Effect of Surface Area, Particle Size and Acid Washing on the Quality of Activated Carbon Derived from Lower Rank Coal by KOH Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Activation of Coal
2.3. Analytical Methods
2.3.1. Calorimetry
2.3.2. Proximate and Ultimate Analyses
2.3.3. Size Analysis
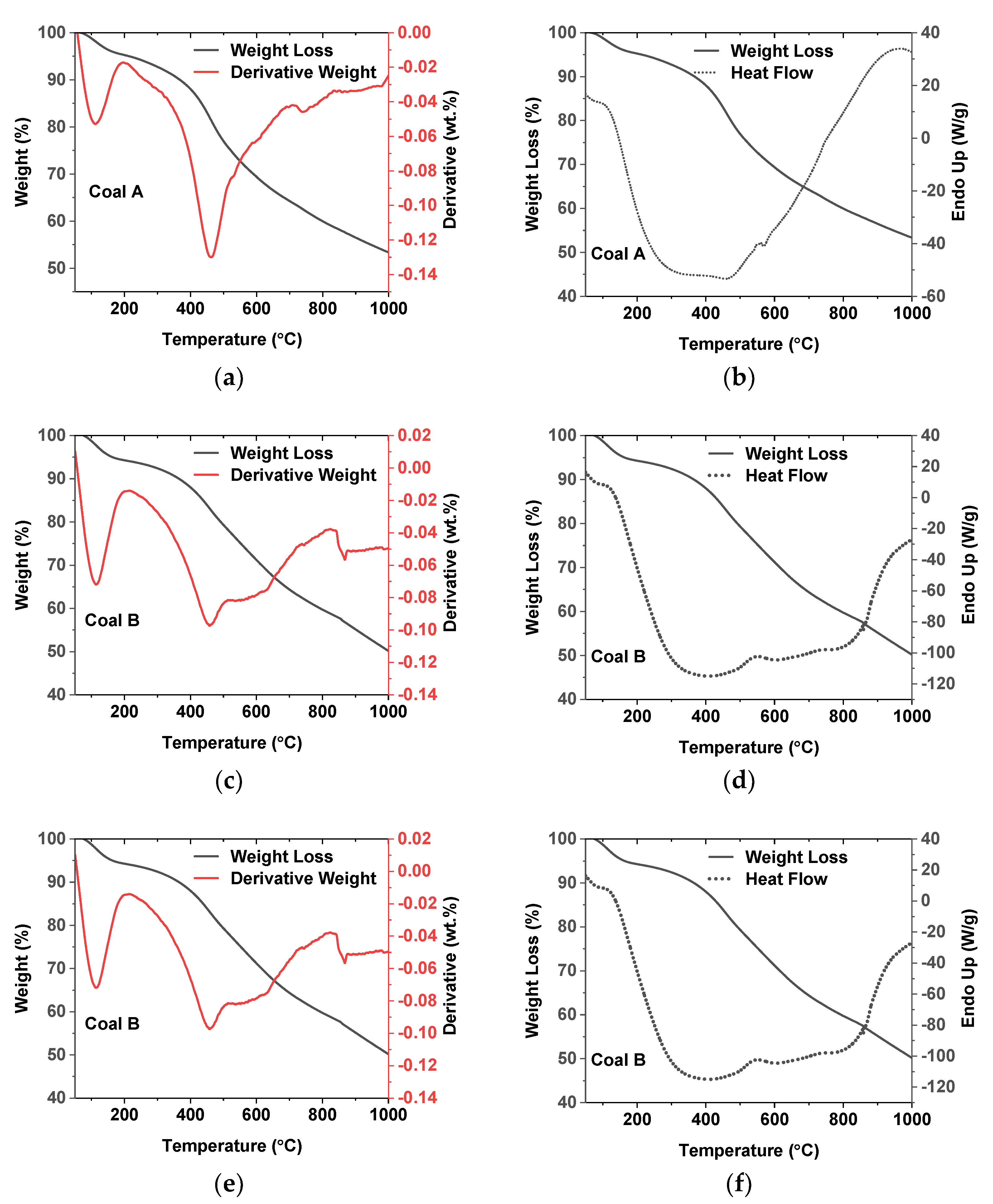
2.3.4. Thermal Analysis
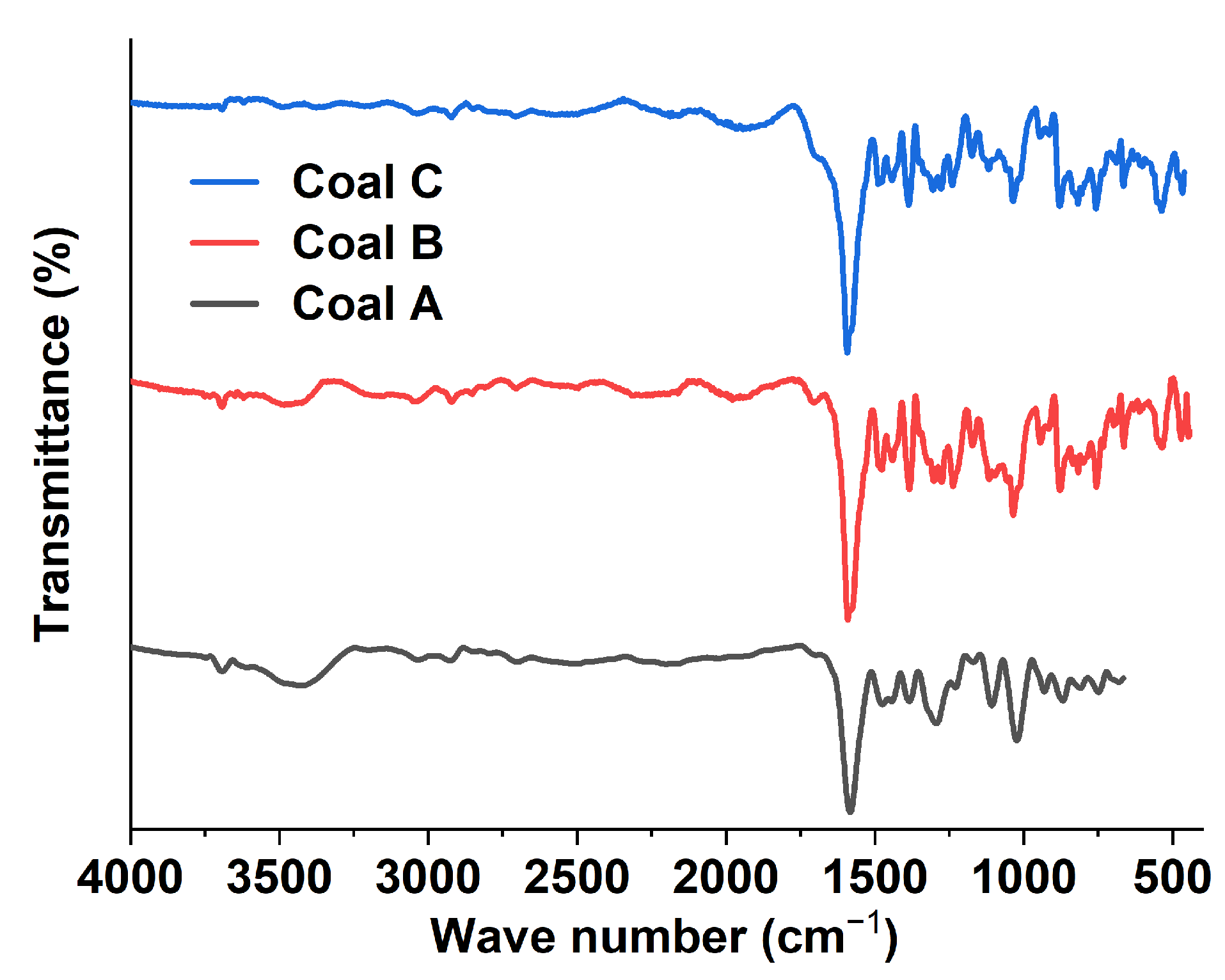
2.3.5. Fourier Transform Infrared Analysis
2.3.6. Surface Area Analysis
2.3.7. Iodine Analysis
2.3.8. Mineralogical Analyses
3. Results and Discussion
3.1. Characterisation of the Coal Samples
3.1.1. Calorimetry
3.1.2. Proximate and Ultimate Analyses
3.1.3. Particle Size Analysis
3.1.4. Thermal Analyses
3.1.5. Fourier Transform Infrared Analysis
3.1.6. Surface Area Analysis
3.1.7. Iodine Analysis
3.1.8. Chemical Analysis of the Coal Ash
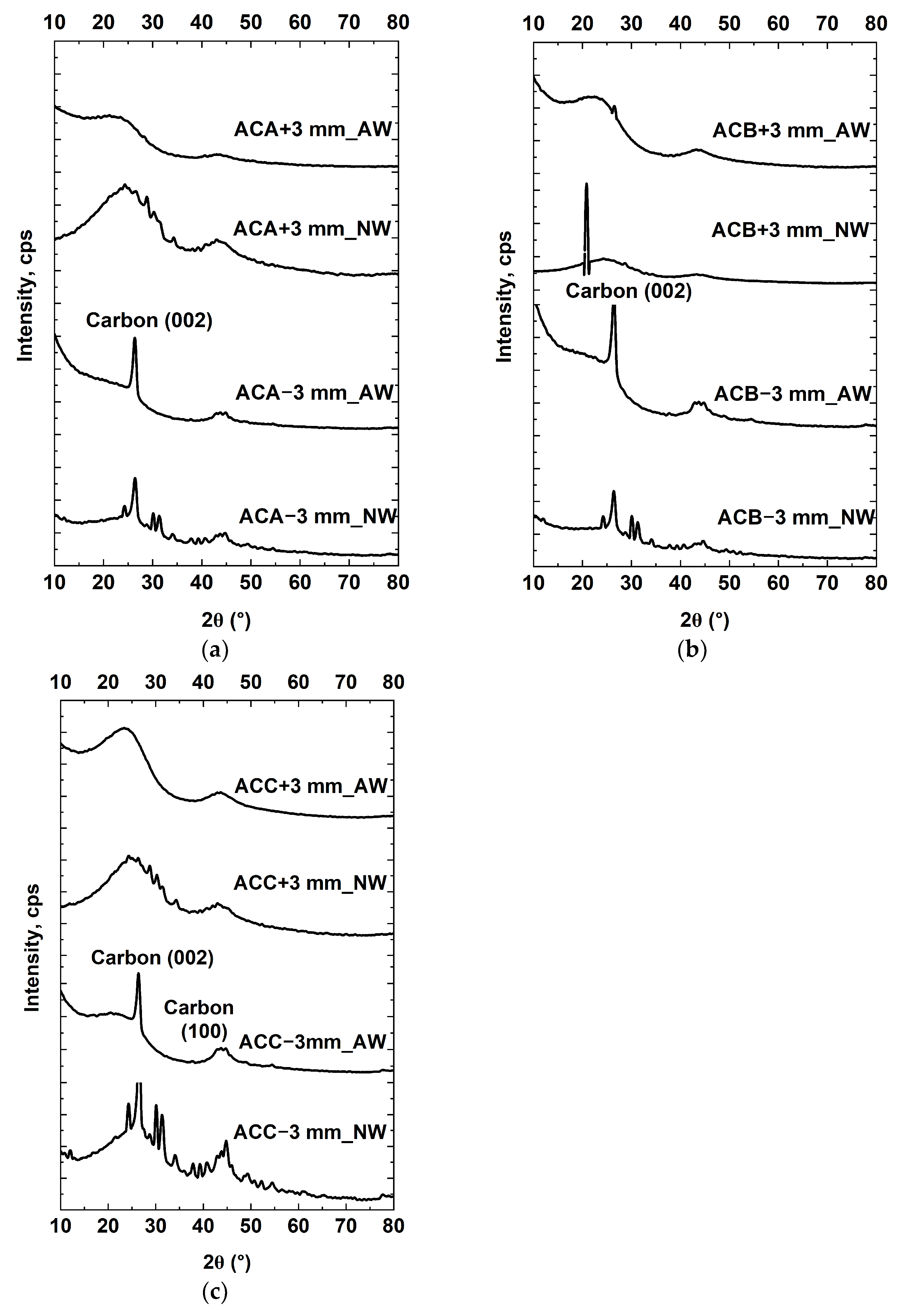
3.1.9. Mineralogical Analyses of Coal Samples
3.2. Characterisation of Activated Carbons
