
Casein is a heterogeneous family of four major components, including αs1-casein, αs2-casein, β-casein, and χ-casein, with molecular weights in the range 19,006–25,226 Da. Whey proteins mainly contain α-lactalbumin (14,178 Da) and β-lactoglobulin (18,277 and 18,363 Da, depending on the genetic variant), which is approximately 70–80%. Among the other dominant types are immunoglobulins (161–1,000,000 Da), serum albumin (66,399 Da), and lactoferrin (76,110 Da) [26]. The smallest milk proteins have molecular weights much larger than the cut-off values of the ultrafiltration modules (5000 Da and 10,000 Da), which determines their very high separation efficiency. A factor supporting the separation is the change in the protein system by the denaturation of whey proteins in the presence of strong alkalis (pH = 12.85) [20,27]. The large clusters that form are separated by the membrane more effectively than the small primary aggregates and, together with casein, accumulate on the membrane surface, forming an additional sieving layer. The structure of casein micelles does not change significantly [28]; however, strong alkaline solutions increase their solubility and the net negative charge, due to the complete deprotonation of carboxyl groups, leads to strong electrostatic repulsion (ζ-potential of −30 mV for pH = 11) [29]. This caused the deposits to swell and redisperse [30] facilitating the transport of the solvent through the polarization layer. Electrostatic repulsions between the negatively charged casein micelles and the negatively charged ultrafiltration membranes contribute to reducing membrane fouling and cause negatively charged proteins to be rejected by electrostatic repulsion, and not only because of simple size sieving [31].
Lactose rejection varied significantly, depending on the type of ultrafiltration module. Ceramic UF modules (with a cut-off value of 5 and 10 kDa) ensured lactose separation of 70%, while the polysulfone PM5 module (with a cut-off value of 5 kDa) separated only 45% of this compound. It should be assumed that the level of lactose separation is primarily responsible for the additional layer of accumulated proteins that forms on the membrane surface. The main reason why the separation of lactose is much greater for ceramic modules is most likely because of the thicker additional layer on the membrane surface (which results from a higher amount of accumulated organic matter (mainly proteins)) and thus a greater share of the sorption in the separation mechanism. An additional contribution to the lactose separation is its sorption in the porous structure of the modules. The large difference between the molecular weight of lactose (342.3 Da) and the module cut-off value excludes the involvement of the simple sieve mechanism in the separation. The relatively high level of lactose separation results from the role of sorption processes during solution purification, with a low concentration of this compound in the purified solution (approx. 0.07% w/v vs. 5% w/v in milk).
Surfactants (organic ingredients of detergent origin) were removed with an efficiency of 66–77%, depending on the type of UF module. As in the case of lactose, the separation of these low-molecular-weight compounds is primarily due to the adsorption in the additional filtration layer at the membrane surface and in the porous structure of the module. The anionic surfactant molecules present in the purified solution interact with both the membrane surface and the components of the cleaning solution. Due to the amphiphilic molecules, the hydrophobic chain adsorbs on the membrane structure through hydrophobic interaction, and the hydrophilic head orients away from the membrane surface through electrostatic repulsion [32], thus improving hydrophilicity and increasing the negative charge of the membrane, which enhances electrostatic repulsion with negatively charged casein micelles. The other surfactant molecules that penetrate the membrane structure are deposited in its pores as a result of hydrophobic and electrostatic interactions, filling pores that expand at an alkaline pH [32,33,34] and limit the swelling effect induced by alkali, which also reduces the accumulation of other small molecular pollutants (lactose) in the pores.
Surfactants may interact with proteins concentrated in the polarization layer of the membrane by binding to them, which can lead to substantial changes in protein conformation [35] and influence the loss of surfactant in the permeate. The binding of the surfactant to proteins involves two stages [36]: the ionic interaction of the head groups of the surfactant with the ionic sites in the proteins, and the hydrophobic interaction of the surfactant alkyl chain with the hydrophobic patches in the protein. The presence of surfactants changes the physical properties of the protein, i.e., unfolds proteins and causes aggregation and adsorption of proteins onto surfaces [35,37].