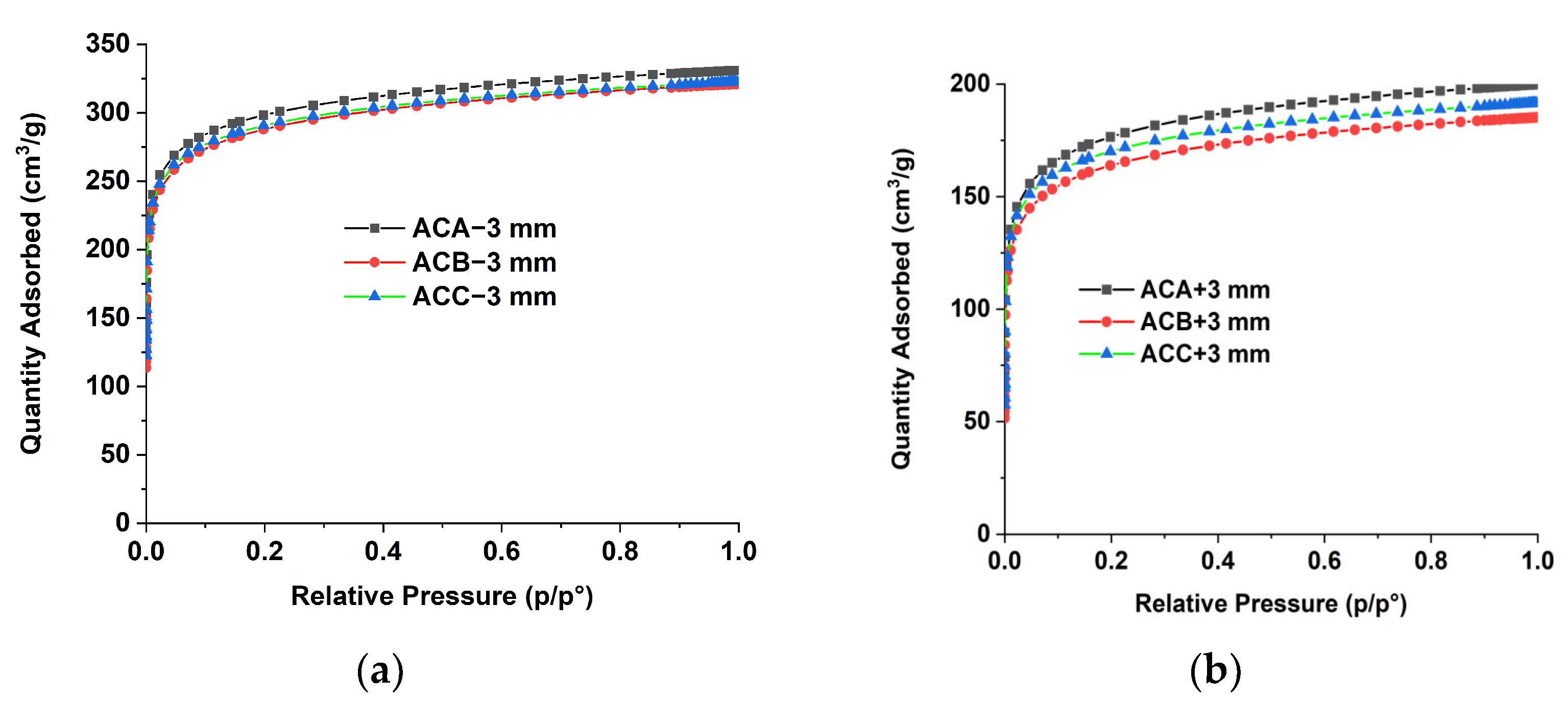
3.2.1. Surface Area Analysis
3.2.2. Iodine Analysis
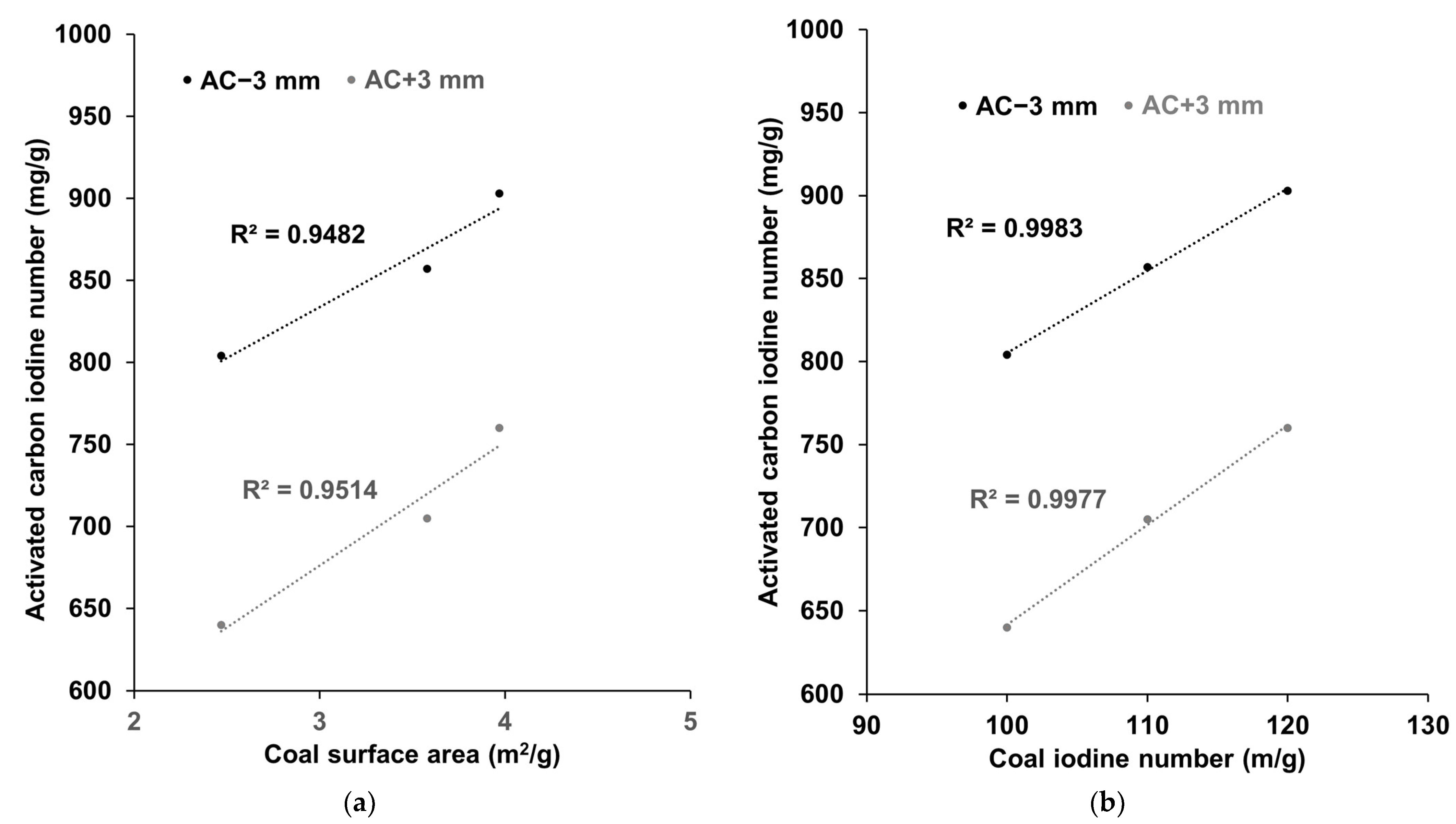
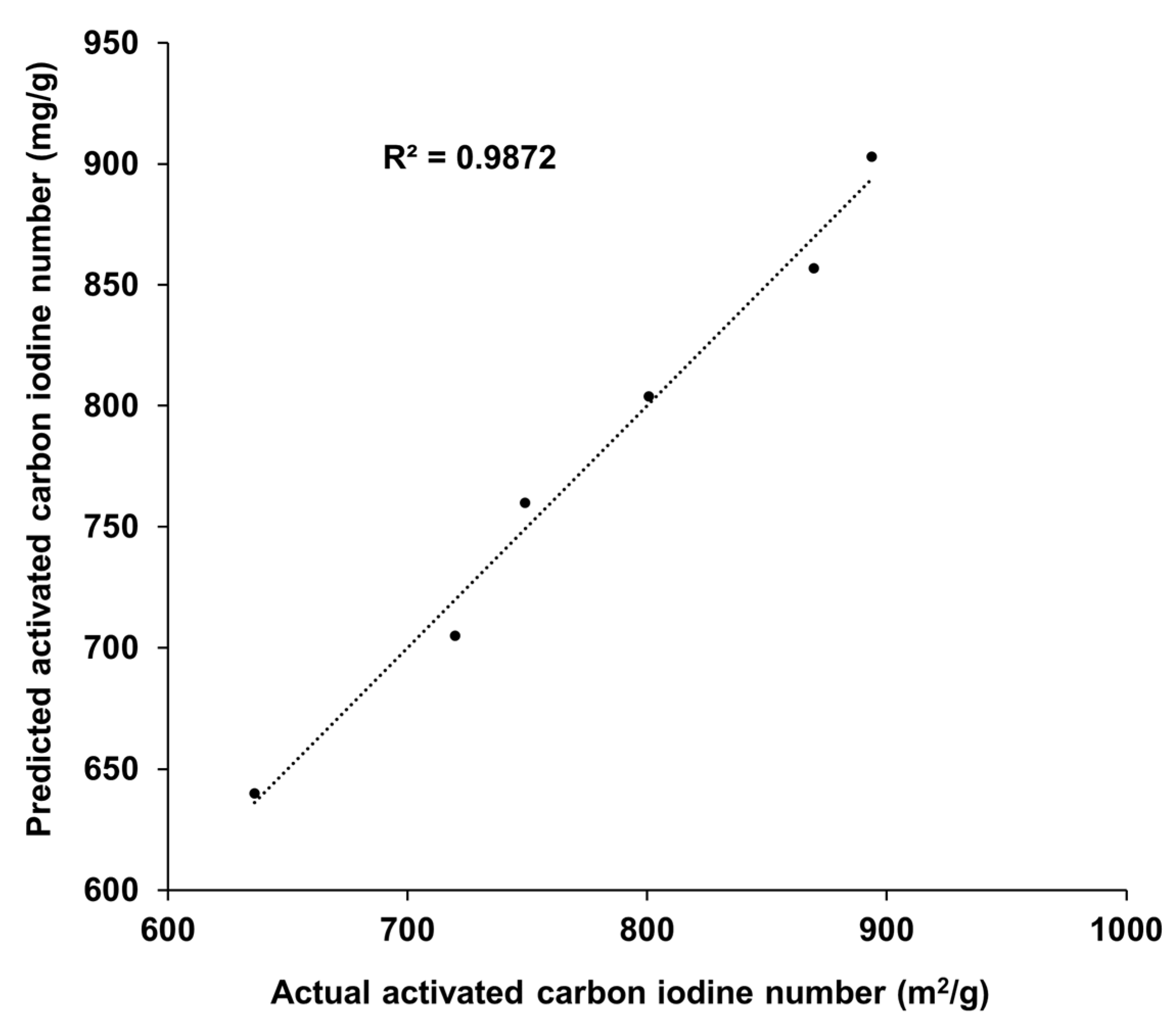
3.3. Effect of Coal Surface Area on Activated Carbon Iodine Number
3.4. Effect of Acid Washing on Activated Quality
3.5. Effect of Coal Particle Size on Activated Carbon Quality and Yield
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. FTIR Functional Groups Identified in the Coal Samples
| Organic/Inorganic Functional Groups | Wave Number (cm−1) | Coal A | Coal B | Coal C | References |
|---|---|---|---|---|---|
| Transmittance (%) | |||||
| Surface hydroxyl groups | 3694 | 93 | [47] | ||
| O-H stretching | 3335 | 93 | [48] | ||
| C=O stretching | 1640 | 93 | 94 | [48] | |
| Aromatic C=C stretching | 1585 | 88 | 93 | [49] | |
| Alcohols, C-O stretching | 1180 | 84 | 90 | 92 | [50] |
| Kaolinite, Al2(Si2O5)(OH)4 | 1095 | 86 | [48] | ||
| C-O-H deformation in cellulose | 1034 | 83 | [51] | ||
| Kaolinite OH bending | 913 | 87 | [48] | ||
| Si-O-Al of clay minerals | 535 | 66 | 80 | 83 | [48] |
| Si-O-Si of clay minerals | 467 | 63 | 76 | [48] | |
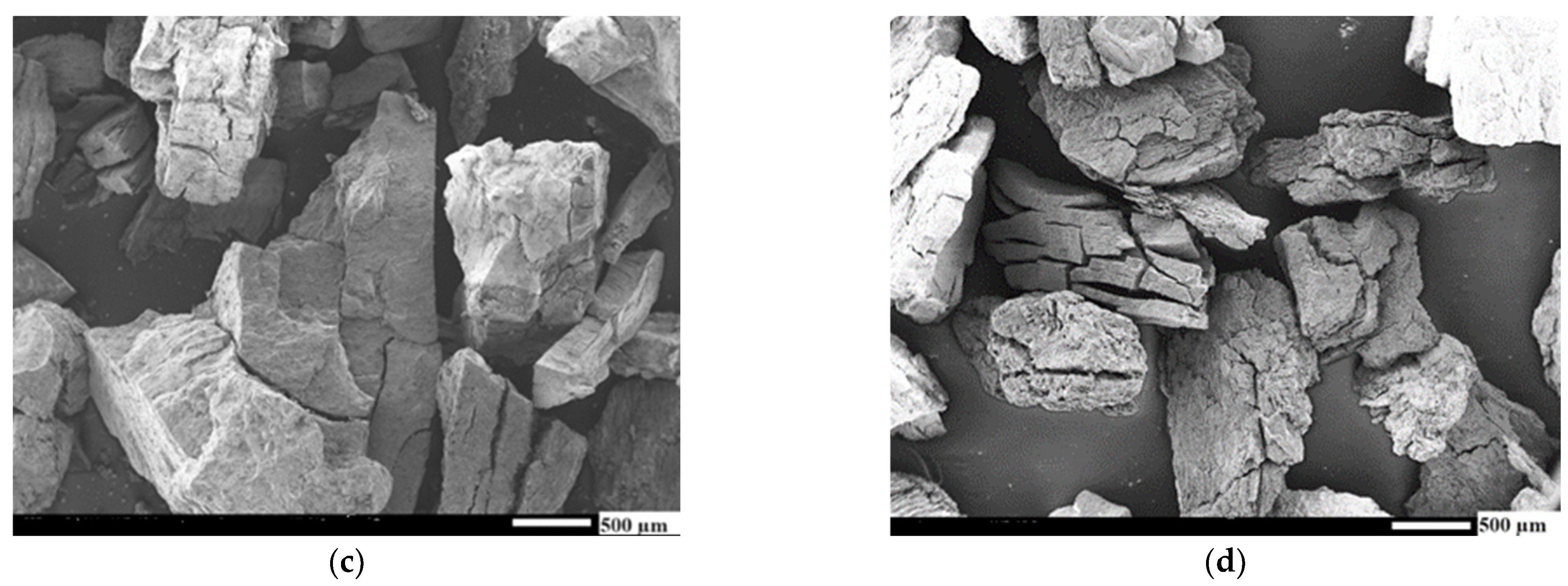
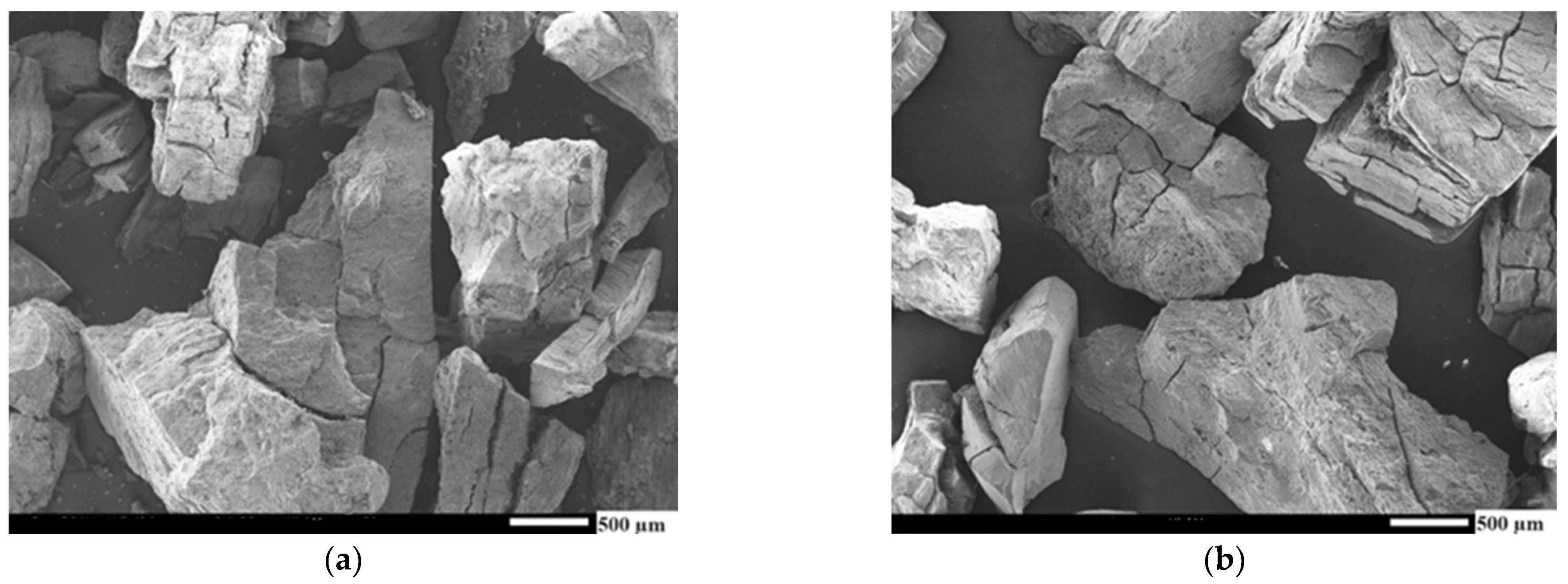
Appendix B. SEM Images of AC (–3 mm) Samples

Appendix C. SEM Images of AC (+3 mm) Samples

References
- Simate, G.S.; Maledi, N.; Ochieng, A.; Ndlovu, S.; Zhang, J.; Walubita, L.F. Coal-based adsorbents for water and wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 2291–2312. [Google Scholar] [CrossRef]
- Edahbi, M.; Mermillod-Blondin, R.; Plante, B.; Benzaazoua, M. CIL gold loss characterization within oxidized leach tails: Creating a synergistic approach between mineralogical characterization, diagnostic leach tests, and preg-robbing tests. Minerals 2019, 9, 557. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Bowtell, L.; Hancock, K.; Preston, S. Addition of activated carbon into a cattle diet to mitigate GHG emissions and improve production. Sustainability 2021, 13, 8254. [Google Scholar] [CrossRef]