Due to the relatively high cut-off values of the UF modules, a slight separation of sodium hydroxide (as the main component of the cleaning composition) was observed at a level of 3% for C10 and PM5, and 5% for C5. The decrease in sodium hydroxide concentrations in the permeate correlated with the decrease in pH and conductivity.
Permeates after the ultrafiltration process were characterized by slightly higher surface tension values (45 ± 0.5 mN/m) compared to the feed solutions (38.1 ± 1.3 mN/m), which is primarily due to the partial rejection of surfactants. The increase in surface tension can also be associated with the removal of proteins, which shows an ability to reduce the surface tension of solutions [38,39].
The implementation of nanofiltration as a post-treatment process for UF permeates contributed to a further reduction in the content of organic compounds. Integrated purification processes ensured 100% protein separation and more than 99% low-molecular-weight lactose separation. In relation to the feed solution, 10–24% of the original surfactant content remained and the increase in the surface tension value was 12–18 mN/m.
With the use of the NF module, partial separation of NaOH occurred (approximately 11.5% of separation compared to the permeate after the ultrafiltration process), resulting in a decrease in the pH value of the solutions, together with the conductivity level.
In summarizing the results, it can be stated that the use of integrated UF-NF purification systems allowed the removal of both basic milk components and single-phase detergent components with very high efficiency. The higher selectivity of the nanofiltration module contributed to a much greater modification of the composition of the single-phase detergent compared to the unit UF process. The permeates obtained from the individual integrated purification variants can be recognized as sodium hydroxide solutions of similar concentrations (0.45–0.52% in permeates and 0.52–0.61% in feed solutions) with a small admixture of surfactants.
4. Conclusions
The recovery of cleaning solutions from spent single-phase detergents by ultrafiltration is not fully satisfactory. Modules significantly reduced the concentration of organic matter (a 70–85% reduction in COD) mainly through the very high separation (99%) of milk proteins; however, the removal efficiency of lactose due to its low molecular weight was 45–70%. The loss of sodium hydroxide in the ultrafiltration process was only slight (3–5%).
A significant reduction in the concentration of organics in the pre-treatment stage contributed to the fouling reduction of NF modules and their relatively stable flux throughout the concentration cycle. This made it possible to achieve a 75% recovery rate without the need to turn off the system to regenerate the NF module.
The implementation of nanofiltration as a second purification stage (due to its higher selectivity) contributed to a further reduction in organics in the purified solutions; therefore, the integrated purification processes ensured a total separation of 100% for proteins and over 99% for lactose. The recovered cleaning solutions still contained surfactant at a concentration of approximately 3 mg/L, which had a positive effect on their detergent properties.
The solutions recovered from the proposed purification variants were recognized as sodium hydroxide solutions with a surfactant admixture. Despite the partial loss of surfactants and a slight increase in surface tension, the cleaning properties of the composition are retained and allow it to be reused for hygienization purposes directly or after minor composition adjustments. Alternatively, these compositions can be used for other cleaning purposes at the production plant (tanks, floors) or for the alkaline regeneration of membrane modules commonly used in milk standardization processes and dairy production.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented within the article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Morseletto, P.; Eline Mooren, C.; Munaretto, S. Circular economy of water: Definition, strategies and challenges. Circ. Econ. Sustain. 2022, 2, 1463–1477. [Google Scholar] [CrossRef]
- Ajiero, I.; Campbell, D. Benchmarking water use in the UK food and drink sector: Case study of three water-intensive dairy products. Water Conserv. Sci. Eng. 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Pant, K.J.; Cotter, P.D.; Wilkinson, M.G.; Sheehan, J.J. Towards sustainable Cleaning-in-Place (CIP) in dairy processing: Exploring enzyme-based approaches to cleaning in the Cheese industry. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3602–3619. [Google Scholar] [CrossRef] [PubMed]
- AEB Cleaning Procedures. Available online: https://www.aeb-group.com/media/procedure-lavaggio/depliant/SINGLE-PHASE_PROCEDURES_AEB_LR.pdf (accessed on 25 January 2024).
- CIP Cleaning Operations by Single-Phase Acid Procedure, Italian Food Tech, February 2018. Available online: https://www.italianfoodtech.com/cip-cleaning-operations-by-single-phase-acid-procedure/ (accessed on 25 January 2024).