- Turkyilmaz, A.; Isinkaralar, K.; Dogan, M.; Kizilduman, B.K.; Bicil, Z. Production, characterization, and hydrogen storage properties of activated carbon from horse chestnut shell. Sustain. Chem. Pharm. 2024, 40, 101634. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, M.; Li, J.; Wang, X.; Zhu, M.; Sun, G. The optimal micro-and meso-pores oriented development of Eucommia ulmoides oliver wood derived activated carbons for capacitive performance. Renew. Energy 2024, 225, 120209. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, Y.; Lian, H.; Wang, Y.; Xue, X.; Zhang, G.; Zhang, Y. Enhancement of CH4/N2 separation capacity of coal-based porous carbons via hydrothermal coupled KOH activation. J. Environ. Chem. Eng. 2024, 12, 112477. [Google Scholar] [CrossRef]
- Xia, Y.; Zuo, H.; Lv, J.; Wei, S.; Yao, Y.; Liu, Z.; Lin, Q.; Yu, Y.; Yu, W.; Huang, Y. Preparation of multi-layered microcapsule-shaped activated biomass carbon with ultrahigh surface area from bamboo parenchyma cells for energy storage and cationic dyes removal. J. Clean. Prod. 2023, 396, 136517. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Karaman, I.; Yagmur, E.; Banford, A.; Aktas, Z. The effect of process parameters on the carbon dioxide based production of activated carbon from lignite in a rotary reactor. Fuel Process. Technol. 2014, 118, 34–41. [Google Scholar] [CrossRef]
- Oglou, R.C.; Gokce, Y.; Yagmur, E.; Aktas, Z. Production of demineralised high quality hierarchical activated carbon from lignite and determination of adsorption performance using methylene blue and p-nitrophenol: The role of surface functionality, accessible pore size and surface area. J. Environ. Manag. 2023, 345, 118812. [Google Scholar] [CrossRef]
- Spencer, W.; Senanayake, G.; Altarawneh, M.; Ibana, D.; Nikoloski, A.N. Review of the effects of coal properties and activation parameters on activated carbon production and quality. Miner. Eng. 2024, 212, 108712. [Google Scholar] [CrossRef]
- Lord, J.H. Mineral Deposits of Western Australia. In Proceedings of the Annual Conference the AusIMM, Perth, Australia, 18 July 1966. [Google Scholar]
- Moody, W. Overview of Western Australia’s coal industry. Energy News 2017, 35, 14–16. [Google Scholar]
- Spencer, W.; Ibana, D.; Singh, P.; Nikoloski, A.N. Effect of ilmenite properties on synthetic rutile quality. Miner. Eng. 2022, 177, 107365. [Google Scholar] [CrossRef]
- Spencer, W.; Ibana, D.; Singh, P.; Nikoloski, A.N. Biofuels as renewable reductants for the processing of ilmenite to produce synthetic rutile. Miner. Eng. 2022, 187, 107808. [Google Scholar] [CrossRef]
- Sappal, K. Organic Petrology and Geochemistry of Coal in the Muja Coal Measures, Collie Basin, Western Australia. 1996. Available online: https://www.osti.gov/etdeweb/biblio/431903 (accessed on 19 June 2024).