- Single-Step Acid Wash Cleaner to Improve Cleaning Efficiency. Available online: https://www.food-safety.com/articles/2521-single-step-acid-wash-cleaner-to-improve-cleaning-efficiency (accessed on 25 January 2024).
- Nagappan, S.; Phinney, D.M.; Heldman, D.R. Management of waste streams from dairy manufacturing operations using membrane filtration and dissolved air flotation. Appl. Sci. 2018, 8, 2694. [Google Scholar] [CrossRef]
- Kolev Slavov, A. Dairy wastewater treatment review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Alalam, S.; Ben-Souilah, F.; Lessard, M.-H.; Chamberland, J.; Perreault, V.; Pouliot, Y.; Labrie, S.; Doyen, A. Characterization of chemical and bacterial compositions of dairy wastewaters. Dairy 2021, 2, 179–190. [Google Scholar] [CrossRef]
- Merin, U.; Gésan-Guiziou, G.; Boyaval, E.; Daufin, G. Cleaning-in-place in the dairy industry: Criteria for reuse of caustic (NaOH) solutions. Lait 2002, 82, 357–366. [Google Scholar] [CrossRef]
- Silva, L.D.; Aguiar, M.M.; Paiva, A.D.; Bernardes, P.C.; Gedraite, R.; Araújo Naves, E.A. Optimization of clean-in-place (CIP) procedure of pipelines contaminated with Bacillus cereus by applying pulsed flow. Food Control 2023, 147, 109565. [Google Scholar] [CrossRef]
- Fernández, P.; Riera, F.A.; Álvarez, R.; Álvarez, S. Nanofiltration regeneration of contaminated single-phase detergents used in the dairy industry. J. Food Eng. 2010, 97, 319–328. [Google Scholar] [CrossRef]
- Suárez, L.; Diez, M.A.; Riera, F.A. Recovery of detergents in food industry: An industrial approach. Desalin. Water Treat. 2015, 56, 967–976. [Google Scholar] [CrossRef]
- Kowalska, I. Concentration of contaminated single-phase detergents by means of unit and integrated membrane processes. Sep. Sci. Technol. 2016, 51, 1199–1209. [Google Scholar] [CrossRef]
- Kim, W.J.; Huellemeier, H.; Heldman, D.R. Recovery of cleaning agents from clean-in-place (CIP) wastewater using nanofiltration (NF) and forward osmosis (FO). J. Water Process Eng. 2023, 53, 103617. [Google Scholar] [CrossRef]
- Product Data Sheet of the Inside CeRam Modules. Available online: https://www.tami-industries.com/en/produits/inside-ceram-4/ (accessed on 25 January 2024).
- Product Data Sheet of the Romicon® Modules. Available online: https://www.kovalus.com/wp-content/uploads/2020/10/Romicon-5-inch-hollow-fiber-cartridges.pdf (accessed on 25 January 2024).
- Product Data Sheet of the PCI Modules. Available online: https://www.pcimembranes.com/wp-content/uploads/2019/10/Membrane-Data-Sheet-AFC30AFC40.pdf (accessed on 25 January 2024).