- Zhou, Y.; Li, S.; Bai, Y.; Long, H.; Cai, Y.; Zhang, J. Joint Characterization and Fractal Laws of Pore Structure in Low-Rank Coal. Sustainability 2023, 15, 9599. [Google Scholar] [CrossRef]
- Hanson, S.; Patrick, J.; Walker, A. The effect of coal particle size on pyrolysis and steam gasification. Fuel 2002, 81, 531–537. [Google Scholar] [CrossRef]
- Zhu, W.; Song, W.; Lin, W. Effect of the coal particle size on pyrolysis and char reactivity for two types of coal and demineralized coal. Energy Fuels 2008, 22, 2482–2487. [Google Scholar] [CrossRef]
- Kim, D.-G.; Kim, T.-H.; Ko, S.-O. Enhanced catalytic activity of a coal-based powdered activated carbon by thermal treatment. Water 2022, 14, 3308. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.-E.; Baek, S.-H.; Choi, U.; Bae, H.-J. Preparation of Activated Carbon from Korean Anthracite: Simultaneous Control of Ash Reduction and Pore Development. Processes 2023, 11, 2877. [Google Scholar] [CrossRef]
- Quan, C.; Wang, H.; Jia, X.; Gao, N. Effect of carbonization temperature on CO2 adsorption behavior of activated coal char. J. Energy Inst. 2021, 97, 92–99. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q. Fabrication and characterization of activated carbon from phyllostachys edulis using single-step KOH activation with different temperatures. Processes 2022, 10, 1712. [Google Scholar] [CrossRef]
- Carneiro, M.T.; Morais, A.Í.S.; de Carvalho Melo, A.L.F.; Ferreira, F.J.L.; Santos, F.E.P.; Viana, B.C.; Osajima, J.A.; Bezerra, R.D.; Del Mar Orta Cuevas, M.; Peña-Garcia, R.R. Biochar derived from water hyacinth biomass chemically activated for dye removal in aqueous solution. Sustainability 2023, 15, 14578. [Google Scholar] [CrossRef]
- Dobele, G.; Volperts, A.; Plavniece, A.; Zhurinsh, A.; Upskuviene, D.; Balciunaite, A.; Niaura, G.; Colmenares-Rausseo, L.C.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Thermochemical Activation of Wood with NaOH, KOH and H3PO4 for the Synthesis of Nitrogen-Doped Nanoporous Carbon for Oxygen Reduction Reaction. Molecules 2024, 29, 2238. [Google Scholar] [CrossRef] [PubMed]
- Labus, K.; Gryglewicz, S.; Machnikowski, J. Granular KOH-activated carbons from coal-based cokes and their CO2 adsorption capacity. Fuel 2014, 118, 9–15. [Google Scholar] [CrossRef]
- Montes, V.; Hill, J.M. Activated carbon production: Recycling KOH to minimize waste. Mater. Lett. 2018, 220, 238–240. [Google Scholar] [CrossRef]
- Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A. Hydrothermal carbonization vs. pyrolysis: Effect on the porosity of the activated carbon materials. Sustainability 2022, 14, 15982. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Asavamongkolkul, T.; Perngyai, T.; Jongsomjit, B.; Phisalaphong, M. Interconnected micro, meso, and macro porous activated carbon from bacterial nanocellulose for superior adsorption properties and effective catalytic performance. Molecules 2020, 25, 4063. [Google Scholar] [CrossRef]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar] [CrossRef]
- Baresel, C.; Harding, M.; Fang, J. Ultrafiltration/granulated active carbon-biofilter: Efficient removal of a broad range of micropollutants. Appl. Sci. 2019, 9, 710. [Google Scholar] [CrossRef]
- ISO 18125; Solid Biofuels—Determination of Calorific Value. ISO: Geneva, Switzerland, 2017.
- ASTM International Standard D4607; Standard Test Method for Determination of Iodine Number of Activated Carbon. ASTM: West Conshohocken, PA, USA, 2021.
- Li, L.; Sun, G.; Qi, W.; Chen, Q.; Sun, Z.; Hao, D.; Duan, L. NOx and SO2 emission of oxygen carrier aided combustion in fluidized bed. J. Energy Inst. 2024, 112, 101463. [Google Scholar] [CrossRef]
- USDOE Office of Fossil Energy. Clean Coal Technology Demonstration Program: Project Fact Sheets 2000, Status as of June 30, 2000; American Water Works Association: Denver, CO, USA, 2000; Medium: P; 195p. [Google Scholar]
- Zhang, Y.; Jia, W.; Wang, R.; Guo, Y.; Guo, F.; Wu, J.; Dai, B. Investigation of the characteristics of catalysis synergy during co-combustion for coal gasification fine slag with bituminous coal and bamboo residue. Catalysts 2021, 11, 1152. [Google Scholar] [CrossRef]
- Bilgen, S.; Kaygusuz, K. The calculation of the chemical exergies of coal-based fuels by using the higher heating values. Appl. Energy 2008, 85, 776–785. [Google Scholar] [CrossRef]
- Dormans, H.; Huntjens, F.; Van Krevelen, D. Chemical structure and properties of coal XX-composition of the individual macerals (vitrinites, fusinites, micrinites and exinites). Fuel 1957, 36, 321–339. [Google Scholar]
- Davini, P.; Ghetti, P.; Bonfanti, L.; De Michele, G. Investigation of the combustion of particles of coal. Fuel 1996, 75, 1083–1088. [Google Scholar] [CrossRef]
- AWWA. AWWA B604-18 Granular Activated Carbon. American Water Works Association. 2018. Available online: https://engage.awwa.org/PersonifyEbusiness/Bookstore/Product-Details/productId/69295320 (accessed on 24 November 2023).
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shafiullah, A.Z.; Pal, A.; Islam, M.A.; Jahan, I.; Saha, B.B. Study on optimum IUPAC adsorption isotherm models employing sensitivity of parameters for rigorous adsorption system performance evaluation. Energies 2021, 14, 7478. [Google Scholar] [CrossRef]
- Shimizu, S.; Matubayasi, N. Surface Area Estimation: Replacing the Brunauer–Emmett–Teller Model with the Statistical Thermodynamic Fluctuation Theory. Langmuir 2022, 38, 7989–8002. [Google Scholar] [CrossRef] [PubMed]
- Ashtaputrey, P.; Ashtaputrey, S. Preparation and characterization of activated charcoal derived from wood apple fruit shell. J. Sci. Res 2020, 64, 236–240. [Google Scholar] [CrossRef]
- Oglou, R.C.; Gokce, Y.; Yagmur, E.; Ghobadi, T.G.U.; Aktas, Z. Highly stable Megalopolis lignite based N and S self-doped hierarchically porous activated carbons for high performance supercapacitors and ash content effects on performance. J. Energy Storage 2022, 46, 103817. [Google Scholar] [CrossRef]
- Zou, Y.; Han, B.-X. High-surface-area activated carbon from Chinese coal. Energy Fuels 2001, 15, 1383–1386. [Google Scholar] [CrossRef]
- Yuan, P.; Wu, D.; He, H.; Lin, Z. The hydroxyl species and acid sites on diatomite surface: A combined IR and Raman study. Appl. Surf. Sci. 2004, 227, 30–39. [Google Scholar] [CrossRef]
- Fujioka, Y.; Nishifuji, M.; Saito, K.; Kato, K. Analysis of Thermal Decomposition Behavior of Coals Using High Temperature Infrared Spectrophotometer System. SHINNITTETSU GIHO 2006, 384, 53. [Google Scholar]
- Ibarra, J.; Munoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G.; Gryglewicz, S. Development of mesoporosity in activated carbons via coal modification using Ca-and Fe-exchange. Microporous Mesoporous Mater. 2004, 76, 193–201. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]





| Coal A | Coal B | Coal C | |
|---|---|---|---|
| Proximate analysis (wt.%, air-dried) | |||
| Moisture | 3.57 ± 0.2 | 4.34 ± 0.3 | 3.64 ± 0.2 |
| Volatile matter | 40.77 ± 0.9 | 37.00 ± 0.7 | 37.34 ± 0.8 |
| Ash | 4.07 ± 0.4 | 5.01 ± 0.5 | 2.48 ± 0.3 |
| Fixed carbon | 51.6 ± 0.8 | 53.7 ± 0.9 | 56.5 ± 0.9 |
| Ultimate analysis (wt.%, air-dried) | |||
| C | 59.78 ± 0.3 | 61.31 ± 0.4 | 63.42 ± 0.4 |
| H | 6.14 ± 0.1 | 6.74 ± 0.1 | 7.08 ± 0.2 |
| N | 0.77 ± 0.1 | 1.36 ± 0.1 | 1.26 ± 0.1 |
| O * | 30.44 ± 0.2 | 29.88 ± 0.2 | 27.33 ± 0.2 |
| S | 2.87 ± 0.1 | 0.70 ± 0.1 | 0.91 ± 0.1 |
| Property | Units | Coal A | Coal B | Coal C |
|---|---|---|---|---|
| BET surface area | m2/g | 2.5 | 3.6 | 4.0 |
| Langmuir surface area | m2/g | 3.6 | 5.3 | 5.8 |
| t-Plot micropore area | m2/g | 0.2 | 0.1 | 0.1 |
| Oxides | Coal A | Coal B | Coal C |
|---|---|---|---|
| % (w/w) | |||
| SiO2 | 43.32 | 55.05 | 29.08 |
| Al2O3 | 26.93 | 22.15 | 33.95 |
| Fe2O3 | 16.15 | 12.00 | 18.69 |
| MgO | 2.02 | 1.87 | 3.46 |
| TiO2 | 1.24 | 1.57 | 2.44 |
| CaO | 1.98 | 1.32 | 2.71 |
| Cr2O3 | 0.13 | 0.02 | 0.03 |
| MnO | 0.17 | 0.11 | 0.16 |
| ZrO2 | 0.07 | 0.07 | 0.09 |
| CeO2 | 0.06 | 0.06 | 0.12 |
| V2O5 | 0.07 | 0.01 | 0.02 |
| P2O5 | 0.04 | 0.27 | 1.55 |
| Others | 7.82 | 5.49 | 7.68 |
| Surface Area | ACA −3 mm | ACA +3 mm | ACB −3 mm | ACB +3 mm | ACC −3 mm | ACC +3 mm |
|---|---|---|---|---|---|---|
| AW | ||||||
| BET (m2/g) | 973 ± 19 | 538 ± 10 | 940 ± 15 | 514 ± 10 | 935 ± 21 | 539 ± 10 |
| Langmuir (m2/g) | 1392 ± 9 | 737 ± 2 | 1330 ± 7 | 712 ± 3 | 1334 ± 7 | 739 ± 2 |
| Pore size (Å) | ||||||
| Adsorption average pore diameter (4 V/A by BET) | 14.65 | 16.61 | 14.74 | 16.67 | 15.13 | 16.73 |
| Desorption average pore diameter (4 V/A by BET) | 18.48 | 20.37 | 15.96 | 13.61 | 14.87 | 15.71 |
| Micropore volume (cm3/g) | 0.33 | 0.22 | 0.33 | 0.21 | 0.33 | 0.22 |
| Micropore area (m2/g) | 658 | 457 | 665 | 427 | 674 | 465 |
| Sample | ACA −3 mm | ACA +3 mm | ACB −3 mm | ACB +3 mm | ACC −3 mm | ACC +3 mm |
|---|---|---|---|---|---|---|
| AW | ||||||
| Yield (%) | 58.6 | 58.4 | 48.5 | 64.4 | 57.1 | 65.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spencer, W.; Ibana, D.; Singh, P.; Nikoloski, A.N. Effect of Surface Area, Particle Size and Acid Washing on the Quality of Activated Carbon Derived from Lower Rank Coal by KOH Activation. Sustainability 2024, 16, 5876. https://doi.org/10.3390/su16145876
Spencer W, Ibana D, Singh P, Nikoloski AN. Effect of Surface Area, Particle Size and Acid Washing on the Quality of Activated Carbon Derived from Lower Rank Coal by KOH Activation. Sustainability. 2024; 16(14):5876. https://doi.org/10.3390/su16145876
Chicago/Turabian StyleSpencer, William, Don Ibana, Pritam Singh, and Aleksandar N. Nikoloski. 2024. "Effect of Surface Area, Particle Size and Acid Washing on the Quality of Activated Carbon Derived from Lower Rank Coal by KOH Activation" Sustainability 16, no. 14: 5876. https://doi.org/10.3390/su16145876
APA StyleSpencer, W., Ibana, D., Singh, P., & Nikoloski, A. N. (2024). Effect of Surface Area, Particle Size and Acid Washing on the Quality of Activated Carbon Derived from Lower Rank Coal by KOH Activation. Sustainability, 16(14), 5876. https://doi.org/10.3390/su16145876