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Simon, A.; Price, W.E.; Nghiem, L.D. Influence of formulated chemical cleaning reagents on the surface properties and separation efficiency of nanofiltration membranes. J. Membr. Sci. 2013, 432, 73. [Google Scholar] [CrossRef]
- Oh, N.-W.; Jegal, J.; Lee, K.-H. Preparation and characterization of nanofiltration composite membranes using polyacrylonitrile (PAN). I. Preparation and modification of PAN supports. J. Appl. Polym. Sci. 2001, 80, 1854–1862. [Google Scholar] [CrossRef]
- Gul, A.; Hruza, J.; Dvorak, L.; Yalcinkaya, F. Chemical cleaning process of polymeric nanofibrous membranes. Polymers 2022, 14, 1102. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.-M.; Yoon, Y.; Park, C.M. Post-Treatment of Nanofiltration Polyamide Membrane through Alkali-Catalyzed Hydrolysis to Treat Dyes in Model Wastewater. Water 2019, 11, 1645. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Moreno, I.; Vilas-Vilela, J.L. Characterization and Optimization of the Alkaline Hydrolysis of Polyacrylonitrile Membranes. Polymers 2019, 11, 1843. [Google Scholar] [CrossRef]
- Davoodi, S.H.; Shahbazi, R.; Esmaeili, S.; Sohrabvandi, S.; Mortazavian, A.; Jazayeri, S.; Taslimi, A. Health-related aspects of milk proteins. Iran. J. Pharm. Res. 2016, 15, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.M., Jr.; Jimenez-Flores, R.; Bleck, G.T.; Brown, E.M.; Butler, J.E.; Creamer, L.K.; Hicks, C.L.; Hollar, C.M.; Ng-Kwai-Hang, K.F.; Swaisgood, H.E. Nomenclature of the proteins of cows’ milk—Sixth revision. J. Dairy Sci. 2006, 87, 1641–1674. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Isobe, S.; Tomasula, P.M.; Cooke, P.H. Properties of whey protein isolates extruded under acidic and alkaline conditions. J. Dairy Sci. 2006, 89, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Sun, J.; Cao, D.; Tuo, Y.; Jiang, S.; Mu, G. Experimental and modelling study of the denaturation of milk protein by heat treatment. Korean J. Food Sci. Anim. Resour. 2017, 37, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Post, A.E.; Arnold, B.; Weiss, J.; Hinrichs, J. Effect of temperature and pH on the solubility of caseins: Environmental influences on the dissociation of αS-and β-casein. J. Dairy Sci. 2012, 95, 1603–1616. [Google Scholar] [CrossRef]
- Loginov, M.; Doudiès, F.; Hengl, N.; Karrouch, M.; Leconte, N.; Garnier-Lambrouin, F.; Pérez, J.; Pignon, F.; Gésan-Guiziou, G. On the reversibility of membrane fouling by deposits produced during crossflow ultrafiltration of casein micelle suspensions. J. Membr. Sci. 2021, 630, 119289. [Google Scholar] [CrossRef]
- Arunkumar, A.; Etzel, M.R. Milk protein concentration using negatively charged ultrafiltration membranes. Foods 2018, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, A.S.; Jönsson, B. The influence of nonionic and ionic surfactants on hydrophobic and hydrophilic ultrafiltration membranes. J. Membr. Sci. 1991, 56, 49. [Google Scholar] [CrossRef]
- Huang, j.; Luo, J.; Chen, X.; Feng, S.; Wan, Y. New insights into effect of alkaline cleaning on fouling behavior of polyamide nanofiltration membrane for wastewater treatment. Sci. Total Environ. 2021, 780, 146632. [Google Scholar] [CrossRef]
- Kowalska, I. Dead-end and cross-flow ultrafiltration of ionic and non-ionic surfactants. Des. Water Treat. 2012, 50, 397–410. [Google Scholar] [CrossRef]
- Sharma, A. Equilibrium dialysis studies on the binding of anionic surfactants with bovine milk casein. J. Appl. Pharm. Sci. 2012, 2, 102–105. [Google Scholar]
- Madaeni, S.S.; Rostami, E.; Rahimpour, A. Surfactant cleaning of ultrafiltration membranes fouled by whey. Int. J. Dairy Technol. 2010, 63, 273–283. [Google Scholar] [CrossRef]
- Jelińska, A.; Zagożdżon, A.; Górecki, M.; Wisniewska, A.; Frelek, J.; Holyst, R. Denaturation of proteins by surfactants studied by the Taylor dispersion analysis. PLoS ONE 2017, 20, e175838. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.Y.; Hussain, S.; Tsay, R.Y.; Noskov, B.A.; Akentiev, A.; Lin, S.Y. On the equilibrium surface tension of aqueous protein solutions—Bovine serum albumin. J. Mol. Liq. 2022, 347, 118305. [Google Scholar] [CrossRef]
- Marinova, K.G.; Basheva, E.S.; Nenova, B.; Temelska, M.; Mirarefi, A.Y.; Campbell, B.; Ivanov, I.B. Physico-chemical factors controlling the foamability and foam stability of milk proteins: Sodium caseinate and whey protein concentrates. Food Hydrocoll. 2009, 23, 1864–1876. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).